Nerve Growth Factor Modulates Regulatory Cell Volume Behavior via Stimulating TRPV1, TRPM8 Channels and Inducing Ca2+ Signaling in Human Conjunctival Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions
2.2. IOBA-NHC Cell Cultivation
2.3. Fura-2 Fluorescence Calcium Imaging
2.4. Calcein Fluorescence Cell Volume Imaging
2.5. Statistical Data Analyses
3. Results
3.1. TRPV1 and TRPM8 Regulate Intracellular Ca2+ in IOBA-NHC Cells
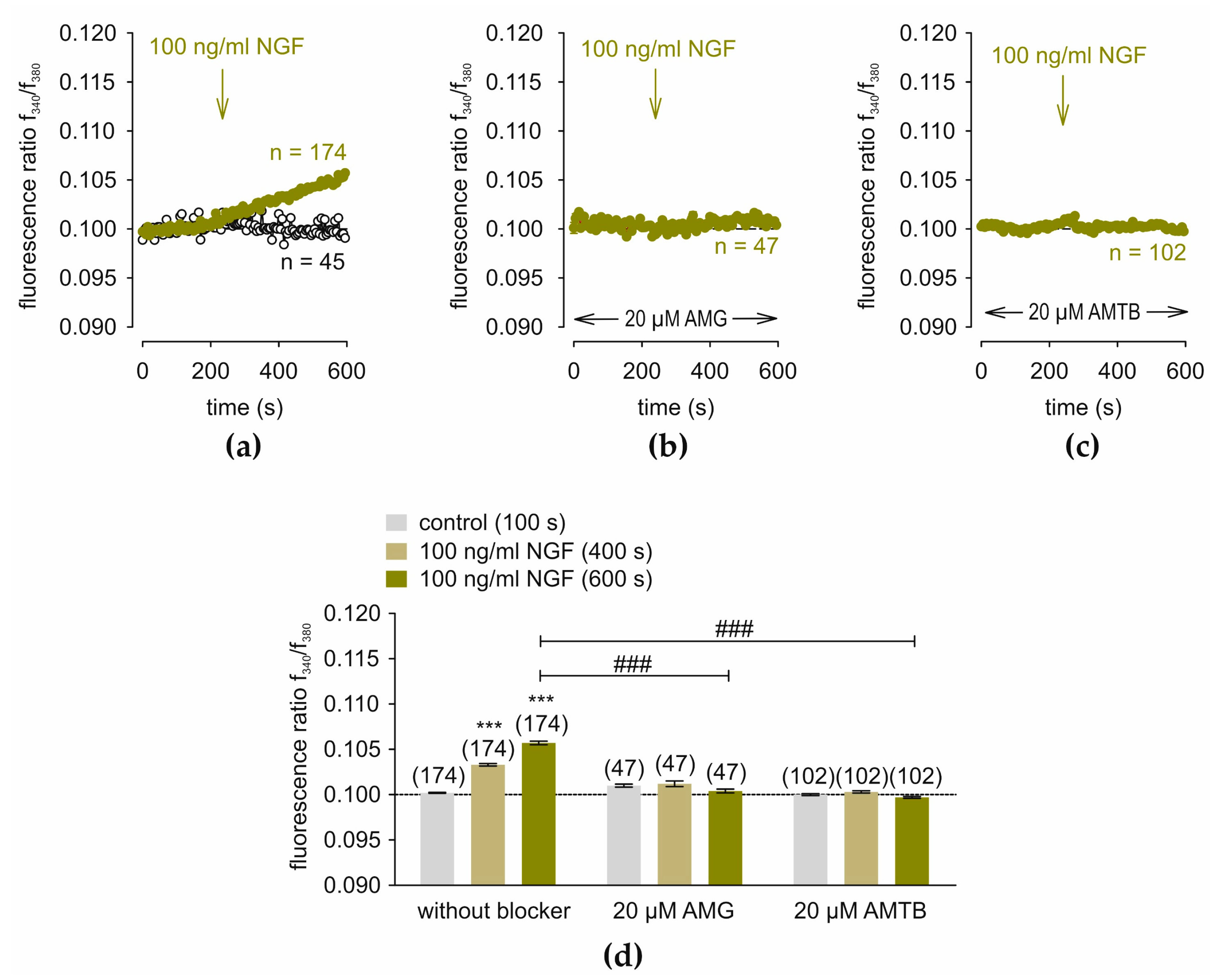
3.2. NGF-Induces Ca2+ Increase via TRPV1 and TRPM8
3.3. NGF-Induces Ca2+ Influx After Passive Store Depletion
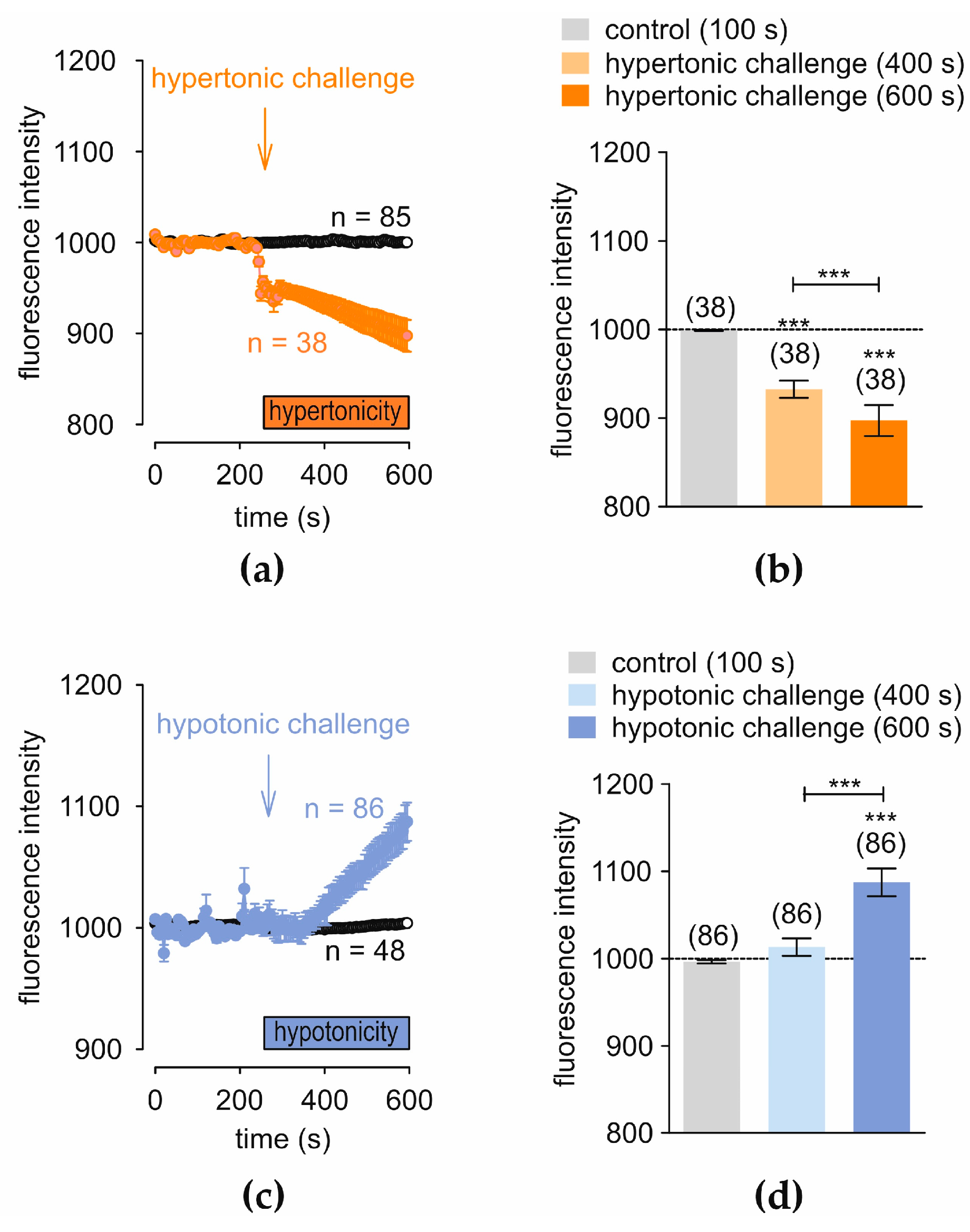
3.4. Hyper- and Hypotonicity-Induce Opposite Changes in Regulatory Cell Volume
3.5. TRPV1 and TRPM8 Regulate Cell Volume in IOBA-NHC Cells
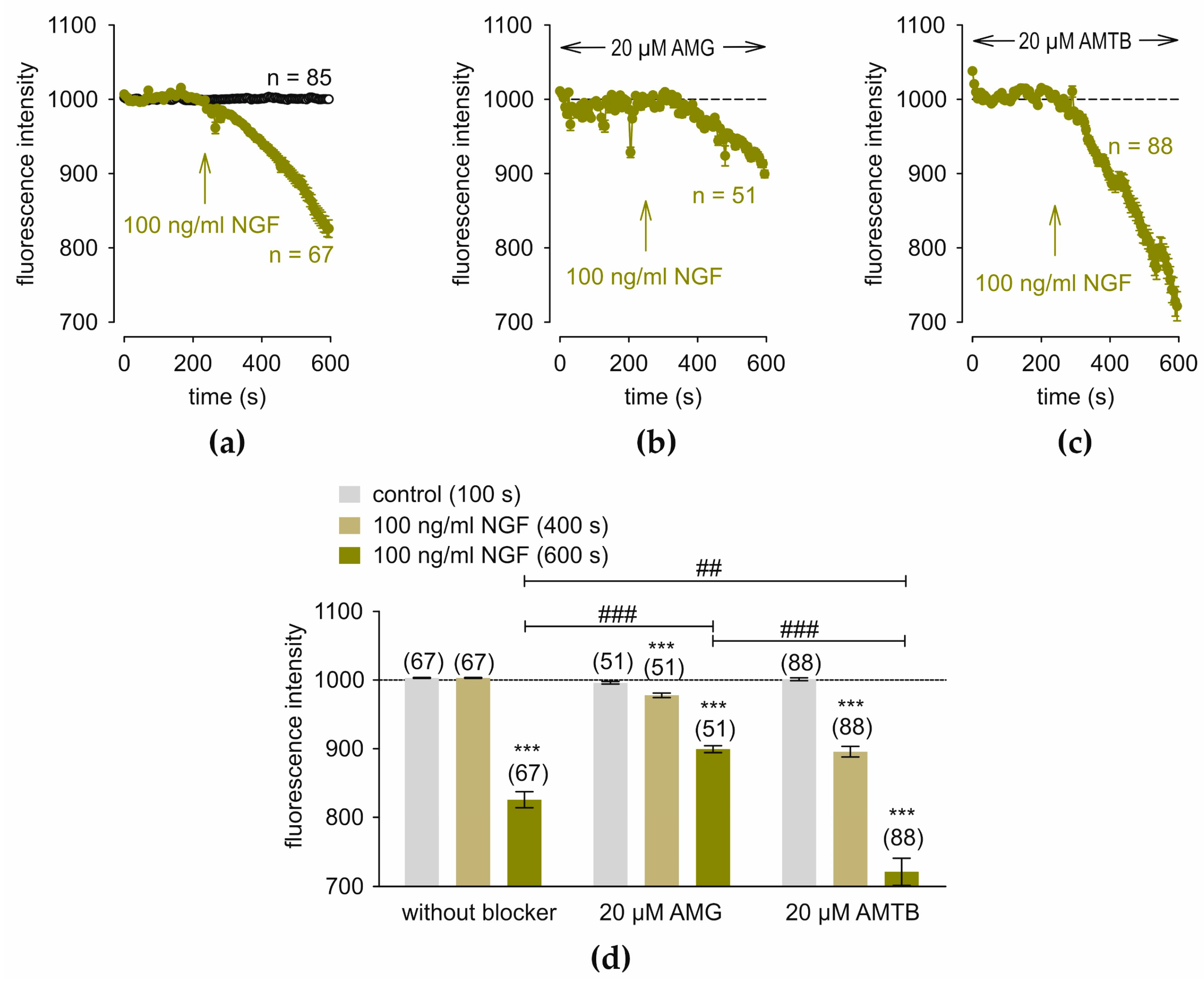
3.6. NGF Induces Changes in Regulatory Cell Volume via TRPV1 and TRPM8
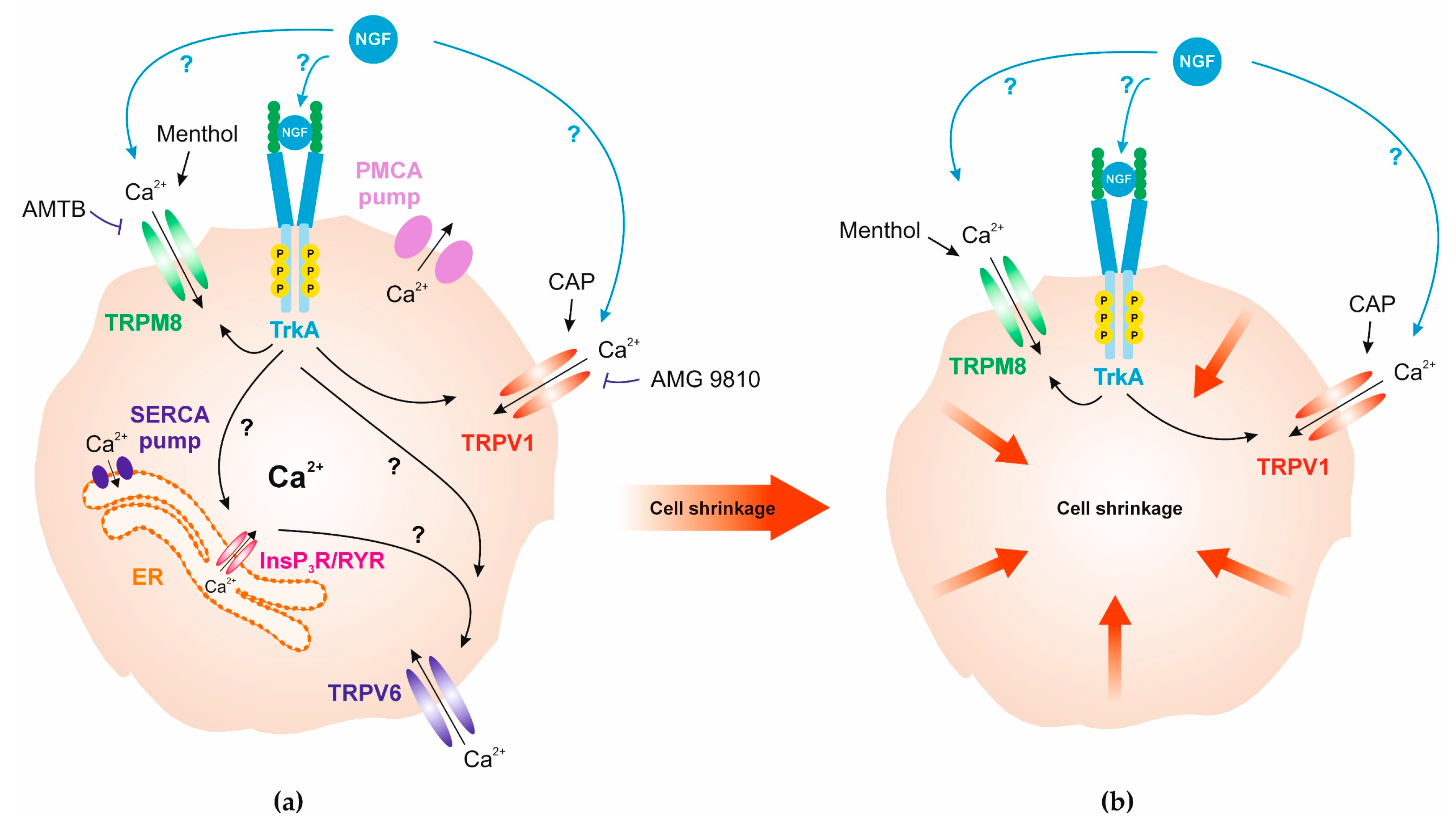
4. Discussion
4.1. Clinical Implications
4.2. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, Y.; Jin, X.; Zhang, N.; Shi, Y.; Zhu, R.; Wang, J.; Dong, Y.; Zhang, H. Observation of Conjunctiva-Associated Lymphoid Tissue with In Vivo Confocal Microscopy in Healthy Patients and Patients with Meibomian Gland Dysfunction. Cornea 2022, 41, 1129–1136. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N. Anatomy and immunology of the ocular surface. Chem. Immunol. Allergy 2007, 92, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Karpecki, P.M.; Meske, M.E.; Reissman, D. Evolving knowledge of the unmet needs in dry eye disease. Am. J. Manag. Care 2021, 27, S23–S32. [Google Scholar] [CrossRef]
- Ruskell, G.L. Innervation of the conjunctiva. Trans. Ophthalmol. Soc. UK (1962) 1985, 104 Pt 4, 390–395. [Google Scholar]
- Dartt, D.A.; Willcox, M.D. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Messmer, E.M.; Bulgen, M.; Kampik, A. Hyperosmolarity of the tear film in dry eye syndrome. Dev. Ophthalmol. 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Liu, H.; Begley, C.; Chen, M.; Bradley, A.; Bonanno, J.; McNamara, N.A.; Nelson, J.D.; Simpson, T. A link between tear instability and hyperosmolarity in dry eye. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3671–3679. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Nichols, K.K. Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape. Ophthalmol. Ther. 2023, 12, 1397–1418. [Google Scholar] [CrossRef]
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069. [Google Scholar]
- Yavuz Saricay, L.; Gonzalez Monroy, J.E.; Fulton, A.B. Can Nerve Growth Factor (NGF) Be a Treatment Option for Pediatric Eye Diseases? Semin. Ophthalmol. 2023, 38, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Mantelli, F.; Sacchetti, M.; Rossi, S.; Aloe, L.; Bonini, S. Clinical applications of NGF in ocular diseases. Arch. Ital. Biol. 2011, 149, 283–292. [Google Scholar] [CrossRef]
- Blanco-Vazquez, M.; Vazquez, A.; Fernandez, I.; Novo-Diez, A.; Martinez-Plaza, E.; Garcia-Vazquez, C.; Gonzalez-Garcia, M.J.; Sobas, E.M.; Calonge, M.; Enriquez-de-Salamanca, A. Inflammation-related molecules in tears of patients with chronic ocular pain and dry eye disease. Exp. Eye Res. 2022, 219, 109057. [Google Scholar] [CrossRef]
- Jain, P.; Li, R.; Lama, T.; Saragovi, H.U.; Cumberlidge, G.; Meerovitch, K. An NGF mimetic, MIM-D3, stimulates conjunctival cell glycoconjugate secretion and demonstrates therapeutic efficacy in a rat model of dry eye. Exp. Eye Res. 2011, 93, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.; Kalangara, J.; Lee, F.E.E. Neuropathic Pain and Itch Mechanisms Underlying Allergic Conjunctivitis. J. Investig. Allergol. Clin. Immunol. 2019, 29, 349–356. [Google Scholar] [CrossRef]
- Sacchetti, M.; Segatto, M.; Bruscolini, A.; Abicca, I.; Cavaliere, C.; Lambiase, A. Changes of NGF pathway in allergic rhinoconjunctivitis: A conjunctival allergen challenge study. Allergy 2019, 74, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Micera, A.; Pellegrini, G.; Merlo, D.; Rama, P.; De Luca, M.; Bonini, S.; Bonini, S. In vitro evidence of nerve growth factor effects on human conjunctival epithelial cell differentiation and mucin gene expression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4622–4630. [Google Scholar] [CrossRef]
- Li, W.; Sun, X.; Wang, Z.; Li, R.; Li, L. The effect of nerve growth factor on differentiation of corneal limbal epithelial cells to conjunctival goblet cells in vitro. Mol. Vis. 2010, 16, 2739–2744. [Google Scholar] [PubMed]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Sluysmans, S.; Salmaso, A.; Rouaud, F.; Mean, I.; Brini, M.; Citi, S. The PLEKHA7-PDZD11 complex regulates the localization of the calcium pump PMCA and calcium handling in cultured cells. J. Biol. Chem. 2022, 298, 102138. [Google Scholar] [CrossRef]
- Bazan, J.F.; Wiesmann, C. Chapter 41—The Mechanism of NGF Signaling Suggested by the p75 and TrkA Receptor Complexes. In Handbook of Cell Signaling, 2nd ed.; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 277–285. [Google Scholar]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Dembla, S.; Hasan, N.; Becker, A.; Beck, A.; Philipp, S.E. Transient receptor potential A1 channels regulate epithelial cell barriers formed by MDCK cells. FEBS Lett. 2016, 590, 1509–1520. [Google Scholar] [CrossRef]
- Brazhe, A.R.; Verisokin, A.Y.; Verveyko, D.V.; Postnov, D.E. Sodium-Calcium Exchanger Can Account for Regenerative Ca(2+) Entry in Thin Astrocyte Processes. Front. Cell Neurosci. 2018, 12, 250. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Docampo, R. Membrane Proteins in Trypanosomatids Involved in Ca(2+) Homeostasis and Signaling. Genes 2018, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Mergler, S.; Garreis, F.; Sahlmuller, M.; Lyras, E.M.; Reinach, P.S.; Dwarakanath, A.; Paulsen, F.; Pleyer, U. Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells. Histochem. Cell Biol. 2012, 137, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Kashio, M. Thermosensation involving thermo-TRPs. Mol. Cell Endocrinol. 2021, 520, 111089. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Caterina, M.J. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem. Neurosci. 2014, 5, 1107–1116. [Google Scholar] [CrossRef]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Brit J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- O’Neil, R.G.; Heller, S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005, 451, 193–203. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell. Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef]
- Gou, Q.; Song, Z.; Gong, Y.; Li, J. TRPV1 in Dry Eye Disease. Front. Biosci. 2024, 29, 175. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K. TRPV4 ion channel as important cell sensors. J. Anesth. 2016, 30, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Terada, Y.; Yamanaka, R.; Enoyoshi, M.; Ito, K. TRPV4 activation in human corneal epithelial cells promotes membrane mucin production. Biochem. Biophys. Res. Commun. 2024, 731, 150402. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ortar, G.; Schiano Moriello, A.; Serum, E.M.; Rusterholz, D.B. Structure-activity relationships of the prototypical TRPM8 agonist icilin. Bioorg. Med. Chem. Lett. 2015, 25, 2285–2290. [Google Scholar] [CrossRef]
- Nealen, M.L.; Gold, M.S.; Thut, P.D.; Caterina, M.J. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J. Neurophysiol. 2003, 90, 515–520. [Google Scholar] [CrossRef]
- Zakharian, E.; Cao, C.; Rohacs, T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 2010, 30, 12526–12534. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Andersson, D.A.; Chase, H.W.; Bevan, S. TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J. Neurosci. 2004, 24, 5364–5369. [Google Scholar] [CrossRef]
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat. Commun. 2015, 6, 7150. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Merayo-Lloves, J.; Gallar, J. What Causes Eye Pain? Curr. Ophthalmol. Rep. 2015, 3, 111–121. [Google Scholar] [CrossRef]
- Khajavi, N.; Reinach, P.S.; Slavi, N.; Skrzypski, M.; Lucius, A.; Strauss, O.; Kohrle, J.; Mergler, S. Thyronamine induces TRPM8 channel activation in human conjunctival epithelial cells. Cell Signal 2015, 27, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef]
- Yang, J.M.; Wei, E.T.; Kim, S.J.; Yoon, K.C. TRPM8 Channels and Dry Eye. Pharmaceuticals 2018, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, N.; Reinach, P.S.; Skrzypski, M.; Lude, A.; Mergler, S. L-carnitine reduces in human conjunctival epithelial cells hypertonic-induced shrinkage through interacting with TRPV1 channels. Cell Physiol. Biochem. 2014, 34, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Gonzalez-Gonzalez, O.; Gallar, J.; Belmonte, C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain 2014, 155, 1481–1491. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef]
- Diebold, Y.; Calonge, M.; Enriquez de Salamanca, A.; Callejo, S.; Corrales, R.M.; Saez, V.; Siemasko, K.F.; Stern, M.E. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4263–4274. [Google Scholar] [CrossRef][Green Version]
- Rech, L.; Dietrich-Ntoukas, T.; Reinach, P.S.; Brockmann, T.; Pleyer, U.; Mergler, S. Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells. Cells 2024, 13, 1339. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Mergler, S.; Mertens, C.; Valtink, M.; Reinach, P.S.; Szekely, V.C.; Slavi, N.; Garreis, F.; Abdelmessih, S.; Turker, E.; Fels, G.; et al. Functional significance of thermosensitive transient receptor potential melastatin channel 8 (TRPM8) expression in immortalized human corneal endothelial cells. Exp. Eye Res. 2013, 116, 337–349. [Google Scholar] [CrossRef]
- Lucius, A.; Chhatwal, S.; Valtink, M.; Reinach, P.S.; Li, A.; Pleyer, U.; Mergler, S. L-Carnitine Suppresses Transient Receptor Potential Vanilloid Type 1 Activation in Human Corneal Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 11815. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef]
- Liu, Y.; Leng, A.; Li, L.; Yang, B.; Shen, S.; Chen, H.; Zhu, E.; Xu, Q.; Ma, X.; Shi, P.; et al. AMTB, a TRPM8 antagonist, suppresses growth and metastasis of osteosarcoma through repressing the TGFbeta signaling pathway. Cell Death Dis. 2022, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Lashinger, E.S.; Steiginga, M.S.; Hieble, J.P.; Leon, L.A.; Gardner, S.D.; Nagilla, R.; Davenport, E.A.; Hoffman, B.E.; Laping, N.J.; Su, X. AMTB, a TRPM8 channel blocker: Evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Renal Physiol. 2008, 295, F803–F810. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, T.; Chen, J.; Cai, X.; Fu, Y. Uncovering the role of transient receptor potential channels in pterygium: A machine learning approach. Inflamm. Res. 2023, 72, 589–602. [Google Scholar] [CrossRef]
- Garreis, F.; Schroder, A.; Reinach, P.S.; Zoll, S.; Khajavi, N.; Dhandapani, P.; Lucius, A.; Pleyer, U.; Paulsen, F.; Mergler, S. Upregulation of Transient Receptor Potential Vanilloid Type-1 Channel Activity and Ca2+ Influx Dysfunction in Human Pterygial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2564–2577. [Google Scholar] [CrossRef]
- Tuylu, Y.; Okumus, S.; Gul, R.; Erbagci, I. High-throughput screening of transient receptor potential (TRP) channels in pterygium. Int. Ophthalmol. 2024, 44, 63. [Google Scholar] [CrossRef]
- Shahraki, T.; Arabi, A.; Feizi, S. Pterygium: An update on pathophysiology, clinical features, and management. Ther. Adv. Ophthalmol. 2021, 13, 25158414211020152. [Google Scholar] [CrossRef]
- Weil, A.; Moore, S.E.; Waite, N.J.; Randall, A.; Gunthorpe, M.J. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol. Pharmacol. 2005, 68, 518–527. [Google Scholar] [CrossRef]
- Zhang, L.; Barritt, G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004, 64, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fang, Z.; Wang, G.; Shi, M.; Wang, X.; Jiang, K.; Yang, Z.; Cao, R.; Tao, H.; Wang, X.; et al. Anti-tumor activity of the TRPM8 inhibitor BCTC in prostate cancer DU145 cells. Oncol. Lett. 2016, 11, 182–188. [Google Scholar] [CrossRef]
- Yapa, K.; Deuis, J.; Peters, A.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Vetter, I.; Monteith, G.R. Assessment of the TRPM8 inhibitor AMTB in breast cancer cells and its identification as an inhibitor of voltage gated sodium channels. Life Sci. 2018, 198, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, H.; Wang, Z.; Mergler, S.; Liu, H.; Kawakita, T.; Tachado, S.D.; Pan, Z.; Capo-Aponte, J.E.; Pleyer, U.; et al. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J. Cell Physiol. 2007, 213, 730–739. [Google Scholar] [CrossRef]
- Lucius, A.; Khajavi, N.; Reinach, P.S.; Kohrle, J.; Dhandapani, P.; Huimann, P.; Ljubojevic, N.; Grotzinger, C.; Mergler, S. 3-Iodothyronamine increases transient receptor potential melastatin channel 8 (TRPM8) activity in immortalized human corneal epithelial cells. Cell Signal 2016, 28, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Mergler, S.; Li, A.; Zahn, I.; Paulsen, F.; Garreis, F. Thermosensitive TRP Channels Are Functionally Expressed and Influence the Lipogenesis in Human Meibomian Gland Cells. Int. J. Mol. Sci. 2024, 25, 4043. [Google Scholar] [CrossRef]
- Duan, X.; Ju, M.; Liu, X.; Hu, J. Efficacy and safety of transient receptor potential channel modulators for dry eye: A systematic review and meta-analysis. Cont. Lens Anterior Eye 2025, 1–9. [Google Scholar] [CrossRef]
- Ashok, N.; Khamar, P.; D’Souza, S.; Gijs, M.; Ghosh, A.; Sethu, S.; Shetty, R. Ion channels in dry eye disease. Indian. J. Ophthalmol. 2023, 71, 1215–1226. [Google Scholar] [CrossRef]
- Bereiter, D.A.; Rahman, M.; Thompson, R.; Stephenson, P.; Saito, H. TRPV1 and TRPM8 Channels and Nocifensive Behavior in a Rat Model for Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3739–3746. [Google Scholar] [CrossRef]
- Jiang, H.; Takeda, K.; Lazarovici, P.; Katagiri, Y.; Yu, Z.X.; Dickens, G.; Chabuk, A.; Liu, X.W.; Ferrans, V.; Guroff, G. Nerve growth factor (NGF)-induced calcium influx and intracellular calcium mobilization in 3T3 cells expressing NGF receptors. J. Biol. Chem. 1999, 274, 26209–26216. [Google Scholar] [CrossRef] [PubMed]
- Milman, T.; Hu, D.N.; McCormick, S.A.; Eagle, R.C., Jr.; Crawford, J.B.; Chin, K.; Shields, C.L.; Shields, J.A.; Char, D.H.; Finger, P.T. Expression of neurotrophin receptors by retinoinvasive uveal melanoma. Melanoma Res. 2012, 22, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D.; Sefton, M.; Rodriguez-Tebar, A.; Grantyn, R. An abnormal response of retinoblastoma cells (Y-79) to neurotrophins. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1932–1939. [Google Scholar]
- Aubert, L.; Guilbert, M.; Corbet, C.; Genot, E.; Adriaenssens, E.; Chassat, T.; Bertucci, F.; Daubon, T.; Magne, N.; Le Bourhis, X.; et al. NGF-induced TrkA/CD44 association is involved in tumor aggressiveness and resistance to lestaurtinib. Oncotarget 2015, 6, 9807–9819. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm. Sin. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lubin, M.L.; Reitz, T.L.; Wang, Y.; Colburn, R.W.; Flores, C.M.; Qin, N. Molecular identification and functional characterization of a temperature-sensitive transient receptor potential channel (TRPM8) from canine. Eur. J. Pharmacol. 2006, 530, 23–32. [Google Scholar] [CrossRef]
- Bourque, C.W.; Ciura, S.; Trudel, E.; Stachniak, T.J.; Sharif-Naeini, R. Neurophysiological characterization of mammalian osmosensitive neurones. Exp. Physiol. 2007, 92, 499–505. [Google Scholar] [CrossRef]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 is activated by both acidic and basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef]
- Homuth, G.; Lietzow, J.; Schanze, N.; Golchert, J.; Kohrle, J. Endocrine, Metabolic and Pharmacological Effects of Thyronamines (TAM), Thyroacetic Acids (TA) and Thyroid Hormone Metabolites (THM) - Evidence from in vitro, Cellular, Experimental Animal and Human Studies. Exp. Clin. Endocrinol. Diabetes 2020, 128, 401–413. [Google Scholar] [CrossRef]
- Strauss, O.; Wienrich, M. Cultured retinal pigment epithelial cells from RCS rats express an increased calcium conductance compared with cells from non-dystrophic rats. Pflugers Arch. 1993, 425, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mergler, S.; Skrzypski, M.; Sassek, M.; Pietrzak, P.; Pucci, C.; Wiedenmann, B.; Strowski, M.Z. Thermo-sensitive transient receptor potential vanilloid channel-1 regulates intracellular calcium and triggers chromogranin A secretion in pancreatic neuroendocrine BON-1 tumor cells. Cell Signal 2012, 24, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Pigozzi, D.; Ducret, T.; Tajeddine, N.; Gala, J.L.; Tombal, B.; Gailly, P. Calcium store contents control the expression of TRPC1, TRPC3 and TRPV6 proteins in LNCaP prostate cancer cell line. Cell Calcium 2006, 39, 401–415. [Google Scholar] [CrossRef]
- Skrzypski, M.; Khajavi, N.; Mergler, S.; Szczepankiewicz, D.; Kolodziejski, P.A.; Metzke, D.; Wojciechowicz, T.; Billert, M.; Nowak, K.W.; Strowski, M.Z. TRPV6 channel modulates proliferation of insulin secreting INS-1E beta cell line. Biochim. Biophys. Acta 2015, 1853, 3202–3210. [Google Scholar] [CrossRef][Green Version]
- Lehen’kyi, V.; Raphael, M.; Oulidi, A.; Flourakis, M.; Khalimonchyk, S.; Kondratskyi, A.; Gordienko, D.V.; Mauroy, B.; Bonnal, J.L.; Skryma, R.; et al. TRPV6 determines the effect of vitamin D3 on prostate cancer cell growth. PLoS ONE 2011, 6, e16856. [Google Scholar] [CrossRef][Green Version]
- Bolanz, K.A.; Kovacs, G.G.; Landowski, C.P.; Hediger, M.A. Tamoxifen inhibits TRPV6 activity via estrogen receptor-independent pathways in TRPV6-expressing MCF-7 breast cancer cells. Mol. Cancer Res. 2009, 7, 2000–2010. [Google Scholar] [CrossRef]
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new “hot” cancer treatment. Biochim. Biophys. Acta 2007, 1773, 565–576. [Google Scholar] [CrossRef]
- Skrzypski, M.; Kolodziejski, P.A.; Mergler, S.; Khajavi, N.; Nowak, K.W.; Strowski, M.Z. TRPV6 modulates proliferation of human pancreatic neuroendocrine BON-1 tumour cells. Biosci. Rep. 2016, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vanden Abeele, F.; Shuba, Y.; Roudbaraki, M.; Lemonnier, L.; Vanoverberghe, K.; Mariot, P.; Skryma, R.; Prevarskaya, N. Store-operated Ca2+ channels in prostate cancer epithelial cells: Function, regulation, and role in carcinogenesis. Cell Calcium 2003, 33, 357–373. [Google Scholar] [CrossRef]
- Bodding, M.; Wissenbach, U.; Flockerzi, V. The recombinant human TRPV6 channel functions as Ca2+ sensor in human embryonic kidney and rat basophilic leukemia cells. J. Biol. Chem. 2002, 277, 36656–36664. [Google Scholar] [CrossRef]
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; Benitez Del Castillo, J.M.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 2011, 151, 792–798.e791. [Google Scholar] [CrossRef]
- Versura, P.; Profazio, V.; Campos, E.C. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr. Eye Res. 2010, 35, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Capo-Aponte, J.E.; Iserovich, P.; Reinach, P.S. Characterization of regulatory volume behavior by fluorescence quenching in human corneal epithelial cells. J. Membr. Biol. 2005, 207, 11–22. [Google Scholar] [CrossRef]
- Sidhaye, V.K.; Guler, A.D.; Schweitzer, K.S.; D’Alessio, F.; Caterina, M.J.; King, L.S. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc. Natl. Acad. Sci. USA 2006, 103, 4747–4752. [Google Scholar] [CrossRef] [PubMed]
- Troiano, P.; Monaco, G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: A cross-over study. Cornea 2008, 27, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Li, Z.; Park, S.H.; Yoon, K.C. Effect of hypotonic 0.18% sodium hyaluronate eyedrops on inflammation of the ocular surface in experimental dry eye. J. Ocul. Pharmacol. Ther. 2014, 30, 533–542. [Google Scholar] [CrossRef]
- Izquierdo, C.; Martin-Martinez, M.; Gomez-Monterrey, I.; Gonzalez-Muniz, R. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. Int. J. Mol. Sci. 2021, 22, 8502. [Google Scholar] [CrossRef]
- Lee, P.R.; Lee, J.Y.; Kim, H.B.; Lee, J.H.; Oh, S.B. TRPM8 Mediates Hyperosmotic Stimuli-Induced Nociception in Dental Afferents. J. Dent. Res. 2020, 99, 107–114. [Google Scholar] [CrossRef]
- Genova, T.; Grolez, G.P.; Camillo, C.; Bernardini, M.; Bokhobza, A.; Richard, E.; Scianna, M.; Lemonnier, L.; Valdembri, D.; Munaron, L.; et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J. Cell Biol. 2017, 216, 2107–2130. [Google Scholar] [CrossRef]
- Heumann, R.; Kachel, V.; Thoenen, H. Relationship between NGF-mediated volume increase and “priming effect” in fast and slow reacting clones of PC12 pheochromocytoma cells. Role of cAMP. Exp. Cell Res. 1983, 145, 179–190. [Google Scholar] [CrossRef]
- Leung, S.; O’Donnell, M.E.; Martinez, A.; Palfrey, H.C. Regulation by nerve growth factor and protein phosphorylation of Na/K/2Cl cotransport and cell volume in PC12 cells. J. Biol. Chem. 1994, 269, 10581–10589. [Google Scholar] [CrossRef] [PubMed]
- Tandrup, T.; Vestergaard, S.; Tomlinson, D.R.; Diemel, L.T.; Jakobsen, J. The structural effect of systemic NGF treatment on permanently axotomised dorsal root ganglion cells in adult rats. J. Anat. 1999, 194 Pt 3, 373–379. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Cell shrinkage and monovalent cation fluxes: Role in apoptosis. Arch. Biochem. Biophys. 2007, 462, 176–188. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.V.; Cotter, T.G. Cell shrinkage and apoptosis: A role for potassium and sodium ion efflux. Cell Death Differ. 1997, 4, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Bodding, M.; Fecher-Trost, C.; Flockerzi, V. Store-operated Ca2+ current and TRPV6 channels in lymph node prostate cancer cells. J. Biol. Chem. 2003, 278, 50872–50879. [Google Scholar] [CrossRef]
- Avila-Medina, J.; Mayoral-Gonzalez, I.; Dominguez-Rodriguez, A.; Gallardo-Castillo, I.; Ribas, J.; Ordonez, A.; Rosado, J.A.; Smani, T. The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells. Front. Physiol. 2018, 9, 257. [Google Scholar] [CrossRef]
- Song, S.; Ayon, R.J.; Yuan, J.X. Ryanodine receptor-2: A necessity for gating store-operated Ca2+ channels. Cardiovasc. Res. 2016, 111, 13–15. [Google Scholar] [CrossRef][Green Version]
- Okada, Y.; Maeno, E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 377–383. [Google Scholar] [CrossRef]
- Wei, L.; Xiao, A.Y.; Jin, C.; Yang, A.; Lu, Z.Y.; Yu, S.P. Effects of chloride and potassium channel blockers on apoptotic cell shrinkage and apoptosis in cortical neurons. Pflugers Arch. 2004, 448, 325–334. [Google Scholar] [CrossRef]
- Roumeau, S.; Dutheil, F.; Sapin, V.; Baker, J.S.; Watson, S.L.; Pereira, B.; Chiambaretta, F.; Navel, V. Efficacy of treatments for neurotrophic keratopathy: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2623–2637. [Google Scholar] [CrossRef]
- Lambiase, A.; Aloe, L.; Centofanti, M.; Parisi, V.; Bao, S.N.; Mantelli, F.; Colafrancesco, V.; Manni, G.L.; Bucci, M.G.; Bonini, S.; et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc. Natl. Acad. Sci. USA 2009, 106, 13469–13474. [Google Scholar] [CrossRef] [PubMed]
- Napoli, D.; Orsini, N.; Salamone, G.; Calvello, M.A.; Capsoni, S.; Cattaneo, A.; Strettoi, E. Human NGF “Painless” Ocular Delivery for Retinitis Pigmentosa: An In Vivo Study. eNeuro 2024, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Bonini, S.; Bonini, S.; Micera, A.; Magrini, L.; Bracci-Laudiero, L.; Aloe, L. Increased plasma levels of nerve growth factor in vernal keratoconjunctivitis and relationship to conjunctival mast cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2127–2132. [Google Scholar]
- Lambiase, A.; Bonini, S.; Micera, A.; Tirassa, P.; Magrini, L.; Bonini, S.; Aloe, L. Increased plasma levels of substance P in vernal keratoconjunctivitis. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2161–2164. [Google Scholar]
- Lee, H.K.; Ryu, I.H.; Seo, K.Y.; Hong, S.; Kim, H.C.; Kim, E.K. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology 2006, 113, 198–205. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, J.; Lee, Y.I.; Kim, J.; Choi, Y.S.; Ham, S.; Lee, J.H. Cutaneous neurogenic inflammation mediated by TRPV1-NGF-TRKA pathway activation in rosacea is exacerbated by the presence of Demodex mites. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2589–2600. [Google Scholar] [CrossRef]
- Evans, R.M.; Scott, R.H.; Ross, R.A. Chronic exposure of sensory neurones to increased levels of nerve growth factor modulates CB1/TRPV1 receptor crosstalk. Br. J. Pharmacol. 2007, 152, 404–413. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Chen, G.L.; Lei, M.; Zhou, L.P.; Zeng, B.; Zou, F. Borneol Is a TRPM8 Agonist that Increases Ocular Surface Wetness. PLoS ONE 2016, 11, e0158868. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Abir-Awan, M.; Al-Jubair, T.; Tornroth-Horsefield, S.; Conner, A.C.; Bill, R.M. Calcein Fluorescence Quenching to Measure Plasma Membrane Water Flux in Live Mammalian Cells. STAR Protoc. 2020, 1, 100157. [Google Scholar] [CrossRef]
- Davidson, A.F.; Higgins, A.Z. Detection of volume changes in calcein-stained cells using confocal microscopy. J. Fluoresc. 2013, 23, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, F.; Dietrich-Ntoukas, T.; Reinach, P.S.; Pleyer, U.; Mergler, S. Nerve Growth Factor Modulates Regulatory Cell Volume Behavior via Stimulating TRPV1, TRPM8 Channels and Inducing Ca2+ Signaling in Human Conjunctival Epithelial Cells. Cells 2025, 14, 719. https://doi.org/10.3390/cells14100719
Wolf F, Dietrich-Ntoukas T, Reinach PS, Pleyer U, Mergler S. Nerve Growth Factor Modulates Regulatory Cell Volume Behavior via Stimulating TRPV1, TRPM8 Channels and Inducing Ca2+ Signaling in Human Conjunctival Epithelial Cells. Cells. 2025; 14(10):719. https://doi.org/10.3390/cells14100719
Chicago/Turabian StyleWolf, Friedrich, Tina Dietrich-Ntoukas, Peter S. Reinach, Uwe Pleyer, and Stefan Mergler. 2025. "Nerve Growth Factor Modulates Regulatory Cell Volume Behavior via Stimulating TRPV1, TRPM8 Channels and Inducing Ca2+ Signaling in Human Conjunctival Epithelial Cells" Cells 14, no. 10: 719. https://doi.org/10.3390/cells14100719
APA StyleWolf, F., Dietrich-Ntoukas, T., Reinach, P. S., Pleyer, U., & Mergler, S. (2025). Nerve Growth Factor Modulates Regulatory Cell Volume Behavior via Stimulating TRPV1, TRPM8 Channels and Inducing Ca2+ Signaling in Human Conjunctival Epithelial Cells. Cells, 14(10), 719. https://doi.org/10.3390/cells14100719







