Immunological Mechanisms and Effects of Bacterial Infections in Acute-on-Chronic Liver Failure
Abstract
1. Introduction
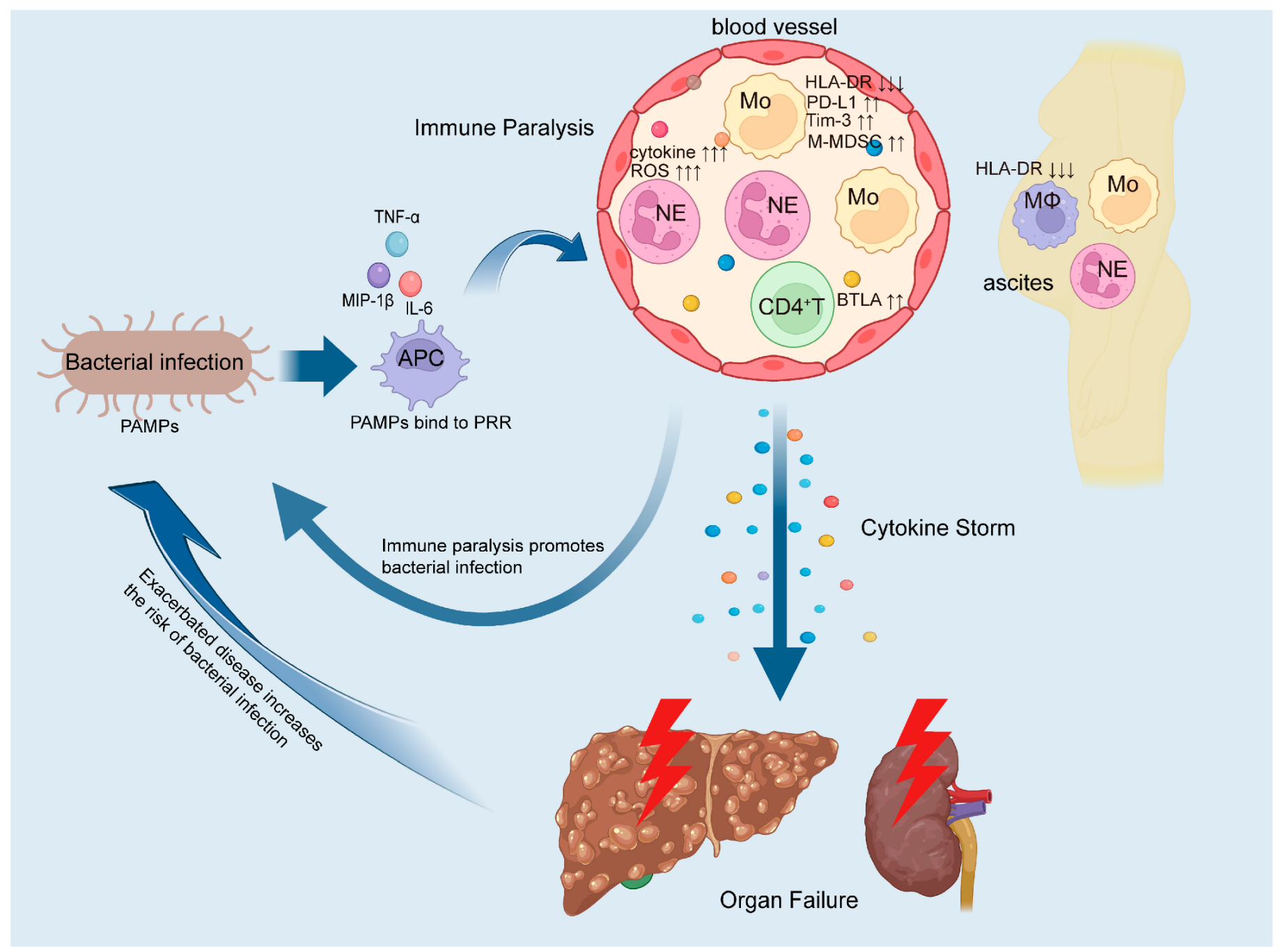
2. Immune Paralysis in ACLF Increases the Risk of Bacterial Infections
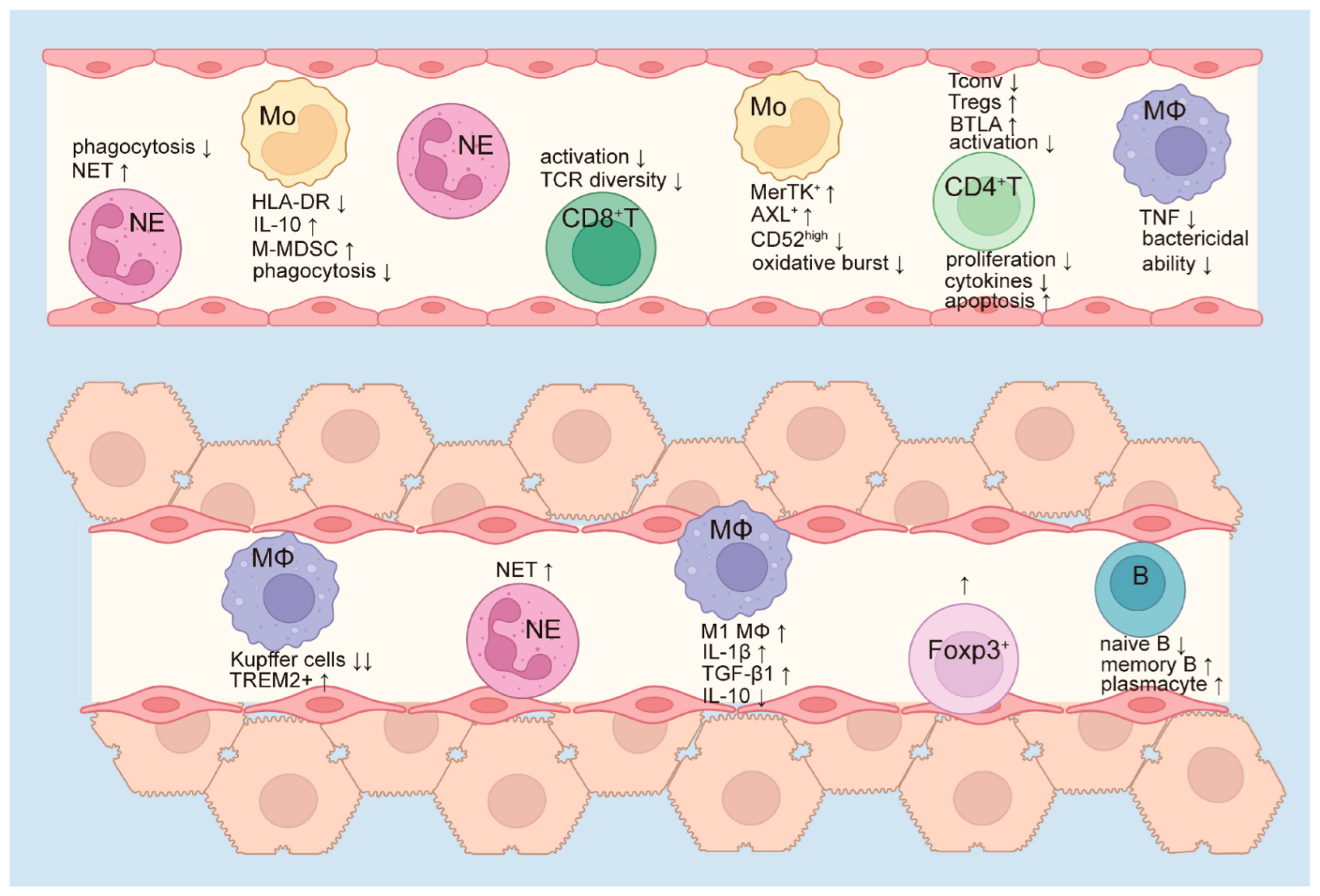
3. Innate Immune Dysfunction
4. Monocytes
5. Macrophages
6. Neutrophils
7. Adaptive Immune Suppression
8. T Cells
9. B Cells
10. Impact of Bacterial Infections on ACLF Immunity
11. Immunomodulatory Therapies
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bernal, W.; Jalan, R.; Quaglia, A.; Simpson, K.; Wendon, J.; Burroughs, A. Acute-on-chronic liver failure. Lancet 2015, 386, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Arroyo, V.; Moreau, R. Roles of systemic inflammatory and metabolic responses in the pathophysiology of acute-on-chronic liver failure. JHEP Rep. 2023, 5, 100807. [Google Scholar] [CrossRef] [PubMed]
- Feio-Azevedo, R.; Boesch, M.; Radenkovic, S.; van Melkebeke, L.; Smets, L.; Wallays, M.; Boeckx, B.; Philips, G.; de Oliveira, J.P.; Ghorbani, M.; et al. Distinct immunometabolic signatures in circulating immune cells define disease outcome in acute-on-chronic liver failure. Hepatology 2025, 81, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Gines, P.; Olson, J.C.; Mookerjee, R.P.; Moreau, R.; Garcia-Tsao, G.; Arroyo, V.; Kamath, P.S. Acute-on chronic liver failure. J. Hepatol. 2012, 57, 1336–1348. [Google Scholar] [CrossRef]
- Hernaez, R.; Kramer, J.R.; Liu, Y.; Tansel, A.; Natarajan, Y.; Hussain, K.B.; Ginès, P.; Solà, E.; Moreau, R.; Gerbes, A.; et al. Prevalence and short-term mortality of acute-on-chronic liver failure: A national cohort study from the USA. J. Hepatol. 2019, 70, 639–647. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Shao, L.; Xin, J.; Jiang, L.; Zhou, Q.; Shi, D.; Jiang, J.; Sun, S.; Jin, L.; et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2018, 67, 2181–2191. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef]
- Yu, X.; Yang, F.; Shen, Z.; Zhang, Y.; Sun, J.; Qiu, C.; Zheng, Y.; Zhao, W.; Yuan, S.; Zeng, D.; et al. BTLA contributes to acute-on-chronic liver failure infection and mortality through CD4(+) T-cell exhaustion. Nat. Commun. 2024, 15, 1835. [Google Scholar] [CrossRef]
- Mücke, M.M.; Rumyantseva, T.; Mücke, V.T.; Schwarzkopf, K.; Joshi, S.; Kempf, V.A.; Welsch, C.; Zeuzem, S.; Lange, C.M. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018, 38, 645–653. [Google Scholar] [CrossRef]
- Wong, F.; Piano, S.; Singh, V.; Bartoletti, M.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; et al. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J. Hepatol. 2021, 74, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, S.; Chokshi, S.; Bernsmeier, C.; Albillos, A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J. Hepatol. 2021, 75 (Suppl. 1), S82–S100. [Google Scholar] [CrossRef]
- Albillos, A.; Martin-Mateos, R.; Van der Merwe, S.; Wiest, R.; Jalan, R.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 112–134. [Google Scholar] [CrossRef]
- Bernsmeier, C.; van Der Merwe, S.; Périanin, A. Innate immune cells in cirrhosis. J. Hepatol. 2020, 73, 186–201. [Google Scholar] [CrossRef]
- Mezzano, G.; Juanola, A.; Cardenas, A.; Mezey, E.; Hamilton, J.P.; Pose, E.; Graupera, I.; Ginès, P.; Solà, E.; Hernaez, R. Global burden of disease: Acute-on-chronic liver failure, a systematic review and meta-analysis. Gut 2022, 71, 148–155. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef]
- Piano, S.; Singh, V.; Caraceni, P.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; Marino, M.; et al. Epidemiology and Effects of Bacterial Infections in Patients with Cirrhosis Worldwide. Gastroenterology 2019, 156, 1368–1380.e10. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Castro, M.; Garcia, O.; de Lope, C.R.; Roca, D.; Pavesi, M.; Sola, E.; Moreira, L.; Silva, A.; et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology 2012, 55, 1551–1561. [Google Scholar] [CrossRef]
- Fernández, J.; Prado, V.; Trebicka, J.; Amoros, A.; Gustot, T.; Wiest, R.; Deulofeu, C.; Garcia, E.; Acevedo, J.; Fuhrmann, V.; et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J. Hepatol. 2019, 70, 398–411. [Google Scholar] [CrossRef]
- Ariza, X.; Castellote, J.; Lora-Tamayo, J.; Girbau, A.; Salord, S.; Rota, R.; Ariza, J.; Xiol, X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J. Hepatol. 2012, 56, 825–832. [Google Scholar] [CrossRef]
- Arvaniti, V.; D’Amico, G.; Fede, G.; Manousou, P.; Tsochatzis, E.; Pleguezuelo, M.; Burroughs, A.K. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010, 139, 1246–1256.e5. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; O’Leary, J.G.; Reddy, K.R.; Wong, F.; Olson, J.C.; Subramanian, R.M.; Brown, G.; Noble, N.A.; Thacker, L.R.; Kamath, P.S.; et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: The North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology 2012, 56, 2328–2335. [Google Scholar] [CrossRef]
- Dionigi, E.; Garcovich, M.; Borzio, M.; Leandro, G.; Majumdar, A.; Tsami, A.; Arvaniti, V.; Roccarina, D.; Pinzani, M.; Burroughs, A.K.; et al. Bacterial Infections Change Natural History of Cirrhosis Irrespective of Liver Disease Severity. Am. J. Gastroenterol. 2017, 112, 588–596. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Triantafyllou, E.; Brenig, R.; Lebosse, F.J.; Singanayagam, A.; Patel, V.C.; Pop, O.T.; Khamri, W.; Nathwani, R.; Tidswell, R.; et al. CD14(+) CD15(-) HLA-DR(-) myeloid-derived suppressor cells impair antimicrobial responses in patients with acute-on-chronic liver failure. Gut 2018, 67, 1155–1167. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Pop, O.T.; Singanayagam, A.; Triantafyllou, E.; Patel, V.C.; Weston, C.J.; Curbishley, S.; Sadiq, F.; Vergis, N.; Khamri, W.; et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology 2015, 148, 603–615.e14. [Google Scholar] [CrossRef]
- Khamri, W.; Abeles, R.D.; Hou, T.Z.; Anderson, A.E.; El-Masry, A.; Triantafyllou, E.; Bernsmeier, C.; Larsen, F.S.; Singanayagam, A.; Kudo, N.; et al. Increased Expression of Cytotoxic T-Lymphocyte-Associated Protein 4 by T Cells, Induced by B7 in Sera, Reduces Adaptive Immunity in Patients with Acute Liver Failure. Gastroenterology 2017, 153, 263–276.e8. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Triantafyllou, E.; Woollard, K.J.; McPhail, M.J.W.; Antoniades, C.G.; Possamai, L.A. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front. Immunol. 2018, 9, 2948. [Google Scholar] [CrossRef]
- Antoniades, C.G.; Quaglia, A.; Taams, L.S.; Mitry, R.R.; Hussain, M.; Abeles, R.; Possamai, L.A.; Bruce, M.; McPhail, M.; Starling, C.; et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 2012, 56, 735–746. [Google Scholar] [CrossRef]
- Triantafyllou, E.; Pop, O.T.; Possamai, L.A.; Wilhelm, A.; Liaskou, E.; Singanayagam, A.; Bernsmeier, C.; Khamri, W.; Petts, G.; Dargue, R.; et al. MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure. Gut 2018, 67, 333–347. [Google Scholar] [CrossRef]
- Wu, W.; Yan, H.; Zhao, H.; Sun, W.; Yang, Q.; Sheng, J.; Shi, Y. Characteristics of systemic inflammation in hepatitis B-precipitated ACLF: Differentiate it from No-ACLF. Liver Int. 2018, 38, 248–257. [Google Scholar] [CrossRef]
- Thoma, C. ACLF monocyte dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-derived suppressor cells: An emerging target for anticancer immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef]
- Lu, Y.; Xin, J.; Liang, X.; Luo, J.; Li, P.; Zhou, X.; Yang, H.; Li, J.; Wang, Y. Plasma MERTK Is a Promising Biomarker for the Diagnosis and Prognosis of Hepatitis B Virus–Related Acute-on-Chronic Liver Failure. J. Infect. Dis. 2024, 230, 957–969. [Google Scholar] [CrossRef]
- Brenig, R.; Pop, O.T.; Triantafyllou, E.; Geng, A.; Singanayagam, A.; Perez-Shibayama, C.; Besse, L.; Cupovic, J.; Künzler, P.; Boldanova, T.; et al. Expression of AXL receptor tyrosine kinase relates to monocyte dysfunction and severity of cirrhosis. Life Sci. Alliance 2020, 3, e201900465. [Google Scholar] [CrossRef]
- Geng, A.; Brenig, R.G.; Roux, J.; Lütge, M.; Cheng, H.-W.; Flint, E.E.; Lussier, P.O.; Meier, M.-A.; Pop, O.T.; Künzler-Heule, P.; et al. Circulating monocytes upregulate CD52 and sustain innate immune function in cirrhosis unless acute decompensation emerges. J. Hepatol. 2025; in press. [Google Scholar] [CrossRef]
- Qiu, X.; Li, J.; Bonenfant, J.; Jaroszewski, L.; Mittal, A.; Klein, W.; Godzik, A.; Nair, M.G. Dynamic changes in human single-cell transcriptional signatures during fatal sepsis. J. Leukoc. Biol. 2021, 110, 1253–1268. [Google Scholar] [CrossRef]
- Rashidi, M.; Bandala-Sanchez, E.; Lawlor, K.E.; Zhang, Y.; Neale, A.M.; Vijayaraj, S.L.; O’Donoghue, R.; Wentworth, J.M.; E Adams, T.; Vince, J.E.; et al. CD52 inhibits Toll-like receptor activation of NF-κB and triggers apoptosis to suppress inflammation. Cell Death Differ. 2018, 25, 392–405. [Google Scholar] [CrossRef]
- O’Brien, A.J.; Fullerton, J.N.; Massey, K.A.; Auld, G.; Sewell, G.; James, S.; Newson, J.; Karra, E.; Winstanley, A.; Alazawi, W.; et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat. Med. 2014, 20, 518–523. [Google Scholar] [CrossRef]
- Korf, H.; Du Plessis, J.; Van Pelt, J.; De Groote, S.; Cassiman, D.; Verbeke, L.; Ghesquière, B.; Fendt, S.M.; Bird, M.J.; Talebi, A.; et al. Inhibition of glutamine synthetase in monocytes from patients with acute-on-chronic liver failure resuscitates their antibacterial and inflammatory capacity. Gut 2019, 68, 1872–1883. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, K.; Liu, S.; Zhao, Y.; Wang, Q. IGF2BP3 regulates macrophage-induced inflammation and liver damage in acute-on-chronic liver failure via the RORα-NF-κB signaling axis. Int. Immunopharmacol. 2024, 142, 113030. [Google Scholar] [CrossRef] [PubMed]
- China, L.; Maini, A.; Skene, S.S.; Shabir, Z.; Sylvestre, Y.; Colas, R.A.; Ly, L.; Salles, N.B.; Belloti, V.; Dalli, J.; et al. Albumin Counteracts Immune-Suppressive Effects of Lipid Mediators in Patients with Advanced Liver Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 738–747.e7. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.C.; Sánchez, E.; Romero, C.; Román, E.; Poca, M.; Guarner, C.; Juárez, C.; Soriano, G.; Vidal, S. Impaired innate immune response of leukocytes from ascitic fluid of patients with spontaneous bacterial peritonitis. J. Leukoc. Biol. 2015, 98, 819–825. [Google Scholar] [CrossRef]
- Nieto, J.C.; Perea, L.; Soriano, G.; Zamora, C.; Cantó, E.; Medina, A.; Poca, M.; Sanchez, E.; Roman, E.; Julià, G.; et al. Ascitic fluid regulates the local innate immune response of patients with cirrhosis. J. Leukoc. Biol. 2018, 104, 833–841. [Google Scholar] [CrossRef]
- Peng, B.; Li, H.; Liu, K.; Zhang, P.; Zhuang, Q.; Li, J.; Yang, M.; Cheng, K.; Ming, Y. Intrahepatic macrophage reprogramming associated with lipid metabolism in hepatitis B virus-related acute-on-chronic liver failure. J. Transl. Med. 2023, 21, 419. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.-L.; Li, J.-Q.; Wu, D.-S.; Li, Q.; Chen, B. Mitochondrial dysfunction affects hepatic immune and metabolic remodeling in patients with hepatitis B virus-related acute-on-chronic liver failure. World J. Gastroenterol. 2024, 30, 881–900. [Google Scholar] [CrossRef]
- Li, H.; Feng, D.; Cai, Y.; Liu, Y.; Xu, M.; Xiang, X.; Zhou, Z.; Xia, Q.; Kaplan, M.J.; Kong, X.; et al. Hepatocytes and neutrophils cooperatively suppress bacterial infection by differentially regulating lipocalin-2 and neutrophil extracellular traps. Hepatology 2018, 68, 1604–1620. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Wu, W.; Sun, S.; Wang, Y.; Zhao, R.; Ren, H.; Li, Z.; Zhao, H.; Zhang, Y.; Sheng, J.; Chen, Z.; et al. Circulating Neutrophil Dysfunction in HBV-Related Acute-on-Chronic Liver Failure. Front. Immunol. 2021, 12, 620365. [Google Scholar] [CrossRef]
- Rice, J.; Dodge, J.L.; Bambha, K.M.; Bajaj, J.S.; Reddy, K.R.; Gralla, J.; Ganapathy, D.; Mitrani, R.; Reuter, B.; Palecki, J.; et al. Neutrophil-to-Lymphocyte Ratio Associates Independently with Mortality in Hospitalized Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2018, 16, 1786–1791.e1. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Trehanpati, N.; Riese, P.; Rastogi, A.; Guzman, C.A.; Sarin, S.K. Blockade of Neutrophil’s Chemokine Receptors CXCR1/2 Abrogate Liver Damage in Acute-on-Chronic Liver Failure. Front. Immunol. 2017, 8, 464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, D.-P.; Chen, H.-D.; Wang, Y.-Z.; Shi, W.; Lu, Y.-T.; Ren, Y.-Z.; Wu, Y.-K.; Pang, Y.-H.; Deng, H.; et al. NK-cell–elicited gasdermin-D–dependent hepatocyte pyroptosis induces neutrophil extracellular traps that facilitate HBV-related acute-on-chronic liver failure. Hepatology 2025, 81, 917–931. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- McDonald, B.; Urrutia, R.; Yipp, B.G.; Jenne, C.N.; Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12, 324–333. [Google Scholar] [CrossRef]
- Burgener, S.S.; Schroder, K. Neutrophil Extracellular Traps in Host Defense. Cold Spring Harb. Perspect. Biol. 2020, 12, a037028. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef]
- Zhong, H.; Lu, R.-Y.; Wang, Y. Neutrophil extracellular traps in fungal infections: A seesaw battle in hosts. Front. Immunol. 2022, 13, 977493. [Google Scholar] [CrossRef]
- Schultz, B.M.; Acevedo, O.A.; Kalergis, A.M.; Bueno, S.M. Role of Extracellular Trap Release During Bacterial and Viral Infection. Front. Microbiol. 2022, 13, 798853. [Google Scholar] [CrossRef]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil extracellular traps: From physiology to pathology. Cardiovasc. Res. 2022, 118, 2737–2753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, K.; Zhu, B.; Feng, Y.; Liu, Y.; Wang, X. Neutrophil Extracellular Trap Scores Predict 90-Day Mortality in Hepatitis B-Related Acute-on-Chronic Liver Failure. Biomedicines 2024, 12, 2048. [Google Scholar] [CrossRef]
- Weiss, E.; de la Grange, P.; Defaye, M.; Lozano, J.J.; Aguilar, F.; Hegde, P.; Jolly, A.; Moga, L.; Sukriti, S.; Agarwal, B.; et al. Characterization of Blood Immune Cells in Patients with Decompensated Cirrhosis Including ACLF. Front. Immunol. 2020, 11, 619039. [Google Scholar] [CrossRef]
- Langer, M.; Sichelschmidt, S.; Bauschen, A.; Bornemann, L.; Guckenbiehl, S.; Gunzer, M.; Lange, C.M. Pathological neutrophil migration predicts adverse outcomes in hospitalized patients with liver cirrhosis. Liver Int. 2023, 43, 896–905. [Google Scholar] [CrossRef]
- Makkar, K.; Tomer, S.; Verma, N.; Rathi, S.; Arora, S.K.; Taneja, S.; Duseja, A.; Chawla, Y.K.; Dhiman, R.K. Neutrophil dysfunction predicts 90-day survival in patients with acute on chronic liver failure: A longitudinal case–control study. JGH Open 2020, 4, 595–602. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, J.; Lai, Q.; Zhao, Q.; Peng, L.; Xie, C.; Zhang, G.; Zhang, S.; Zhang, Y.; Zhu, J.; et al. Decreases in activated CD8+ T cells in patients with severe hepatitis B are related to outcomes. Dig. Dis. Sci. 2015, 60, 136–145. [Google Scholar] [CrossRef]
- Shen, C.; Yan, W.-Z.; Zhao, C.-Y.; Che, H.-H.; Liu, X.-Y.; Liu, Z.-Z.; Wang, Y.-D.; Wang, W.; Li, M.; Gao, J. Increased CD4+CD25+ regulatory T cells correlate with poor short-term outcomes in hepatitis B virus-related acute-on-chronic liver failure patients. J. Microbiol. Immunol. Infect. 2015, 48, 137–146. [Google Scholar] [CrossRef][Green Version]
- Dong, X.; Gong, Y.; Zeng, H.; Hao, Y.; Wang, X.; Hou, J.; Wang, J.; Li, J.; Zhu, Y.; Liu, H.; et al. Imbalance between circulating CD4+ regulatory T and conventional T lymphocytes in patients with HBV-related acute-on-chronic liver failure. Liver Int. 2013, 33, 1517–1526. [Google Scholar] [CrossRef]
- Yang, J.; Yi, P.; Wei, L.; Xu, Z.; Chen, Y.; Tang, L.; Li, L. Phenotypes and clinical significance of circulating CD4+CD25+ regulatory T cells (Tregs) in patients with acute-on-chronic liver failure (ACLF). J. Transl. Med. 2012, 10, 193. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Guo, G.; Li, H.; Cao, D.; Xu, H.; Guo, S.; Fei, L.; Yan, W.; Ning, Q.; et al. Expression of B and T lymphocyte attenuator (BTLA) in macrophages contributes to the fulminant hepatitis caused by murine hepatitis virus strain-3. Gut 2013, 62, 1204–1213. [Google Scholar] [CrossRef]
- Shen, G.; Sun, S.; Huang, J.; Deng, H.; Xu, Y.; Wang, Z.; Tang, X.; Gong, X. Dynamic changes of T cell receptor repertoires in patients with hepatitis B virus-related acute-on-chronic liver failure. Hepatol. Int. 2020, 14, 47–56. [Google Scholar] [CrossRef]
- Abdelbary, M.; Hobbs, S.J.; Gibbs, J.S.; Yewdell, J.W.; Nolz, J.C. T cell receptor signaling strength establishes the chemotactic properties of effector CD8+ T cells that control tissue-residency. Nat. Commun. 2023, 14, 3928. [Google Scholar] [CrossRef]
- Zikherman, J.; Au-Yeung, B. The role of T cell receptor signaling thresholds in guiding T cell fate decisions. Curr. Opin. Immunol. 2015, 33, 43–48. [Google Scholar] [CrossRef]
- Amin, A.M.; O’leary, J.G. To recover or not to recover from ACLF: Ask the monocytes. Hepatology 2025, 81, 396–398. [Google Scholar] [CrossRef]
- Lebossé, F.; Gudd, C.; Tunc, E.; Singanayagam, A.; Nathwani, R.; Triantafyllou, E.; Pop, O.; Kumar, N.; Mukherjee, S.; Hou, T.Z.; et al. CD8+ T cells from patients with cirrhosis display a phenotype that may contribute to cirrhosis-associated immune dysfunction. EBioMedicine 2019, 49, 258–268. [Google Scholar] [CrossRef]
- Zhao, Y.; He, W.; Wang, C.; Cui, N.; Yang, C.; You, Z.; Shi, B.; Xia, L.; Chen, X. Characterization of intrahepatic B cells in acute-on-chronic liver failure. Front. Immunol. 2022, 13, 1041176. [Google Scholar] [CrossRef]
- Francés, R.; Rodríguez, E.; Muñoz, C.; Zapater, P.; De La, M.L.; Ndongo, M.; Pérez-Mateo, M.; Such, J. Intracellular cytokine expression in peritoneal monocyte/macrophages obtained from patients with cirrhosis and presence of bacterial DNA. Eur. J. Gastroenterol. Hepatol. 2005, 17, 45–51. [Google Scholar] [CrossRef]
- Frances, R.; Muñoz, C.; Zapater, P.; Uceda, F.; Gascón, I.; Pascual, S.; Pérez-Mateo, M.; Such, J. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut 2004, 53, 860–864. [Google Scholar] [CrossRef]
- Fagan, K.J.; Rogers, G.B.; Melino, M.; Arthur, D.M.; Costello, M.-E.; Morrison, M.; Powell, E.E.; Irvine, K.M. Ascites bacterial burden and immune cell profile are associated with poor clinical outcomes in the absence of overt infection. PLoS ONE 2015, 10, e0120642. [Google Scholar] [CrossRef]
- Lesińska, M.; Hartleb, M.; Gutkowski, K.; Nowakowska-Duława, E. Procalcitonin and macrophage inflammatory protein-1 beta (MIP-1β) in serum and peritoneal fluid of patients with decompensated cirrhosis and spontaneous bacterial peritonitis. Adv. Med. Sci. 2014, 59, 52–56. [Google Scholar] [CrossRef]
- Hadjivasilis, A.; Tzanis, A.; Ioakim, K.J.; Poupoutsi, I.; Agouridis, A.P.; Kouis, P. The diagnostic accuracy of ascitic calprotectin for the early diagnosis of spontaneous bacterial peritonitis: Systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 312–318. [Google Scholar] [CrossRef]
- Singanayagam, A.; Triantafyllou, E. Macrophages in Chronic Liver Failure: Diversity, Plasticity and Therapeutic Targeting. Front. Immunol. 2021, 12, 661182. [Google Scholar] [CrossRef]
- Yadav, P.; Trehanpati, N.; Maiwall, R.; Sehgal, R.; Singh, R.; Islam, M.; Jagdish, R.K.; Vijayaraghavan, R.; Maheshwari, D.; Bhat, S.; et al. Soluble factors and suppressive monocytes can predict early development of sepsis in acute-on-chronic liver failure. Hepatol. Commun. 2022, 6, 2105–2120. [Google Scholar] [CrossRef]
- Bunt, S.K.; Clements, V.K.; Hanson, E.M.; Sinha, P.; Ostrand-Rosenberg, S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukoc. Biol. 2009, 85, 996–1004. [Google Scholar] [CrossRef]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R.; et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef]
- D’amico, G.; Bernardi, M.; Angeli, P. Towards a new definition of decompensated cirrhosis. J. Hepatol. 2022, 76, 202–207. [Google Scholar] [CrossRef]
- Maiwall, R.; Piano, S.; Singh, V.; Caraceni, P.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; Marino, M.; et al. Determinants of clinical response to empirical antibiotic treatment in patients with cirrhosis and bacterial and fungal infections-Results from the ICA “Global Study” (EABCIR-Global Study). Hepatology 2024, 79, 1019–1032. [Google Scholar] [CrossRef]
- Olona, A.; Hateley, C.; Muralidharan, S.; Wenk, M.R.; Torta, F.; Behmoaras, J. Sphingolipid metabolism during Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br. J. Pharmacol. 2021, 178, 4575–4587. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, D.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Diseases. Front. Immunol. 2019, 10, 2852. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Lu, C.; Wu, B.; Lan, C.; Mo, L.; Chen, C.; Wang, X.; Zhang, N.; Lan, L.; Wang, Q.; et al. Taurine Antagonizes Macrophages M1 Polarization by Mitophagy-Glycolysis Switch Blockage via Dragging SAM-PP2Ac Transmethylation. Front. Immunol. 2021, 12, 648913. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Akbari, P.; Wierenga, K.A.; Bates, M.A.; Gilley, K.N.; Wagner, J.G.; Lewandowski, R.P.; Rajasinghe, L.D.; Chauhan, P.S.; Lock, A.L.; et al. Omega-3 Polyunsaturated Fatty Acid Intervention Against Established Autoimmunity in a Murine Model of Toxicant-Triggered Lupus. Front. Immunol. 2021, 12, 653464. [Google Scholar] [CrossRef]
- Wang, S.; Liu, F.; Tan, K.S.; Ser, H.; Tan, L.T.; Lee, L.; Tan, W. Effect of (R)-salbutamol on the switch of phenotype and metabolic pattern in LPS-induced macrophage cells. J. Cell. Mol. Med. 2020, 24, 722–736. [Google Scholar] [CrossRef]
- Li, Z.H.; Chen, J.F.; Zhang, J.; Lei, Z.Y.; Wu, L.L.; Meng, S.B.; Wang, J.L.; Xiong, J.; Lin, D.N.; Wang, J.Y.; et al. Mesenchymal Stem Cells Promote Polarization of M2 Macrophages in Mice with Acute-On-Chronic Liver Failure via Mertk/JAK1/STAT6 Signaling. Stem Cells 2023, 41, 1171–1184. [Google Scholar] [CrossRef]
- Bonilha, C.S.; Veras, F.P.; de Queiroz Cunha, F. NET-targeted therapy: Effects, limitations, and potential strategies to enhance treatment efficacy. Trends Pharmacol. Sci. 2023, 44, 622–634. [Google Scholar] [CrossRef]
- Wang, H.; Yao, W.; Wang, Y.; Dong, H.; Dong, T.; Zhou, W.; Cui, L.; Zhao, L.; Zhang, Y.; Shi, L.; et al. Meta-analysis on last ten years of clinical injection of bone marrow-derived and umbilical cord MSC to reverse cirrhosis or rescue patients with acute-on-chronic liver failure. Stem Cell Res. Ther. 2023, 14, 267. [Google Scholar] [CrossRef]
- Lin, B.L.; Chen, J.F.; Qiu, W.H.; Wang, K.W.; Xie, D.Y.; Chen, X.Y.; Liu, Q.L.; Peng, L.; Li, J.G.; Mei, Y.Y.; et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology 2017, 66, 209–219. [Google Scholar] [CrossRef]
- Xu, W.-X.; He, H.-L.; Pan, S.-W.; Chen, Y.-L.; Zhang, M.-L.; Zhu, S.; Gao, Z.-L.; Peng, L.; Li, J.-G. Combination Treatments of Plasma Exchange and Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation for Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure: A Clinical Trial in China. Stem Cells Int. 2019, 2019, 4130757. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, H.; Shi, M.; Xu, R.; Fu, J.; Lv, J.; Chen, L.; Lv, S.; Li, Y.; Yu, S.; et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012, 27 (Suppl. 2), 112–120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-P.; Jiang, Y.-Z.; Sun, L.-Y.; Zhu, Z.-J. Therapeutic effect and safety of stem cell therapy for chronic liver disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2020, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef]
- Cuadra, B.; Silva, V.; Huang, Y.-L.; Diaz, Y.; Rivas, C.; Molina, C.; Simon, V.; Bono, M.R.; Morales, B.; Rosemblatt, M.; et al. The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model. Int. J. Mol. Sci. 2024, 25, 2073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, J.; Wu, J.; Zheng, X. Immunological Mechanisms and Effects of Bacterial Infections in Acute-on-Chronic Liver Failure. Cells 2025, 14, 718. https://doi.org/10.3390/cells14100718
Li S, Liu J, Wu J, Zheng X. Immunological Mechanisms and Effects of Bacterial Infections in Acute-on-Chronic Liver Failure. Cells. 2025; 14(10):718. https://doi.org/10.3390/cells14100718
Chicago/Turabian StyleLi, Sumeng, Jing Liu, Jun Wu, and Xin Zheng. 2025. "Immunological Mechanisms and Effects of Bacterial Infections in Acute-on-Chronic Liver Failure" Cells 14, no. 10: 718. https://doi.org/10.3390/cells14100718
APA StyleLi, S., Liu, J., Wu, J., & Zheng, X. (2025). Immunological Mechanisms and Effects of Bacterial Infections in Acute-on-Chronic Liver Failure. Cells, 14(10), 718. https://doi.org/10.3390/cells14100718






