Transcriptomic Profile of Early Antral Follicles: Predictive Somatic Gene Markers of Oocyte Maturation Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ovary Collection
2.2. Ovarian Surface Epithelium (OSE) Cell Collection for Follicle-Enclosed Oocyte (FEO) Coculture System
2.3. FEO In Vitro Maturation from EAfs
2.4. Oocyte Nuclear Stage Assessment and FW Collection
2.5. In Vitro Embryo Production
2.6. Microarray Transcriptomic Analysis
2.7. Network Creation, Visualization, and Analysis
2.8. Identification of Highly Interconnected Regions (Modules) Using the MCODE Algorithm
2.9. Identification of Drivers Within Network Modules
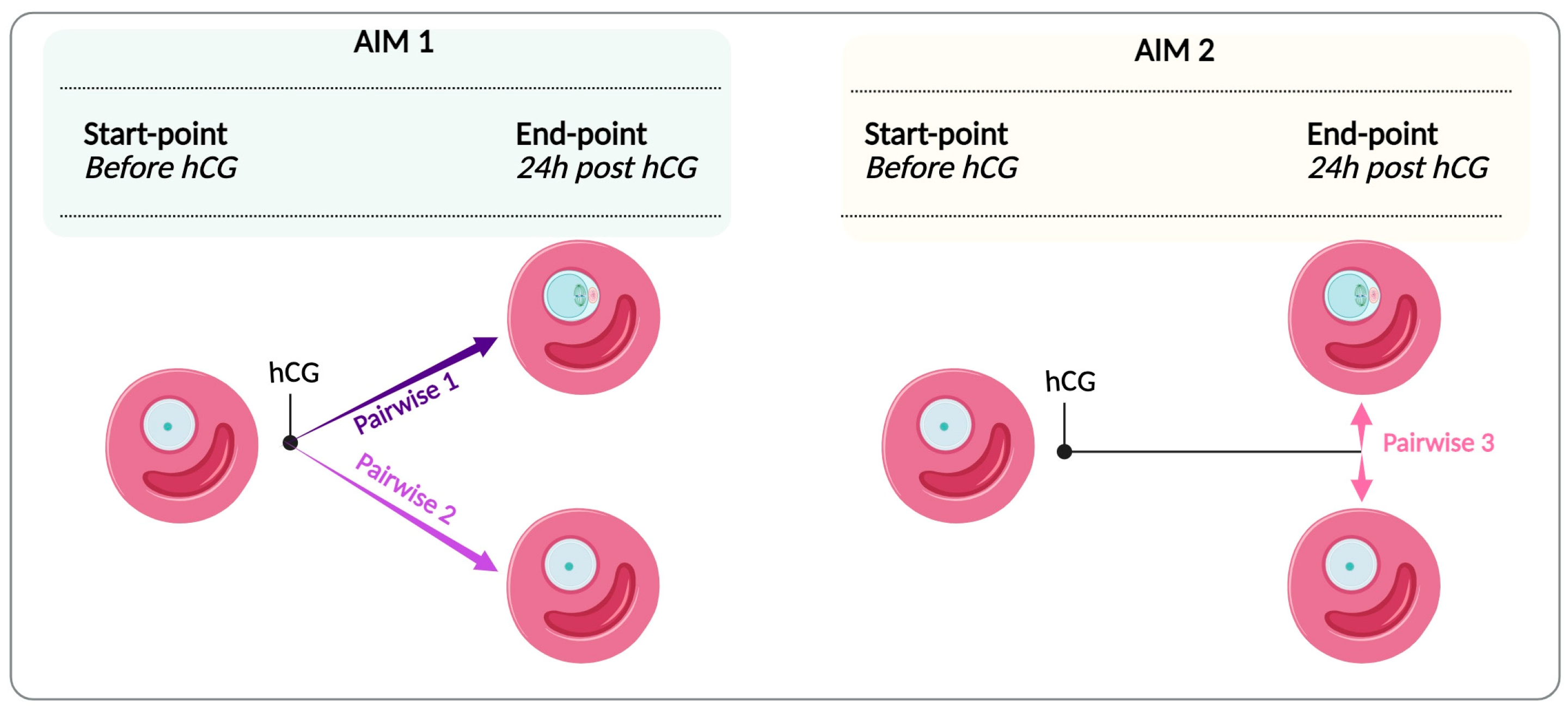
2.10. Venn Diagram
2.11. Microarray Validation Through Real-Time qPCR
2.12. Statistical Analysis
3. Results
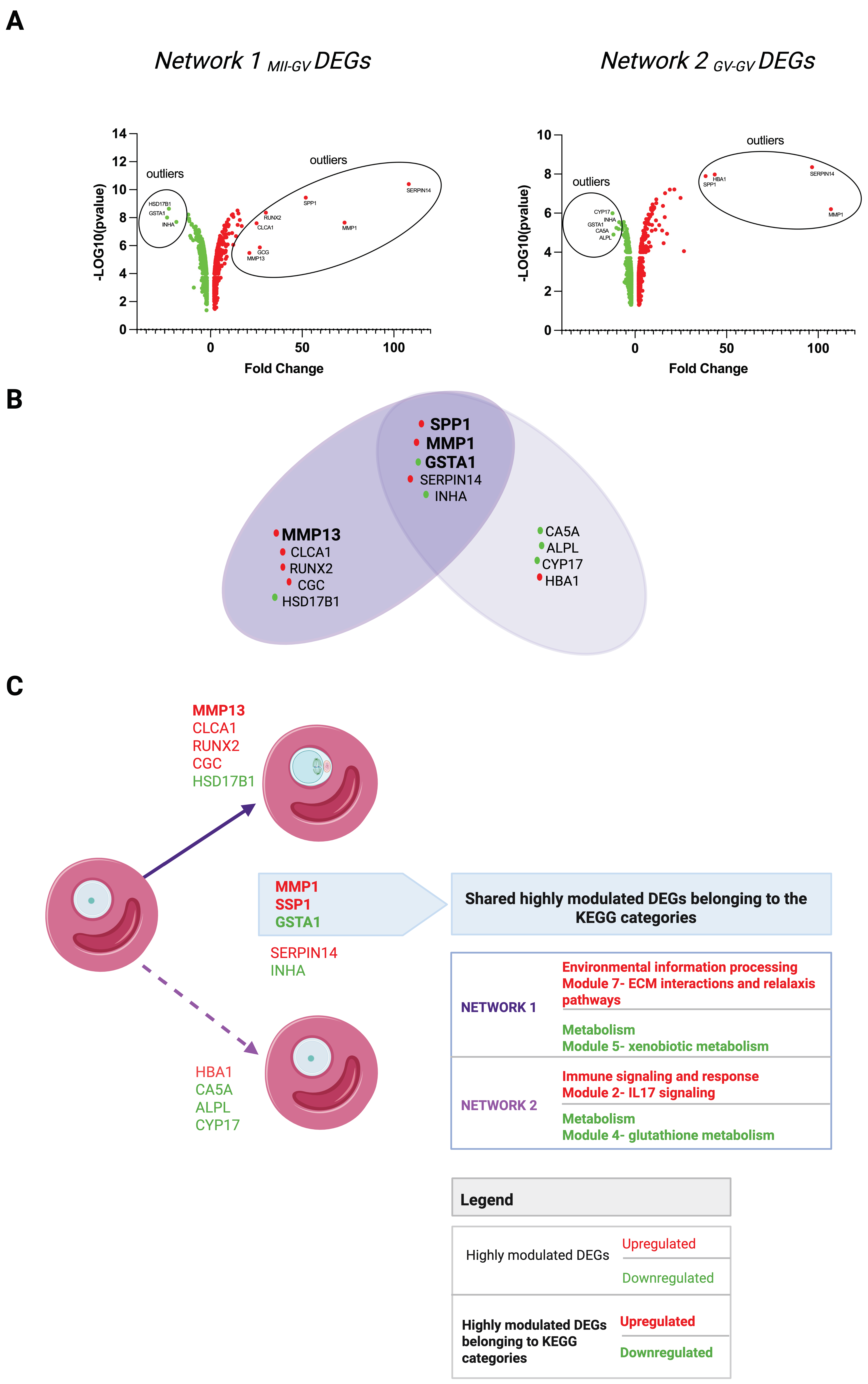
3.1. Comparative Transcriptomic Analysis of FW Compartment from EAfs Enclosing Competent and Incompetent Oocytes
3.2. FW Driver Genes Promoting Maturation in EAfs
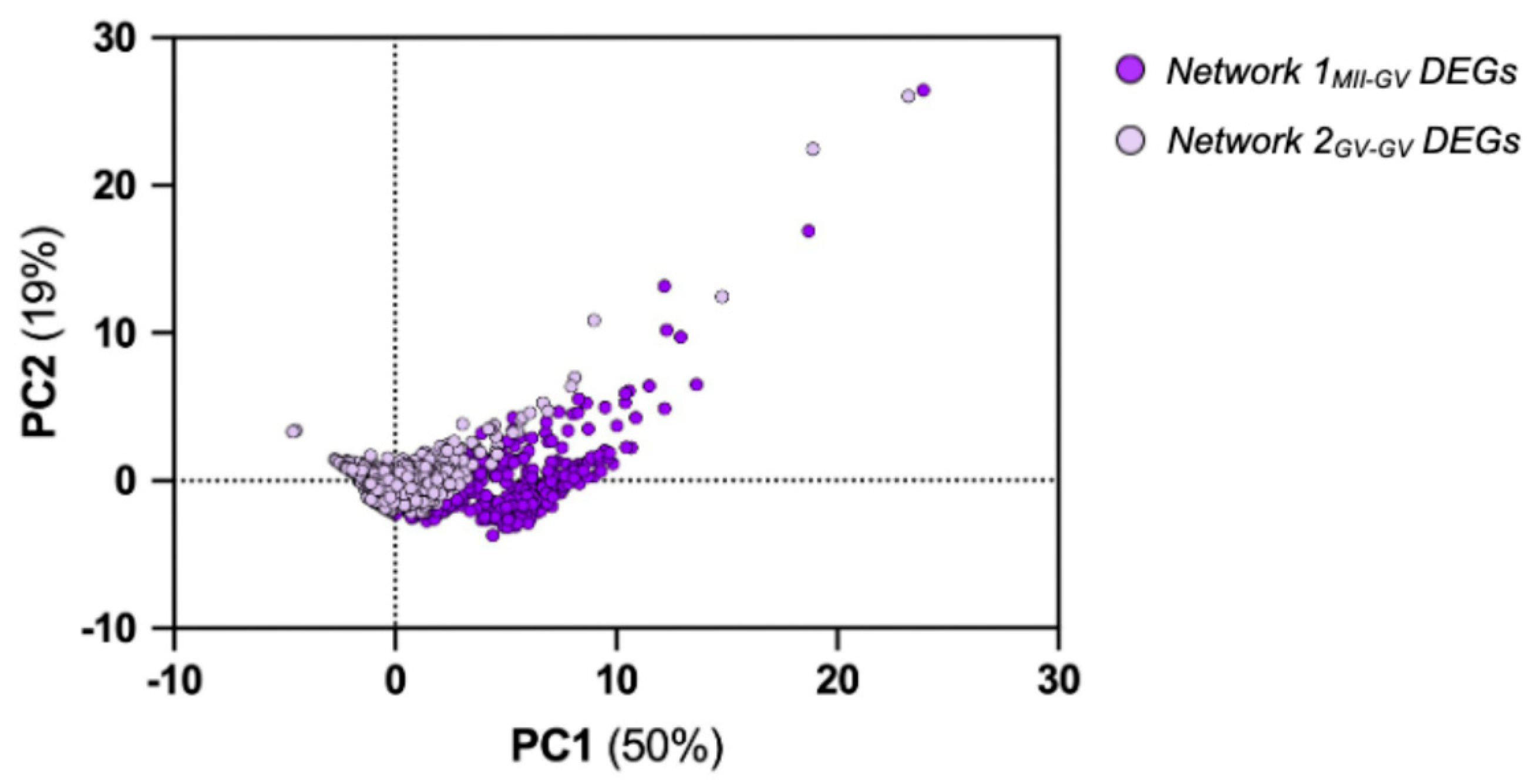
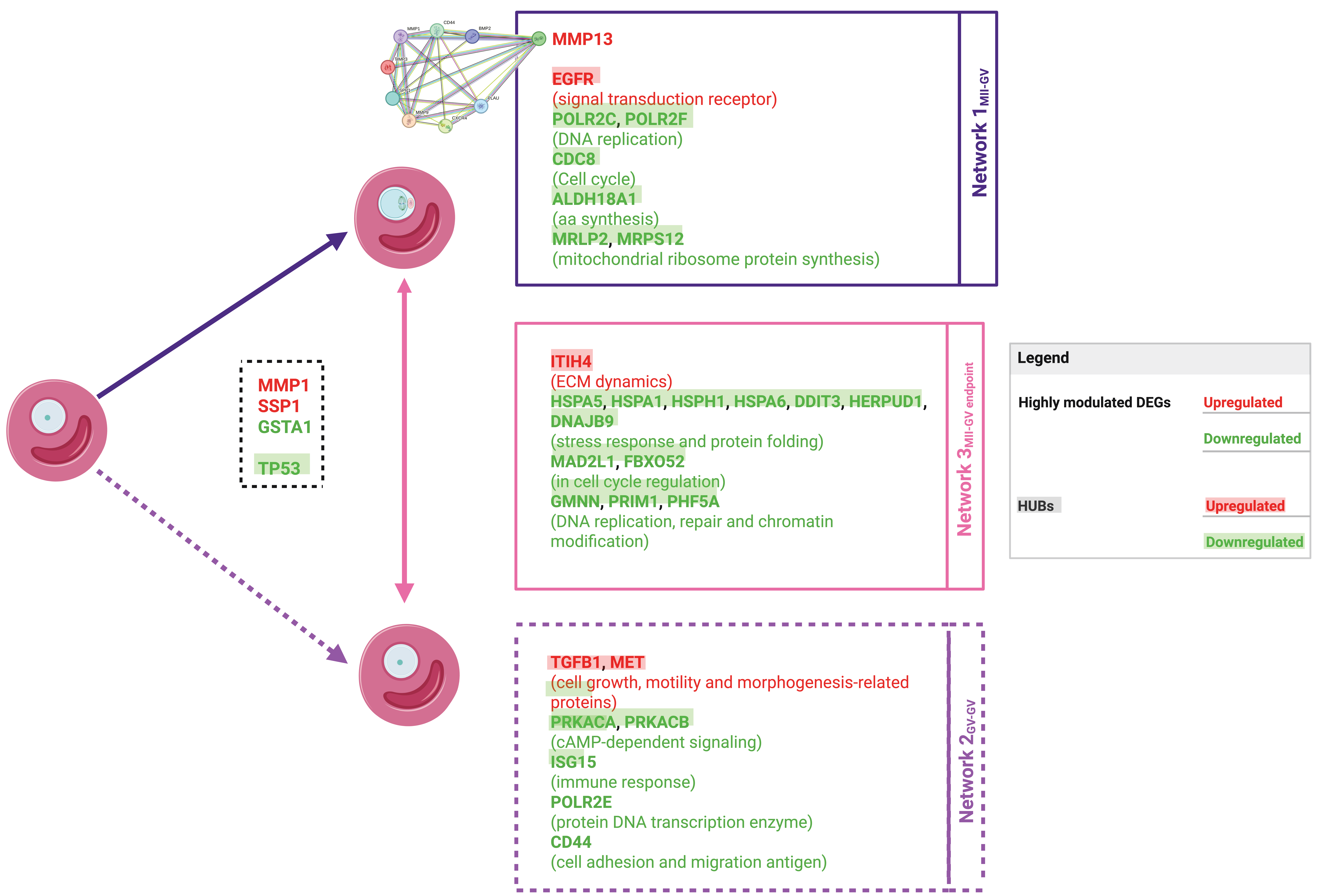
3.2.1. Network 1MII-GV and Network 2GV-GV Signaling Modules
3.2.2. Driver DEGs of Network 1MII-GV and Network 2GV-GV
Highly Modulated DEGs of Network 1MII-GV and Network 2GV-GV
- −
- Network 1MII-GV: Seven upregulated (MMP1, SPP1, CLCA1, MMP13, SERPIN14, GCG, RUNX2) and three downregulated (GSTA1, HSD17B, INHA);
- −
- Network 2GV-GV: Four upregulated (MMP1, SPP1, HBA1, SERPIN14) and five downregulated (GSTA1, CYP17, ALPL, CA5A, INHA).
HUBs of Network 1MII-GV and Network 2GV-GV
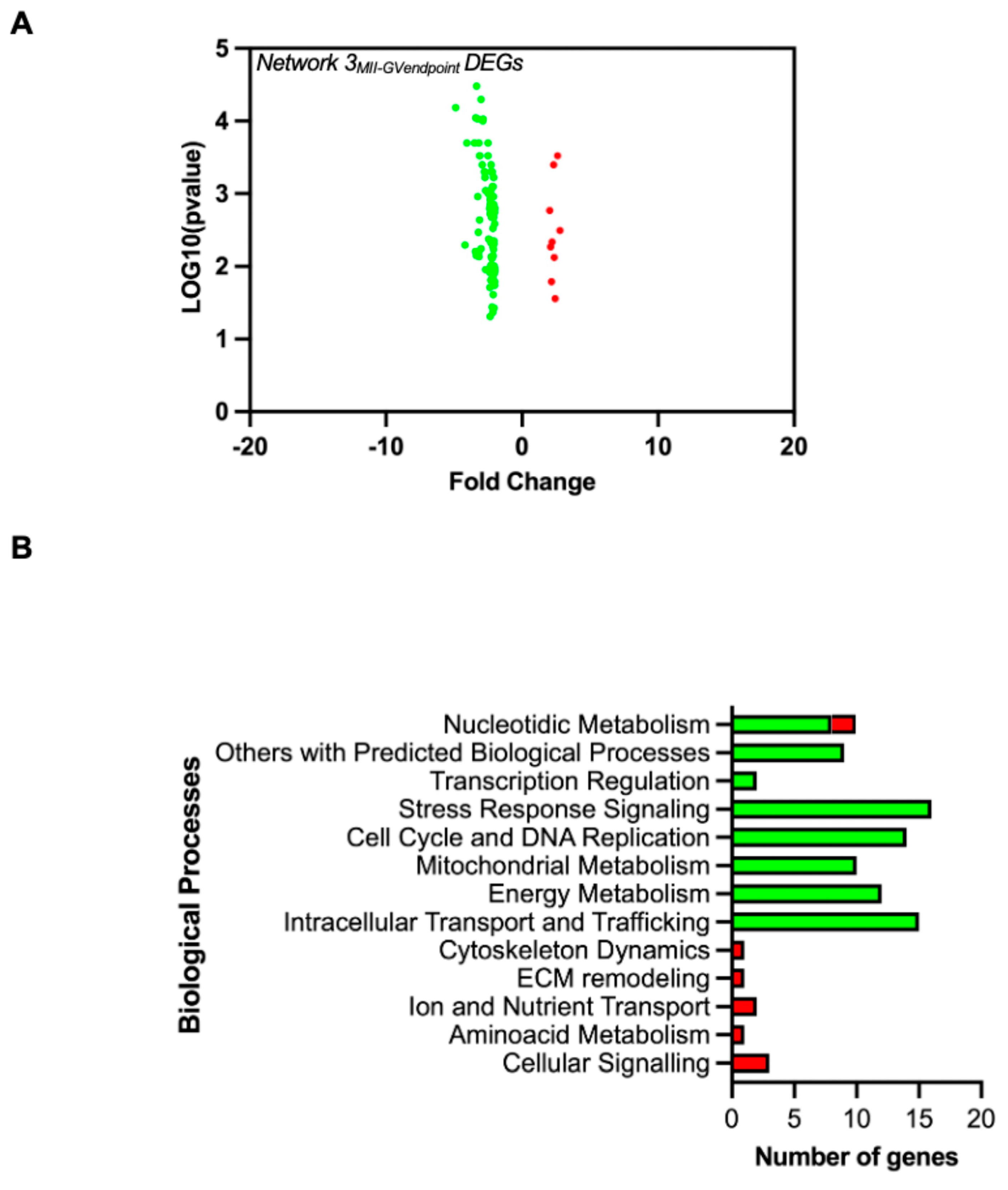
3.3. Signature Genes Distinguishing FWs from Successful Versus Unsuccessful EAfs at the End of the Maturation Phase
3.4. Validation of Predicted Driver Genes in Oocyte Maturation: qRT-PCR Confirmation and Literature Review
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peserico, A.; Di Berardino, C.; Capacchietti, G.; Camerano Spelta Rapini, C.; Liverani, L.; Boccaccini, A.R.; Russo, V.; Mauro, A.; Barboni, B. IVM Advances for Early Antral Follicle-Enclosed Oocytes Coupling Reproductive Tissue Engineering to Inductive Influences of Human Chorionic Gonadotropin and Ovarian Surface Epithelium Coculture. Int. J. Mol. Sci. 2023, 24, 6626. [Google Scholar] [CrossRef]
- Di Berardino, C.; Peserico, A.; Camerano Spelta Rapini, C.; Liverani, L.; Capacchietti, G.; Russo, V.; Berardinelli, P.; Unalan, I.; Damian-Buda, A.I.; Boccaccini, A.R.; et al. Bioengineered 3D ovarian model for long-term multiple development of preantral follicle: Bridging the gap for poly(ε-caprolactone) (PCL)-based scaffold reproductive applications. Reprod. Biol. Endocrinol. 2024, 22, 95. [Google Scholar] [CrossRef]
- Chansaenroj, A.; Songsasen, N.; Chatdarong, K. Equine chorionic gonadotropin induces in vitro follicular growth from the multi-layered secondary developmental stage in cats. Theriogenology 2019, 123, 116–122. [Google Scholar] [CrossRef]
- Barboni, B.; Russo, V.; Cecconi, S.; Curini, V.; Colosimo, A.; Garofalo, M.L.A.; Capacchietti, G.; Di Giacinto, O.; Mattioli, M. In vitro grown sheep preantral follicles yield oocytes with normal nuclear-epigenetic maturation. PLoS ONE 2011, 6, e27550. [Google Scholar] [CrossRef]
- Bernabò, N.; Di Berardino, C.; Capacchietti, G.; Peserico, A.; Buoncuore, G.; Tosi, U.; Crociati, M.; Monaci, M.; Barboni, B. In Vitro Folliculogenesis in Mammalian Models: A Computational Biology Study. Front. Mol. Biosci. 2021, 8, 737912. [Google Scholar] [CrossRef]
- Cortvrindt, R.; Smitz, J.; Van Steirteghem, A.C. A morphological and functional study of the effect of slow freezing followed by complete in-vitro maturation of primary mouse ovarian follicles. Hum. Reprod. 1996, 11, 2648–2655. [Google Scholar] [CrossRef]
- Eppig, J.J.; O’Brien, M.J. Development in Vitro of Mouse Oocytes from Primordial Follicles. Biol. Reprod. 1996, 54, 197–207. [Google Scholar] [CrossRef]
- Cecconi, S.; Barboni, B.; Coccia, M.; Mattioli, M. In vitro development of sheep preantral follicles. Biol. Reprod. 1999, 60, 594–601. [Google Scholar] [CrossRef]
- Di Berardino, C.; Liverani, L.; Peserico, A.; Capacchietti, G.; Russo, V.; Bernabò, N.; Tosi, U.; Boccaccini, A.R.; Barboni, B. When Electrospun Fiber Support Matters: In Vitro Ovine Long-Term Folliculogenesis on Poly (Epsilon Caprolactone) (PCL)-Patterned Fibers. Cells 2022, 11, 1968. [Google Scholar] [CrossRef]
- Di Berardino, C.; Peserico, A.; Capacchietti, G.; Crociati, M.; Monaci, M.; Tosi, U.; Mauro, A.; Russo, V.; Bernabò, N.; Barboni, B. Equine Chorionic Gonadotropin as an Effective FSH Replacement for In Vitro Ovine Follicle and Oocyte Development. Int. J. Mol. Sci. 2021, 22, 12422. [Google Scholar] [CrossRef]
- Barros, V.R.P.; Monte, A.P.O.; Lins, T.L.B.G.; Santos, J.M.; Menezes, V.G.; Cavalcante, A.Y.P.; Arujo, V.R.; Gouveia, B.B.; Matos, M.H.T. In vitro survival, growth, and maturation of sheep oocytes from secondary follicles cultured in serum-free conditions: Impact of a constant or a sequential medium containing recombinant human FSH. Domest. Anim. Endocrinol. 2019, 67, 71–79. [Google Scholar] [CrossRef]
- Sun, J.; Li, X. Growth and antrum formation of bovine primary follicles in long-term culture in vitro. Reprod. Biol. 2013, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.S.P.; Ramesh, H.S.; Manjunatha, B.M.; Nandi, S.; Ravindra, J.P. Production of buffalo embryos using oocytes from in vitro grown preantral follicles. Zygote 2008, 16, 57–63. [Google Scholar] [CrossRef]
- Da Silva, G.M.; Rossetto, R.; Chaves, R.N.; Duarte, A.B.G.; Araújo, V.R.; Feltrin, C.; Bernuci, M.P.; Anselmo-Franci, J.A.; Xu, M.; Woodruff, T.K.; et al. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three-dimensional systems. Zygote 2015, 23, 475–484. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Duarte, A.B.G.; Araújo, V.R.; Brito, I.R.; Soares, T.G.; Lima, I.M.T.; Lopes, C.A.P.; Campello, C.C.; Rodrigues, A.P.R.; Figueiredo, J.R. In vitro production of a caprine embryo from a preantral follicle cultured in media supplemented with growth hormone. Theriogenology 2011, 75, 182–188. [Google Scholar] [CrossRef]
- Costa, S.L.; Costa, E.P.; Pereira, E.C.M.; Benjamin, L.A.; Rodrigues, M.T.; Mendes, V.R.A.; Silva, T.F. Influence of insulin-like growth factor I (IGF-I) on the survival and the in vitro development of caprine preantral follicles. Pesquisa Veterinaria Brasileira 2014, 34, 1037–1044. [Google Scholar] [CrossRef]
- Chaves, R.N.; Duarte, A.B.G.; Rodrigues, G.Q.; Celestino, J.J.H.; Silva, G.M.; Lopes, C.A.P.; Almeida, A.P.; Donato, M.A.M.; Peixoto, C.A.; Moura, A.A.A.; et al. The Effects of Insulin and Follicle-Simulating Hormone (FSH) During In Vitro Development of Ovarian Goat Preantral Follicles and the Relative mRNA Expression for Insulin and FSH Receptors and Cytochrome P450 Aromatase in Cultured Follicles. Biol. Reprod. 2012, 87, 69. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Dattena, M.; Luciano, A.M.; Succu, S.; Gadau, S.D.; Mara, L.; Chessa, F.; Berlinguer, F. In vitro culture of sheep early-antral follicles: Milestones, challenges and future perspectives. Theriogenology 2024, 213, 114–123. [Google Scholar] [CrossRef]
- de Figueiredo, J.R.; Cadenas, J.; de Lima, L.F.; Santos, R.R. Advances in in vitro folliculogenesis in domestic ruminants. Anim. Reprod. 2020, 16, 52–65. [Google Scholar] [CrossRef]
- Xu, J.; Zelinski, M.B. Oocyte quality following in vitro follicle development†. Biol. Reprod. 2022, 106, 291–315. [Google Scholar] [CrossRef]
- Luciano, A.M.; Barros, R.G.; Soares, A.C.S.; Buratini, J.; Lodde, V.; Franciosi, F. Recreating the Follicular Environment: A Customized Approach for In Vitro Culture of Bovine Oocytes Based on the Origin and Differentiation State. Methods Mol. Biol. 2021, 2273, 1–15. [Google Scholar]
- Russo, V.; Martelli, A.; Berardinelli, P.; Di Giacinto, O.; Bernabò, N.; Fantasia, D.; Mattioli, M.; Barboni, B. Modifications in chromatin morphology and organization during sheep oogenesis. Microsc. Res. Tech. 2007, 70, 733–744. [Google Scholar] [CrossRef]
- Colosimo, A.; Di Rocco, G.; Curini, V.; Russo, V.; Capacchietti, G.; Berardinelli, P.; Mattioli, M.; Barboni, B. Characterization of the methylation status of five imprinted genes in sheep gametes. Anim. Genet. 2009, 40, 900–908. [Google Scholar] [CrossRef]
- Russo, V.; Bernabò, N.; Di Giacinto, O.; Martelli, A.; Mauro, A.; Berardinelli, P.; Curini, V.; Nardinocchi, D.; Mattioli, M.; Barboni, B. H3K9 trimethylation precedes DNA methylation during sheep oogenesis: HDAC1, SUV39H1, G9a, HP1, and Dnmts are involved in these epigenetic events. J. Histochem. Cytochem. 2013, 61, 75–89. [Google Scholar] [CrossRef]
- Russo, V.; Berardinelli, P.; Martelli, A.; Di Giacinto, O.; Nardinocchi, D.; Fantasia, D.; Barboni, B. Expression of telomerase reverse transcriptase subunit (TERT) and telomere sizing in pig ovarian follicles. J. Histochem. Cytochem. 2006, 54, 443–455. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Dattena, M.; Mara, L.; Pasciu, V.; Sotgiu, F.D.; Chessa, F.; Luciano, A.M.; Berlinguer, F. In vitro production of meiotically competent oocytes from early antral follicles in sheep. Theriogenology 2024, 226, 253–262. [Google Scholar] [CrossRef]
- Alam, M.H.; Lee, J.; Miyano, T. Inhibition of PDE3A sustains meiotic arrest and gap junction of bovine growing oocytes in in vitro growth culture. Theriogenology 2018, 118, 110–118. [Google Scholar] [CrossRef]
- Garcia Barros, R.; Lodde, V.; Franciosi, F.; Luciano, A.M. A refined culture system of oocytes from early antral follicles promotes oocyte maturation and embryo development in cattle. Reproduction 2023, 165, 221–233. [Google Scholar] [CrossRef]
- Luciano, A.M.; Franciosi, F.; Modina, S.C.; Lodde, V. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s). Biol. Reprod. 2011, 85, 1252–1259. [Google Scholar] [CrossRef]
- de Sá, N.A.R.; Ferreira, A.C.A.; Sousa, F.G.C.; Duarte, A.B.G.; Paes, V.M.; Cadenas, J.; Anjos, J.C.; Fernandes, C.C.; Rosseto, R.; Cibin, F.W.; et al. First pregnancy after in vitro culture of early antral follicles in goats: Positive effects of anethole on follicle development and steroidogenesis. Mol. Reprod. Dev. 2020, 87, 966–977. [Google Scholar] [CrossRef]
- Buratini, J.; Soares, A.C.S.; Barros, R.G.; Dellaqua, T.T.; Lodde, V.; Franciosi, F.; Dal Canto, M.; Mignini Renzini, M.; Luciano, A.M. Physiological parameters related to oocyte nuclear differentiation for the improvement of IVM/IVF outcomes in women and cattle. Reprod. Fertil. Dev. 2021, 34, 27–35. [Google Scholar] [CrossRef]
- Luciano, A.M.; Franciosi, F.; Barros, R.G.; Dieci, C.; Lodde, V. The variable success of in vitro maturation: Can we do better? Anim. Reprod. 2018, 15, 727–736. [Google Scholar] [CrossRef]
- Dieci, C.; Lodde, V.; Labreque, R.; Dufort, I.; Tessaro, I.; Sirard, M.A.; Luciano, A.M. Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production. Mol. Hum. Reprod. 2016, 22, 882–897. [Google Scholar]
- Cecconi, S.; Mauro, A.; Capacchietti, G.; Berardinelli, P.; Bernabò, N.; Di Vincenzo, A.R.; Mattioli, M.; Barboni, B. Meiotic maturation of incompetent prepubertal sheep oocytes is induced by paracrine factor(s) released by gonadotropin-stimulated oocyte-cumulus cell complexes and involves mitogen-activated protein kinase activation. Endocrinology 2008, 149, 100–107. [Google Scholar] [CrossRef]
- Barros, R.G.; Lodde, V.; Franciosi, F.; Luciano, A.M. In Vitro Culture Strategy for Oocytes from Early Antral Follicle in Cattle. J. Vis. Exp. 2020, 161, e61625. [Google Scholar]
- Hubbard, C.J. The effects of forskolin and LH on cAMP changes and maturation in the follicle-enclosed oocytes of hamsters. Acta Endocrinol. 1985, 110, 413–420. [Google Scholar] [CrossRef]
- Tsafriri, A. Mammalian oocyte maturation: Model systems and their physiological relevance. Adv. Exp. Med. Biol. 1979, 112, 269–281. [Google Scholar]
- Yoshimura, Y.; Nakamura, Y.; Oda, T.; Yamada, H.; Nanno, T.; Ando, M.; Ubukata, Y.; Suzuki, M. Effects of Gonadotropin-Releasing Hormone Agonists on Meiotic Maturation of Follicle-Enclosed Oocytes in Rabbits. Biol. Reprod. 1990, 43, 1012–1018. [Google Scholar] [CrossRef]
- McIntosh, J.E.; Kaethner, M.; Stewart, F.; Allen, W.R.; Moor, R.M. Proceedings: FSH AND LH activites of PMSG fractions isolated from serum and from fetal trophoblast cells maintained in culture. J. Reprod. Fertil. 1976, 46, 521–522. [Google Scholar] [CrossRef]
- Moor, R.M.; Hay, M.F.; McIntosh, J.E.; Caldwell, B.V. Effect of gonadotrophins on the production of steroids by sheep ovarian follicles cultured in vitro. J. Endocrinol. 1973, 58, 599–611. [Google Scholar] [CrossRef]
- Mattioli, M.; Bacci, M.L.; Galeati, G.; Seren, E. Developmental competence of pig oocytes matured and fertilized in vitro. Theriogenology 1989, 31, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef]
- Tamadon, A.; Park, K.-H.; Kim, Y.Y.; Kang, B.-C.; Ku, S.-Y. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng. Regen. Med. 2016, 13, 447–454. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, C.; Zhang, X.; He, X.; Liu, Y. Design and Application Strategies of Natural Polymer Biomaterials in Artificial Ovaries. Ann. Biomed. Eng. 2023, 51, 461–478. [Google Scholar] [CrossRef]
- Gargus, E.S.; Rogers, H.B.; McKinnon, K.E.; Edmonds, M.E.; Woodruff, T.K. Engineered reproductive tissues. Nat. Biomed. Eng. 2020, 4, 381–393. [Google Scholar]
- Raffel, N.; Dittrich, R.; Bäuerle, T.; Seyler, L.; Fattahi, A.; Hoffmann, I.; Leal-Egana, A.; Beckmann, M.W.; Boccaccini, A.R.; Liverani, L. Novel approach for the assessment of ovarian follicles infiltration in polymeric electrospun patterned scaffolds. PLoS ONE 2019, 14, e0215985. [Google Scholar] [CrossRef]
- Liverani, L.; Raffel, N.; Fattahi, A.; Preis, A.; Hoffmann, I.; Boccaccini, A.R.; Beckmann, M.W.; Dittrich, R. Electrospun patterned porous scaffolds for the support of ovarian follicles growth: A feasibility study. Sci. Rep. 2019, 9, 1150. [Google Scholar] [CrossRef]
- Khunmanee, S.; Park, H. Three-Dimensional Culture for In Vitro Folliculogenesis in the Aspect of Methods and Materials. Tissue Eng. Part. B Rev. 2022, 28, 1242–1257. [Google Scholar] [CrossRef]
- Ferronato Gde, A.; Vit, F.F.; da Silveira, J.C. 3D culture applied to reproduction in females: Possibilities and perspectives. Anim. Reprod. 2024, 21, e20230039. [Google Scholar] [CrossRef]
- Carletti, M.Z.; Christenson, L.K. Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction 2009, 137, 843–855. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Kawamura, N.; Takae, S.; Okada, A.; Kawagoe, Y.; Mulders, S.; Terarda, Y.; Hsueh, A.J.W. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 2011, 26, 3094–3101. [Google Scholar] [CrossRef]
- Jaffe, L.A.; Egbert, J.R. Regulation of Mammalian Oocyte Meiosis by Intercellular Communication Within the Ovarian Follicle. Annu. Rev. Physiol. 2017, 79, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Gunewardena, S.; Hong, X.; Spitschak, M.; Baufeld, A.; Vanselow, J. Research resource: Preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol. Endocrinol. 2013, 27, 1153–1171. [Google Scholar] [CrossRef]
- Borgbo, T.; Povlsen, B.B.; Andersen, C.Y.; Borup, R.; Humaidan, P.; Grøndahl, M.L. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil. Steril. 2013, 100, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, I.; Robert, C.; Vigneault, C.; Blondin, P.; Sirard, M.A. Impact of the LH surge on granulosa cell transcript levels as markers of oocyte developmental competence in cattle. Reproduction 2012, 143, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, Y.; Chen, W.; Zi, Y.; Shi, D.; Lu, F. Theca cell-conditioned medium added to in vitro maturation enhances embryo developmental competence of buffalo (Bubalus bubalis) oocytes after parthenogenic activation. Reprod. Domest. Anim. 2020, 55, 1501–1510. [Google Scholar] [CrossRef]
- Richards, J.A.S.; Ren, Y.A.; Candelaria, N.; Adams, J.E.; Rajkovic, A. Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocr. Rev. 2018, 39, 1–20. [Google Scholar] [CrossRef]
- Fragouli, E.; Wells, D. Transcriptomic analysis of follicular cells provides information on the chromosomal status and competence of unfertilized oocytes. Expert. Rev. Mol. Diagn. 2012, 12, 1–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Qin, Q.; Nisenblat, V.; Chang, H.-M.; Yu, Y.; Wang, T.; Lu, C.; Yang, M.; Yang, S.; et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Mol. Cell 2018, 72, 1021–1034.e4. [Google Scholar] [CrossRef]
- D’Aurora, M.; Sperduti, S.; Di Emidio, G.; Stuppia, L.; Artini, P.G.; Gatta, V. Inside the granulosa transcriptome. Gynecol. Endocrinol. 2016, 32, 951–956. [Google Scholar] [CrossRef]
- Huang, Z.; Wells, D. The human oocyte and cumulus cells relationship: New insights from the cumulus cell transcriptome. Mol. Hum. Reprod. 2010, 16, 715–725. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, R.; Zhang, S.; Ji, Y.; Zhou, Q.; Leng, L.; Meng, F.; Gong, F.; Lu, G.; Lin, G.; et al. Transcriptomic profiles reveal the characteristics of oocytes and cumulus cells at GV, MI, and MII in follicles before ovulation. J. Ovarian Res. 2023, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, A.; Cabau, C.; Bouchez, O.; Sarry, J.; Marsaud, N.; Foissac, S.; Woloszyn, F.; Mulsant, P.; Mandon-Pepin, B. An overview of gene expression dynamics during early ovarian folliculogenesis: Specificity of follicular compartments and bi-directional dialog. BMC Genom. 2013, 14, 904. [Google Scholar] [CrossRef] [PubMed]
- Peserico, A.; Barboni, B.; Russo, V.; Bernabò, N.; El Khatib, M.; Prencipe, G.; Cervero-Varona, A.; Haidar-Montes, A.A.; Faydaver, M.; Citeroni, M.R.; et al. Mammal comparative tendon biology: Advances in regulatory mechanisms through a computational modeling. Front. Vet. Sci. 2023, 10, 1175346. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Han, J.; Kamber, M.; Pei, J. Getting to Know Your Data. In Data Mining; Elsevier: Amsterdam, The Netherlands, 2012; pp. 39–82. [Google Scholar]
- Irigoien, I.; Arenas, C. Identification of differentially expressed genes by means of outlier detection. BMC Bioinform. 2018, 19, 317. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Xie, Z.; Xia, T.; Wu, D.; Che, L.; Zhang, W.; Cai, X.; Liu, S. Identification of the key genes in chronic obstructive pulmonary disease by weighted gene co-expression network analysis. Ann. Transl. Med. 2022, 10, 665. [Google Scholar] [CrossRef]
- Qin, D.; Yue, R.; Deng, P.; Wang, X.; Zheng, Z.; Lv, M.; Zhang, Y.; Pu, J.; Xu, J.; Liang, Y.; et al. 8-Formylophiopogonanone B antagonizes doxorubicin-induced cardiotoxicity by suppressing heme oxygenase-1-dependent myocardial inflammation and fibrosis. Biomed. Pharmacother. 2021, 140, 111779. [Google Scholar] [CrossRef]
- Xue, J.; Chen, L.; Cheng, H.; Song, X.; Shi, Y.; Li, L.; Xu, R.; Qin, Q.; Ma, J.; Ge, J. The Identification and Validation of Hub Genes Associated with Acute Myocardial Infarction Using Weighted Gene Co-Expression Network Analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 30. [Google Scholar] [CrossRef]
- Fang, X.; Duan, S.-F.; Gong, Y.-Z.; Wang, F.; Chen, X.-L. Identification of Key Genes Associated with Changes in the Host Response to Severe Burn Shock: A Bioinformatics Analysis with Data from the Gene Expression Omnibus (GEO) Database. J. Inflamm. Res. 2020, 13, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Meng, J.; Li, X.; Zhu, F.; Liu, T.; Wu, G.; Zhang, L. Identification of Hub Genes and Key Pathways Associated with Two Subtypes of Diffuse Large B-Cell Lymphoma Based on Gene Expression Profiling via Integrated Bioinformatics. Biomed. Res. Int. 2018, 2018, 3574534. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.M.; Maniruzzaman, M.; Shin, J. Gene Expression and Metadata Based Identification of Key Genes for Hepatocellular Carcinoma Using Machine Learning and Statistical Models. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 3786–3799. [Google Scholar] [CrossRef]
- Wu, W.; Chen, A.; Lin, S.; Wang, Q.; Lian, G.; Luo, L.; Xie, L. The identification and verification of hub genes associated with pulmonary arterial hypertension using weighted gene co-expression network analysis. BMC Pulm. Med. 2022, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, L.; Leng, L.; Zhou, Q.; Zhang, S.; Gong, F.; Xie, P.; Lin, G. CDCA8 regulates meiotic spindle assembly and chromosome segregation during human oocyte meiosis. Gene 2020, 741, 144495. [Google Scholar] [CrossRef]
- Fang, L.; Yu, Y.; Zhang, R.; He, J.; Sun, Y.-P. Amphiregulin mediates hCG-induced StAR expression and progesterone production in human granulosa cells. Sci. Rep. 2016, 6, 24917. [Google Scholar] [CrossRef]
- Abbassi, L.; El-Hayek, S.; Carvalho, K.F.; Wang, W.; Yang, Q.; Granados-Aparici, S.; Mondadori, R.; Bordignon, V.; Clarke, H.J. Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation. Nat. Commun. 2021, 12, 1438. [Google Scholar] [CrossRef]
- Bakke, L.J.; Li, Q.; Cassar, C.A.; Dow, M.P.D.; Pursley, J.R.; Smith, G.W. Gonadotropin surge-induced differential upregulation of collagenase-1 (MMP-1) and collagenase-3 (MMP-13) mRNA and protein in bovine preovulatory follicles. Biol. Reprod. 2004, 71, 605–612. [Google Scholar] [CrossRef]
- Umehara, T.; Winstanley, Y.E.; Andreas, E.; Morimoto, A.; Williams, E.J.; Smith, K.M.; Carroll, J.; Febbraio, M.A.; Shimada, M.; Russell, D.L.; et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci. Adv. 2022, 8, eabn4564. [Google Scholar] [CrossRef]
- Chaffin, C.L.; Stouffer, R.L. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in macaque periovulatory granulosa cells: Time course and steroid regulation. Biol. Reprod. 1999, 61, 14–21. [Google Scholar] [CrossRef]
- Peluffo, M.C.; Murphy, M.J.; Baughman, S.T.; Stouffer, R.L.; Hennebold, J.D. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: Metalloproteinase involvement in follicle rupture. Endocrinology 2011, 152, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y. Metalloproteases in gonad formation and ovulation. Gen. Comp. Endocrinol. 2021, 314, 113924. [Google Scholar] [CrossRef]
- Rosewell, K.L.; Al-Alem, L.; Zakerkish, F.; McCord, L.; Akin, J.W.; Chaffin, C.L.; Brannstrom, M.; Curry Jr, T.E. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil. Steril. 2015, 103, 826–833. [Google Scholar]
- Foley, C.J.; Fanjul-Fernández, M.; Bohm, A.; Nguyen, N.; Agarwal, A.; Austin, K.; Koukos, G.; Covic, L.; Lopez-Otin, C.; Kuliopulos, A. Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis. Oncogene 2014, 33, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Deady, L.D.; Shen, W.; Mosure, S.A.; Spradling, A.C.; Sun, J. Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in Drosophila. PLoS Genet. 2015, 11, e1004989. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wu, S.; Wang, L.; Chen, G.; Chen, D.; Peng, X.; Miao, Y.L.; Mei, S.; Li, F. ISG15 suppresses ovulation and female fertility by ISGylating ADAMTS1. Cell Biosci. 2023, 13, 84. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Katayama, A.; Tomiyama, R.; Piao, H.; Kurihara, S.; Ono, S.; Mine, K.; Akira, S.; Orimo, H.; Takeshita, T. Gonadotropin regulation and role of ovarian osteopontin in the periovulatory period. J. Endocrinol. 2015, 224, 49–59. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Gao, L.; Ma, H.; Chang, R. Osteopontin/SPP1, a potential mediator between immune cells and vascular calcification. Front. Immunol. Front. Media SA 2024, 15, 1395596. [Google Scholar]
- Skinner, M.K.; Schmidt, M.; Savenkova, M.I.; Sadler-Riggleman, I.; Nilsson, E.E. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol. Reprod. Dev. 2008, 75, 1457–1472. [Google Scholar] [CrossRef]
- Homer, H. The APC/C in female mammalian meiosis I. Reproduction 2013, 146, R61–R71. [Google Scholar] [CrossRef]
- Pei, J.; Song, R.; Bao, P.; Yin, M.; Li, J.; Zhang, G.; Wu, F.; Luo, Z.; Wu, X.; Song, W.; et al. Differential proteomic analysis demonstrates follicle fluid participate immune reaction and protein translation in yak. BMC Vet. Res. 2022, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Ledda, S.; Bogliolo, L.; Leoni, G.; Naitana, S. Follicular size affects the meiotic competence of in vitro matured prepubertal and adult oocytes in sheep. Reprod. Nutr. Dev. 1999, 39, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J.; Schultz, R.M.; O’Brien, M.; Chesnel, F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev. Biol. 1994, 164, 1–9. [Google Scholar] [CrossRef]
- Raghu, H.M.; Nandi, S.; Reddy, S.M. Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes in vitro. Reprod. Fertil. Dev. 2002, 14, 55–61. [Google Scholar] [CrossRef]
- Lonergan, P.; Monaghan, P.; Rizos, D.; Boland, M.P.; Gordon, I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol. Reprod. Dev. 1994, 37, 48–53. [Google Scholar] [CrossRef]
- Reich, R.; Tsafriri, A.; Mechanic, G.L. The involvement of collagenolysis in ovulation in the rat. Endocrinology 1985, 116, 522–527. [Google Scholar] [CrossRef]
- Smith, G.W.; McCrone, S.; Petersen, S.L.; Smith, M.F. Expression of messenger ribonucleic acid encoding tissue inhibitor of metalloproteinases-2 within ovine follicles and corpora lutea. Endocrinology 1995, 136, 570–576. [Google Scholar] [CrossRef]
- McIntush, E.W.; Pletz, J.D.; Smith, G.D.; Long, D.K.; Sawyer, H.R.; Smith, M.F. Immunolocalization of tissue inhibitor of metalloproteinases-1 within ovine periovulatory follicular and luteal tissues. Biol. Reprod. 1996, 54, 871–878. [Google Scholar] [CrossRef]
- Smith, G.W.; Juengel, J.L.; Mclntush, E.W.; Youngquist, R.S.; Garverick, H.A.; Smith, M.F. Ontogenies of messenger RNA encoding tissue inhibitor of metalloproteinases 1 and 2 within bovine periovulatory follicles and luteal tissue. Domest. Anim. Endocrinol. 1996, 13, 151–160. [Google Scholar] [CrossRef]
- Curry, T.E.; Dean, D.D.; Sanders, S.L.; Pedigo, N.G.; Jones, P.B. The role of ovarian proteases and their inhibitors in ovulation. Steroids 1989, 54, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Russell, D.L.; Ochsner, S.; Espey, L.L. Ovulation: New dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 2002, 64, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Balbín, M.; Fueyo, A.; López, J.M.; Díez-Itza, I.; Velasco, G.; López-Otín, C. Expression of collagenase-3 in the rat ovary during the ovulatory process. J. Endocrinol. 1996, 149, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Hrabia, A. Matrix Metalloproteinases (MMPs) and Inhibitors of MMPs in the Avian Reproductive System: An Overview. Int. J. Mol. Sci. 2021, 22, 8056. [Google Scholar] [CrossRef]
- Wolak, D.; Sechman, A.; Hrabia, A. Effect of eCG treatment on gene expression of selected matrix metalloproteinases (MMP-2, MMP-7, MMP-9, MMP-10, and MMP-13) and the tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary. Anim. Reprod. Sci. 2021, 224, 106666. [Google Scholar] [CrossRef]
- Song, L.; Ryan, P.L.; Porter, D.G.; Coomber, B.L. Effects of relaxin on matrix remodeling enzyme activity of cultured equine ovarian stromal cells. Anim. Reprod. Sci. 2001, 66, 239–255. [Google Scholar] [CrossRef]
- Klein, C. The role of relaxin in mare reproductive physiology: A comparative review with other species. Theriogenology 2016, 86, 451–456. [Google Scholar] [CrossRef]
- Brännström, M.; MacLennan, A.H. Relaxin induces ovulations in the in-vitro perfused rat ovary. Hum. Reprod. 1993, 8, 1011–1014. [Google Scholar] [CrossRef]
- Wathes, D.C.; Wardle, P.G.; Rees, J.M.; Mitchell, J.D.; McLaughlin, E.A.; Hull, M.G.; Porter, D.G. Identification of relaxin immunoreactivity in human follicular fluid. Hum. Reprod. 1986, 1, 515–517. [Google Scholar] [CrossRef]
- Ryan, P.L.; Klonisch, T.; Yamashiro, S.; Renaud, R.L.; Wasnidge, C.; Porter, D.G. Expression and localization of relaxin in the ovary of the mare. J. Reprod. Fertil. 1997, 110, 329–338. [Google Scholar] [CrossRef]
- Ohleth, K.M.; Zhang, Q.; Bagnell, C.A. Relaxin protein and gene expression in ovarian follicles of immature pigs. J. Mol. Endocrinol. 1998, 21, 179–187. [Google Scholar] [CrossRef]
- Meidan, R.; Klipper, E.; Zalman, Y.; Yalu, R. The role of hypoxia-induced genes in ovarian angiogenesis. Reprod. Fertil. Dev. 2013, 25, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Thompson, J.G.; Dunning, K.R. HYPOXIA AND REPRODUCTIVE HEALTH: Hypoxia and ovarian function: Follicle development, ovulation, oocyte maturation. Reproduction 2021, 161, F33–F40. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bagchi, I.C.; Bagchi, M.K. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology 2009, 150, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Kim, B.; Yeh, J. Luteinizing Hormone Action in Human Oocyte Maturation and Quality: Signaling Pathways, Regulation, and Clinical Impact. Reprod. Sci. 2020, 27, 1223–1252. [Google Scholar]
- Turathum, B.; Gao, E.-M.; Chian, R.-C. The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar]
- Wyse, B.A.; Fuchs Weizman, N.; Kadish, S.; Balakier, H.; Sangaralingam, M.; Librach, C.L. Transcriptomics of cumulus cells—A window into oocyte maturation in humans. J. Ovarian Res. 2020, 13, 93. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Xu, X.; Li, J.; Yuan, F.; Bo, S.; Qiao, J.; Xia, G.; Su, Y.; Zhang, M. Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. 2019, 10, 558. [Google Scholar] [CrossRef]
- Tsuji, T.; Kiyosu, C.; Akiyama, K.; Kunieda, T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 2012, 79, 795–802. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Wigglesworth, K.; Xia, G.; Eppig, J.J. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology 2011, 152, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Yelamanchi, S.; Kumar, M.; Hinduja, I.; Prasad, T.S.K.; Gowda, H.; Mukherjee, S. Quantitative mass spectrometric analysis to unravel glycoproteomic signature of follicular fluid in women with polycystic ovary syndrome. PLoS ONE 2019, 14, e0214742. [Google Scholar] [CrossRef] [PubMed]
- Rosairo, D.; Kuyznierewicz, I.; Findlay, J.; Drummond, A. Transforming growth factor-beta: Its role in ovarian follicle development. Reproduction 2008, 136, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Pihl, R.; Jensen, R.K.; Poulsen, E.C.; Jensen, L.; Hansen, A.G.; Thøgersen, I.B.; Dobo, J.; Gal, P.; Andersen, G.R.; Enghild, J.J.; et al. ITIH4 acts as a protease inhibitor by a novel inhibitory mechanism. Sci Adv. 2021, 7, eaba7381. [Google Scholar] [CrossRef]
- Chandler, K.B.; Brnakova, Z.; Sanda, M.; Wang, S.; Stalnaker, S.H.; Bridger, R.; Zhao, P.; Wells, L.; Edwards, N.J.; Goldman, R. Site-specific glycan microheterogeneity of inter-alpha-trypsin inhibitor heavy chain H4. J. Proteome Res. 2014, 13, 3314–3329. [Google Scholar] [CrossRef]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: A systematic expression analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef]
- Christians, E.; Boiani, M.; Garagna, S.; Dessy, C.; Redi, C.A.; Renard, J.P.; Zuccotti, M. Gene expression and chromatin organization during mouse oocyte growth. Dev. Biol. 1999, 207, 76–85. [Google Scholar] [CrossRef]

| A | ||||
| Follicle Category | Healthy Oocytes (n°) | Oocyte Nuclear Stage | ||
| GV (%; SD) | GVBD/MI | MII | ||
| EAf − hCG | 48 | 100 | -- | -- |
| EAf + hCG | 236 | 11.0 ± 4.2 | 21.2 ± 5.5 | 67.8 ± 6.3 |
| B | ||||
| Healthy Oocytes (n°) | Post-Fertilization Embryo Development | |||
| Uncleaved (%; SD) | Fertilization Rate (%; SD) | Blastocyst Rate (%; SD) | ||
| EAf | 160 | 32 ± 5.2 | 58.2 ± 5.5 | 9.9 ± 3.3 |
| Pairwise Comparison | Generated Network | Total Number of Genes | Genes passed Filter Criteria (DEG) | Genes with No Changes | Upregulated | Downregulated |
|---|---|---|---|---|---|---|
| Pairwise 1 | Network 1MII-GV | 22,141 | 2526 (11.4%) | 19,615 (88.6%) | 854 (33.8%) | 1672 (66.2%) |
| Pairwise 2 | Network 2GV-GV | 22,141 | 1998 (9%) | 20,143 (91%) | 878 (43.9%) | 1120 (56%) |
| Pairwise 3 | Network 3MII-GV end-point | 22,141 | 97 (0.44%) | 22,044 (99.6%) | 9 (9.3%) | 88 (90.7%) |
| Driver Gene Name | Network | Mcode | DEG Fold Change | p-Value | Female Fertility Effects | ||
|---|---|---|---|---|---|---|---|
| Knockout/Mutant Mice | Effects Related to Maturation | ||||||
| AIM 1: Somatic Markers Distinguishing FW Pre- and Post-hCG Maturation | CDCA8 | 1 | 4 | −2.09 | 0.0014 | N/A | N/A any FWs role Role in oocyte: it regulates meiotic spindle assembly and chromosome segregation during human oocyte meiosis [76] |
| EGFR | 1 | 8 | 2.63 | 0.0001 | Mutant mice (Egfrwa2/EgfrWa5) show infertility (J:92308 from the MGI database. | EGFR activation is crucial for hCG-induced progesterone production in human granulosa cells, with amphiregulin (AREG) mediating this effect by upregulating StAR expression [77]; EGFR signaling in granulosa cells at ovulation triggers filopodia retraction, uncoupling germ and somatic cells [78] | |
| MMP13 | 1 | 7 | 21.18 | 3.37 × 10−6 | N/A | Gonadotropin Surge-Induced MMP-13 mRNA and protein in bovine preovulatory follicles [79]. Upregulation of MMP13, together with M2 macrophage polarization by the antifibrotic drug BGP-15, facilitated ovulation in old and obese mice [80] | |
| MMP1 | 1; 2 | 8; 2 | 73.12; 106.96 | 2.24 × 10−8 6.21 × 10−7 | N/A | Affected reproductive processes, including ovulation and folliculogenesis in chickens, drosophila, macaque, and humans [81,82,83,84,85,86] | |
| ISG15 | 2 | 1 | −2.97 | 0.0009 | Knockout in mice causes hyperfertility along with sensitive ovarian responses to gonadotropin, such as increases in cumulus expansion and ovulation rate [87]. | N/A | |
| SPP1 | 1; 2 | 7; 2 | 51.92; 38.49 | 3.67 × 10−10 1.27 × 10−8 | N/A | SPP1/Opn upregulation in preovulatory granulosa cells, triggered by gonadotropin via EGFR signaling, boosts progesterone synthesis and VEGF expression during the early luteal phase [88]. In antral follicles, SPP1 is responsible for immune processes leading to ovarian follicular atresia [89]. SPP1 is upregulated in response to hormonal cues, particularly during the periovulatory phase, where it contributes to follicular rupture and corpus luteum formation [90] | |
| TGFB1 | 2 | 2 | 2.23 | 0.0009 | Knock out (Tgfb1tm1(Tgfb3)Kul/Tgfb1tm1(Tgfb3)Kul) in mice causes infertility (J:204892 from MGI database). | N/A | |
| AIM 2: Somatic Biomarkers of Follicular and Enclosed Oocyte Competence | HSPA5 | 3 | / | −3.37 | 0.0071 | Knock-out phenotype observed for the master regulator of HSP proteins, HSF1. HSF1 knock out (Hsf1tm1Ijb/Hsf1tm1Ijb) in female mice causes infertility (J:58383, J:65267); abnormal female meiosis and abnormal meiotic spindle assembly checkpoint (J:175085). | N/A |
| HSPA1A | 3 | / | −3.37 | 0.0071 | N/A | ||
| HSPH1 | 3 | / | −2.34 | 0.0019 | N/A | ||
| HSPA6 | 3 | / | −4.19 | 0.0051 | N/A | ||
| MAD2L1 | 3 | / | −3.16 | 0.0074 | Mutant mice (Mad2l2repro22/Mad2l2repro22) show infertility (J:92463). | N/A | |
| GMNN | 3 | / | −2.21 | 0.0078 | Oocyte-specific disruption of geminin (Gdf9-Cre Gmnn fl/fl) results in low fertility in mice. Even though there was no evident anomaly of oogenesis, oocyte meiotic maturation, natural ovulation, or fertilization, early embryo development and implantation were impaired (MGI Database). | N/A | |
| FBXO5 | 3 | / | −2.1 | 0.0006 | No role in FWs Role in oocyte: During oocyte maturation, it plays a role in meiosis through the inactivation of the APC-FZR1 complex. Inhibits APC through RPS6KA2 interaction that increases FBXO5 affinity for CDC20, leading to the metaphase arrest of the second meiotic division before fertilization [91]. | N/A | |
| ITIH4 | 3 | / | 2.09 | 0.0054 | N/A | Involved in the stabilization of the extracellular matrix and inflammatory processes. Found to be upregulated as a protein in FF from mature vs. immature oocytes (bovine) along with other proteins involved in complement activation (ITIH4, AHSG, FN1, HP) [92]. This inflammatory pathway may facilitate the local physiological and inflammatory reaction of ovulation [93]. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peserico, A.; Barboni, B.; Camerano Spelta Rapini, C.; Di Berardino, C.; Capacchietti, G.; Canciello, A.; Konstantinidou, F.; Donato, M.; Stuppia, L.; Gatta, V. Transcriptomic Profile of Early Antral Follicles: Predictive Somatic Gene Markers of Oocyte Maturation Outcome. Cells 2025, 14, 704. https://doi.org/10.3390/cells14100704
Peserico A, Barboni B, Camerano Spelta Rapini C, Di Berardino C, Capacchietti G, Canciello A, Konstantinidou F, Donato M, Stuppia L, Gatta V. Transcriptomic Profile of Early Antral Follicles: Predictive Somatic Gene Markers of Oocyte Maturation Outcome. Cells. 2025; 14(10):704. https://doi.org/10.3390/cells14100704
Chicago/Turabian StylePeserico, Alessia, Barbara Barboni, Chiara Camerano Spelta Rapini, Chiara Di Berardino, Giulia Capacchietti, Angelo Canciello, Fani Konstantinidou, Marisa Donato, Liborio Stuppia, and Valentina Gatta. 2025. "Transcriptomic Profile of Early Antral Follicles: Predictive Somatic Gene Markers of Oocyte Maturation Outcome" Cells 14, no. 10: 704. https://doi.org/10.3390/cells14100704
APA StylePeserico, A., Barboni, B., Camerano Spelta Rapini, C., Di Berardino, C., Capacchietti, G., Canciello, A., Konstantinidou, F., Donato, M., Stuppia, L., & Gatta, V. (2025). Transcriptomic Profile of Early Antral Follicles: Predictive Somatic Gene Markers of Oocyte Maturation Outcome. Cells, 14(10), 704. https://doi.org/10.3390/cells14100704










