Characterization of Extracellular Vesicles from Infrapatellar Fat Pad Mesenchymal Stem/Stromal Cells Expanded Using Regulatory-Compliant Media and Inflammatory/Hormonal Priming
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation, Culture, and Expansion of IFP-MSC
2.2. Inflammatory and Hormonal Priming
2.3. Flow Cytometric Analysis
2.4. IFP-MSC EV Isolation and Characterization
2.5. miRNA Profiling of IFP-MSC EVs
2.6. Macrophage Polarization Assay
2.7. Chondropellet/Synoviocyte Co-Culture Assay
2.8. Statistical Analysis
3. Results and Discussion
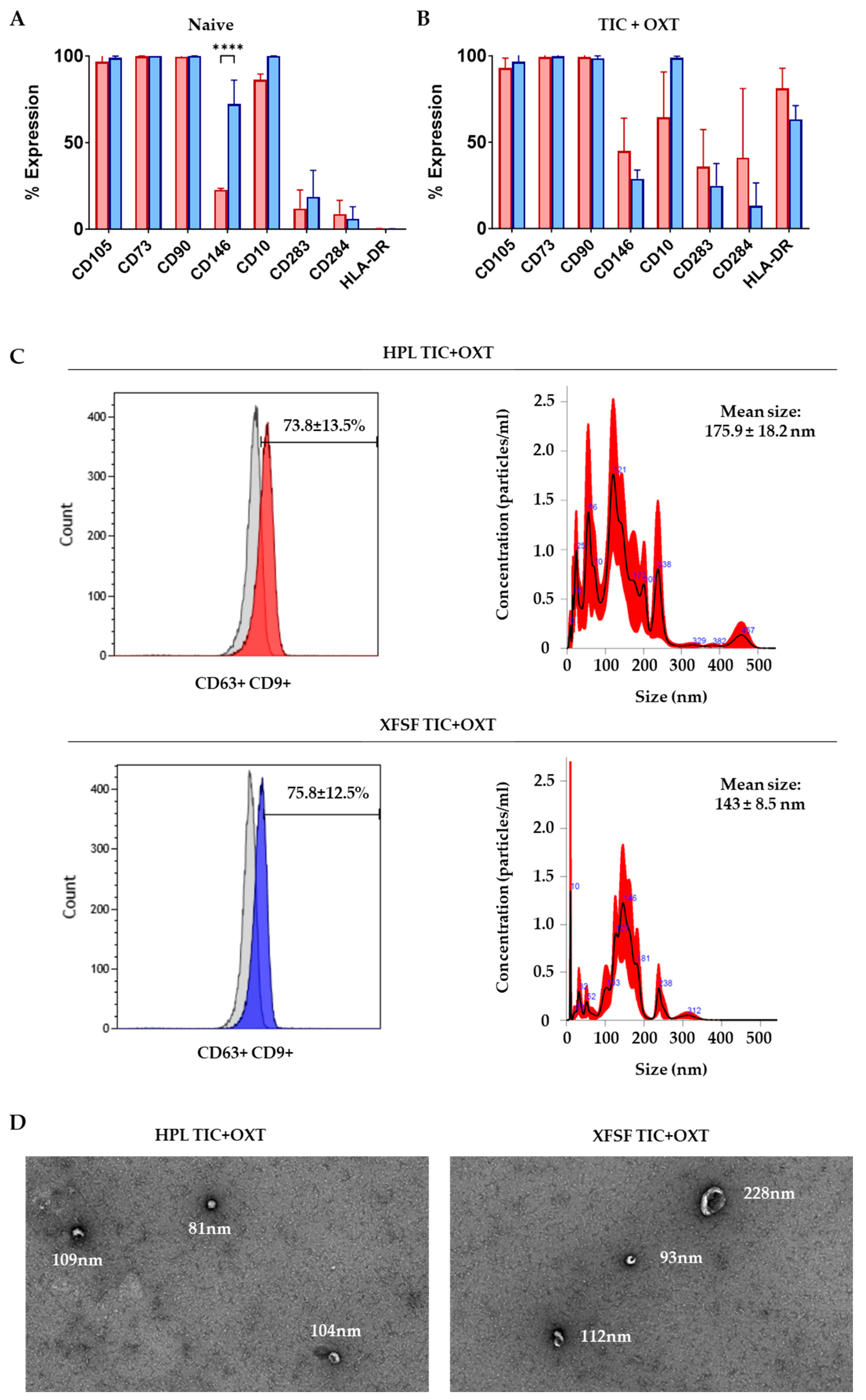
3.1. IFP-MSC Immunophenotyping and IFP-MSC EV Characterization with TIC+OXT Priming
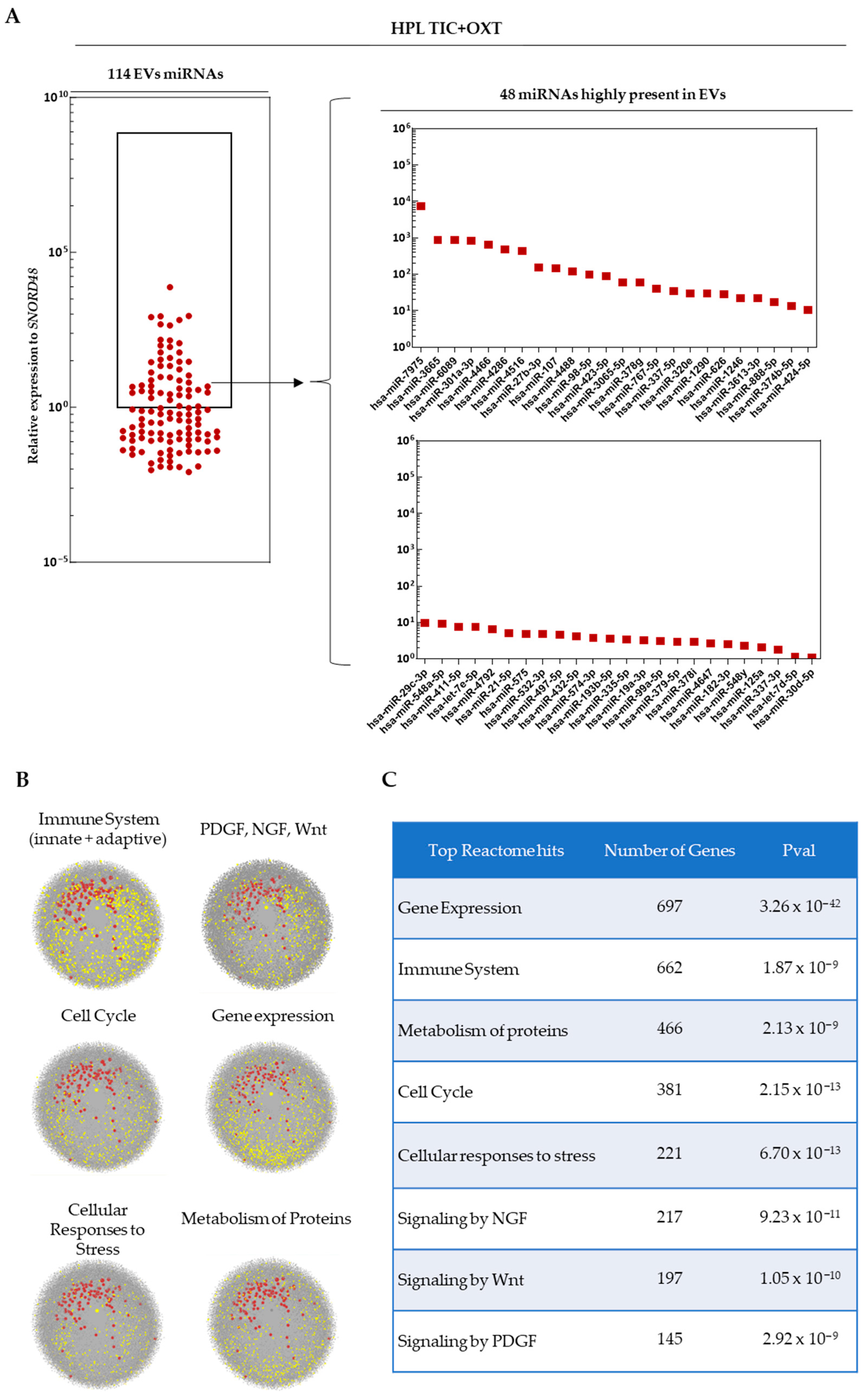
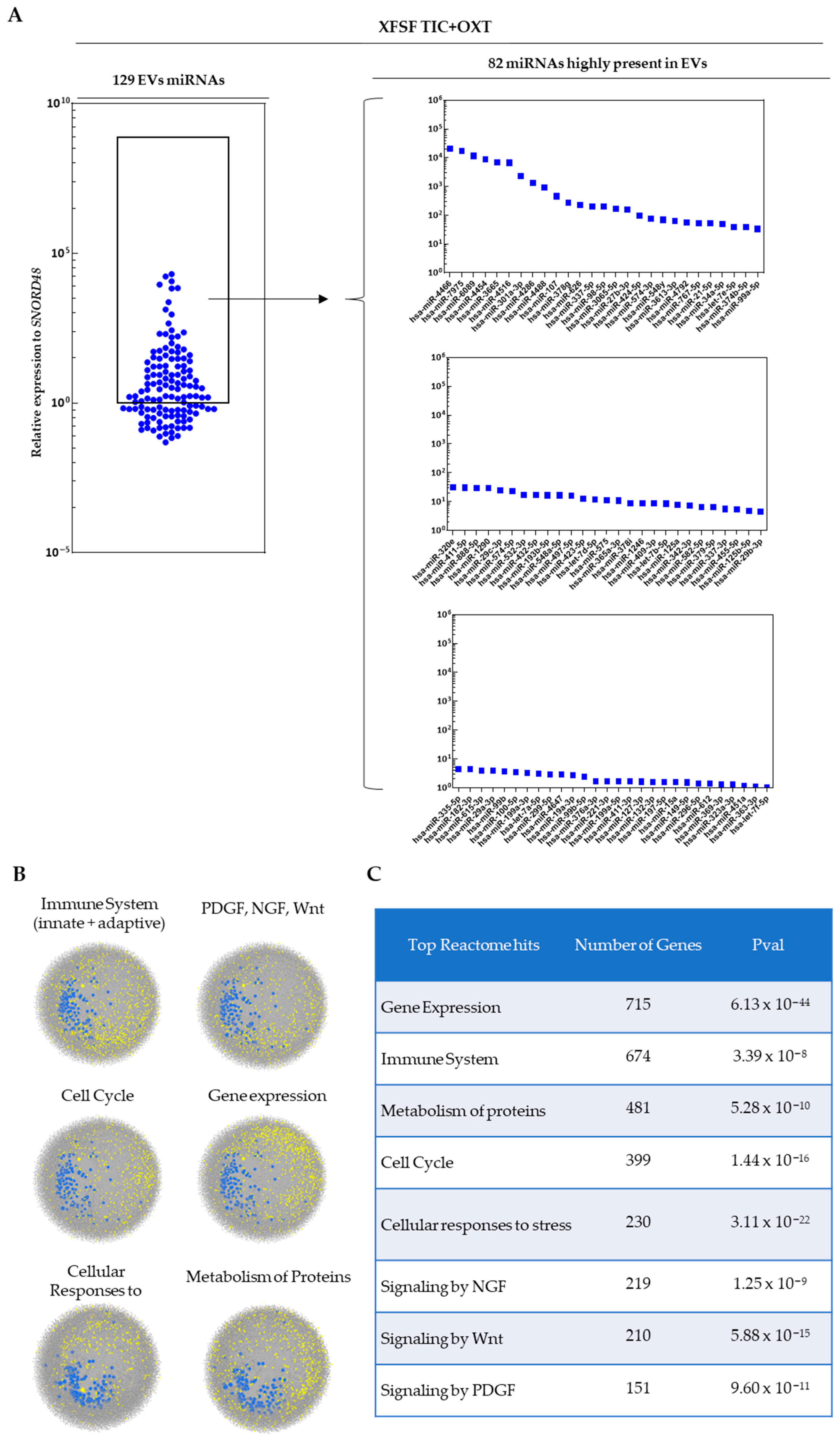
3.2. miRNA Profiling of TIC+OXT-Primed IFP-MSC-Derived EV Cargo
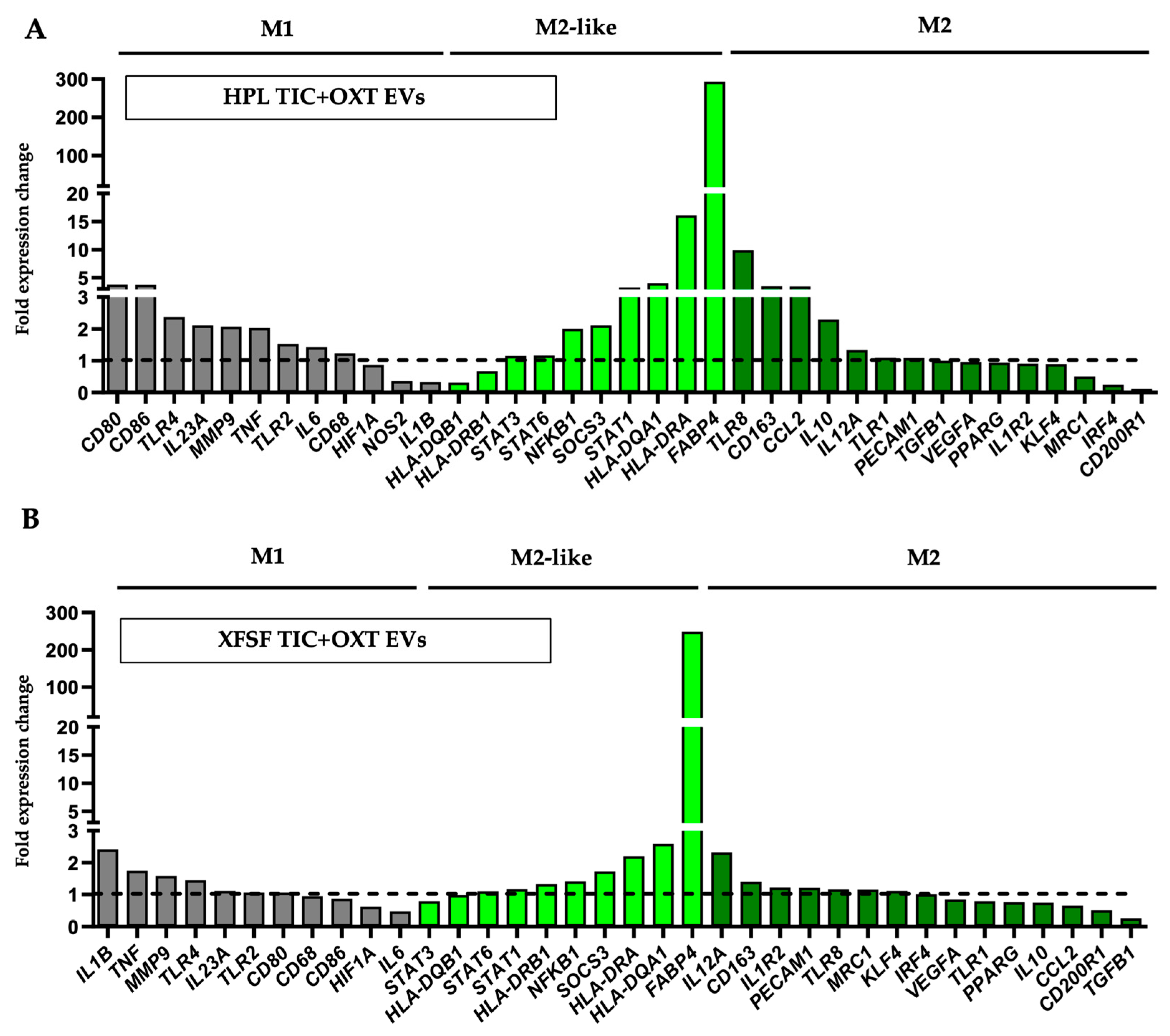
3.3. TIC+OXT-Primed IFP-MSC EVs Modulate Macrophage Polarization
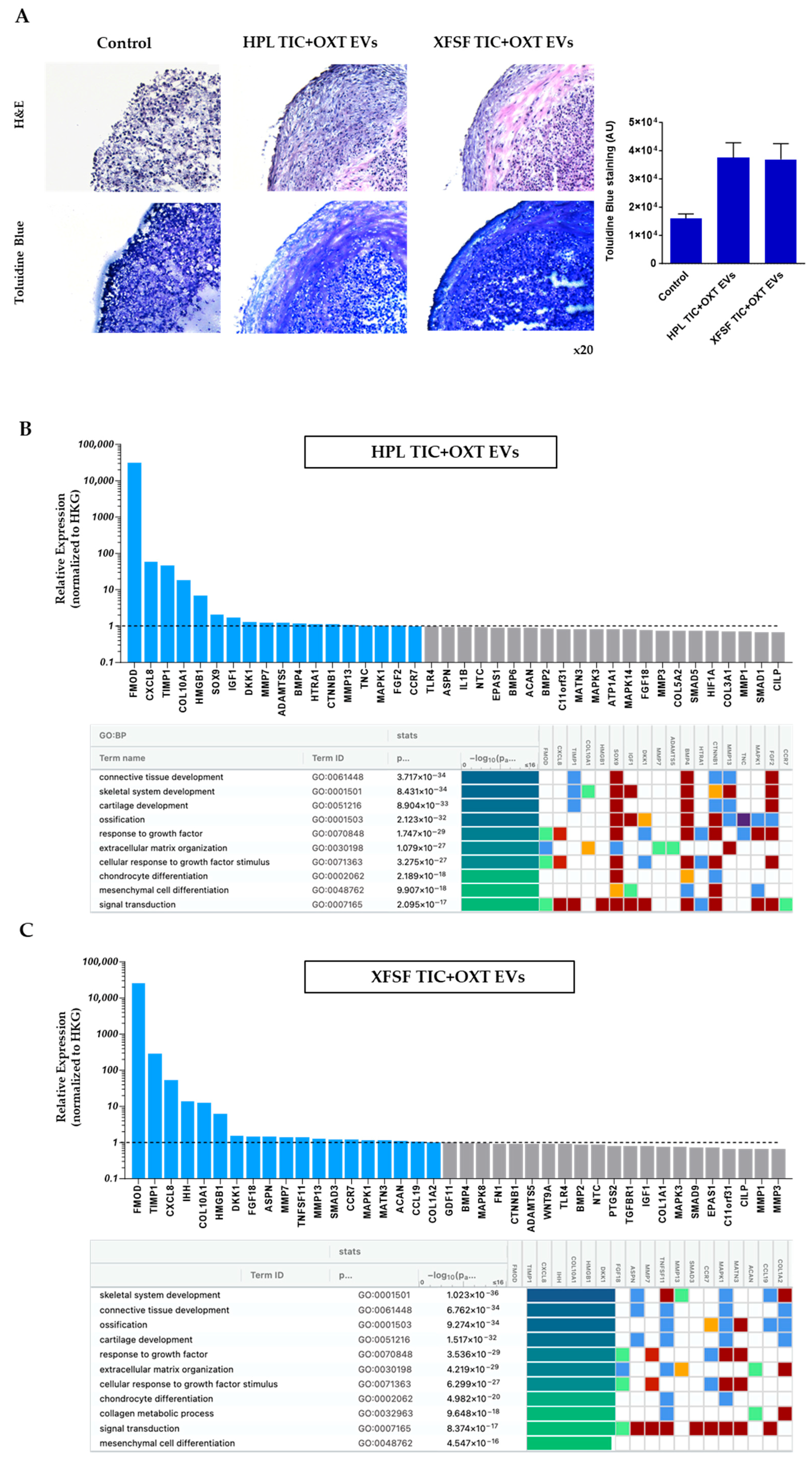
3.4. TIC+OXT-Primed IFP-MSC EVs Modulate Chondrocyte Gene Expression and Cartilage-Associated Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Perruccio, A.V.; Zahid, S.; Yip, C.; Power, J.D.; Canizares, M.; Heckman, G.A.; Badley, E.M. Cardiovascular Risk Profile and Osteoarthritis—Considering Sex and Multisite Joint Involvement: A Canadian Longitudinal Study on Aging. Arthritis Care Res. 2021, 75, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef]
- Barbour, K.E.; Lui, L.; Nevitt, M.C.; Murphy, L.B.; Helmick, C.G.; Theis, K.A.; Hochberg, M.C.; Lane, N.E.; Hootman, J.M.; Cauley, J.A.; et al. Hip Osteoarthritis and the Risk of All-Cause and Disease-Specific Mortality in Older Women: A Population-Based Cohort Study. Arthritis Rheumatol. 2015, 67, 1798–1805. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. Am. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef]
- Bosch, M.v.D.; van Lent, P.; van der Kraan, P. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Favaro, E.; Carpanetto, A.; Lamorte, S.; Fusco, A.; Caorsi, C.; Deregibus, M.C.; Bruno, S.; Amoroso, A.; Giovarelli, M.; Porta, M.; et al. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia 2014, 57, 1664–1673. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yin, X.-M.; Xu, Y.; Xu, C.-C.; Lin, X.; Ye, F.-B.; Cao, Y.; Lin, F.-Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma 2017, 34, 3388–3396. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Colombini, A.; Orfei, C.P.; Kouroupis, D.; Ragni, E.; De Luca, P.; Viganò, M.; Correa, D.; de Girolamo, L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy 2019, 21, 1179–1197. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Q.; Lu, L.; Cui, S.; Ma, K.; Zhang, W.; Ma, F.; Li, H.; Fu, X.; Zhang, C. Immunomodulatory potential of mesenchymal stem cell-derived extracellular vesicles: Targeting immune cells. Front. Immunol. 2023, 14, 1094685. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, Y.; Luo, C.; Cui, W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging 2020, 12, 25138–25152. [Google Scholar] [CrossRef]
- Kouroupis, D.; Kaplan, L.D.; Huard, J.; Best, T.M. CD10-Bound Human Mesenchymal Stem/Stromal Cell-Derived Small Extracellular Vesicles Possess Immunomodulatory Cargo and Maintain Cartilage Homeostasis under Inflammatory Conditions. Cells 2023, 12, 1824. [Google Scholar] [CrossRef]
- Leñero, C.; Kaplan, L.D.; Best, T.M.; Kouroupis, D. CD146+ Endometrial-Derived Mesenchymal Stem/Stromal Cell Subpopulation Possesses Exosomal Secretomes with Strong Immunomodulatory miRNA Attributes. Cells 2022, 11, 4002. [Google Scholar] [CrossRef]
- Kouroupis, D.; Kaplan, L.D.; Best, T.M. Human infrapatellar fat pad mesenchymal stem cells show immunomodulatory exosomal signatures. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, T.; Xu, B.; Xu, X.; Chen, H.; Li, X. Oxytocin prevents cartilage matrix destruction via regulating matrix metalloproteinases. Biochem. Biophys. Res. Commun. 2017, 486, 601–606. [Google Scholar] [CrossRef]
- Ferrero, S.; Amri, E.Z.; Roux, C.H. Relationship between Oxytocin and Osteoarthritis: Hope or Despair? Int. J. Mol. Sci. 2021, 22, 11784. [Google Scholar] [CrossRef]
- Kouroupis, D.; Bowles, A.C.; Greif, D.N.; Leñero, C.; Best, T.M.; Kaplan, L.D.; Correa, D. Regulatory-compliant conditions during cell product manufacturing enhance in vitro immunomodulatory properties of infrapatellar fat pad-derived mesenchymal stem/stromal cells. Cytotherapy 2020, 22, 677–689. [Google Scholar] [CrossRef]
- Kouroupis, D.; Bowles, A.C.; Best, T.M.; Kaplan, L.D.; Correa, D. CD10/Neprilysin Enrichment in Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells Under Regulatory-Compliant Conditions: Implications for Efficient Synovitis and Fat Pad Fibrosis Reversal. Am. J. Sports Med. 2020, 48, 2013–2027. [Google Scholar] [CrossRef]
- Kouroupis, D.; Best, T.M.; Kaplan, L.D.; Correa, D.; Griswold, A.J. Single-Cell RNA-Sequencing Identifies Infrapatellar Fat Pad Macrophage Polarization in Acute Synovitis/Fat Pad Fibrosis and Cell Therapy. Bioengineering 2021, 8, 166. [Google Scholar] [CrossRef]
- Kouroupis, D.; Bowles, A.C.; Willman, M.A.; Orfei, C.P.; Colombini, A.; Best, T.M.; Kaplan, L.D.; Correa, D. Infrapatellar fat pad-derived MSC response to inflammation and fibrosis induces an immunomodulatory phenotype involving CD10-mediated Substance P degradation. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell. Vesicles 2013, 2, 19861. [Google Scholar] [CrossRef]
- Gardashli, M.; Baron, M.; Drohat, P.; Quintero, D.; Kaplan, L.D.; Szeto, A.; Mendez, A.J.; Best, T.M.; Kouroupis, D. The roles of regulatory-compliant media and inflammatory/oxytocin priming selection in enhancing human mesenchymal stem/stromal cell immunomodulatory properties. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Bowles, A.C.; Kouroupis, D.; Willman, M.A.; Orfei, C.P.; Agarwal, A.; Correa, D. Signature quality attributes of CD146+ mesenchymal stem/stromal cells correlate with high therapeutic and secretory potency. STEM CELLS 2020, 38, 1034–1049. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, S.; Barbara, G.; Qiu, B.; Figini, M.; Geppetti, P.; Gerard, N.; Gerard, C.; Grady, E.F.; Bunnett, N.W.; Collins, S.M. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc. Natl. Acad. Sci. USA 1999, 96, 11653–11658. [Google Scholar] [CrossRef]
- A Shipp, M.; Stefano, G.B.; Switzer, S.N.; Griffin, J.D.; Reinherz, E.L. CD10 (CALLA)/neutral endopeptidase 24.11 modulates inflammatory peptide-induced changes in neutrophil morphology, migration, and adhesion proteins and is itself regulated by neutrophil activation. Blood 1991, 78, 1834–1841. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013, 330, 150–162. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Van Megen, K.M.; van ‘t Wout, E.T.; Lages Motta, J.; Dekker, B.; Nikolic, T.; Roep, B.O. Activated Mesenchymal Stromal Cells Process and Present Antigens Regulating Adaptive Immunity. Front. Immunol. 2019, 10, 694. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.; Gazeau, F. Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; De Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface Functionalization of Exosomes Using Click Chemistry. Bioconjugate Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Zhou, S.; Maleitzke, T.; Geissler, S.; Hildebrandt, A.; Fleckenstein, F.N.; Niemann, M.; Fischer, H.; Perka, C.; Duda, G.N.; Winkler, T. Source and hub of inflammation: The infrapatellar fat pad and its interactions with articular tissues during knee osteoarthritis. J. Orthop. Res. 2022, 40, 1492–1504. [Google Scholar] [CrossRef]
- Greif, D.N.; Kouroupis, D.; Murdock, C.J.; Griswold, A.J.; Kaplan, L.D.; Best, T.M.; Correa, D. Infrapatellar Fat Pad/Synovium Complex in Early-Stage Knee Osteoarthritis: Potential New Target and Source of Therapeutic Mesenchymal Stem/Stromal Cells. Front. Bioeng. Biotechnol. 2020, 8, 860. [Google Scholar] [CrossRef]
- Prieto-Potin, I.; Largo, R.; A Roman-Blas, J.; Herrero-Beaumont, G.; A Walsh, D. Characterization of multinucleated giant cells in synovium and subchondral bone in knee osteoarthritis and rheumatoid arthritis. BMC Musculoskelet. Disord. 2015, 16, 226. [Google Scholar] [CrossRef]
- Qian, Y.; Chu, G.; Zhang, L.; Wu, Z.; Wang, Q.; Guo, J.J.; Zhou, F. M2 macrophage-derived exosomal miR-26b-5p regulates macrophage polarization and chondrocyte hypertrophy by targeting TLR3 and COL10A1 to alleviate osteoarthritis. J. Nanobiotechnol. 2024, 22, 1–17. [Google Scholar] [CrossRef]
- Wang, L.; He, C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 2022, 13, 967193. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Dijkstra, C.D.; Berg, T.K.v.D. The macrophage scavenger receptor CD163. Immunobiology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Moestrup, S.; Møller, H. CD163: A regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann. Med. 2004, 36, 347–354. [Google Scholar] [CrossRef]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 Triggers Changes in Macrophage Phenotype That Promote Muscle Growth and Regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef]
- Lurier, E.B.; Dalton, D.; Dampier, W.; Raman, P.; Nassiri, S.; Ferraro, N.M.; Rajagopalan, R.; Sarmady, M.; Spiller, K.L. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology 2017, 222, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Bourke, E.; Cassetti, A.; Villa, A.; Fadlon, E.; Colotta, F.; Mantovani, A. IL-1β Scavenging by the Type II IL-1 Decoy Receptor in Human Neutrophils. J. Immunol. 2003, 170, 5999–6005. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.A.; Joesting, J.J.; Freund, G.G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 2013, 32, 1–8. [Google Scholar] [CrossRef]
- Shimizu, K.; Nakajima, A.; Sudo, K.; Liu, Y.; Mizoroki, A.; Ikarashi, T.; Horai, R.; Kakuta, S.; Watanabe, T.; Iwakura, Y. IL-1 Receptor Type 2 Suppresses Collagen-Induced Arthritis by Inhibiting IL-1 Signal on Macrophages. J. Immunol. 2015, 194, 3156–3168. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Kittan, N.A.; Allen, R.M.; Dhaliwal, A.; Cavassani, K.A.; Schaller, M.; Gallagher, K.A.; Carson, W.F.; Mukherjee, S.; Grembecka, J.; Cierpicki, T.; et al. Cytokine Induced Phenotypic and Epigenetic Signatures Are Key to Establishing Specific Macrophage Phenotypes. PLoS ONE 2013, 8, e78045. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Vereyken, E.J.; E Glim, J.; Heijnen, P.D.; Moeton, M.; van der Valk, P.; Amor, S.; E Teunissen, C.; van Horssen, J.; Dijkstra, C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 2013, 10, 801. [Google Scholar] [CrossRef]
- Rodríguez-Morales, P.; Franklin, R.A. Macrophage phenotypes and functions: Resolving inflammation and restoring homeostasis. Trends Immunol. 2023, 44, 986–998. [Google Scholar] [CrossRef]
- Almansour, S.; Dunster, J.L.; Crofts, J.J.; Nelson, M.R. Modelling the continuum of macrophage phenotypes and their role in inflammation. Math. Biosci. 2024, 377, 109289. [Google Scholar] [CrossRef]
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; McGonagle, D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002, 46, 3349–3360. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In VitroChondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Niu, B.; Tsang, K.Y.; Melhado, I.G.; Ohba, S.; He, X.; Huang, Y.; Wang, C.; McMahon, A.P.; Jauch, R.; et al. Synergistic co-regulation and competition by a SOX9-GLI-FOXA phasic transcriptional network coordinate chondrocyte differentiation transitions. PLoS Genet. 2018, 14, e1007346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mehmood, K.; Chang, Y.-F.; Tang, Z.; Li, Y.; Zhang, H. The molecular mechanisms of glycosaminoglycan biosynthesis regulating chondrogenesis and endochondral ossification. Life Sci. 2023, 335, 122243. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ohba, S.; Hojo, H.; McMahon, A.P. AP-1 family members act with Sox9 to promote chondrocyte hypertrophy. Development 2016, 143, 3012–3023. [Google Scholar] [CrossRef]
- Kikuchi, A.; Matsumoto, S.; Sada, R. Dickkopf signaling, beyond Wnt-mediated biology. Semin. Cell Dev. Biol. 2022, 125, 55–65. [Google Scholar] [CrossRef]
- Nalesso, G.; Sherwood, J.; Bertrand, J.; Pap, T.; Ramachandran, M.; De Bari, C.; Pitzalis, C.; Dell’Accio, F. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J. Cell Biol. 2011, 193, 551–564. [Google Scholar] [CrossRef]
- Ohba, S. Hedgehog Signaling in Skeletal Development: Roles of Indian Hedgehog and the Mode of Its Action. Int. J. Mol. Sci. 2020, 21, 6665. [Google Scholar] [CrossRef]
- Amano, K.; Densmore, M.; Nishimura, R.; Lanske, B. Indian Hedgehog Signaling Regulates Transcription and Expression of Collagen Type X via Runx2/Smads Interactions. J. Biol. Chem. 2014, 289, 24898–24910. [Google Scholar] [CrossRef]
- Muttigi, M.S.; Han, I.; Park, H.-K.; Park, H.; Lee, S.-H. Matrilin-3 Role in Cartilage Development and Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 590. [Google Scholar] [CrossRef]
- Vincent, T.L.; McClurg, O.; Troeberg, L. The Extracellular Matrix of Articular Cartilage Controls the Bioavailability of Pericellular Matrix-Bound Growth Factors to Drive Tissue Homeostasis and Repair. Int. J. Mol. Sci. 2022, 23, 6003. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Yu, A.; Jie, S.; Du, X.; Liu, T.; Wang, W.; Luo, Y. Bioinformatics analysis of differentially expressed genes involved in human developmental chondrogenesis. Medicine 2019, 98, e16240. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhou, Y.; Polson, S.W.; Wan, L.Q.; Wang, M.; Han, L.; Wang, L.; Lu, X.L. Identification of Chondrocyte Genes and Signaling Pathways in Response to Acute Joint Inflammation. Sci. Rep. 2019, 9, 93. [Google Scholar] [CrossRef]
- de Kroon, L.M.; Akker, G.G.v.D.; Brachvogel, B.; Narcisi, R.; Belluoccio, D.; Jenner, F.; Bateman, J.F.; Little, C.B.; Brama, P.A.; Davidson, E.N.B.; et al. Identification of TGFβ-related genes regulated in murine osteoarthritis and chondrocyte hypertrophy by comparison of multiple microarray datasets. Bone 2018, 116, 67–77. [Google Scholar] [CrossRef]
- Li, S.; Pritchard, D.M.; Yu, L.-G. Regulation and Function of Matrix Metalloproteinase-13 in Cancer Progression and Metastasis. Cancers 2022, 14, 3263. [Google Scholar] [CrossRef]
- Kudo, Y.; Iizuka, S.; Yoshida, M.; Tsunematsu, T.; Kondo, T.; Subarnbhesaj, A.; Deraz, E.M.; Siriwardena, S.B.S.M.; Tahara, H.; Ishimaru, N.; et al. Matrix Metalloproteinase-13 (MMP-13) Directly and Indirectly Promotes Tumor Angiogenesis. J. Biol. Chem. 2012, 287, 38716–38728. [Google Scholar] [CrossRef]
- Zheng, Z.; Granado, H.; Li, C. Fibromodulin, a Multifunctional Matricellular Modulator. J. Dent. Res. 2022, 102, 125–134. [Google Scholar] [CrossRef]
- Jian, J.; Zheng, Z.; Zhang, K.; Rackohn, T.M.; Hsu, C.; Levin, A.; Enjamuri, D.R.; Zhang, X.; Ting, K.; Soo, C. Fibromodulin promoted in vitro and in vivo angiogenesis. Biochem. Biophys. Res. Commun. 2013, 436, 530–535. [Google Scholar] [CrossRef]
- Han, Z.J.; Li, Y.B.; Yang, L.X.; Cheng, H.J.; Liu, X.; Chen, H. Roles of the CXCL8-CXCR1/2 Axis in the Tumor Microenvironment and Immunotherapy. Molecules 2021, 27, 137. [Google Scholar] [CrossRef]
- Grünwald, B.; Schoeps, B.; Krüger, A. Recognizing the Molecular Multifunctionality and Interactome of TIMP-1. Trends Cell Biol. 2019, 29, 6–19. [Google Scholar] [CrossRef]
- Ries, C. Cytokine functions of TIMP-1. Cell. Mol. Life Sci. 2013, 71, 659–672. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippon, M., Jr.; Labib, R.; Ley, M.B.R.G.; Kaplan, L.D.; Mendez, A.J.; Best, T.M.; Kouroupis, D. Characterization of Extracellular Vesicles from Infrapatellar Fat Pad Mesenchymal Stem/Stromal Cells Expanded Using Regulatory-Compliant Media and Inflammatory/Hormonal Priming. Cells 2025, 14, 706. https://doi.org/10.3390/cells14100706
Philippon M Jr., Labib R, Ley MBRG, Kaplan LD, Mendez AJ, Best TM, Kouroupis D. Characterization of Extracellular Vesicles from Infrapatellar Fat Pad Mesenchymal Stem/Stromal Cells Expanded Using Regulatory-Compliant Media and Inflammatory/Hormonal Priming. Cells. 2025; 14(10):706. https://doi.org/10.3390/cells14100706
Chicago/Turabian StylePhilippon, Marc, Jr., Ramy Labib, Michelle Bellas Romariz Gaudie Ley, Lee D. Kaplan, Armando J. Mendez, Thomas M. Best, and Dimitrios Kouroupis. 2025. "Characterization of Extracellular Vesicles from Infrapatellar Fat Pad Mesenchymal Stem/Stromal Cells Expanded Using Regulatory-Compliant Media and Inflammatory/Hormonal Priming" Cells 14, no. 10: 706. https://doi.org/10.3390/cells14100706
APA StylePhilippon, M., Jr., Labib, R., Ley, M. B. R. G., Kaplan, L. D., Mendez, A. J., Best, T. M., & Kouroupis, D. (2025). Characterization of Extracellular Vesicles from Infrapatellar Fat Pad Mesenchymal Stem/Stromal Cells Expanded Using Regulatory-Compliant Media and Inflammatory/Hormonal Priming. Cells, 14(10), 706. https://doi.org/10.3390/cells14100706








