Endometriosis Cell Spheroids Undergo Mesothelial Clearance in a Similar Manner to Ovarian Cancer Cell Spheroids
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Drug Treatment
2.3. 3D Spheroid Culture
2.4. Cell Viability Staining
2.5. Mesothelial Clearance Assay and Analysis
3. Results
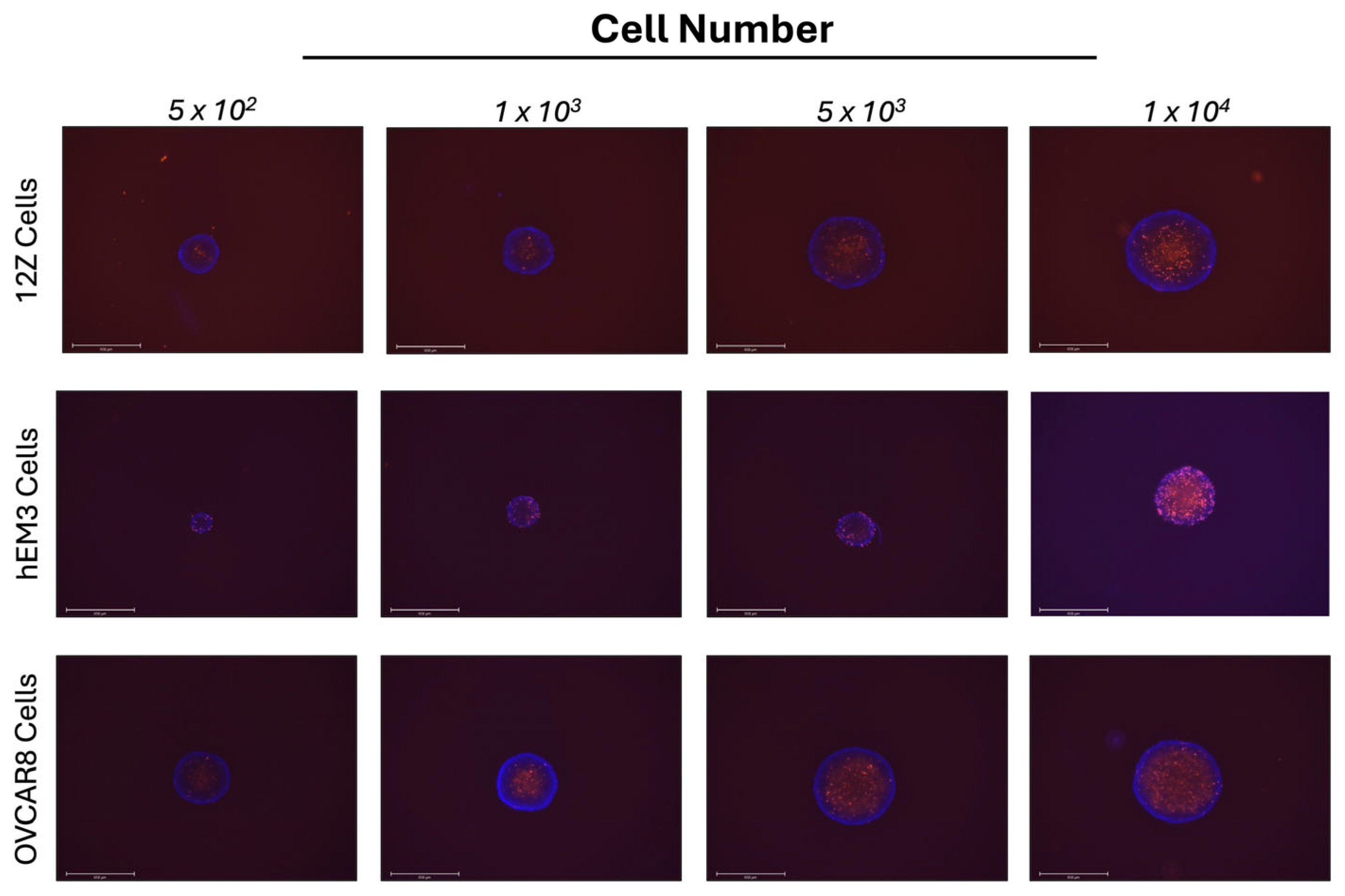
3.1. 12Z Endometriosis and hEM3 Endometrial Cells Form Spheroids in 3D Cell Culture
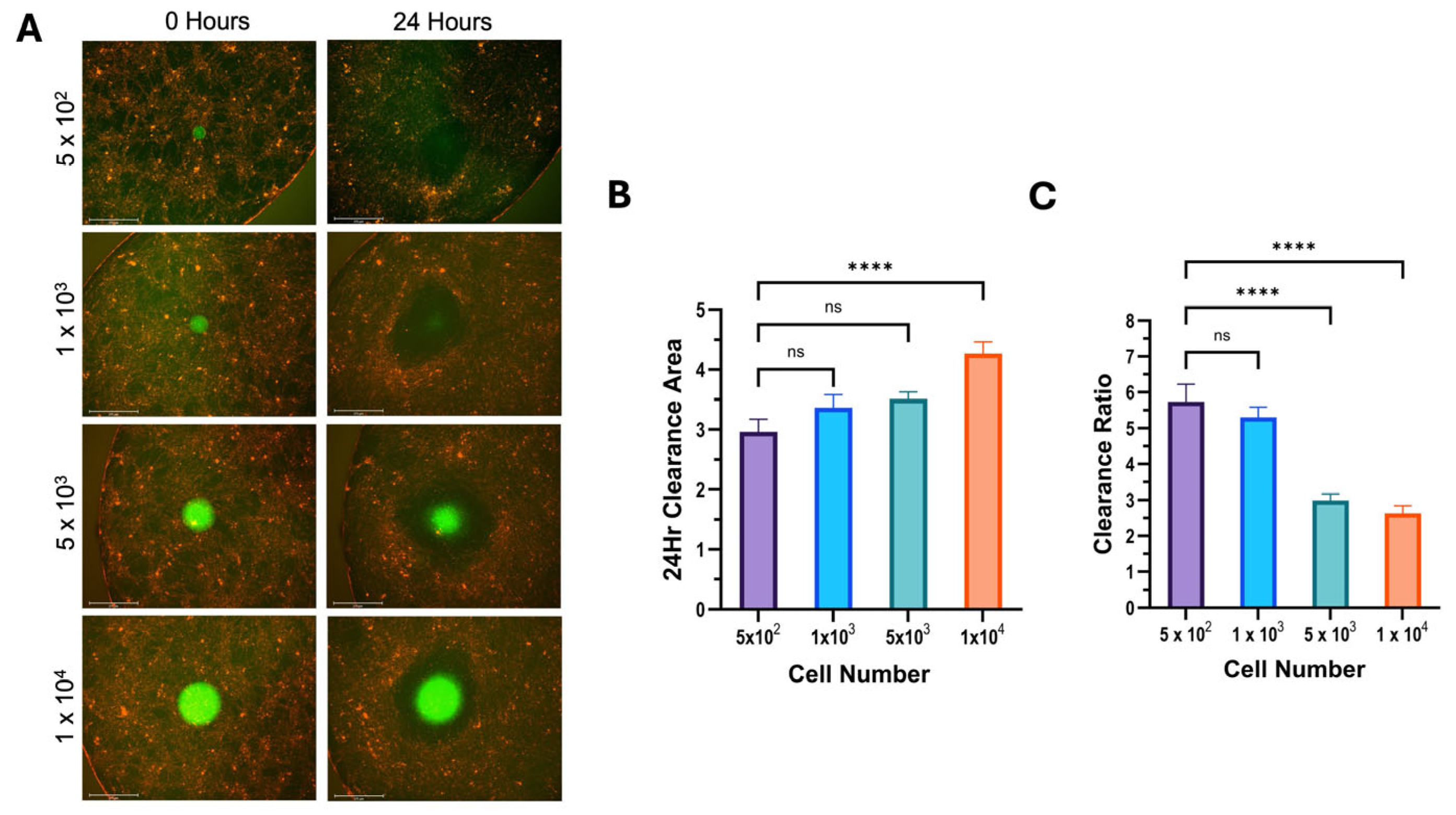
3.2. 12Z and hEM3 Spheroids Can Execute Mesothelial Clearance
3.3. Clearance Ratio Has an Inverse Relationship with Initial Spheroid Size
3.4. Analysis of Potential Pharmacological Inhibitors of Mesothelial Clearance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| 2D | Two-dimensional |
| ULA | Ultra-low attachment |
| PI | Propidium iodide |
| DMSO | Dimethyl sulfoxide |
References
- Horne, A.W.; Missmer, S.A. Pathophysiology, Diagnosis, and Management of Endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- May, K.E.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Endometrial Alterations in Endometriosis: A Systematic Review of Putative Biomarkers. Hum. Reprod. Updat. 2011, 17, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; Koninckx, P.; et al. ESHRE Guideline for the Diagnosis and Treatment of Endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Ramírez-González, J.A.; Vaamonde-Lemos, R.; Cunha-Filho, J.S.; Varghese, A.C.; Swanson, R.J. Overview of the Female Reproductive System. In Exercise and Human Reproduction: Induced Fertility Disorders and Possible Therapies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 19–46. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal Endometriosis Due to the Menstrual Dissemination of Endometrial Tissue into the Peritoneal Cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Witz, C.A.; Monotoya-Rodriguez, I.A.; Schenken, R.S. Whole Explants of Peritoneum and Endometrium: A Novel Model of the Early Endometriosis Lesion. Fertil. Steril. 1999, 71, 56–60. [Google Scholar] [CrossRef]
- Young, V.J.; Brown, J.K.; Saunders, P.T.K.; Horne, A.W. The Role of the Peritoneum in the Pathogenesis of Endometriosis. Hum. Reprod. Update 2013, 19, 558–569. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef]
- Nair, A.S.; Nair, H.B.; Lucidi, R.S.; Kirchner, A.J.; Schenken, R.S.; Tekmal, R.R.; Witz, C.A. Modeling the Early Endometriotic Lesion: Mesothelium-Endometrial Cell Co-Culture Increases Endometrial Invasion and Alters Mesothelial and Endometrial Gene Transcription. Fertil. Steril. 2008, 90, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Burns, G.W.; Joshi, N.R.; Arora, R.; Kim, J.J.; Fazleabas, A.T. Spheroids as a Model for Endometriotic Lesions. JCI Insight 2023, 8, e160815. [Google Scholar] [CrossRef] [PubMed]
- Nezhat, F.R.; Pejovic, T.; Reis, F.M.; Guo, S.W. The Link Between Endometriosis and Ovarian Cancer: Clinical Implications. Int. J. Gynecol. Cancer 2014, 24, 623–628. [Google Scholar] [CrossRef]
- Guo, S.W. The Association of Endometriosis with Ovarian Cancer: A Critical Review of Epidemiological Data. In Endometriosis: Pathogenesis and Treatment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 363–384. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between Endometriosis and Risk of Histological Subtypes of Ovarian Cancer: A Pooled Analysis of Case-Control Studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Yee, C.; Dickson, K.A.; Muntasir, M.N.; Ma, Y.; Marsh, D.J. Three-Dimensional Modelling of Ovarian Cancer: From Cell Lines to Organoids for Discovery and Personalized Medicine. Front. Bioeng. Biotechnol. 2022, 10, 836984. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910. [Google Scholar] [CrossRef]
- Kerslake, R.; Belay, B.; Panfilov, S.; Hall, M.; Kyrou, I.; Randeva, H.S.; Hyttinen, J.; Karteris, E.; Sisu, C. Transcriptional Landscape of 3D vs. 2D Ovarian Cancer Cell Models. Cancers 2023, 15, 3350. [Google Scholar] [CrossRef]
- Brueggmann, D.; Templeman, C.; Starzinski-Powitz, A.; Rao, N.P.; Gayther, S.A.; Lawrenson, K. Novel Three-Dimensional In Vitro Models of Ovarian Endometriosis. J. Ovarian Res. 2014, 7, 17. [Google Scholar] [CrossRef]
- Davidowitz, R.A.; Iwanicki, M.P.; Brugge, J.S. In Vitro Mesothelial Clearance Assay That Models the Early Steps of Ovarian Cancer Metastasis. J. Vis. Exp. 2012, 60, e3888. [Google Scholar] [CrossRef]
- Witz, C.A.; Cho, S.; Centonze, V.E.; Montoya-Rodriguez, I.A.; Schenken, R.S. Time Series Analysis of Transmesothelial Invasion by Endometrial Stromal and Epithelial Cells Using Three-Dimensional Confocal Microscopy. Fertil. Steril. 2003, 79 (Suppl. S1), 770–778. [Google Scholar] [CrossRef]
- Park, Y.; Jung, J.G.; Yu, Z.C.; Asaka, R.; Shen, W.; Wang, Y.; Jung, W.H.; Tomaszewski, A.; Shimberg, G.; Chen, Y.; et al. A Novel Human Endometrial Epithelial Cell Line for Modeling Gynecological Diseases and for Drug Screening. Lab. Investig. 2021, 101, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, N.; Kreeger, P.K.; Notbohm, J. Topological Defects in the Mesothelium Suppress Ovarian Cancer Cell Clearance. APL Bioeng. 2021, 5, 36103. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N.; Khazaei, M.; Ai, J.; Bielecki, R.; Gotlieb, L.; Ryan, E.; Casper, R.F. Effect of a Statin on an in Vitro Model of Endometriosis. Fertil. Steril. 2007, 87, 257–262. [Google Scholar] [CrossRef]
- Young, V.J.; Brown, J.K.; Saunders, P.T.K.; Duncan, W.C.; Horne, A.W. The Peritoneum Is Both a Source and Target of TGF-β in Women with Endometriosis. PLoS ONE 2014, 9, e106773. [Google Scholar] [CrossRef] [PubMed]
- Beniey, M. Peritoneal Metastases from Breast Cancer: A Scoping Review. Cureus 2019, 11, e5367. [Google Scholar] [CrossRef]
- Davidowitz, R.A.; Selfors, L.M.; Iwanicki, M.P.; Elias, K.M.; Karst, A.; Piao, H.; Ince, T.A.; Drage, M.G.; Dering, J.; Konecny, G.E.; et al. Mesenchymal Gene Program–Expressing Ovarian Cancer Spheroids Exhibit Enhanced Mesothelial Clearance. J. Clin. Investig. 2014, 124, 2611–2625. [Google Scholar] [CrossRef]
- Witz, C.A.; Allsup, K.T.; Montoya-Rodriguez, I.A.; Vaughn, S.L.; Centonze, V.E.; Schenken, R.S. Culture of Menstrual Endometrium with Peritoneal Explants and Mesothelial Monolayers Confirms Attachment to Intact Mesothelial Cells. Hum. Reprod. 2002, 17, 2832–2838. [Google Scholar] [CrossRef]
- Kavoussi, S.K.; Witz, C.A.; Binkley, P.A.; Nair, A.S.; Lebovic, D.I. Peroxisome-Proliferator Activator Receptor-Gamma Activation Decreases Attachment of Endometrial Cells to Peritoneal Mesothelial Cells in an in Vitro Model of the Early Endometriotic Lesion. Mol. Hum. Reprod. 2009, 15, 687–692. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A Repurposed Drug to Fight Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Oktem, M.; Esinler, I.; Eroglu, D.; Haberal, N.; Bayraktar, N.; Zeyneloglu, H.B. High-Dose Atorvastatin Causes Regression of Endometriotic Implants: A Rat Model. Hum. Reprod. 2007, 22, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G.; Duleba, A.J. Simvastatin Protects against the Development of Endometriosis in a Nude Mouse Model. J. Clin. Endocrinol. Metab. 2009, 94, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Yuge, A.; Tsuno, A.; Narahara, H. Simvastatin Inhibits the Proliferation and the Contractility of Human Endometriotic Stromal Cells: A Promising Agent for the Treatment of Endometriosis. Fertil. Steril. 2009, 92, 2097–2099. [Google Scholar] [CrossRef]
- Sokalska, A.; Wong, D.H.; Cress, A.; Piotrowski, P.C.; Rzepczynska, I.; Villanueva, J.; Duleba, A.J. Simvastatin Induces Apoptosis and Alters Cytoskeleton in Endometrial Stromal Cells. J. Clin. Endocrinol. Metab. 2010, 95, 3453–3459. [Google Scholar] [CrossRef]
- Gibran, L.; Maranhão, R.C.; Abrão, M.S.; Baracat, E.C.; Podgaec, S. Could Statins Constitute a Novel Treatment for Endometriosis? Systematic Review of the Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 153–158. [Google Scholar] [CrossRef]
- Jiao, X.F.; Li, H.L.; Jiao, X.Y.; Guo, Y.C.; Zhang, C.; Yang, C.S.; Zeng, L.N.; Bo, Z.Y.; Chen, Z.; Song, H.B.; et al. Ovary and Uterus Related Adverse Events Associated with Statin Use: An Analysis of the FDA Adverse Event Reporting System. Sci. Rep. 2020, 10, 11955. [Google Scholar] [CrossRef]
- Cicinelli, E.; Vitagliano, A.; Loizzi, V.; De Ziegler, D.; Fanelli, M.; Bettocchi, S.; Nardelli, C.; Trojano, G.; Cicinelli, R.; Minervini, C.F.; et al. Altered Gene Expression Encoding Cytochines, Grow Factors and Cell Cycle Regulators in the Endometrium of Women with Chronic Endometritis. Diagnostics 2021, 11, 471. [Google Scholar] [CrossRef]
- Oală, I.E.; Mitranovici, M.-I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R.; et al. Endometriosis and the Role of Pro-Inflammatory and Anti-Inflammatory Cytokines in Pathophysiology: A Narrative Review of the Literature. Diagnostics 2024, 14, 312. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pouly, J.-L.; Canis, M. Persistent Activation of Signal Transducer and Activator of Transcription 3 via Interleukin-6 Trans-Signaling Is Involved in Fibrosis of Endometriosis. Hum. Reprod. 2022, 37, 1489–1504. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloeckner, A.A.; Walker, S.R. Endometriosis Cell Spheroids Undergo Mesothelial Clearance in a Similar Manner to Ovarian Cancer Cell Spheroids. Cells 2025, 14, 742. https://doi.org/10.3390/cells14100742
Kloeckner AA, Walker SR. Endometriosis Cell Spheroids Undergo Mesothelial Clearance in a Similar Manner to Ovarian Cancer Cell Spheroids. Cells. 2025; 14(10):742. https://doi.org/10.3390/cells14100742
Chicago/Turabian StyleKloeckner, Allison A., and Sarah R. Walker. 2025. "Endometriosis Cell Spheroids Undergo Mesothelial Clearance in a Similar Manner to Ovarian Cancer Cell Spheroids" Cells 14, no. 10: 742. https://doi.org/10.3390/cells14100742
APA StyleKloeckner, A. A., & Walker, S. R. (2025). Endometriosis Cell Spheroids Undergo Mesothelial Clearance in a Similar Manner to Ovarian Cancer Cell Spheroids. Cells, 14(10), 742. https://doi.org/10.3390/cells14100742






