Extracellular Vesicles in the Crosstalk of Autophagy and Apoptosis: A Role for Lipid Rafts
Abstract
1. Introduction
2. Classification of EVs
3. Functions of EVs
4. Characterization of EVs
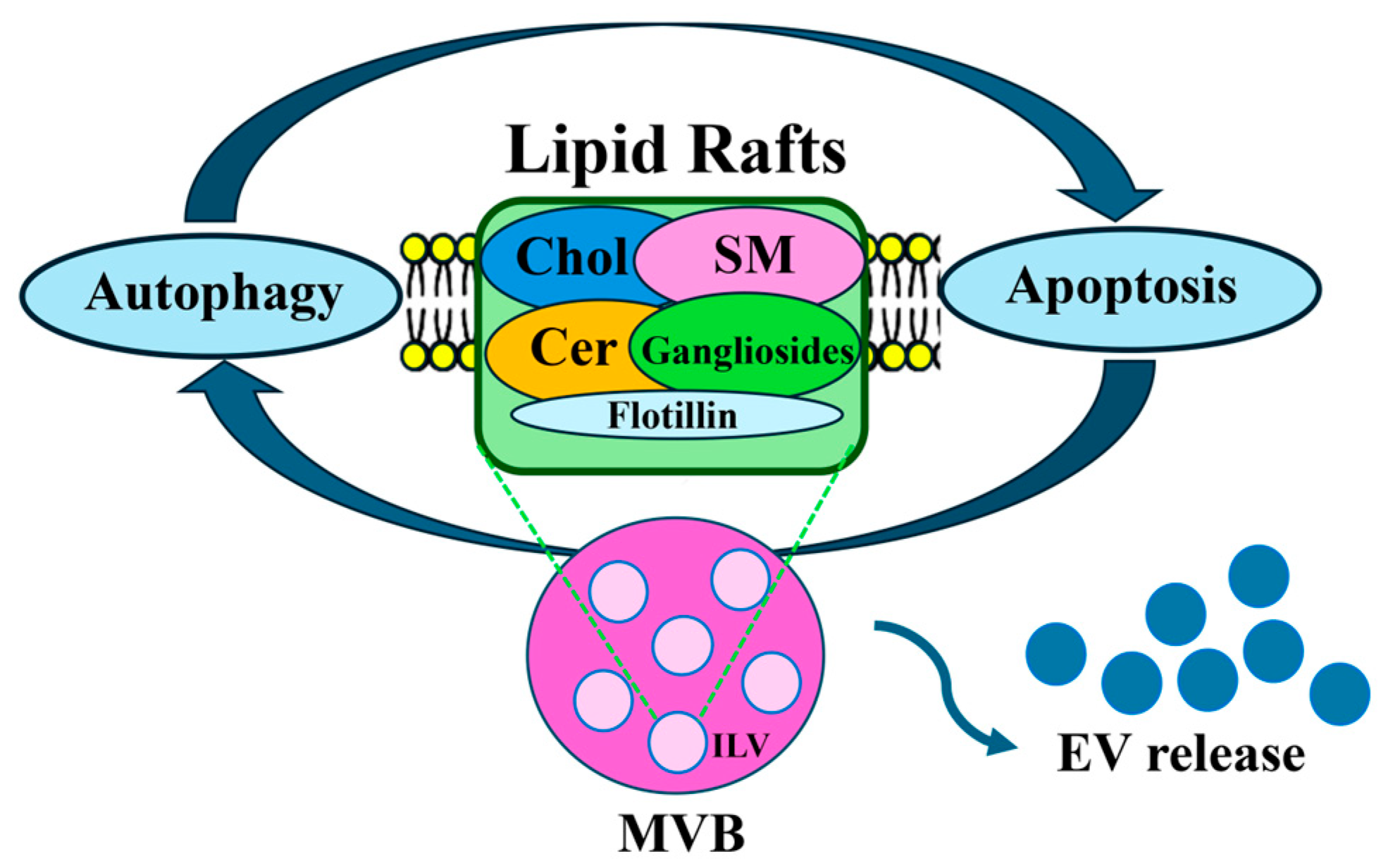
5. Lipids Rafts Involved in EV Biogenesis, Sorting and Secretion
5.1. Lipid Rafts
5.2. Lipid Rafts and EVs
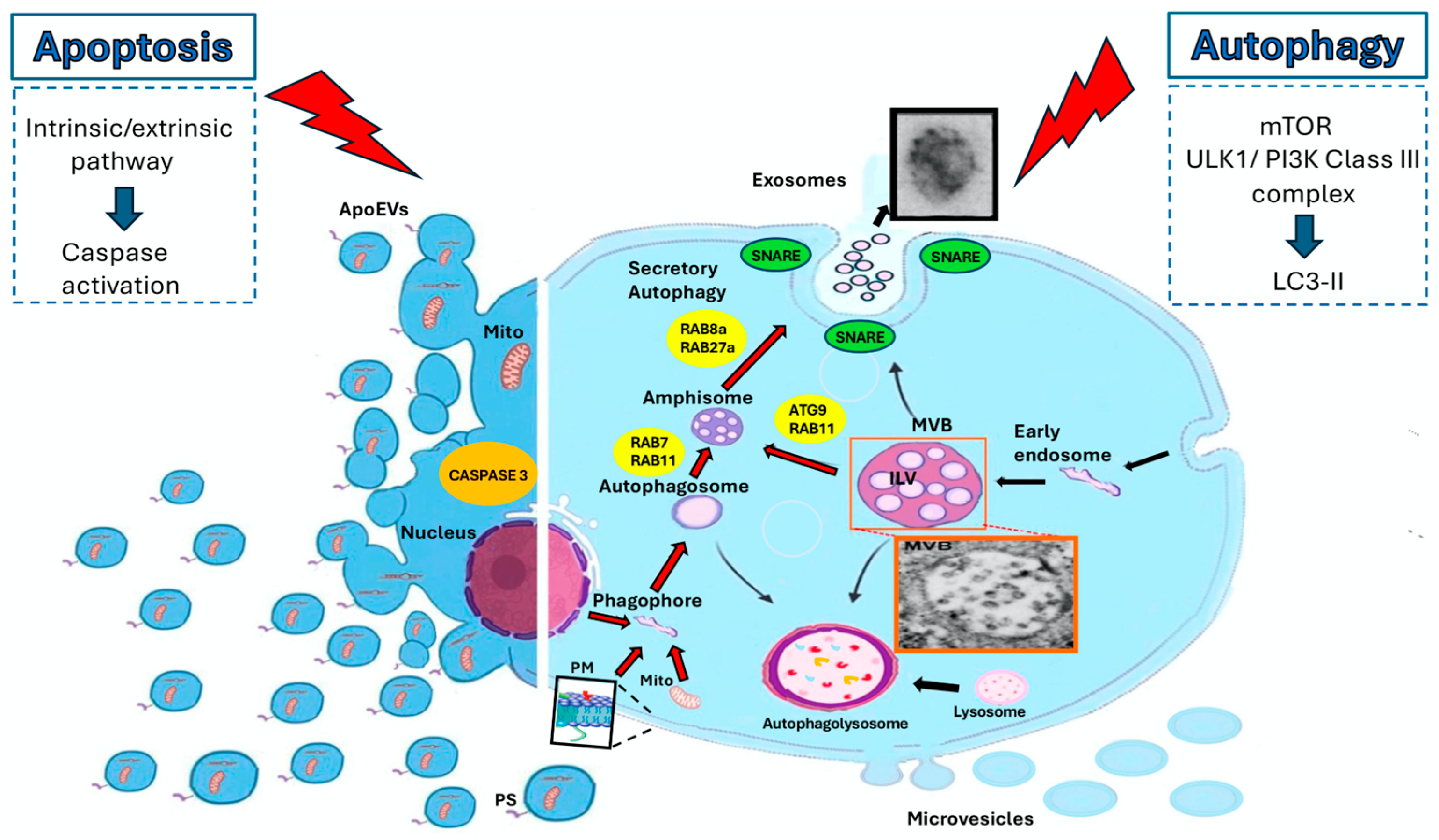
6. EVs and Secretory Autophagy
6.1. Role of Autophagy in EV Release
6.2. Role of Lipid Rafts in EV Release During Secretory Autophagy
7. EVs and Apoptosis
7.1. Role of Apoptosis in EV Release
7.2. Role of Lipid Rafts in EV Release During Apoptosis
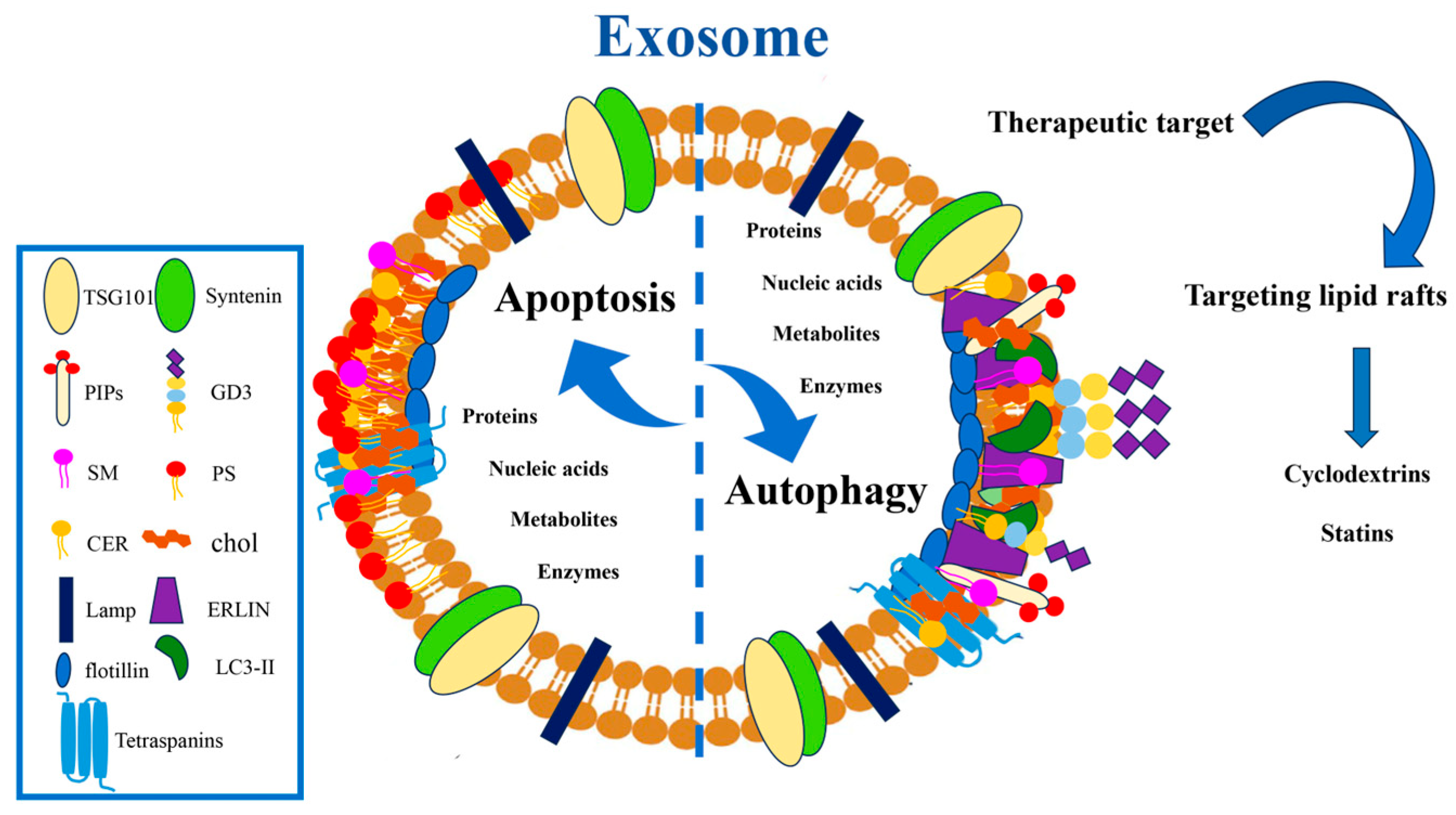
8. EVs in the Crosstalk Between Autophagy and Apoptosis
9. Interconnected Pathways Between Autophagy and Apoptosis: A Role for Lipid Rafts
10. Extracellular Vesicles and Diseases: A Focus on Autoimmune Disorders and Cancer
11. Lipid Raft-Targeting Drugs
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APP-CTFs | Amyloid precursor protein C-terminal fragments |
| ANXA2 | Annexin A2 |
| ACPAs | Anti-citrullinated protein antibodies |
| APS | Antiphospholipid syndrome |
| ApoBDs | Apoptotic bodies |
| ApoEVs: | Apoptotic cell-derived extracellular vesicles |
| CL | Cardiolipin |
| CERT | Ceramide transfer protein |
| C1P | Ceramide-1-phosphate |
| Chol | Cholesterol |
| DAMPs | Danger-associated molecular patterns |
| GD3 | Disialogangliosides |
| ESCRT | Endosomal sorting complexes required for transport |
| ERLIN | ER lipid raft protein |
| ApoExos | Extracellular vesicle-like exosomes |
| EVs | Extracellular vesicles |
| GSLs | Glycosphingolipids |
| Lamp | Lysosomal membrane proteins |
| LBPA | Lysobisphosphatidic acid |
| LPA | Lysophosphatidic Acid |
| LC3-II | Microtubule-associated protein 1B-light chain 3 |
| MAMs | Mitochondria-associated membranes |
| MβCD | Methyl-β-cyclodextrin |
| MP | Microparticle |
| MVs | Microvesicles |
| MVBs | Multivesicular bodies |
| NPC1 | Niemann–Pick type C1 protein |
| PA | Phosphatidic acid |
| PE | Phosphatidylethanolamine |
| PI3P | Phosphatidylinositol 3-phosphate |
| PI4P | Phosphatidylinositol 4-phosphate |
| PI(3,5)P2 | Phosphatidylinositol-3,5-bisphosphate |
| PI3K | Phosphatidylinositol 3-kinase |
| PS | Phosphatidylserine |
| PIPs | Phosphoinositides |
| PBP | Phospholipid bis(mono-acylglycero)phosphate |
| SM | Sphingomyelin |
| SMase | Sphingomyelinase |
| S1P | Sphingosine 1-phosphate |
| mTORC1 | Target of rapamycin complex 1 |
| TDEs | Tumor-derived EVs |
| TRPML1 | Transient receptor potential mucolipin 1 |
References
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Liu, X.M.; Ma, L.; Schekman, R. Selective Sorting of Micrornas into Exosomes by Phase-Separated Ybx1 Condensates. Elife 2021, 10, e71982. [Google Scholar] [CrossRef]
- Shekari, F.; Alibhai, F.J.; Baharvand, H.; Börger, V.; Bruno, S.; Davies, O.; Giebel, B.; Gimona, M.; Salekdeh, G.H.; Martin-Jaular, L.; et al. Cell Culture-derived Extracellular Vesicles: Considerations for Reporting Cell Culturing Parameters. J. Extracell. Biol. 2023, 2, e115. [Google Scholar] [CrossRef] [PubMed]
- Arab, T.; Mallick, E.R.; Huang, Y.; Dong, L.; Liao, Z.; Zhao, Z.; Gololobova, O.; Smith, B.; Haughey, N.J.; Pienta, K.J.; et al. Characterization of Extracellular Vesicles and Synthetic Nanoparticles with Four Orthogonal Single-Particle Analysis Platforms. J. Extracell. Vesicles 2021, 10, e12079. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Shaw, M.; Hole, P.; Smith, J.; Tannetta, D.; Redman, C.W.; Sargent, I.L. Measurement of Refractive Index by Nanoparticle Tracking Analysis Reveals Heterogeneity in Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 25361. [Google Scholar] [CrossRef]
- Gardiner, C.; Vizio, D.D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef]
- Welsh, J.A.; van der Pol, E.; Bettin, B.A.; Carter, D.R.F.; Hendrix, A.; Lenassi, M.; Langlois, M.A.; Llorente, A.; van de Nes, A.S.; Nieuwland, R.; et al. Towards Defining Reference Materials for Measuring Extracellular Vesicle Refractive Index, Epitope Abundance, Size and Concentration. J. Extracell. Vesicles 2020, 9, 1816641. [Google Scholar] [CrossRef]
- Pleet, M.L.; Cook, S.; Tang, V.A.; Stack, E.; Ford, V.J.; Lannigan, J.; Do, N.; Wenger, E.; Fraikin, J.L.; Jacobson, S.; et al. Extracellular Vesicle Refractive Index Derivation Utilizing Orthogonal Characterization. Nano Lett. 2023, 23, 9195–9202. [Google Scholar] [CrossRef]
- Preußer, C.; Stelter, K.; Tertel, T.; Linder, M.; Helmprobst, F.; Szymanski, W.; Graumann, J.; Giebel, B.; Reinartz, S.; Müller, R.; et al. Isolation of Native EVs from Primary Biofluids—Free-flow Electrophoresis as a Novel Approach to Purify Ascites-derived EVs. J. Extracell. Biol. 2022, 1, e71. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and Proteomic Profiling of Exosomes in Human Urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Scicluna, B.J.; Hill, A.F.; Götz, J. Extracellular Vesicles Isolated from the Brains of RTg4510 Mice Seed Tau Protein Aggregation in a Threshold-Dependent Manner. J. Biol. Chem. 2016, 291, 12445–12466. [Google Scholar] [CrossRef] [PubMed]
- Pucci, F.; Garris, C.; Lai, C.P.; Newton, A.; Pfirschke, C.; Engblom, C.; Alvarez, D.; Sprachman, M.; Evavold, C.; Magnuson, A.; et al. SCS Macrophages Suppress Melanoma by Restricting Tumor-Derived Vesicle-B Cell Interactions. Science 2016, 352, 242–246. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Lötvall, J. Isolation and Characterization of Extracellular Vesicle Subpopulations from Tissues. Nat. Protoc. 2021, 16, 1548–1580. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA Profiles in Subpopulations of Extracellular Vesicles: Apoptotic Bodies, Microvesicles and Exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Shlomovitz, I.; Erlich, Z.; Arad, G.; Edry-Botzer, L.; Zargarian, S.; Cohen, H.; Manko, T.; Ofir-Birin, Y.; Cooks, T.; Regev-Rudzki, N.; et al. Proteomic Analysis of Necroptotic Extracellular Vesicles. Cell Death Dis. 2021, 12, 1059. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Ragni, E.; Perucca Orfei, C.; De Luca, P.; Lugano, G.; Viganò, M.; Colombini, A.; Valli, F.; Zacchetti, D.; Bollati, V.; De Girolamo, L. Interaction with Hyaluronan Matrix and MiRNA Cargo as Contributors for in Vitro Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles in a Model of Human Osteoarthritic Synoviocytes. Stem Cell Res. Ther. 2019, 10, 109. [Google Scholar] [CrossRef]
- Record, M.; Poirot, M.; Silvente-Poirot, S. Emerging Concepts on the Role of Exosomes in Lipid Metabolic Diseases. Biochimie 2014, 96, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Didiot, M.C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-Resolution Proteomic and Lipidomic Analysis of Exosomes and Microvesicles from Different Cell Sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, B.; Holovati, J.L.; Acker, J.P. Phospholipidomics Reveals Differences in Glycerophosphoserine Profiles of Hypothermically Stored Red Blood Cells and Microvesicles. Biochim. Biophys. Acta Biomembr. 2013, 1828, 317–326. [Google Scholar] [CrossRef]
- Laurén, E.; Tigistu-Sahle, F.; Valkonen, S.; Westberg, M.; Valkeajärvi, A.; Eronen, J.; Siljander, P.; Pettilä, V.; Käkelä, R.; Laitinen, S.; et al. Phospholipid Composition of Packed Red Blood Cells and That of Extracellular Vesicles Show a High Resemblance and Stability during Storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; Van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of Extracellular Communication during Cancer Progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef]
- Simpson, R.J.; Mathivanan, S. Extracellular Microvesicles: The Need for Internationally Recognised Nomenclature and Stringent Purification Criteria. J. Proteom. Bioinform. 2012, 5, 1. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, J.M.; Kim, J.; Hur, J.; Park, S.; Kim, K.; Shin, H.J.; Chwae, Y.J. Molecular Mechanisms of Biogenesis of Apoptotic Exosome-like Vesicles and Their Roles as Damage-Associated Molecular Patterns. Proc. Natl. Acad. Sci. USA 2018, 115, E11721–E11730. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K.H. A Novel Mechanism of Generating Extracellular Vesicles during Apoptosis via a Beads-on-a-String Membrane Structure. Nat. Commun. 2015, 6, 7439. [Google Scholar] [CrossRef]
- Schiller, M.; Parcina, M.; Heyder, P.; Foermer, S.; Ostrop, J.; Leo, A.; Heeg, K.; Herrmann, M.; Lorenz, H.-M.; Bekeredjian-Ding, I. Induction of Type I IFN Is a Physiological Immune Reaction to Apoptotic Cell-Derived Membrane Microparticles. J. Immunol. 2012, 189, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Ainola, M.; Porola, P.; Takakubo, Y.; Przybyla, B.; Kouri, V.P.; Tolvanen, T.A.; Hänninen, A.; Nordström, D.C. Activation of Plasmacytoid Dendritic Cells by Apoptotic Particles—Mechanism for the Loss of Immunological Tolerance in Sjögren’s Syndrome. Clin. Exp. Immunol. 2018, 191, 301–310. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell–Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Buratta, S.; Urbanelli, L.; Sagini, K.; Giovagnoli, S.; Caponi, S.; Fioretto, D.; Mitro, N.; Caruso, D.; Emiliani, C. Extracellular Vesicles Released by Fibroblasts Undergoing H-Ras Induced Senescence Show Changes in Lipid Profile. PLoS ONE 2017, 12, e0188840. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Mathew, A.; Mason, A.B.; Teng, K. Exosome Formation during Maturation of Mammalian and Avian Reticulocytes: Evidence That Exosome Release Is a Major Route for Externalization of Obsolete Membrane Proteins. J. Cell. Physiol. 1991, 147, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.J.; Fondrie, W.E.; Yang, A.; Mao, L. Triple SILAC Quantitative Proteomic Analysis Reveals Differential Abundance of Cell Signaling Proteins between Normal and Lung Cancer-Derived Exosomes. J. Proteom. 2016, 133, 161–169. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.Q.; Xu, H.; Xiang, Q.Y.; Zhao, Y.; Zhan, J.K.; He, J.Y.; Li, S.; Liu, Y.S. Roles and Mechanisms of Exosomal Non-Coding RNAs in Human Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef]
- Pizzirani, C.; Ferrari, D.; Chiozzi, P.; Adinolfi, E.; Sandonà, D.; Savaglio, E.; Di Virgilio, F. Stimulation of P2 Receptors Causes Release of IL-1β-Loaded Microvesicles from Human Dendritic Cells. Blood 2007, 109, 3856–3864. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes Are Released by Cultured Cortical Neurones. Mol. Cell. Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Gaur, A.; Minetti, G. Membrane Remodelling and Vesicle Formation during Ageing of Human Red Blood Cells. Cell. Physiol. Biochem. 2017, 42, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Bouchareychas, L.; Duong, P.; Covarrubias, S.; Alsop, E.; Phu, T.A.; Chung, A.; Gomes, M.; Wong, D.; Meechoovet, B.; Capili, A.; et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020, 32, 107881. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G. Exosomes and MicroRNA-223 at the Intersection of Inflammation and Fibrosis in NAFLD. Hepatology 2021, 74, 5–8. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging Role of Exosomes in Cancer Progression and Tumor Microenvironment Remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Moosazadeh Moghaddam, M.; Fazel, P.; Fallah, A.; Sedighian, H.; Kachuei, R.; Behzadi, E.; Imani Fooladi, A.A. Host and Pathogen-Directed Therapies against Microbial Infections Using Exosome- and Antimicrobial Peptide-Derived Stem Cells with a Special Look at Pulmonary Infections and Sepsis. Stem Cell Rev. Rep. 2023, 19, 2166–2191. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Manganelli, V.; Dini, L.; Tacconi, S.; Dinarelli, S.; Capozzi, A.; Riitano, G.; Recalchi, S.; Caglar, T.R.; Fratini, F.; Misasi, R.; et al. Autophagy Promotes Enrichment of Raft Components within Extracellular Vesicles Secreted by Human 2FTGH Cells. Int. J. Mol. Sci. 2024, 25, 6175. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics, Transcriptomics and Lipidomics of Exosomes and Ectosomes. Proteomics 2013, 13, 1554–1571. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Carayon, K.; Chaoui, K.; Ronzier, E.; Lazar, I.; Bertrand-Michel, J.; Roques, V.; Balor, S.; Terce, F.; Lopez, A.; Salomé, L.; et al. Proteolipidic Composition of Exosomes Changes during Reticulocyte Maturation. J. Biol. Chem. 2011, 286, 34426–34439. [Google Scholar] [CrossRef]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular Lipidomics of Exosomes Released by PC-3 Prostate Cancer Cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Laulagnier, K.; Grand, D.; Dujardin, A.; Hamdi, S.; Vincent-Schneider, H.; Lankar, D.; Salles, J.P.; Bonnerot, C.; Perret, B.; Record, M. PLD2 Is Enriched on Exosomes and Its Activity Is Correlated to the Release of Exosomes. FEBS Lett. 2004, 572, 11–14. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Subra, C.; Laulagnier, K.; Perret, B.; Record, M. Exosome Lipidomics Unravels Lipid Sorting at the Level of Multivesicular Bodies. Biochimie 2007, 89, 205–212. [Google Scholar] [CrossRef]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of Sphingolipids in the Biogenesis and Biological Activity of Extracellular Vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef]
- Sorice, M.; Garofalo, T.; Misasi, R.; Manganelli, V.; Vona, R.; Malorni, W. Ganglioside GD3 as a Raft Component in Cell Death Regulation. Anti-Cancer Agents Med. Chem. 2012, 12, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Santacroce, C.; Tasciotti, V.; Martellucci, S.; Santilli, F.; Manganelli, V.; Piccoli, L.; Misasi, R.; Sorice, M.; Garofalo, T. Role of Lipid Rafts in Neuronal Differentiation of Dental Pulp-Derived Stem Cells. Exp. Cell Res. 2015, 339, 231–240. [Google Scholar] [CrossRef] [PubMed]
- De Gassart, A.; Géminard, C.; Février, B.; Raposo, G.; Vidal, M. Lipid Raft-Associated Protein Sorting in Exosomes. Blood 2003, 102, 4336–4344. [Google Scholar] [CrossRef]
- Salzer, U.; Hinterdorfer, P.; Hunger, U.; Borken, C.; Prohaska, R. Ca++-Dependent Vesicle Release from Erythrocytes Involves Stomatin-Specific Lipid Rafts, Synexin (Annexin VII), and Sorcin. Blood 2002, 99, 2569–2577. [Google Scholar] [CrossRef]
- Kajimoto, T.; Okada, T.; Miya, S.; Zhang, L.; Nakamura, S.I. Ongoing Activation of Sphingosine 1-Phosphate Receptors Mediates Maturation of Exosomal Multivesicular Endosomes. Nat. Commun. 2013, 4, 2712. [Google Scholar] [CrossRef]
- Pike, L.J. Lipid Rafts: Bringing Order to Chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Barbat, C.; Trucy, M.; Sorice, M.; Garofalo, T.; Manganelli, V.; Fischer, A.; Mazerolles, F. P56lck, LFA-1 and PI3K but Not SHP-2 Interact with G M1- or GM3-Enriched Microdomains in a CD4-P56 Lck Association-Dependent Manner. Biochem. J. 2007, 402, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Garofalo, T.; Misasi, R.; Gizzi, C.; Mascellino, M.T.; Dolo, V.; Pontieri, G.M.; Sorice, M.; Pavan, A. Association of Cellular Prion Protein with Gangliosides in Plasma Membrane Microdomains of Neural and Lymphocytic Cells. Neurochem. Res. 2002, 27, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Bharti, D.; Levental, I. Membrane Heterogeneity Beyond the Plasma Membrane. Front. Cell Dev. Biol. 2020, 8, 580814. [Google Scholar] [CrossRef]
- Ciarlo, L.; Manganelli, V.; Matarrese, P.; Garofalo, T.; Tinari, A.; Gambardella, L.; Marconi, M.; Grasso, M.; Misasi, R.; Sorice, M.; et al. Raft-like Microdomains Play a Key Role in Mitochondrial Impairment in Lymphoid Cells from Patients with Huntington’s Disease. J. Lipid Res. 2012, 53, 2057–2068. [Google Scholar] [CrossRef]
- Sorice, M.; Mattei, V.; Matarrese, P.; Garofalo, T.; Tinari, A.; Gambardella, L.; Ciarlo, L.; Manganelli, V.; Tasciotti, V.; Misasi, R.; et al. Dynamics of Mitochondrial Raft-like Microdomains in Cell Life and Death. Commun. Integr. Biol. 2012, 5, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.B.; Zurzolo, C. Lipids as Targeting Signals: Lipid Rafts and Intracellular Trafficking. Traffic 2004, 5, 247–254. [Google Scholar] [CrossRef]
- Sorice, M.; Mattei, V.; Tasciotti, V.; Manganelli, V.; Garofalo, T.; Misasi, R. Trafficking of PrPCto Mitochondrial Raft-like Microdomains during Cell Apoptosis. Prion 2012, 6, 354–358. [Google Scholar] [CrossRef]
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The Ins and Outs of Lipid Rafts: Functions in Intracellular Cholesterol Homeostasis, Microparticles, and Cell Membranes. J. Lipid Res. 2020, 61, 676–686. [Google Scholar] [CrossRef]
- Manganelli, V.; Capozzi, A.; Recalchi, S.; Riitano, G.; Mattei, V.; Longo, A.; Misasi, R.; Garofalo, T.; Sorice, M. The Role of Cardiolipin as a Scaffold Mitochondrial Phospholipid in Autophagosome Formation: In Vitro Evidence. Biomolecules 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, C.; Morales, A.; Fernández-Checa, J.C. Glycosphingolipids and Cell Death: One Aim, Many Ways. Apoptosis 2015, 20, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Sorice, M.; Matarrese, P.; Manganelli, V.; Tinari, A.; Giammarioli, A.M.; Mattei, V.; Misasi, R.; Garofalo, T.; Malorni, W. Role of GD3-CLIPR-59 Association in Lymphoblastoid T Cell Apoptosis Triggered by CD95/Fas. PLoS ONE 2010, 5, e8567. [Google Scholar] [CrossRef]
- Raiborg, C.; Stenmark, H. The ESCRT Machinery in Endosomal Sorting of Ubiquitylated Membrane Proteins. Nature 2009, 458, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W.; Vitale, N. Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of Their Biogenesis and Functions Cholesterol and the Journey of Extracellular Vesicles. J. Lipid Res. 2018, 59, 2255–2261. [Google Scholar] [CrossRef]
- Möbius, W.; van Donselaar, E.; Ohno-Iwashita, Y.; Shimada, Y.; Heijnen, H.F.G.; Slot, J.W.; Geuze, H.J. Recycling Compartments and the Internal Vesicles of Multivesicular Bodies Harbor Most of the Cholesterol Found in the Endocytic Pathway. Traffic 2003, 4, 222–231. [Google Scholar] [CrossRef]
- Palmulli, R.; Couty, M.; Piontek, M.C.; Ponnaiah, M.; Dingli, F.; Verweij, F.J.; Charrin, S.; Tantucci, M.; Sasidharan, S.; Rubinstein, E.; et al. CD63 Sorts Cholesterol into Endosomes for Storage and Distribution via Exosomes. Nat. Cell Biol. 2024, 26, 1093–1109. [Google Scholar] [CrossRef]
- Charrin, S.; Manié, S.; Thiele, C.; Billard, M.; Gerlier, D.; Boucheix, C.; Rubinstein, E. A Physical and Functional Link between Cholesterol and Tetraspanins. Eur. J. Immunol. 2003, 33, 2479–2489. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral Sphingomyelinases Control Extracellular Vesicles Budding from the Plasma Membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Fukushima, M.; Dasgupta, D.; Mauer, A.S.; Kakazu, E.; Nakao, K.; Malhi, H. StAR-Related Lipid Transfer Domain 11 (STARD11)-Mediated Ceramide Transport Mediates Extracellular Vesicle Biogenesis. J. Biol. Chem. 2018, 293, 15277–15289. [Google Scholar] [CrossRef]
- Rao, R.P.; Yuan, C.; Allegood, J.C.; Rawat, S.S.; Edwards, M.B.; Wang, X.; Merrill, A.H.; Acharya, U.; Acharya, J.K. Ceramide Transfer Protein Function Is Essential for Normal Oxidative Stress Response and Lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 11364–11369. [Google Scholar] [CrossRef] [PubMed]
- Barman, B.; Sung, B.H.; Krystofiak, E.; Ping, J.; Ramirez, M.; Millis, B.; Allen, R.; Prasad, N.; Chetyrkin, S.; Calcutt, M.W.; et al. VAP-A and Its Binding Partner CERT Drive Biogenesis of RNA-Containing Extracellular Vesicles at ER Membrane Contact Sites. Dev. Cell 2022, 57, 974–994.e8. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; Giovagnoni, C.; Zhu, Z.; Tripathi, P.; Elsherbini, A.; Quadri, Z.; Pu, J.; Zhang, L.; Ferko, B.; Berkes, D.; et al. Function of Ceramide Transfer Protein for Biogenesis and Sphingolipid Composition of Extracellular Vesicles. J. Extracell. Vesicles 2022, 11, e12233. [Google Scholar] [CrossRef]
- Matsuo, H.; Chevallier, J.; Mayran, N.; Le Blanc, I.; Ferguson, C.; Fauré, J.; Blanc, N.S.; Matile, S.; Dubochet, J.; Sadoul, R.; et al. Role of LBPA and Alix in Multivesicular Liposome Formation and Endosome Organization. Science 2004, 303, 531–534. [Google Scholar] [CrossRef] [PubMed]
- van der Goot, F.G.; Gruenberg, J. Intra-Endosomal Membrane Traffic. Trends Cell Biol. 2006, 16, 514–521. [Google Scholar] [CrossRef]
- Peruzzi, J.A.; Gunnels, T.F.; Edelstein, H.I.; Lu, P.; Baker, D.; Leonard, J.N.; Kamat, N.P. Enhancing Extracellular Vesicle Cargo Loading and Functional Delivery by Engineering Protein-Lipid Interactions. Nat. Commun. 2024, 15, 5618. [Google Scholar] [CrossRef]
- Kim, S.; Alsaidan, O.A.; Goodwin, O.; Li, Q.; Sulejmani, E.; Han, Z.; Bai, A.; Albers, T.; Beharry, Z.; Zheng, Y.G.; et al. Blocking Myristoylation of Src Inhibits Its Kinase Activity and Suppresses Prostate Cancer Progression. Cancer Res. 2017, 77, 6950–6962. [Google Scholar] [CrossRef]
- Kim, S.; Yang, X.; Li, Q.; Wu, M.; Costyn, L.; Beharry, Z.; Bartlett, M.G.; Cai, H. Myristoylation of Src Kinase Mediates Src-Induced and High-Fat Diet–Accelerated Prostate Tumor Progression in Mice. J. Biol. Chem. 2017, 292, 18422–18433. [Google Scholar] [CrossRef]
- Ye, C.; Gosser, C.; Runyon, E.D.; Zha, J.; Cai, J.; Beharry, Z.; Bowes Rickman, C.; Klingeborn, M.; Liu, Y.; Xie, J.; et al. Src Family Kinases Engage Differential Pathways for Encapsulation into Extracellular Vesicles. J. Extracell. Biol. 2023, 2, e96. [Google Scholar] [CrossRef]
- Mariscal, J.; Vagner, T.; Kim, M.; Zhou, B.; Chin, A.; Zandian, M.; Freeman, M.R.; You, S.; Zijlstra, A.; Yang, W.; et al. Comprehensive Palmitoyl-Proteomic Analysis Identifies Distinct Protein Signatures for Large and Small Cancer-Derived Extracellular Vesicles. J. Extracell. Vesicles 2020, 9, 1764192. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular Definitions of Autophagy and Related Processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Yu, L.; Alva, A.; Su, H.; Dutt, P.; Freundt, E.; Welsh, S.; Baehrecke, E.H.; Lenardo, M.J. Regulation of an ATG7-Beclin 1 Program of Autophaglic Cell Death by Caspase-8. Science 2004, 304, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Jun, C.B.; Ro, S.H.; Kim, Y.M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.H. ULK-Atg13-FIP200 Complexes Mediate MTOR Signaling to the Autophagy Machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef]
- Kihara, A.; Kabeya, Y.; Ohsumi, Y.; Yoshimori, T. Beclin-Phosphatidylinositol 3-Kinase Complex Functions at the Trans-Golgi Network. EMBO Rep. 2001, 2, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy Pathway: Cellular and Molecular Mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.B.; Hoyvik, H.; Seglen, P.O. Prelysosomal and Lysosomal Connections between Autophagy and Endocytosis. Biochem. J. 1992, 283, 361–369. [Google Scholar] [CrossRef]
- Liou, W.; Geuze, H.J.; Geelen, M.J.H.; Slot, J.W. The Autophagic and Endocytic Pathways Converge at the Nascent Autophagic Vacuoles. J. Cell Biol. 1997, 136, 61–70. [Google Scholar] [CrossRef]
- Guo, H.; Sadoul, R.; Gibbings, D. Autophagy-Independent Effects of Autophagy-Related-5 (Atg5) on Exosome Production and Metastasis. Mol. Cell. Oncol. 2018, 5, e1445941. [Google Scholar] [CrossRef]
- Fader, C.M.; Sánchez, D.; Furlán, M.; Colombo, M.I. Induction of Autophagy Promotes Fusion of Multivesicular Bodies with Autophagic Vacuoles in K562 Cells. Traffic 2008, 9, 230–250. [Google Scholar] [CrossRef]
- Bader, C.A.; Shandala, T.; Ng, Y.S.; Johnson, I.R.D.; Brooks, D.A. Atg9 Is Required for Intraluminal Vesicles in Amphisomes and Autolysosomes. Biol. Open 2015, 4, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, D.; Cai, Q. Understanding Amphisomes. Biochem. J. 2021, 478, 1959–1976. [Google Scholar] [CrossRef]
- Dupont, N.; Jiang, S.; Pilli, M.; Ornatowski, W.; Bhattacharya, D.; Deretic, V. Autophagy-Based Unconventional Secretory Pathway for Extracellular Delivery of IL-1β. EMBO J. 2011, 30, 4701–4711. [Google Scholar] [CrossRef]
- Solvik, T.A.; Nguyen, T.A.; Lin, Y.H.T.; Marsh, T.; Huang, E.J.; Wiita, A.P.; Debnath, J.; Leidal, A.M. Secretory Autophagy Maintains Proteostasis upon Lysosome Inhibition. J. Cell Biol. 2022, 221, e202110151. [Google Scholar] [CrossRef]
- Ejlerskov, P.; Rasmussen, I.; Nielsen, T.T.; Bergström, A.L.; Tohyama, Y.; Jensen, P.H.; Vilhardt, F. Tubulin Polymerization-Promoting Protein (TPPP/P25α) Promotes Unconventional Secretion of α-Synuclein through Exophagy by Impairing Autophagosome-Lysosome Fusion. J. Biol. Chem. 2013, 288, 17313–17335. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, G.; Liu, H.; Wang, L.; Lu, R.; Li, L. Molecular Mechanisms of Secretory Autophagy and Its Potential Role in Diseases. Life Sci. 2024, 347, 122653. [Google Scholar] [CrossRef]
- Debnath, J.; Leidal, A.M. Secretory Autophagy during Lysosome Inhibition (SALI). Autophagy 2022, 18, 2498–2499. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Ching, K.L.; Liang, F.X.; Dhabaria, A.; Tam, K.; Ueberheide, B.M.; Unutmaz, D.; Torres, V.J.; Cadwell, K. Decoy Exosomes Provide Protection against Bacterial Toxins. Nature 2020, 579, 260–264. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated HnRNPA2B1 Controls the Sorting of MiRNAs into Exosomes through Binding to Specific Motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef]
- Murrow, L.; Malhotra, R.; Debnath, J. ATG12-ATG3 Interacts with Alix to Promote Basal Autophagic Flux and Late Endosome Function. Nat. Cell Biol. 2015, 17, 300–310. [Google Scholar] [CrossRef]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-Syntenin-ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Fang, Y.T.; Cheng, Y.L.; Lin, C.F.; Hsu, L.J.; Wang, S.Y.; Anderson, R.; Chang, C.P.; Lin, Y.S. Exophagy of Annexin A2 via RAB11, RAB8A and RAB27A in IFN-γ-Stimulated Lung Epithelial Cells. Sci. Rep. 2017, 7, 5676. [Google Scholar] [CrossRef] [PubMed]
- Tooze, S.A.; Abada, A.; Elazar, Z. Endocytosis and Autophagy: Exploitation or Cooperation? Cold Spring Harb. Perspect. Biol. 2014, 6, a018358. [Google Scholar] [CrossRef]
- Leidal, A.M.; Huang, H.H.; Marsh, T.; Solvik, T.; Zhang, D.; Ye, J.; Kai, F.B.; Goldsmith, J.; Liu, J.Y.; Huang, Y.H.; et al. The LC3-Conjugation Machinery Specifies the Loading of RNA-Binding Proteins into Extracellular Vesicles. Nat. Cell Biol. 2020, 22, 187–199. [Google Scholar] [CrossRef] [PubMed]
- James, H.H. ESCRTs Are Everywhere. Embo J. 2015, 34, 2398–2407. [Google Scholar] [CrossRef]
- Lin, P.W.; Chu, M.L.; Liu, Y.W.; Chen, Y.C.; Shih, Y.H.; Lan, S.H.; Wu, S.Y.; Kuo, I.Y.; Chang, H.Y.; Liu, H.S.; et al. Revealing Potential Rab Proteins Participate in Regulation of Secretory Autophagy Machinery. Kaohsiung J. Med. Sci. 2024, 40, 642–649. [Google Scholar] [CrossRef]
- de Medina, P.; Bunay, J.; Poirot, M.; Record, M.; Silvente-Poirot, S. Targeting NR1H/Liver X Receptor with Dendrogenin A Differentiates Tumor Cells to Activate a New Secretory Pathway Releasing Immunogenic Anti-Tumor Vesicles Enriched in LC3-II-Associated Exosomes. Autophagy 2023, 19, 1036–1038. [Google Scholar] [CrossRef]
- Fader, C.M.; Sánchez, D.G.; Mestre, M.B.; Colombo, M.I. TI-VAMP/VAMP7 and VAMP3/Cellubrevin: Two v-SNARE Proteins Involved in Specific Steps of the Autophagy/Multivesicular Body Pathways. Biochim Biophys Acta Mol. Cell Res. 2009, 1793, 1901–1916. [Google Scholar] [CrossRef]
- Janas, T.; Janas, M.M.; Sapoń, K.; Janas, T. Mechanisms of RNA Loading into Exosomes. FEBS Lett. 2015, 589, 1391–1398. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Yamazaki, A.; Kawashima, A.; Honda, T.; Kohama, T.; Murakami, C.; Sakane, F.; Murayama, T.; Nakamura, H. Identification and Characterization of Diacylglycerol Kinase ζ as a Novel Enzyme Producing Ceramide-1-Phosphate. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159307. [Google Scholar] [CrossRef]
- Schink, K.O.; Tan, K.W.; Stenmark, H. Phosphoinositides in Control of Membrane Dynamics. Annu. Rev. Cell Dev. Biol. 2016, 32, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Mccartney, A.J.; Zhang, Y.; Weisman, L.S. Phosphatidylinositol 3,5-Bisphosphate: Low Abundance, High Significance. BioEssays 2014, 36, 52–64. [Google Scholar] [CrossRef]
- Boya, P.; Reggiori, F.; Codogno, P. Emerging Regulation and Functions of Autophagy. Nat. Cell Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H.; Suzuki, K.; Kamada, Y.; Ohsumi, Y. Dynamics and Diversity in Autophagy Mechanisms: Lessons from Yeast. Nat. Rev. Mol. Cell Biol. 2009, 10, 458–467. [Google Scholar] [CrossRef]
- Zolov, S.N.; Bridges, D.; Zhang, Y.; Lee, W.W.; Riehle, E.; Verma, R.; Lenk, G.M.; Converso-Baran, K.; Weide, T.; Albin, R.L.; et al. In Vivo, Pikfyve Generates PI(3,5)P2, Which Serves as Both a Signaling Lipid and the Major Precursor for PI5P. Proc. Natl. Acad. Sci. USA 2012, 109, 17472–17477. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Øverbye, A.; Brech, A.; Torgersen, M.L.; Jakobsen, I.S.; Sandvig, K.; Llorente, A. PIKfyve Inhibition Increases Exosome Release and Induces Secretory Autophagy. Cell. Mol. Life Sci. 2016, 73, 4717–4737. [Google Scholar] [CrossRef]
- Samie, M.A.; Xu, H. Lysosomal Exocytosis and Lipid Storage Disorders. J. Lipid Res. 2014, 55, 995–1009. [Google Scholar] [CrossRef]
- Dayam, R.M.; Saric, A.; Shilliday, R.E.; Botelho, R.J. The Phosphoinositide-Gated Lysosomal Ca2+ Channel, TRPML1, Is Required for Phagosome Maturation. Traffic 2015, 16, 1010–1026. [Google Scholar] [CrossRef]
- Ju, R.; Zhuang, Z.W.; Zhang, J.; Lanahan, A.A.; Kyriakides, T.; Sessa, W.C.; Simons, M. Angiopoietin-2 Secretion by Endothelial Cell Exosomes: Regulation by the Phosphatidylinositol 3-Kinase (PI3K)/Akt/Endothelial Nitric Oxide Synthase (ENOS) and Syndecan-4/Syntenin Pathways. J. Biol. Chem. 2014, 289, 510–519. [Google Scholar] [CrossRef]
- Polson, H.E.J.; De Lartigue, J.; Rigden, D.J.; Reedijk, M.; Urbé, S.; Clague, M.J.; Tooze, S.A. Mammalian Atg18 (WIPI2) Localizes to Omegasome-Anchored Phagophores and Positively Regulates LC3 Lipidation. Autophagy 2010, 6, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Lasiecka, Z.M.; Xu, Y.; Neufeld, J.; Shahriar, S.; Simoes, S.; Chan, R.B.; Oliveira, T.G.; Small, S.A.; Di Paolo, G. Neuronal Lysosomal Dysfunction Releases Exosomes Harboring APP C-Terminal Fragments and Unique Lipid Signatures. Nat. Commun. 2018, 9, 291. [Google Scholar] [CrossRef]
- Matarrese, P.; Garofalo, T.; Manganelli, V.; Gambardella, L.; Marconi, M.; Grasso, M.; Tinari, A.; Misasi, R.; Malorni, W.; Sorice, M. Evidence for the Involvement of GD3 Ganglioside in Autophagosome Formation and Maturation. Autophagy 2014, 10, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Paone, S.; Caruso, S.; Atkin-Smith, G.K.; Phan, T.K.; Hulett, M.D.; Poon, I.K.H. Determining the Contents and Cell Origins of Apoptotic Bodies by Flow Cytometry. Sci. Rep. 2017, 7, 14444. [Google Scholar] [CrossRef]
- Lane, J.D.; Allan, V.J.; Woodman, P.G. Active Relocation of Chromatin and Endoplasmic Reticulum into Blebs in Late Apoptotic Cells. J. Cell Sci. 2005, 118, 4059–4071. [Google Scholar] [CrossRef] [PubMed]
- Dieudé, M.; Turgeon, J.; Karakeussian Rimbaud, A.; Beillevaire, D.; Qi, S.; Patey, N.; Gaboury, L.A.; Boilard, É.; Hébert, M.J. Extracellular Vesicles Derived from Injured Vascular Tissue Promote the Formation of Tertiary Lymphoid Structures in Vascular Allografts. Am. J. Transplant. 2020, 20, 726–738. [Google Scholar] [CrossRef]
- Kakarla, R.; Hur, J.; Kim, Y.J.; Kim, J.; Chwae, Y.J. Apoptotic Cell-Derived Exosomes: Messages from Dying Cells. Exp. Mol. Med. 2020, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Beillevaire, D.; Migneault, F.; Turgeon, J.; Gingras, D.; Rimbaud, A.K.; Marcoux, G.; Spino, C.; Thibodeau, N.; Bonneil, E.; Thibault, P.; et al. Autolysosomes and Caspase-3 Control the Biogenesis and Release of Immunogenic Apoptotic Exosomes. Cell Death Dis. 2022, 13, 145. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Marconi, M.; Ascione, B.; Ciarlo, L.; Vona, R.; Garofalo, T.; Sorice, M.; Gianni, A.M.; Locatelli, S.L.; Carlo-Stella, C.; Malorni, W.; et al. Constitutive Localization of DR4 in Lipid Rafts Is Mandatory for TRAIL-Induced Apoptosis in B-Cell Hematologic Malignancies. Cell Death Dis. 2013, 4, e863. [Google Scholar] [CrossRef]
- Motoyama, K.; Kameyama, K.; Onodera, R.; Araki, N.; Hirayama, F.; Uekama, K.; Arima, H. Involvement of PI3K-Akt-Bad Pathway in Apoptosis Induced by 2,6-Di-O-Methyl-β-Cyclodextrin, Not 2,6-Di-O-Methyl-α-Cyclodextrin, through Cholesterol Depletion from Lipid Rafts on Plasma Membranes in Cells. Eur. J. Pharm. Sci. 2009, 38, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Ouasti, S.; Matarrese, P.; Paddon, R.; Khosravi-Far, R.; Sorice, M.; Tinari, A.; Malorni, W.; Degli Esposti, M. Death receptor ligation triggers membrane scrambling between Golgi and mitochondria. Cell Death Differ. 2007, 14, 453. [Google Scholar] [CrossRef][Green Version]
- Colombini, M. Ceramide Channels and Their Role in Mitochondria-Mediated Apoptosis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1239–1244. [Google Scholar] [CrossRef]
- Manganelli, V.; Capozzi, A.; Recalchi, S.; Signore, M.; Mattei, V.; Garofalo, T.; Misasi, R.; Degli Esposti, M.; Sorice, M. Altered Traffic of Cardiolipin during Apoptosis: Exposure on the Cell Surface as a Trigger for “Antiphospholipid Antibodies”. J. Immunol. Res. 2015, 2015, 847985. [Google Scholar] [CrossRef]
- Alessandri, C.; Sorice, M.; Bombardieri, M.; Conigliaro, P.; Longo, A.; Garofalo, T.; Manganelli, V.; Conti, F.; Degli Esposti, M.; Valesini, G. Antiphospholipid Reactivity against Cardiolipin Metabolites Occurring during Endothelial Cell Apoptosis. Arthritis Res. Ther. 2006, 8, R180. [Google Scholar] [CrossRef]
- Hengartner, M.O. Apoptosis: Corralling the Corpses. Cell 2001, 104, 325–328. [Google Scholar] [CrossRef]
- Kurihara, H.; Yang, D.J.; Cristofanilli, M.; Erwin, W.D.; Yu, D.F.; Kohanim, S.; Mendez, R.; Kim, E.E. Imaging and Dosimetry of 99mTc EC Annexin V: Preliminary Clinical Study Targeting Apoptosis in Breast Tumors. Appl. Radiat. Isot. 2008, 66, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Sirois, I.; Bell, C.; Hanafi, L.A.; Hamelin, K.; Dieudé, M.; Rondeau, C.; Thibault, P.; Desjardins, M.; Hebert, M.J. A Comprehensive Characterization of Membrane Vesicles Released by Autophagic Human Endothelial Cells. Proteomics 2013, 13, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Sirois, I.; Groleau, J.; Pallet, N.; Brassard, N.; Hamelin, K.; Londono, I.; Pshezhetsky, A.V.; Bendayan, M.; Hébert, M.J. Caspase Activation Regulates the Extracellular Export of Autophagic Vacuoles. Autophagy 2012, 8, 927–937. [Google Scholar] [CrossRef]
- Prerna, K.; Dubey, V.K. Beclin1-Mediated Interplay between Autophagy and Apoptosis: New Understanding. Int. J. Biol. Macromol. 2022, 204, 258–273. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural Stem Cell-Derived Small Extracellular Vesicles Attenuate Apoptosis and Neuroinflammation after Traumatic Spinal Cord Injury by Activating Autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef]
- Mnich, K.; Koryga, I.; Pakos-Zebrucka, K.; Thomas, M.; Logue, S.E.; Eriksson, L.A.; Gorman, A.M.; Samali, A. The Stressosome, a Caspase-8-Activating Signalling Complex Assembled in Response to Cell Stress in an ATG5-Mediated Manner. J. Cell. Mol. Med. 2021, 25, 8809–8820. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Che, X.; Zheng, Q.; Wu, A.; Pan, K.; Shao, A.; Wu, Q.; Zhang, J.; Hong, Y. Caspases: A Molecular Switch Node in the Crosstalk between Autophagy and Apoptosis. Int. J. Biol. Sci. 2014, 10, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Deegan, S.; Saveljeva, S.; Logue, S.E.; Pakos-Zebrucka, K.; Gupta, S.; Vandenabeele, P.; Bertrand, M.J.; Samali, A. Deficiency in the Mitochondrial Apoptotic Pathway Reveals the Toxic Potential of Autophagy under ER Stress Conditions. Autophagy 2014, 10, 1921–1936. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chitiprolu, M.; Roncevic, L.; Javalet, C.; Hemming, F.J.; Trung, M.T.; Meng, L.; Latreille, E.; Tanese de Souza, C.; McCulloch, D.; et al. Atg5 Disassociates the V1V0-ATPase to Promote Exosome Production and Tumor Metastasis Independent of Canonical Macroautophagy. Dev. Cell 2017, 43, 716–730.e7. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.D.; Rimmer, M.P. Extracellular Vesicles Arising from Apoptosis: Forms, Functions, and Applications. J. Pathol. 2023, 260, 592–608. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Young, M.M.; Kester, M.; Wang, H.G. Sphingolipids: Regulators of Crosstalk between Apoptosis and Autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Cosot, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of Ceramide-Mediated Programmed Cell Death by Sphingosine- 1-Phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Guenther, G.G.; Peralta, E.R.; Rosales, K.R.; Wong, S.Y.; Siskind, L.J.; Edinger, A.L. Ceramide Starves Cells to Death by Downregulating Nutrient Transporter Proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 17402–17407. [Google Scholar] [CrossRef]
- Lépine, S.; Allegood, J.C.; Edmonds, Y.; Milstien, S.; Spiegel, S. Autophagy Induced by Deficiency of Sphingosine-1-Phosphate Phosphohydrolase 1 Is Switched to Apoptosis by Calpain-Mediated Autophagy-Related Gene 5 (Atg5) Cleavage. J. Biol. Chem. 2011, 286, 44380–44390. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Kitatani, K.; Kondo, T.; Hashimoto-Nishimura, M.; Asano, S.; Hayashi, A.; Mitsutake, S.; Igarashi, Y.; Umehara, H.; Takeya, H.; et al. Regulation of Autophagy and Its Associated Cell Death by “Sphingolipid Rheostat”: Reciprocal Role of Ceramide and Sphingosine 1-Phosphate in the Mammalian Target of Rapamycin Pathway. J. Biol. Chem. 2012, 287, 39898–39910. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Spiegel, S. Sphingosine-1-Phosphate as Second Messenger in Cell Proliferation Induced by PDGF and FCS Mitogens. Nature 1993, 365, 557–560. [Google Scholar] [CrossRef]
- Lavieu, G.; Scarlatti, F.; Sala, G.; Carpentier, S.; Levade, T.; Ghidoni, R.; Botti, J.; Codogno, P. Regulation of Autophagy by Sphingosine Kinase 1 and Its Role in Cell Survival during Nutrient Starvation. J. Biol. Chem. 2006, 281, 8518–8527. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, H.J.; Lee, W.H.; Suk, K. NF-B as a Common Signaling Pathway in Ganglioside-Induced Autophagic Cell Death and Activation of Astrocytes. J. Neuroimmunol. 2010, 226, 66–72. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.; Lee, J.T.; Kwon, T.K.; Kim, D.R.; Kim, H.; Park, H.C.; Suk, K. Gangliosides Induce Autophagic Cell Death in Astrocytes: RESEARCH PAPER. Br. J. Pharmacol. 2010, 159, 586–603. [Google Scholar] [CrossRef]
- Varela, L.; van de Lest, C.H.A.; René van Weeren, P.; Wauben, M.H.M. Synovial Fluid Extracellular Vesicles as Arthritis Biomarkers: The Added Value of Lipid-Profiling and Integrated Omics. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 276–296. [Google Scholar] [CrossRef]
- Buttari, B.; Recalchi, S.; Riitano, G.; Capozzi, A.; Ucci, F.M.; Manganelli, V.; Fratini, F.; Profumo, E.; Garofalo, T.; Alessandri, C.; et al. Extracellular Microvesicles from Patients with Rheumatoid Arthritis Promote Dendritic Cell Activation in Vitro. Front. Immunol. 2025, 16, 1532114. [Google Scholar] [CrossRef]
- Ucci, F.M.; Recalchi, S.; Barbati, C.; Manganelli, V.; Capozzi, A.; Riitano, G.; Buoncuore, G.; Garofalo, T.; Ceccarelli, F.; Spinelli, F.R.; et al. Citrullinated and Carbamylated Proteins in Extracellular Microvesicles from Plasma of Patients with Rheumatoid Arthritis. Rheumatology 2023, 62, 2312–2319. [Google Scholar] [CrossRef]
- Capozzi, A.; Manganelli, V.; Riitano, G.; Caissutti, D.; Longo, A.; Garofalo, T.; Sorice, M.; Misasi, R. Advances in the Pathophysiology of Thrombosis in Antiphospholipid Syndrome: Molecular Mechanisms and Signaling through Lipid Rafts. J. Clin. Med. 2023, 12, 891. [Google Scholar] [CrossRef]
- Štok, U.; Čučnik, S.; Sodin-šemrl, S.; Žigon, P. Extracellular Vesicles and Antiphospholipid Syndrome: State-of-the-art and Future Challenges. Int. J. Mol. Sci. 2021, 22, 4689. [Google Scholar] [CrossRef]
- McNamara, R.P.; Dittmer, D.P. Extracellular Vesicles in Virus Infection and Pathogenesis. Curr. Opin. Virol. 2020, 44, 129–138. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-Derived Exosomes Induce N2 Polarization of Neutrophils to Promote Gastric Cancer Cell Migration. Mol. Cancer 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, V.; Sahu, A.; Taware, R. Stress-Induced Extracellular Vesicles: Insight into Their Altered Proteomic Composition and Probable Physiological Role in Cancer. Mol. Cell. Biochem. 2024, 480, 2025–2041. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The Role of Lipids in Exosome Biology and Intercellular Communication: Function, Analytics and Applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.M.; Angelova, M.I.; Kinnunen, P.K.J. Vectorial Budding of Vesicles by Asymmetrical Enzymatic Formation of Ceramide in Giant Liposomes. Biophys. J. 2000, 78, 830–838. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory Mechanisms and Intercellular Transfer of MicroRNAs in Living Cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.C. Blockade of Exosome Generation with GW4869 Dampens the Sepsis-Induced Inflammation and Cardiac Dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 2362–2371. [Google Scholar] [CrossRef]
- Li, J.; Kemper, T.; Broering, R.; Lin, Y.; Wang, X.; Lu, M. Amphisome Plays a Role in HBV Production and Release through the Endosomal and Autophagic Pathways. Hepatol. Commun. 2025, 9, e0654. [Google Scholar] [CrossRef]
- Sanada, T.; Hirata, Y.; Naito, Y.; Yamamoto, N.; Kikkawa, Y.; Ishida, Y.; Yamasaki, C.; Tateno, C.; Ochiya, T.; Kohara, M. Transmission of HBV DNA Mediated by Ceramide-Triggered Extracellular Vesicles. CMGH 2017, 3, 272–283. [Google Scholar] [CrossRef]
- Ranganathan, S.; Jackson, R.L.; Harmony, J.A.K. Effect of Pantethine on the Biosynthesis of Cholesterol in Human Skin Fibroblasts. Atherosclerosis 1982, 44, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Marut, W.; Servettaz, A.; Nicco, C.; Chéreau, C.; Lemaréchal, H.; Guilpain, P.; Chimini, G.; Galland, F.; Weill, B.; et al. Pantethine Prevents Murine Systemic Sclerosis through the Inhibition of Microparticle Shedding. Arthritis Rheumatol. 2015, 67, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Peng, Y.; Jiang, Y.; Wu, Y.; Ding, Y.; Wang, Y.; Xu, D.; Fu, Q. Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles. Int. J. Mol. Sci. 2017, 18, 1013. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Singh, S.; Ahmad, M.; Khanna, K.; Ahmad, T.; Agrawal, A.; Ghosh, B. Simvastatin Mediates Inhibition of Exosome Synthesis, Localization and Secretion via Multicomponent Interventions. Sci. Rep. 2019, 9, 16373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, A.; Manganelli, V.; Misasi, R.; Riitano, G.; Caglar, T.R.; Fasciolo, E.; Recalchi, S.; Sorice, M.; Garofalo, T. Extracellular Vesicles in the Crosstalk of Autophagy and Apoptosis: A Role for Lipid Rafts. Cells 2025, 14, 749. https://doi.org/10.3390/cells14100749
Longo A, Manganelli V, Misasi R, Riitano G, Caglar TR, Fasciolo E, Recalchi S, Sorice M, Garofalo T. Extracellular Vesicles in the Crosstalk of Autophagy and Apoptosis: A Role for Lipid Rafts. Cells. 2025; 14(10):749. https://doi.org/10.3390/cells14100749
Chicago/Turabian StyleLongo, Agostina, Valeria Manganelli, Roberta Misasi, Gloria Riitano, Tuba Rana Caglar, Elena Fasciolo, Serena Recalchi, Maurizio Sorice, and Tina Garofalo. 2025. "Extracellular Vesicles in the Crosstalk of Autophagy and Apoptosis: A Role for Lipid Rafts" Cells 14, no. 10: 749. https://doi.org/10.3390/cells14100749
APA StyleLongo, A., Manganelli, V., Misasi, R., Riitano, G., Caglar, T. R., Fasciolo, E., Recalchi, S., Sorice, M., & Garofalo, T. (2025). Extracellular Vesicles in the Crosstalk of Autophagy and Apoptosis: A Role for Lipid Rafts. Cells, 14(10), 749. https://doi.org/10.3390/cells14100749







