Jun N-Terminal Kinase Inhibitor Suppresses CASK Deficiency-Induced Cerebellar Granular Cell Death in MICPCH Syndrome Model Mice
Abstract
1. Introduction
2. Materials/Subjects and Methods
2.1. Animals and Housing Conditions
2.2. Plasmid and Lentivirus Preparation
2.3. Cell Culture
2.4. RNA Extraction and Purification
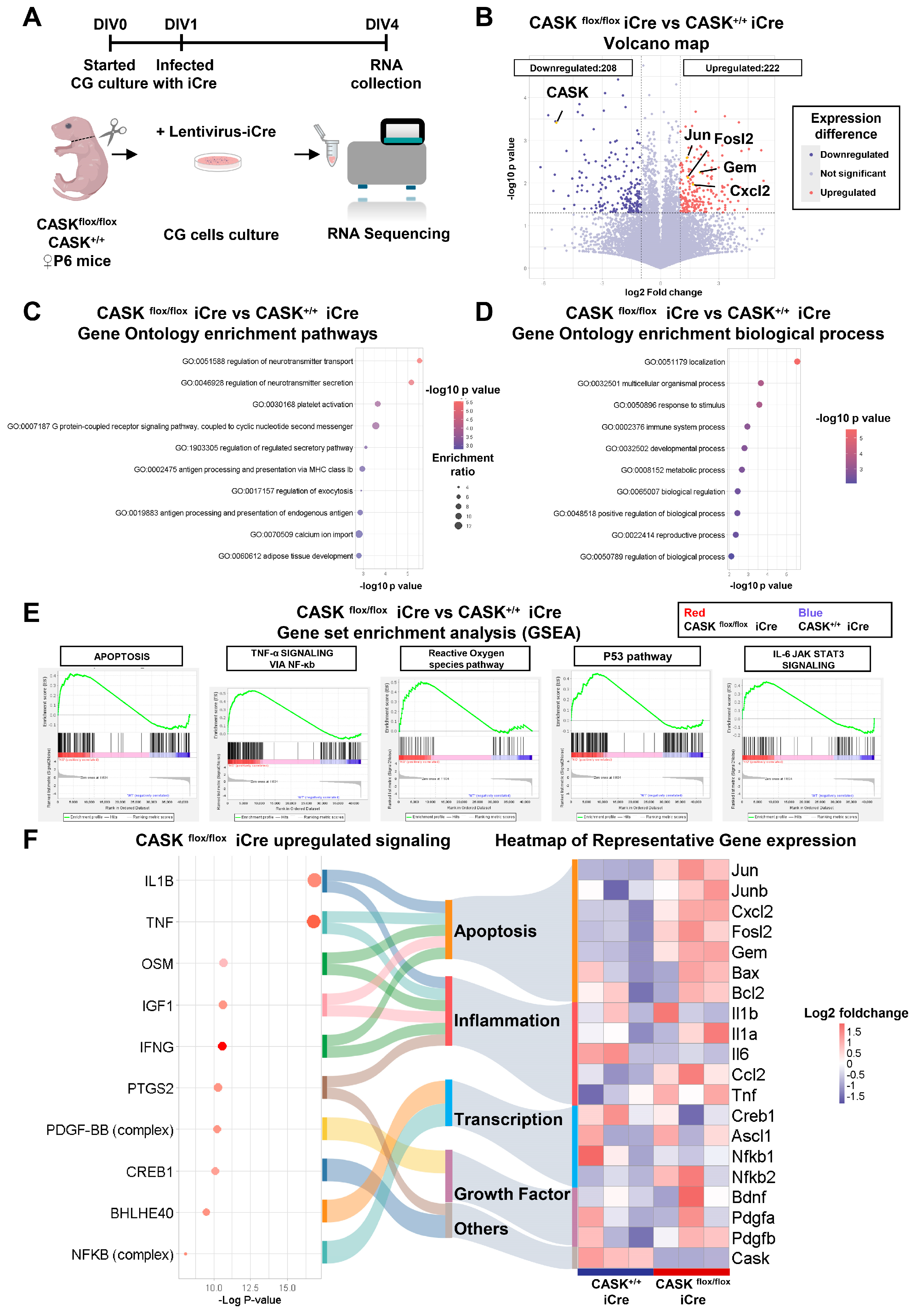
2.5. RNA-Sequencing Analysis
2.6. Stereotaxic Surgery
2.7. Limb-Clasping Scoring
2.8. Mouse Foot Gait Analysis by Ink
2.9. Data Visualization and Statistical Analyses
2.10. Additional Materials and Methods
3. Results
3.1. CASK Deletion Causes Activation of JNK Signaling and Apoptosis Pathway in CG Cells
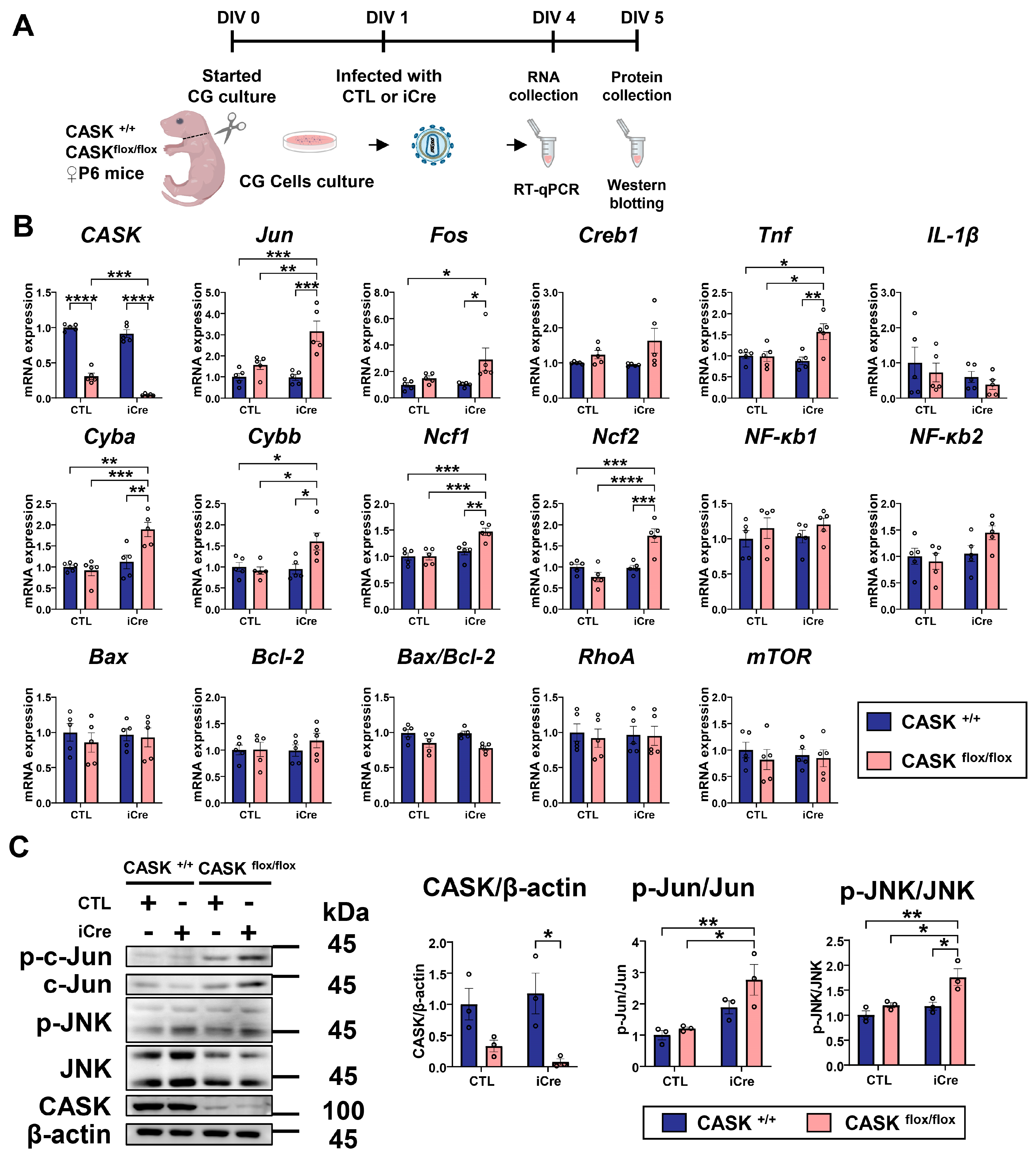
3.2. The Levels of p-c-Jun and p-JNK in CASKflox/flox iCre CG Cells Are Significantly Increased
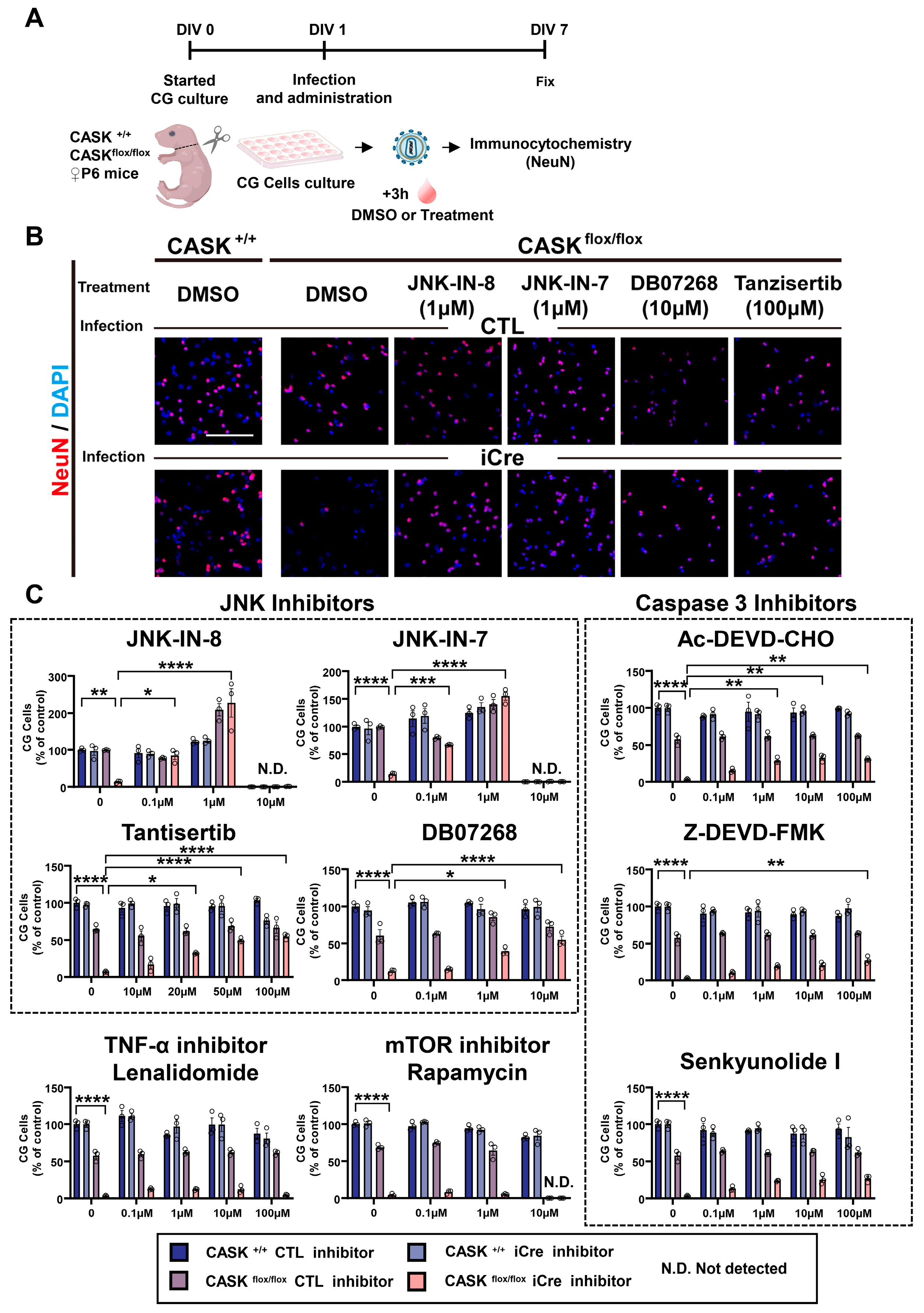
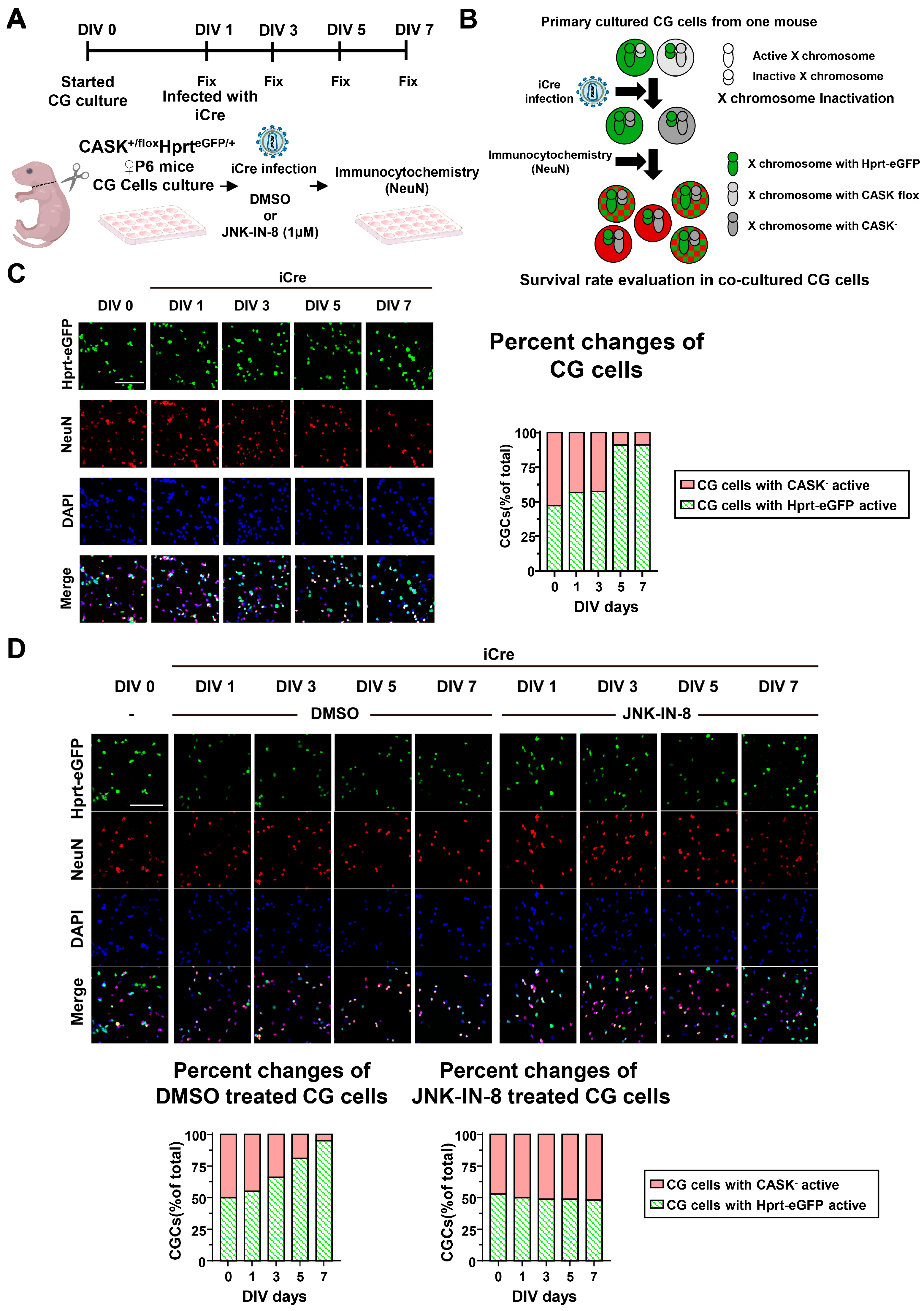
3.3. Administration of JNK Inhibitors Promotes the Survival Rate in CASKflox/flox iCre CG Cells In Vitro
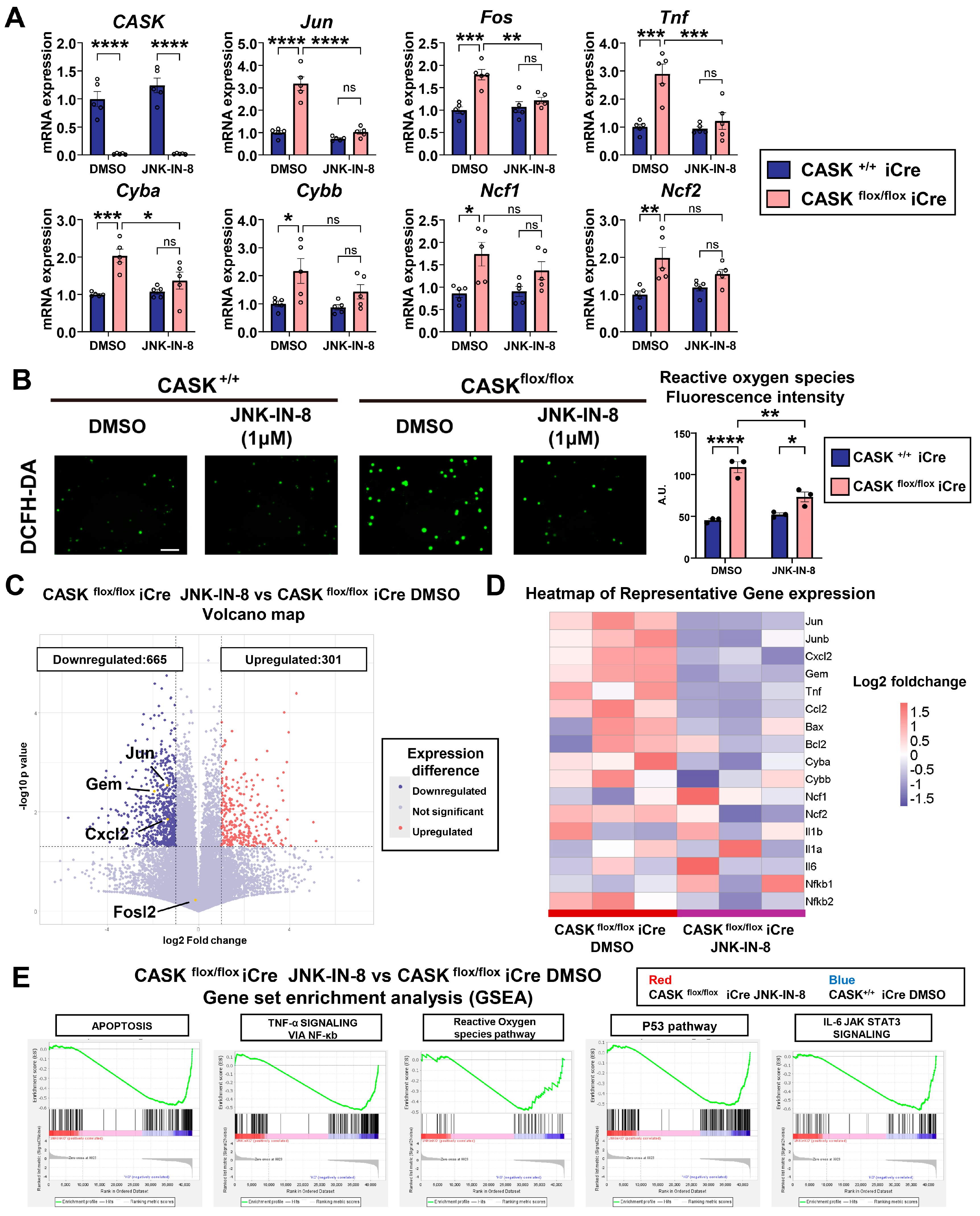
3.4. Gene Expression Analyses Indicate That JNK Inhibition Specifically Reduced Apoptosis in CASKflox/flox iCre CG Cells
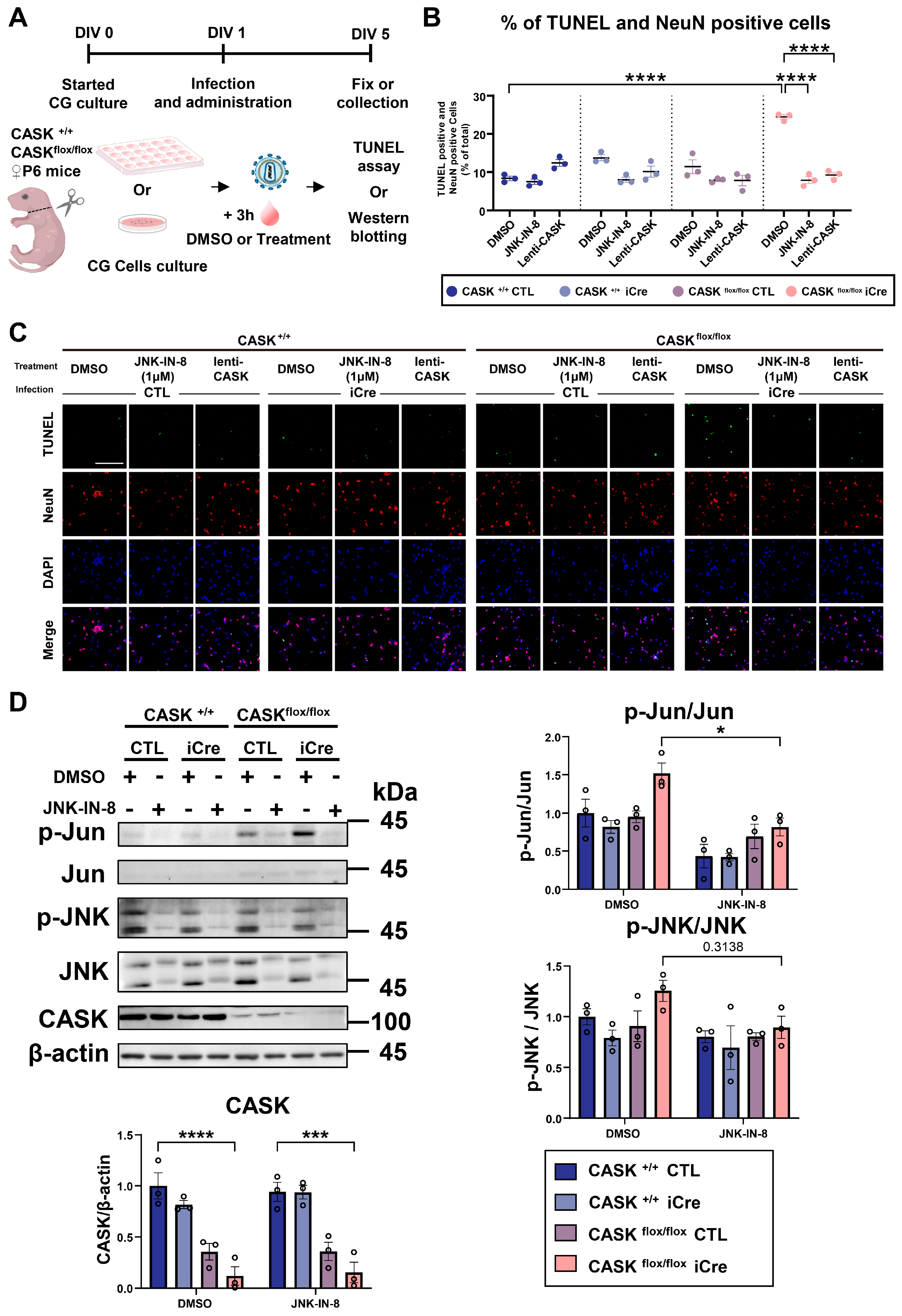
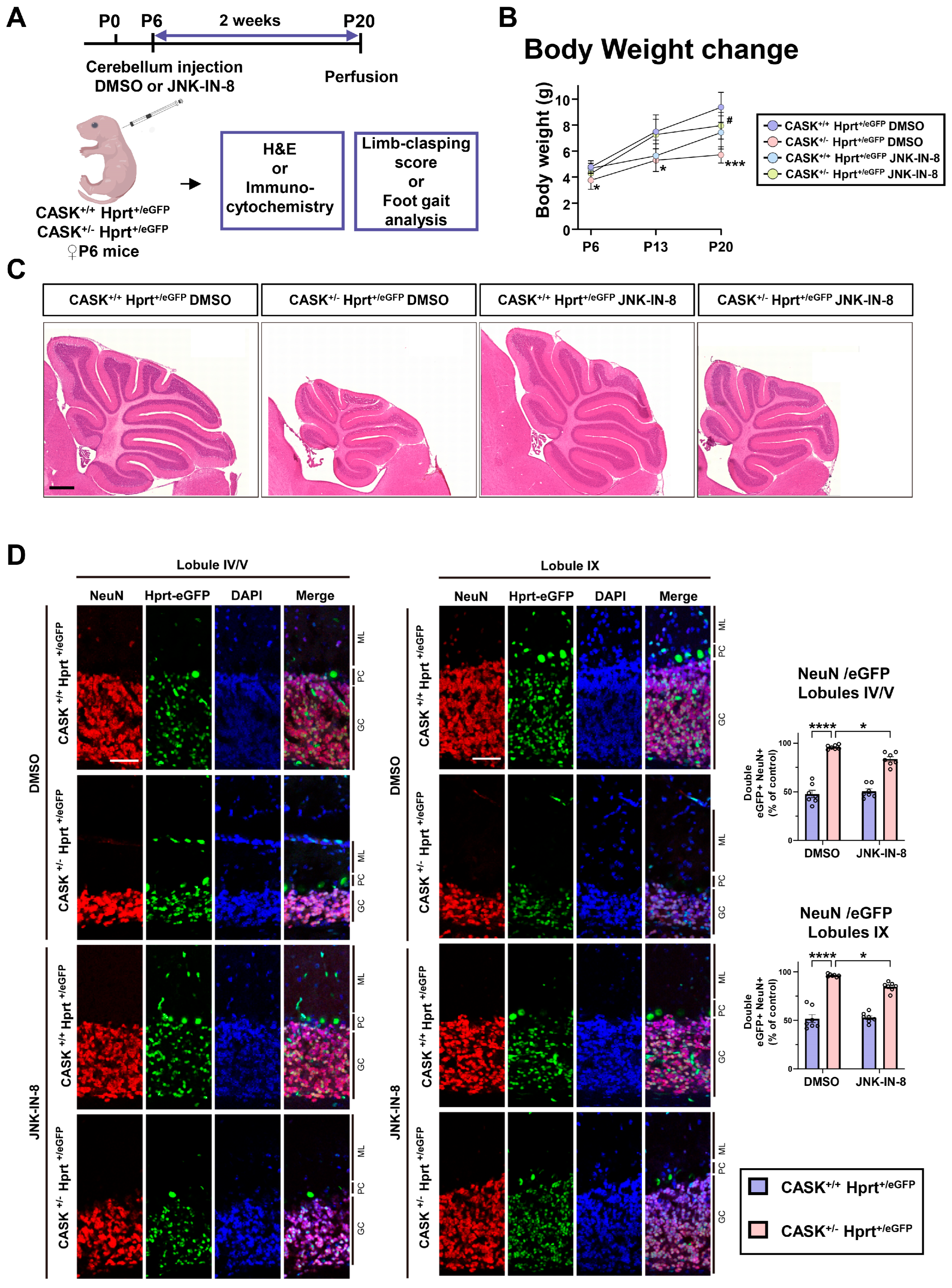
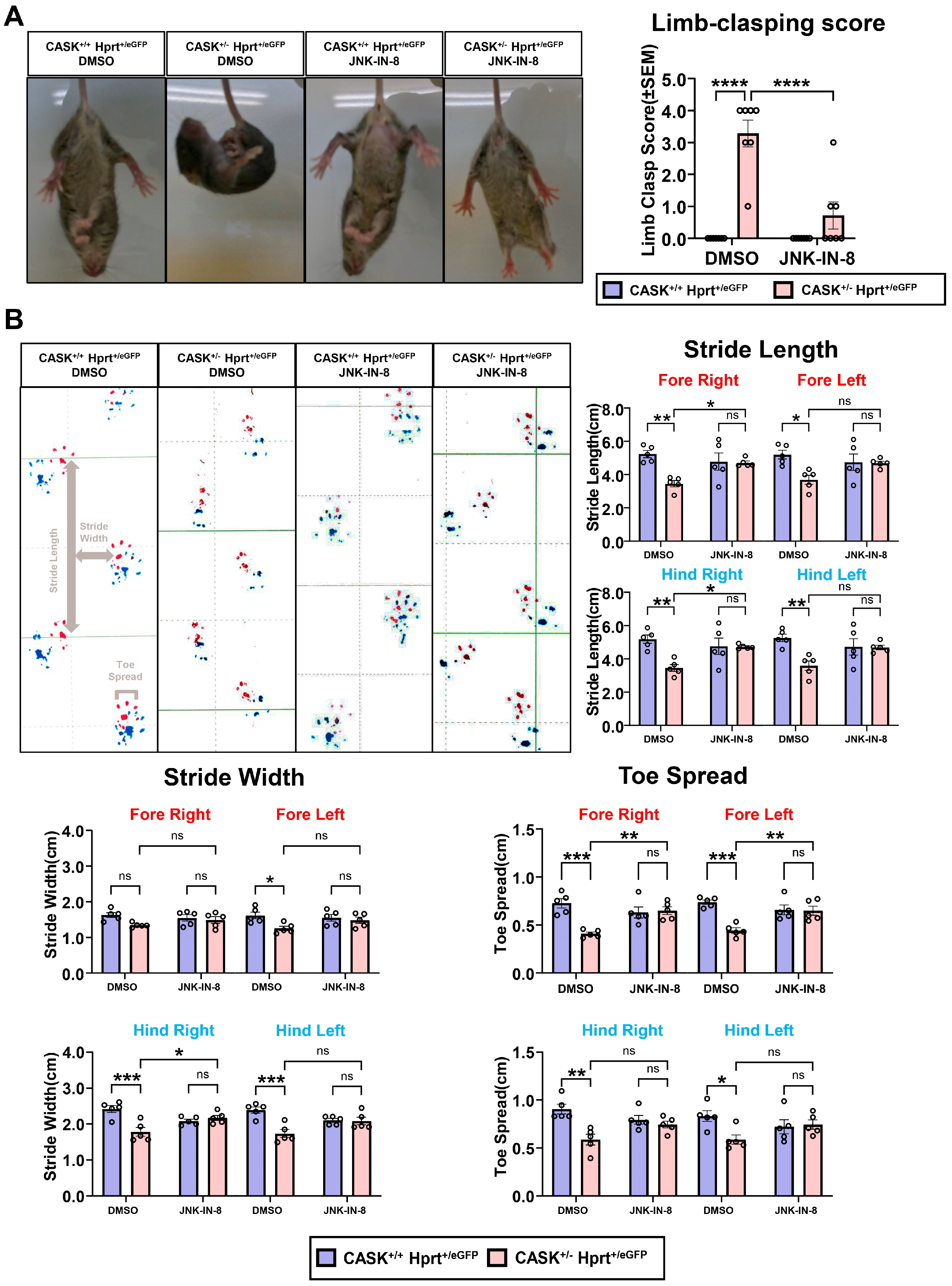
3.5. The Administration of the JNK Inhibitor In Vivo Shows Preventive Effect Against Neurodegeneration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishio, Y.; Kidokoro, H.; Takeo, T.; Narita, H.; Sawamura, F.; Narita, K.; Kawano, Y.; Nakata, T.; Muramatsu, H.; Hara, S.; et al. The eldest case of MICPCH with CASK mutation exhibiting gross motor regression. Brain Dev. 2021, 43, 459–463. [Google Scholar] [CrossRef]
- Piluso, G.; D’Amico, F.; Saccone, V.; Bismuto, E.; Rotundo, I.L.; Di Domenico, M.; Aurino, S.; Schwartz, C.E.; Neri, G.; Nigro, V. A missense mutation in CASK causes FG syndrome in an Italian family. Am. J. Hum. Genet. 2009, 84, 162–177. [Google Scholar] [CrossRef][Green Version]
- Moog, U.; Kutsche, K.; Kortum, F.; Chilian, B.; Bierhals, T.; Apeshiotis, N.; Balg, S.; Chassaing, N.; Coubes, C.; Das, S.; et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J. Med. Genet. 2011, 48, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.P. Calcium/calmodulin-dependent serine protein kinase and mental retardation. Ann. Neurol. 2009, 66, 438–443. [Google Scholar] [CrossRef]
- Hata, Y.; Butz, S.; Sudhof, T.C. CASK: A novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J. Neurosci. 1996, 16, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Olsen, O.; Moore, K.A.; Nicoll, R.A.; Bredt, D.S. Synaptic transmission regulated by a presynaptic MALS/Liprin-alpha protein complex. Curr. Opin. Cell Biol. 2006, 18, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Schoch, S.; Ho, A.; Nadasy, K.A.; Liu, X.; Zhang, W.; Mukherjee, K.; Nosyreva, E.D.; Fernandez-Chacon, R.; Missler, M.; et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc. Natl. Acad. Sci. USA 2007, 104, 2525–2530. [Google Scholar] [CrossRef]
- Mori, T.; Kasem, E.A.; Suzuki-Kouyama, E.; Cao, X.; Li, X.; Kurihara, T.; Uemura, T.; Yanagawa, T.; Tabuchi, K. Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B. Mol. Psychiatry 2019, 24, 1079–1092. [Google Scholar] [CrossRef]
- Guo, Q.; Kouyama-Suzuki, E.; Shirai, Y.; Cao, X.; Yanagawa, T.; Mori, T.; Tabuchi, K. Structural Analysis Implicates CASK-Liprin-alpha2 Interaction in Cerebellar Granular Cell Death in MICPCH Syndrome. Cells 2023, 12, 1177. [Google Scholar] [CrossRef]
- Ding, B.; Bao, C.; Jin, L.; Xu, L.; Fan, W.; Lou, W. CASK Silence Overcomes Sorafenib Resistance of Hepatocellular Carcinoma Through Activating Apoptosis and Autophagic Cell Death. Front. Oncol. 2021, 11, 681683. [Google Scholar] [CrossRef]
- Shen, H.M.; Liu, Z.G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006, 40, 928–939. [Google Scholar] [CrossRef]
- Nakano, H.; Nakajima, A.; Sakon-Komazawa, S.; Piao, J.H.; Xue, X.; Okumura, K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006, 13, 730–737. [Google Scholar] [CrossRef]
- Wu, H.; Luo, J.; Yu, H.; Rattner, A.; Mo, A.; Wang, Y.; Smallwood, P.M.; Erlanger, B.; Wheelan, S.J.; Nathans, J. Cellular resolution maps of X chromosome inactivation: Implications for neural development, function, and disease. Neuron 2014, 81, 103–119. [Google Scholar] [CrossRef]
- Uemura, T.; Suzuki-Kouyama, E.; Kawase, S.; Kurihara, T.; Yasumura, M.; Yoshida, T.; Fukai, S.; Yamazaki, M.; Fei, P.; Abe, M.; et al. Neurexins play a crucial role in cerebellar granule cell survival by organizing autocrine machinery for neurotrophins. Cell Rep. 2022, 39, 110624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Guo, Q.; Wang, P.; Li, P.; Du, Q.; Xu, H.; Yu, Q.; Zhao, X.; Zhang, W.; et al. Sophoricoside reduces inflammation in type II collagen-induced arthritis by downregulating NLRP3 signaling. Biochem. Biophys. Rep. 2024, 40, 101867. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sakurai, T.; Kamiyoshi, A.; Tanaka, M.; Ichikawa-Shindo, Y.; Kawate, H.; Matsuda, Y.; Zhang, Y.; Guo, Q.; Li, P.; et al. Adrenomedullin 2/Intermedin Exerts Cardioprotective Effects by Regulating Cardiomyocyte Mitochondrial Function. Hypertension 2025, 82, e6–e21. [Google Scholar] [CrossRef]
- Miedel, C.J.; Patton, J.M.; Miedel, A.N.; Miedel, E.S.; Levenson, J.M. Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-beta and Tau Neuropathology. J. Vis. Exp. 2017, 123, e55523. [Google Scholar]
- Wertman, V.; Gromova, A.; La Spada, A.R.; Cortes, C.J. Low-Cost Gait Analysis for Behavioral Phenotyping of Mouse Models of Neuromuscular Disease. J. Vis. Exp. 2019, 149, e59878. [Google Scholar]
- Kobayashi, S.; Hosoi, Y.; Shiura, H.; Yamagata, K.; Takahashi, S.; Fujihara, Y.; Kohda, T.; Okabe, M.; Ishino, F. Live imaging of X chromosome reactivation dynamics in early mouse development can discriminate naive from primed pluripotent stem cells. Development 2016, 143, 2958–2964. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Smith, R.; Pleasance, E.; Whibley, A.; Edkins, S.; Hardy, C.; O’Meara, S.; Latimer, C.; Dicks, E.; Menzies, A.; et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat. Genet. 2009, 41, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Hong, C.J.; Yen, T.Y.; Huang, H.Y.; Ou, Y.; Huang, T.N.; Jung, W.G.; Kuo, T.Y.; Sheng, M.; Wang, T.F.; et al. Transcriptional modification by a CASK-interacting nucleosome assembly protein. Neuron 2004, 42, 113–128. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Borsello, T.; Forloni, G. JNK signalling: A possible target to prevent neurodegeneration. Curr. Pharm. Des. 2007, 13, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Hegert, J.V.; Cristian, I.; Kerr, A.; LaConte, L.E.; Fox, M.A.; Srivastava, S.; Mukherjee, K. Complete loss of the X-linked gene CASK causes severe cerebellar degeneration. J. Med. Genet. 2022, 59, 1044–1057. [Google Scholar] [CrossRef]
- Tibbe, D.; Pan, Y.E.; Reissner, C.; Harms, F.L.; Kreienkamp, H.J. Functional analysis of CASK transcript variants expressed in human brain. PLoS ONE 2021, 16, e0253223. [Google Scholar] [CrossRef]
- Busquets, O.; Parcerisas, A.; Verdaguer, E.; Ettcheto, M.; Camins, A.; Beas-Zarate, C.; Castro-Torres, R.D.; Auladell, C. c-Jun N-Terminal Kinases in Alzheimer’s Disease: A Possible Target for the Modulation of the Earliest Alterations. J. Alzheimers Dis. 2021, 82, S127–S139. [Google Scholar] [CrossRef]
- Ahmed, T.; Zulfiqar, A.; Arguelles, S.; Rasekhian, M.; Nabavi, S.F.; Silva, A.S.; Nabavi, S.M. Map kinase signaling as therapeutic target for neurodegeneration. Pharmacol. Res. 2020, 160, 105090. [Google Scholar] [CrossRef]
- Murata, H.; Phoo, M.T.Z.; Ochi, T.; Tomonobu, N.; Yamamoto, K.-I.; Kinoshita, R.; Miyazaki, I.; Nishibori, M.; Asanuma, M.; Sakaguchi, M. Phosphorylated SARM1 is involved in the pathological process of rotenone-induced neurodegeneration. J. Biochem. 2023, 174, 533–548. [Google Scholar] [CrossRef]
- Yadav, R.K.; Minz, E.; Mehan, S. Understanding Abnormal c-JNK/p38MAPK Signaling in Amyotrophic Lateral Sclerosis: Potential Drug Targets and Influences on Neurological Disorders. CNS Neurol. Disord. Drug Targets 2021, 20, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, W.; Yang, G.; Lu, X.; Qi, X.; Wang, S.; Cao, C.; Zhang, P.; Ren, J.; Zhao, J.; et al. CASK modulates the assembly and function of the Mint1/Munc18-1 complex to regulate insulin secretion. Cell Discov. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; McMillan, R.; Willis, J.; Clark, H.; Chavan, V.; Liang, C.; Zhang, H.; Hulver, M.; Mukherjee, K. X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta Neuropathol. Commun. 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Kouyama-Suzuki, E.; Shirai, Y.; Tabuchi, K. Jun N-Terminal Kinase Inhibitor Suppresses CASK Deficiency-Induced Cerebellar Granular Cell Death in MICPCH Syndrome Model Mice. Cells 2025, 14, 750. https://doi.org/10.3390/cells14100750
Guo Q, Kouyama-Suzuki E, Shirai Y, Tabuchi K. Jun N-Terminal Kinase Inhibitor Suppresses CASK Deficiency-Induced Cerebellar Granular Cell Death in MICPCH Syndrome Model Mice. Cells. 2025; 14(10):750. https://doi.org/10.3390/cells14100750
Chicago/Turabian StyleGuo, Qi, Emi Kouyama-Suzuki, Yoshinori Shirai, and Katsuhiko Tabuchi. 2025. "Jun N-Terminal Kinase Inhibitor Suppresses CASK Deficiency-Induced Cerebellar Granular Cell Death in MICPCH Syndrome Model Mice" Cells 14, no. 10: 750. https://doi.org/10.3390/cells14100750
APA StyleGuo, Q., Kouyama-Suzuki, E., Shirai, Y., & Tabuchi, K. (2025). Jun N-Terminal Kinase Inhibitor Suppresses CASK Deficiency-Induced Cerebellar Granular Cell Death in MICPCH Syndrome Model Mice. Cells, 14(10), 750. https://doi.org/10.3390/cells14100750







