Cooperative Role of Carbonic Anhydrase IX/XII in Driving Tumor Invasion and Metastasis: A Novel Targeted Therapeutic Strategy
Abstract
1. Introduction
2. CA IX and CA XII in Cancer Metastasis
2.1. CA IX in Cancer Metastasis
2.2. CA XII in CANCER Metastasis
3. Synergistic Effect of CA IX and CA XII
4. Clinical Significance of CA IX and CA XII
4.1. Compartmentalization of Metabolic Regions Coupled to Core-Invasion Fronts
4.2. Clinical Application of CA IX and CA XII
| Drugs | Targets | Indications | Development Stage | Drug Type | Refs. |
|---|---|---|---|---|---|
| Girentuximab | CA IX | Clear Cell Renal Cell Carcinoma | Phase III Clinical Trial | Monoclonal Antibody | [115,118] |
| SLC-0111 | CA IX/CA XII | Pancreatic Cancer, Multiple Solid Tumors | Phase I Clinical Trial | Small Molecule Inhibitor | [124] |
| 177Lu-cG250 | CA IX | Clear Cell Renal Cell Carcinoma, Triple-Negative Breast Cancer, etc | Phase II Clinical Trial | Diagnostic Radioisotope Conjugated Drug | [136,137] |
| CA IX-PMTE | CA IX | Locally advanced clear cell renal cell carcinoma | Preclinical | Bispecific T-cell engager | [138] |
| ITM-31 (LuCaFab) | CA XII | Malignant glioblastoma | Phase I Clinical Trial | Antibody-drug conjugate | [139] |
| CA IXhu-1 | CA IX | Non-small cell lung cancer | Phase I Clinical Trial | Monoclonal Antibody | [47] |
| SLC-149 | CA IX | Various tumors | Preclinical | Small Molecule Inhibitor | [140] |
| JS-403 | CA XIICA IXMMP2 | Various tumors | Preclinical | Small molecule chemical drugs | [141] |
| Anti-CA XII antibody | CA XII | Liver cancer, kidney cancer, etc. | In development | Monoclonal Antibody | [108] |
| Bispecific antibody (CA IX + CA XII) | CA IX, CA XII | Various tumors | In development | Bispecific antibody | [138] |
| Antibody-drug conjugate (ADC) | CA IX, CA XII | Various tumors | In development | Antibody-drug conjugate | [120] |
| Immune checkpoint inhibitor combination therapy | CA IX, CA XII | Various tumors | Clinical trial | Immunotherapy combination therapy | [142] |
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CA IX | Carbonic Anhydrase IX. |
| CA XII | Carbonic Anhydrase XII. |
| TME | Tumor Microenvironment. |
| HIF | Hypoxia-Inducible Factor. |
| VHL | Von Hippel-Lindau (gene). |
| TCGA | The Cancer Genome Atlas. |
| EMT | Epithelial-Mesenchymal Transition. |
| ECM | Extracellular Matrix. |
| MMP | Matrix Metalloproteinase. |
| MT1-MMP | Membrane-Type Matrix Metalloproteinase 1. |
| FAK | Focal Adhesion Kinase. |
| CSCs | Cancer Stem Cells. |
| EGFR | Epidermal Growth Factor Receptor. |
| PI3K | Phosphatidylinositol 3-Kinase. |
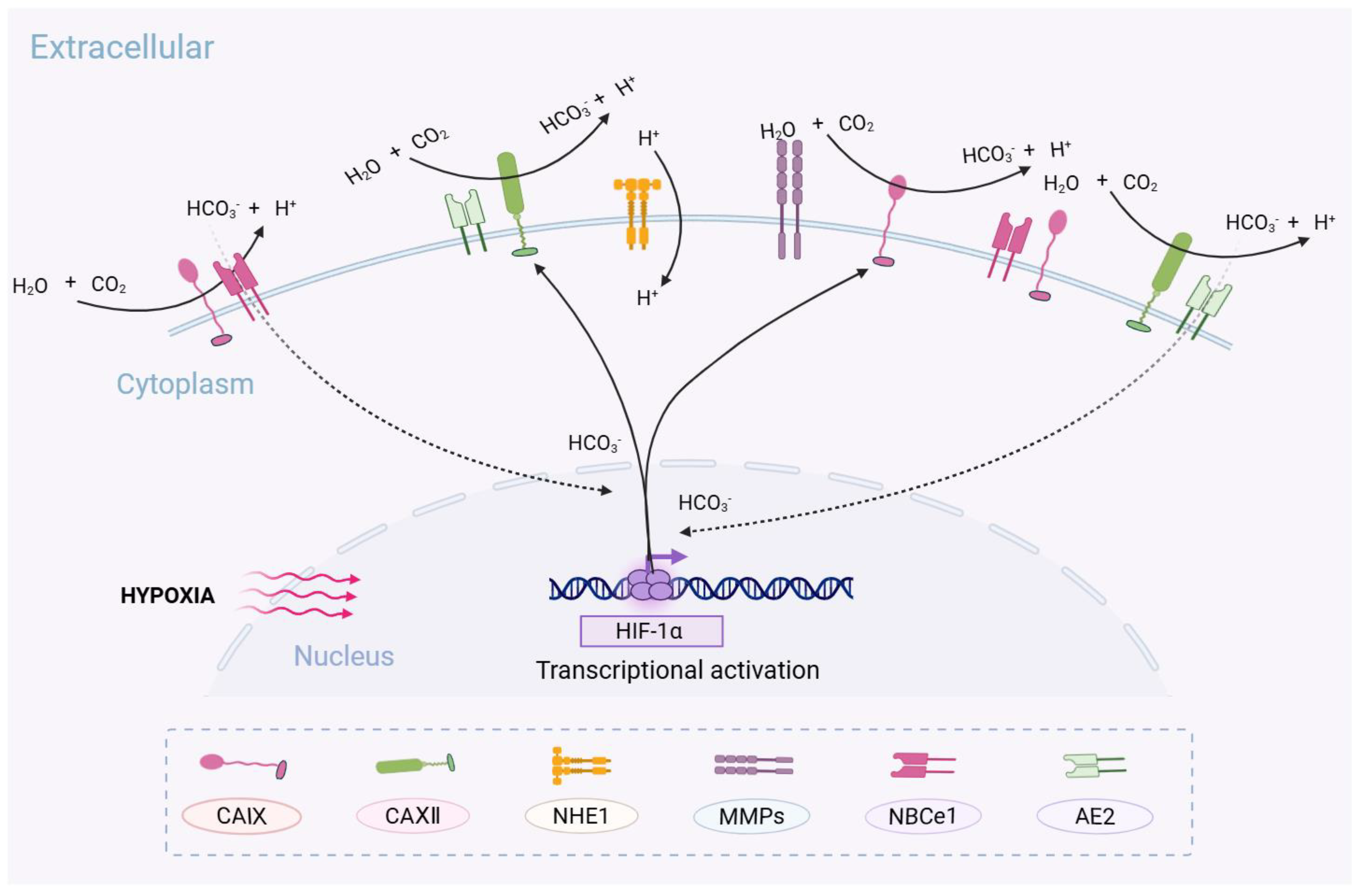
| NHE1 | Na+/H+ Exchanger 1. |
| TGF-β | Transforming Growth Factor Beta. |
| IGF-1 | Insulin-like Growth Factor 1. |
| EV | Extracellular Vesicles. |
| MVBs | Multivesicular Bodies. |
| HUVECs | Human Umbilical Vein Endothelial Cells. |
| TAMs | Tumor-Associated Macrophages. |
| CCL8 | C-C Motif Chemokine Ligand 8. |
| NF-κB | Nuclear Factor Kappa B. |
| PAAD | Pancreatic Adenocarcinoma. |
| PDAC | Pancreatic Ductal Adenocarcinoma. |
| AF | Auranofin. |
| NSCLC | Non-Small Cell Lung Cancer. |
| MCT1 | Monocarboxylate Transporter 1. |
| MCT4 | Monocarboxylate Transporter 4. |
| LDHA | Lactate Dehydrogenase A. |
| LDHB | Lactate Dehydrogenase B. |
| OXPHOS | Oxidative Phosphorylation. |
| 5-FU | 5-Fluorouracil. |
| CAR-T | Chimeric Antigen Receptor T-Cell Therapy. |
| BLA | Biologics License Application. |
| ADC | Antibody-Drug Conjugate. |
| SMDCs | Small Molecule-Drug Conjugates. |
| PET | Positron Emission Tomography. |
| P-gp | P-glycoprotein. |
| VEGF | Vascular Endothelial Growth Factor. |
| RCC | Renal Cell Carcinoma. |
| ccRCC | Clear Cell Renal Cell Carcinoma. |
| HCC | Hepatocellular Carcinoma. |
| HDI | Human Development Index. |
| HRE | Hypoxia Response Element. |
| TCA | Tricarboxylic Acid (Cycle). |
| ACC | Acetyl-CoA Carboxylase. |
| CAFs | Cancer-Associated Fibroblasts. |
| DFS | Disease-Free Survival. |
| OS | Overall Survival. |
References
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The extracellular matrix as hallmark of cancer and metastasis: From biomechanics to therapeutic targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.; Lauffenburger, D.; Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 2022, 22, 157–173. [Google Scholar] [CrossRef]
- Meldrum, N.U.; Roughton, F.J.W. Carbonic anhydrase. Its preparation and properties. J. Physiol. 1933, 80, 113–142. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin. Ther. Pat. 2018, 28, 709–712. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase activators. Future Med. Chem. 2018, 10, 561–573. [Google Scholar] [CrossRef]
- Pospelov, A.S.; Ala-Kurikka, T.; Kurki, S.; Voipio, J.; Kaila, K. Carbonic anhydrase inhibitors suppress seizures in a rat model of birth asphyxia. Epilepsia 2021, 62, 1971–1984. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Stoner, A.; Harris, A.; Oddone, F.; Belamkar, A.; Verticchio Vercellin, A.C.; Shin, J.; Januleviciene, I.; Siesky, B. Topical carbonic anhydrase inhibitors and glaucoma in 2021: Where do we stand? Br. J. Ophthalmol. 2022, 106, 1332–1337. [Google Scholar] [CrossRef]
- Supuran, C.T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 135, 1233–1249. [Google Scholar] [CrossRef] [PubMed]
- Guttler, A.; Eiselt, Y.; Funtan, A.; Thiel, A.; Petrenko, M.; Kessler, J.; Thondorf, I.; Paschke, R.; Vordermark, D.; Bache, M. Betulin Sulfonamides as Carbonic Anhydrase Inhibitors and Anticancer Agents in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 8808. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.M.; Gu, V.W.; Grimm, B.G.; Cassady, V.C.; White, J.R.; Weigel, R.J.; Kulak, M.V. TFAP2C regulates carbonic anhydrase XII in human breast cancer. Oncogene 2020, 39, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Barnett, D.H.; Sheng, S.; Charn, T.H.; Waheed, A.; Sly, W.S.; Lin, C.Y.; Liu, E.T.; Katzenellenbogen, B.S. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008, 68, 3505–3515. [Google Scholar] [CrossRef]
- Ong, C.H.C.; Lee, D.Y.; Lee, B.; Li, H.; Lim, J.C.T.; Lim, J.X.; Yeong, J.P.S.; Lau, H.Y.; Thike, A.A.; Tan, P.H.; et al. Hypoxia-regulated carbonic anhydrase IX (CAIX) protein is an independent prognostic indicator in triple negative breast cancer. Breast Cancer Res. 2022, 24, 38. [Google Scholar] [CrossRef]
- Janoniene, A.; Mazutis, L.; Matulis, D.; Petrikaite, V. Inhibition of Carbonic Anhydrase IX Suppresses Breast Cancer Cell Motility at the Single-Cell Level. Int. J. Mol. Sci. 2021, 22, 11571. [Google Scholar] [CrossRef]
- Daunys, S.; Petrikaite, V. The roles of carbonic anhydrases IX and XII in cancer cell adhesion, migration, invasion and metastasis. Biol. Cell. 2020, 112, 383–397. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Sarnella, A.; Ferrara, Y.; Auletta, L.; Albanese, S.; Cerchia, L.; Alterio, V.; De Simone, G.; Supuran, C.T.; Zannetti, A. Inhibition of carbonic anhydrases IX/XII by SLC-0111 boosts cisplatin effects in hampering head and neck squamous carcinoma cell growth and invasion. J. Exp. Clin. Cancer Res. 2022, 41, 122. [Google Scholar] [CrossRef]
- Logozzi, M.; Capasso, C.; Di Raimo, R.; Del Prete, S.; Mizzoni, D.; Falchi, M.; Supuran, C.T.; Fais, S. Prostate cancer cells and exosomes in acidic condition show increased carbonic anhydrase IX expression and activity. J. Enzym. Inhib. Med. Chem. 2019, 34, 272–278. [Google Scholar] [CrossRef]
- De Luca, L.; Mancuso, F.; Ferro, S.; Buemi, M.R.; Angeli, A.; Del Prete, S.; Capasso, C.; Supuran, C.T.; Gitto, R. Inhibitory effects and structural insights for a novel series of coumarin-based compounds that selectively target human CA IX and CA XII carbonic anhydrases. Eur. J. Med. Chem. 2018, 143, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Hilvo, M.; Di Fiore, A.; Supuran, C.T.; Pan, P.; Parkkila, S.; Scaloni, A.; Pastorek, J.; Parkkila, S.; Pedone, C.; et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. USA 2009, 106, 16233–16238. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Vergara, D.; Ravaioli, S.; Fonzi, E.; Adamo, L.; Damato, M.; Bravaccini, S.; Pirini, F.; Gaballo, A.; Barbano, R.; Pasculli, B.; et al. Carbonic Anhydrase XII Expression Is Modulated during Epithelial Mesenchymal Transition and Regulated through Protein Kinase C Signaling. Int. J. Mol. Sci. 2020, 21, 715. [Google Scholar] [CrossRef]
- Kivela, A.J.; Parkkila, S.; Saarnio, J.; Karttunen, T.J.; Kivela, J.; Parkkila, A.K.; Bartosova, M.; Mucha, V.; Novak, M.; Waheed, A.; et al. Expression of von Hippel-Lindau tumor suppressor and tumor-associated carbonic anhydrases IX and XII in normal and neoplastic colorectal mucosa. World J. Gastroenterol. 2005, 11, 2616–2625. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Linehan, W.M.; Ricketts, C.J. The metabolic basis of kidney cancer. Semin. Cancer Biol. 2013, 23, 46–55. [Google Scholar] [CrossRef]
- Potter, C.P.; Harris, A.L. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br. J. Cancer 2003, 89, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Pastorekova, S.; Zatovicova, M.; Pastorek, J. Cancer-associated carbonic anhydrases and their inhibition. Curr. Pharm. Des. 2008, 14, 685–698. [Google Scholar] [CrossRef]
- McDonald, P.C.; Chafe, S.C.; Brown, W.S.; Saberi, S.; Swayampakula, M.; Venkateswaran, G.; Nemirovsky, O.; Gillespie, J.A.; Karasinska, J.M.; Kalloger, S.E.; et al. Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells With Activated KRAS in Response to Hypoxia. Gastroenterology 2019, 157, 823–837. [Google Scholar] [CrossRef]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007, 26, 299–310. [Google Scholar] [CrossRef]
- Rohani, N.; Hao, L.; Alexis, M.S.; Joughin, B.A.; Krismer, K.; Moufarrej, M.N.; Soltis, A.R.; Lauffenburger, D.A.; Yaffe, M.B.; Burge, C.B.; et al. Acidification of Tumor at Stromal Boundaries Drives Transcriptome Alterations Associated with Aggressive Phenotypes. Cancer Res. 2019, 79, 1952–1966. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, J.; Zheng, X.; Chen, T.; Zhang, R.; Chen, R.; Cao, T.; Zeng, F.; Liu, Q. The Hippo Pathway in Breast Cancer: The Extracellular Matrix and Hypoxia. Int. J. Mol. Sci. 2024, 25, 12868. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Tanaka, N.; Sakamoto, T. MT1-MMP as a Key Regulator of Metastasis. Cells 2023, 12, 2187. [Google Scholar] [CrossRef] [PubMed]
- Maybee, D.V.; Ink, N.L.; Ali, M.A.M. Novel Roles of MT1-MMP and MMP-2: Beyond the Extracellular Milieu. Int. J. Mol. Sci. 2022, 23, 9513. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, S.; Xu, L.; Liu, X.; Ding, W.; Wang, Q.; Chen, Y.; Deng, H. CA9 Silencing Promotes Mitochondrial Biogenesis, Increases Putrescine Toxicity and Decreases Cell Motility to Suppress ccRCC Progression. Int. J. Mol. Sci. 2020, 21, 5939. [Google Scholar] [CrossRef] [PubMed]
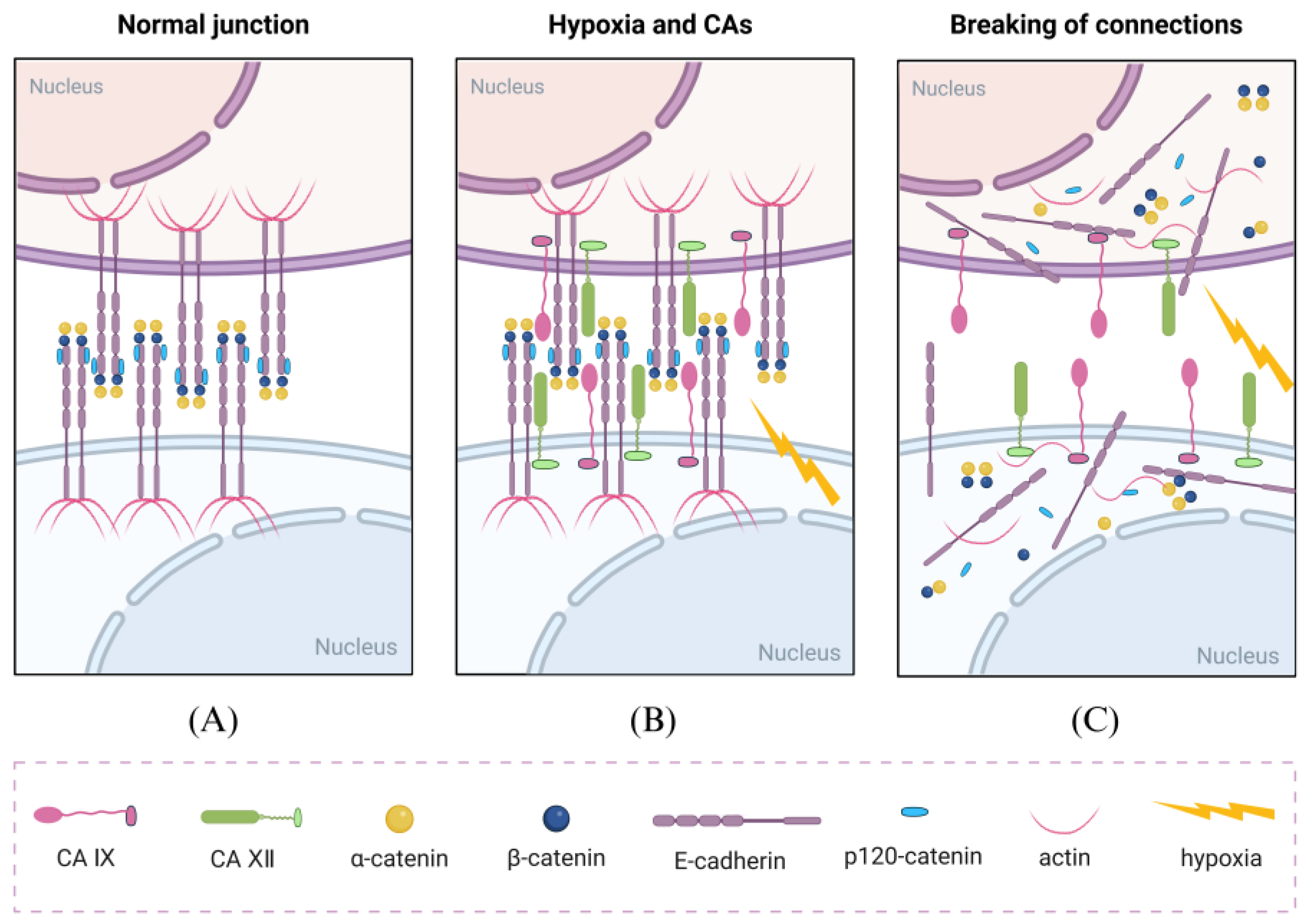
- Švastová, E.; Žilka, N.; Zat’ovičová, M.; Gibadulinová, A.; Čiampor, F.; Pastorek, J.; Pastoreková, S. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with β-catenin. Exp. Cell Res. 2003, 290, 332–345. [Google Scholar] [CrossRef]
- Lock, F.E.; McDonald, P.C.; Lou, Y.; Serrano, I.; Chafe, S.C.; Ostlund, C.; Aparicio, S.; Winum, J.-Y.; Supuran, C.T.; Dedhar, S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 2013, 32, 5210–5219. [Google Scholar] [CrossRef]
- Thiry, A.; Dogne, J.M.; Masereel, B.; Supuran, C.T. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol. Sci. 2006, 27, 566–573. [Google Scholar] [CrossRef]
- Zatovicova, M.; Kajanova, I.; Barathova, M.; Takacova, M.; Labudova, M.; Csaderova, L.; Jelenska, L.; Svastova, E.; Pastorekova, S.; Harris, A.L.; et al. Novel humanized monoclonal antibodies for targeting hypoxic human tumors via two distinct extracellular domains of carbonic anhydrase IX. Cancer Metab. 2022, 10, 3. [Google Scholar] [CrossRef]
- Stock, C.; Pedersen, S.F. Roles of pH and the Na(+)/H(+) exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin. Cancer Biol. 2017, 43, 5–16. [Google Scholar] [CrossRef]
- Xia, Y.; Inoue, K.; Du, Y.; Baker, S.J.; Reddy, E.P.; Greenblatt, M.B.; Zhao, B. TGFβ reprograms TNF stimulation of macrophages towards a non-canonical pathway driving inflammatory osteoclastogenesis. Nat. Commun. 2022, 13, 3920. [Google Scholar] [CrossRef]
- Izadi, N.; Solár, P.; Hašanová, K.; Zamani, A.; Akbar, M.S.; Mrázová, K.; Bartošík, M.; Kazda, T.; Hrstka, R.; Joukal, M. Breaking boundaries: Role of the brain barriers in metastatic process. Fluids Barriers CNS 2025, 22, 3. [Google Scholar] [CrossRef]
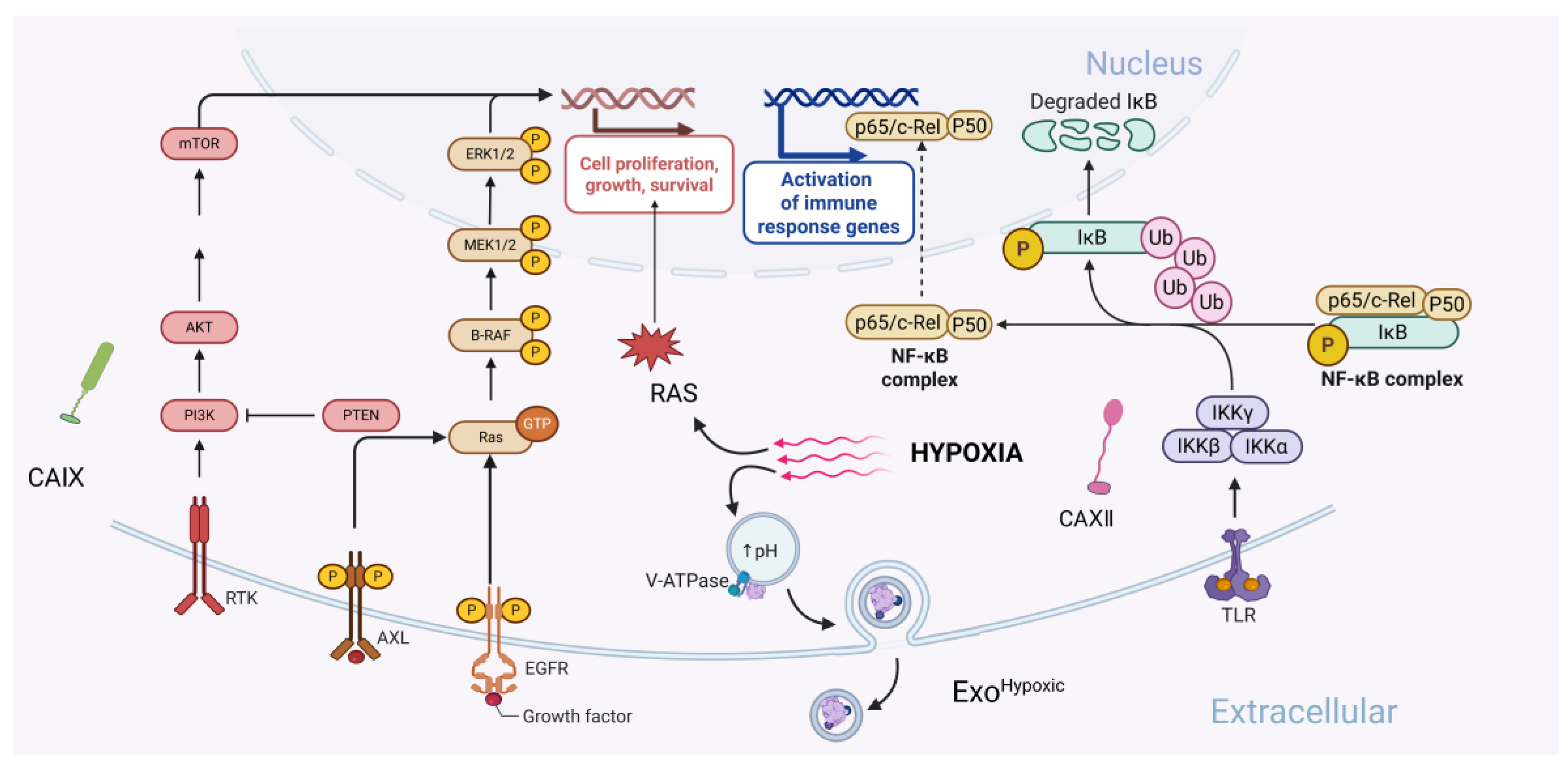
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Yu, C.C. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int. J. Nanomed. 2020, 15, 8019–8036. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Kawakami, K.; Fujita, Y.; Sugaya, M.; Kameyama, K.; Mizutani, K.; Deguchi, T.; Ito, M. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem. Biophys. Res. Commun. 2017, 492, 356–361. [Google Scholar] [CrossRef]
- Morrissey, S.M.; Zhang, F.; Ding, C.; Montoya-Durango, D.E.; Hu, X.; Yang, C.; Wang, Z.; Yuan, F.; Fox, M.; Zhang, H.-G.; et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021, 33, 2040–2058 e10. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Lee, S.; Toft, N.J.; Axelsen, T.V.; Espejo, M.S.; Pedersen, T.M.; Mele, M.; Pedersen, H.L.; Balling, E.; Johansen, T.; Burton, M.; et al. Carbonic anhydrases reduce the acidity of the tumor microenvironment, promote immune infiltration, decelerate tumor growth, and improve survival in ErbB2/HER2-enriched breast cancer. Breast Cancer Res. 2023, 25, 46. [Google Scholar] [CrossRef]
- Ning, W.R.; Jiang, D.; Liu, X.C.; Huang, Y.F.; Peng, Z.P.; Jiang, Z.Z.; Kang, T.; Zhuang, S.-M.; Wu, Y.; Zheng, L. Carbonic anhydrase XII mediates the survival and prometastatic functions of macrophages in human hepatocellular carcinoma. J. Clin. Investig. 2022, 132, 153110. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602 e10. [Google Scholar] [CrossRef]
- Graham, N.; Pollard, J.W. An acid trip activates protumoral macrophages to promote hepatocellular carcinoma malignancy. J. Clin. Investig. 2022, 132, 158562. [Google Scholar]
- Du, Y.; Xin, Z.; Liu, T.; Xu, P.; Mao, F.; Yao, J. Overexpressed CA12 has prognostic value in pancreatic cancer and promotes tumor cell apoptosis via NF-kappaB signaling. J. Cancer Res. Clin. Oncol. 2021, 147, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Deben, C.; Boullosa, L.F.; Fortes, F.R.; De La Hoz, E.C.; Le Compte, M.; Seghers, S.; Peeters, M.; Vanlanduit, S.; Lin, A.; Dijkstra, K.K.; et al. Auranofin repurposing for lung and pancreatic cancer: Low CA12 expression as a marker of sensitivity in patient-derived organoids, with potentiated efficacy by AKT inhibition. J. Exp. Clin. Cancer Res. 2024, 43, 88. [Google Scholar] [CrossRef]
- Chen, X.; Ding, J.C.; Hu, G.S.; Shu, X.Y.; Liu, Y.; Du, J.; Wen, Z.J.; Liu, J.Y.; Huang, H.H.; Tang, G.H.; et al. Estrogen-Induced LncRNA, LINC02568, Promotes Estrogen Receptor-Positive Breast Cancer Development and Drug Resistance Through Both In Trans and In Cis Mechanisms. Adv. Sci. 2023, 10, e2206663. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hong, J.H. Trafficking of carbonic anhydrase 12 and bicarbonate transporters by histamine stimulation mediates intracellular acidic scenario in lung cancer cells. J. Enzym. Inhib. Med. Chem. 2023, 38, 2247181. [Google Scholar] [CrossRef]
- Pena-Romero, A.C.; Orenes-Pinero, E. Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers 2022, 14, 1681. [Google Scholar] [CrossRef]
- Chiche, J.; Ilc, K.; Laferriere, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, C.; Pouysségur, J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009, 69, 358–368. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Seneff, S. Explaining deuterium-depleted water as a cancer therapy: A narrative review. Eur. J. Cancer Prev. 2025, 10, 586069. [Google Scholar] [CrossRef]
- Somlyai, G.; Papp, A.; Somlyai, I.; Kovács, B.Z.; Debrődi, M. Real-World Data Confirm That the Integration of Deuterium Depletion into Conventional Cancer Therapy Multiplies the Survival Probability of Patients. Biomedicines 2025, 13, 876. [Google Scholar] [CrossRef]
- Kim, S.J.; Rabbani, Z.N.; Dewhirst, M.W.; Vujaskovic, Z.; Vollmer, R.T.; Schreiber, E.G.; Oosterwijk, E.; Kelley, M.J. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer 2005, 49, 325–335. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Beasley, N.; Watson, P.H.; Campo, L.; Chia, S.K.; English, R.; Pastorek, J.; Sly, W.S.; Ratcliffe, P.; Harris, A.L. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am. J. Pathol. 2001, 158, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Hyuga, S.; Wada, H.; Eguchi, H.; Otsuru, T.; Iwgami, Y.; Yamada, D.; Noda, T.; Asaoka, T.; Kawamoto, K.; Gotoh, K.; et al. Expression of carbonic anhydrase IX is associated with poor prognosis through regulation of the epithelial-mesenchymal transition in hepatocellular carcinoma. Int. J. Oncol. 2017, 51, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, C.; Irshad, K.; Dikshit, B.; Chattopadhyay, P.; Sarkar, C.; Gupta, D.K.; Sinha, S.; Chosdol, K. FAT1 modulates EMT and stemness genes expression in hypoxic glioblastoma. Int. J. Cancer 2018, 142, 805–812. [Google Scholar] [CrossRef]
- da Motta, L.L.; Ledaki, I.; Purshouse, K.; Haider, S.; De Bastiani, M.A.; Baban, D.; Morotti, M.; Steers, G.; Wigfield, S.; Bridges, E.; et al. The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer. Oncogene 2016, 36, 122–132. [Google Scholar] [CrossRef]
- Kim, E.S.; Nam, S.M.; Song, H.K.; Lee, S.; Kim, K.; Lim, H.K.; Lee, H.; Kang, K.-T.; Kwon, Y.-J.; Chun, Y.-J.; et al. CCL8 mediates crosstalk between endothelial colony forming cells and triple-negative breast cancer cells through IL-8, aggravating invasion and tumorigenicity. Oncogene 2021, 40, 3245–3259. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, J.; Guo, X.; Yu, Q.; Ding, S.; Xu, X.; Peng, Y.; Zhu, L.; Zou, G.; Zhang, X. Possible involvement of crosstalk between endometrial cells and mast cells in the development of endometriosis via CCL8/CCR1. Biomed. Pharmacother. 2020, 129, 110476. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, X.; Jin, Y.; Zhu, X.; Zhou, T.; Yu, Y.; Ji, X.; Chang, Y.; Luo, J.; Ni, X.; et al. LncRNA ZNF674-AS1 drives cell growth and inhibits cisplatin-induced pyroptosis via up-regulating CA9 in neuroblastoma. Cell Death Dis. 2024, 15, 5. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, C.E.; Wu, S.C.; Yang, Z.Y.; Chiang, Y.F.; Huang, K.C.; Wang, K.L.; Ali, M.; Shieh, T.M.; Chang, H.Y.; et al. Para-toluenesulfonamide, a novel potent carbonic anhydrase inhibitor, improves hypoxia-induced metastatic breast cancer cell viability and prevents resistance to alphaPD-1 therapy in triple-negative breast cancer. Biomed. Pharmacother. 2023, 167, 115533. [Google Scholar] [CrossRef]
- Teixeira, S.A.; Viapiano, M.S.; Andrade, A.F.; Nandhu, M.S.; Pezuk, J.A.; Bidinotto, L.T.; Suazo, V.K.; Neder, L.; Carlotti, C.G.; Becker, A.P.; et al. The Carbonic Anhydrase Inhibitor E7070 Sensitizes Glioblastoma Cells to Radio- and Chemotherapy and Reduces Tumor Growth. Mol. Neurobiol. 2021, 58, 4520–4534. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.X.; Zhu, Y.J.; Fu, J.; Zhao, X.F.; Zhang, Y.N.; Wang, S.; Wu, J.; Wang, K.; Wu, R.; et al. Single-Cell Transcriptome Analysis Uncovers Intratumoral Heterogeneity and Underlying Mechanisms for Drug Resistance in Hepatobiliary Tumor Organoids. Adv. Sci. 2021, 8, e2003897. [Google Scholar] [CrossRef]
- Huang, T.; Tang, L.; Wang, H.; Lin, L.; Fu, J. Carbonic anhydrase 12 gene silencing reverses the sensitivity of paclitaxel in drug-resistant breast cancer cells. Bioengineered 2021, 12, 9806–9818. [Google Scholar] [CrossRef] [PubMed]
- Teodori, E.; Braconi, L.; Bua, S.; Lapucci, A.; Bartolucci, G.; Manetti, D.; Romanelli, M.N.; Dei, S.; Supuran, C.T.; Coronnello, M. Dual P-Glycoprotein and CA XII Inhibitors: A New Strategy to Reverse the P-gp Mediated Multidrug Resistance (MDR) in Cancer Cells. Molecules 2020, 25, 1748. [Google Scholar] [CrossRef]
- Ronca, R.; Supuran, C.T. Carbonic anhydrase IX: An atypical target for innovative therapies in cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2024, 1879, 189120. [Google Scholar] [CrossRef] [PubMed]
- Jarman, E.J.; Ward, C.; Turnbull, A.K.; Martinez-Perez, C.; Meehan, J.; Xintaropoulou, C.; Sims, A.H.; Langdon, S.P. HER2 regulates HIF-2alpha and drives an increased hypoxic response in breast cancer. Breast Cancer Res. 2019, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhao, J.H.; Wang, X.L.; Guo, X.J.; Yang, J.; Bai, X.; Jin, S.Y.; Ge, R.L. Correlation between carbonic anhydrase IX (CA-9), XII (CA-12) and hypoxia inducible factor-2alpha (HIF-2alpha) in breast cancer. Neoplasma 2015, 62, 456–463. [Google Scholar] [CrossRef][Green Version]
- Lin, C.; Lai, S.W.; Shen, C.K.; Chen, C.W.; Tsai, C.F.; Liu, Y.S.; Lu, D.Y.; Huang, B.R. Fenofibrate inhibits hypoxia-inducible factor-1 alpha and carbonic anhydrase expression through activation of AMP-activated protein kinase/HO-1/Sirt1 pathway in glioblastoma cells. Environ. Toxicol. 2021, 36, 2551–2561. [Google Scholar] [CrossRef]
- Wu, L.; Yan, J.; Bai, Y.; Chen, F.; Zou, X.; Xu, J.; Huang, A.; Hou, L.; Zhong, Y.; Jing, Z.; et al. An invasive zone in human liver cancer identified by Stereo-seq promotes hepatocyte-tumor cell crosstalk, local immunosuppression and tumor progression. Cell Res. 2023, 33, 585–603. [Google Scholar] [CrossRef]
- Maiques, O.; Sallan, M.C.; Laddach, R.; Pandya, P.; Varela, A.; Crosas-Molist, E.; Barcelo, J.; Courbot, O.; Liu, Y.; Graziani, V.; et al. Matrix mechano-sensing at the invasive front induces a cytoskeletal and transcriptional memory supporting metastasis. Nat. Commun. 2025, 16, 1394. [Google Scholar] [CrossRef]
- Rademakers, S.E.; Lok, J.; van der Kogel, A.J.; Bussink, J.; Kaanders, J.H.A.M. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer 2011, 11, 167. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Chen, Z.; Khokhar, D.; Wolff, A.; Ai, L.; Heldermon, C.D.; Bozdag, M.; Carta, F.; Supuran, C.T.; Brown, K.D.; et al. A non-catalytic function of carbonic anhydrase IX contributes to the glycolytic phenotype and pH regulation in human breast cancer cells. Biochem. J. 2019, 476, 1497–1513. [Google Scholar] [CrossRef]
- Alfarouk, K.O. Tumor metabolism, cancer cell transporters, and microenvironmental resistance. J. Enzym. Inhib. Med. Chem. 2016, 31, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, M.R.; Barar, J.; Pourseif, M.M.; Eskandani, M.; Jafari Niya, M.; Mashayekhi, M.R.; Omidi, Y. Molecular machineries of pH dysregulation in tumor microenvironment: Potential targets for cancer therapy. Bioimpacts 2017, 7, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Babl, N.; Decking, S.M.; Voll, F.; Althammer, M.; Sala-Hojman, A.; Ferretti, R.; Korf, C.; Schmidl, C.; Schmidleithner, L.; Nerb, B.; et al. MCT4 blockade increases the efficacy of immune checkpoint blockade. J. Immunother. Cancer 2023, 11, e007349. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Silagi, E.S.; Schipani, E.; Shapiro, I.M.; Risbud, M.V. The role of HIF proteins in maintaining the metabolic health of the intervertebral disc. Nat. Rev. Rheumatol. 2021, 17, 426–439. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Lin, R.; Huang, Y.; Wang, Z.; Xu, S.; Wang, L.; Chen, F.; Zhang, J.; Pan, K.; et al. Lactate drives epithelial-mesenchymal transition in diabetic kidney disease via the H3K14la/KLF5 pathway. Redox. Biol. 2024, 75, 103246. [Google Scholar] [CrossRef]
- Darmanis, S.; Sloan, S.A.; Croote, D.; Mignardi, M.; Chernikova, S.; Samghababi, P.; Zhang, Y.; Neff, N.; Kowarsky, M.; Caneda, C.; et al. Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma. Cell Rep. 2017, 21, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.T.; Montgomery, M.M.; Biagioni, E.M.; Krassovskaia, P.; Jevtovic, F.; Shookster, D.; Sharma, U.; Tung, K.; Broskey, N.T.; May, L.; et al. Intrinsic adaptations in OXPHOS power output and reduced tumorigenicity characterize doxorubicin resistant ovarian cancer cells. Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148915. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Wang, Z.; Wang, Z.; Cui, Y.; Tian, R.; Zhu, Y.; Han, B.; Liu, H.; Zuo, X.; et al. Mitochondrial Breast Cancer Resistant Protein Sustains the Proliferation and Survival of Drug-Resistant Breast Cancer Cells by Regulating Intracellular Reactive Oxygen Species. Front Cell Dev. Biol. 2021, 9, 719209. [Google Scholar] [CrossRef]
- Hedlund, E.E.; McDonald, P.C.; Nemirovsky, O.; Awrey, S.; Jensen, L.D.E.; Dedhar, S. Harnessing Induced Essentiality: Targeting Carbonic Anhydrase IX and Angiogenesis Reduces Lung Metastasis of Triple Negative Breast Cancer Xenografts. Cancers 2019, 11, 1002. [Google Scholar] [CrossRef]
- Sarnella, A.; Ferrara, Y.; Albanese, S.; Omodei, D.; Cerchia, L.; De Simone, G.; Supuran, C.T.; Zannetti, A. Inhibition of Bone Marrow-Mesenchymal Stem Cell-Induced Carbonic Anhydrase IX Potentiates Chemotherapy Efficacy in Triple-Negative Breast Cancer Cells. Cells 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.K.; Cormerais, Y.; Durivault, J.; Pouyssegur, J. Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget 2016, 8, 10225–10237. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef]
- Schodel, J.; Grampp, S.; Maher, E.R.; Moch, H.; Ratcliffe, P.J.; Russo, P.; Mole, D.R. Hypoxia, Hypoxia-inducible Transcription Factors, and Renal Cancer. Eur. Urol. 2016, 69, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Moncao, C.C.D.; Scrideli, C.A.; Andrade, A.F.; Viapiano, M.S.; Carlotti, C.G.; Moreno, D.A.; Baroni, M.; Tone, L.G.; Teixeira, S.A. Indisulam Reduces Viability and Regulates Apoptotic Gene Expression in Pediatric High-Grade Glioma Cells. Biomedicines 2022, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; Jirstrom, K.; Kronblad, A.S.; Millikan, R.C.; Landberg, G.; Duffy, M.J.; Rydén, L.; Gallagher, W.M.; O’Brien, S.L. CA IX is an Independent Prognostic Marker in Premenopausal Breast Cancer Patients with One to Three Positive Lymph Nodes and a Putative Marker of Radiation Resistance. Clin. Cancer Res. 2006, 12, 6421–6431. [Google Scholar] [CrossRef]
- Chien, M.H.; Ying, T.H.; Hsieh, Y.H.; Lin, C.H.; Shih, C.H.; Wei, L.H.; Yang, S.F. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral. Oncol. 2012, 48, 417–423. [Google Scholar] [CrossRef]
- Haapasalo, J.; Hilvo, M.; Nordfors, K.; Haapasalo, H.; Parkkila, S.; Hyrskyluoto, A.; Rantala, I.; Waheed, A.; Sly, W.S.; Pastorekova, S.; et al. Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astrocytic gliomas. Neuro. Oncol. 2008, 10, 131–138. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Mulders, P.F.; Boerman, O.C.; Oyen, W.J.; Oosterwijk, E. Carbonic anhydrase IX in renal cell carcinoma: Implications for prognosis, diagnosis, and therapy. Eur. Urol. 2010, 58, 75–83. [Google Scholar] [CrossRef]
- Chia, S.K.; Wykoff, C.C.; Watson, P.H.; Han, C.; Leek, R.D.; Pastorek, J.; Gatter, K.C.; Ratcliffe, P.; Harris, A.L. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J. Clin. Oncol. 2001, 19, 3660–3668. [Google Scholar] [CrossRef]
- Sathornsumetee, S.; Cao, Y.; Marcello, J.E.; Herndon, J.E.; McLendon, R.E.; Desjardins, A.; Friedman, H.S.; Dewhrist, M.W.; Vredenburgh, J.J.; Rich, J.N. Tumor Angiogenic and Hypoxic Profiles Predict Radiographic Response and Survival in Malignant Astrocytoma Patients Treated With Bevacizumab and Irinotecan. J. Clin. Oncol. 2008, 26, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; Wolfsberger, S.; Haberler, C.; Breitschopf, H.; Czech, T.; Slavc, I.; Harris, A.L.; Acker, T.; Budka, H.; Hainfellner, J.A. Vascularization and expression of hypoxia-related tissue factors in intracranial ependymoma and their impact on patient survival. Acta Neuropathol. 2005, 109, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Swinson, D.E.; Jones, J.L.; Richardson, D.; Wykoff, C.; Turley, H.; Pastorek, J.; Taub, N.; Harris, L.; O’Byrne, K.J. Carbonic. anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J. Clin. Oncol. 2003, 21, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, S.; Xie, Y.; Liu, J.; Sun, L.; Zeng, D.; Wang, P.; Ma, X.; Kroemer, G.; Bartlett, D.L.; et al. JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice. Gastroenterology 2018, 154, 1480–1493. [Google Scholar] [CrossRef]
- Chamie, K.; Donin, N.M.; Klopfer, P.; Bevan, P.; Fall, B.; Wilhelm, O.; Störkel, S.; Said, J.; Gambla, M.; Hawkins, R.E.; et al. Adjuvant Weekly Girentuximab Following Nephrectomy for High-Risk Renal Cell Carcinoma: The ARISER Randomized Clinical Trial. JAMA Oncol. 2017, 3, 913–920. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Gao, X.; Chen, X.; Li, L.; Li, G.; Liu, C.; Miao, Y.; Wang, R.; Hu, K. Radiopharmaceuticals and their applications in medicine. Signal Transduct. Target. Ther. 2025, 10, 1. [Google Scholar] [CrossRef]
- McDonald, P.C.; Chafe, S.C.; Supuran, C.T.; Dedhar, S. Cancer Therapeutic Targeting of Hypoxia Induced Carbonic Anhydrase IX: From Bench to Bedside. Cancers 2022, 14, 3297. [Google Scholar] [CrossRef]
- Shuch, B.; Pantuck, A.J.; Bernhard, J.C.; Morris, M.A.; Master, V.; Scott, A.M.; van Praet, C.; Bailly, C.; Önal, B.; Aksoy, T.; et al. [(89)Zr]Zr-girentuximab for PET-CT imaging of clear-cell renal cell carcinoma: A prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 2024, 25, 1277–1287. [Google Scholar] [CrossRef]
- Shuch, B.M.; Pantuck, A.J.; Bernhard, J.-C.; Morris, M.A.; Master, V.A.; Scott, A.M.; Van Praet, C.; Bailly, C.; Aksoy, T.; Merkx, R.; et al. Results from phase 3 study of 89Zr-DFO-girentuximab for PET/CT imaging of clear cell renal cell carcinoma (ZIRCON). J. Clin. Oncol. 2023, 41, LBA602. [Google Scholar] [CrossRef]
- Testa, C.; Papini, A.M.; Zeidler, R.; Vullo, D.; Carta, F.; Supuran, C.T.; Rovero, P. First studies on tumor associated carbonic anhydrases IX and XII monoclonal antibodies conjugated to small molecule inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 592–596. [Google Scholar] [CrossRef]
- Lenferink, A.E.G.; McDonald, P.C.; Cantin, C.; Grothe, S.; Gosselin, M.; Baardsnes, J.; Banville, M.; Lachance, P.; Robert, A.; Cepero-Donates, Y.; et al. Isolation and characterization of monoclonal antibodies against human carbonic anhydrase-IX. MAbs 2021, 13, 1999194. [Google Scholar] [CrossRef] [PubMed]
- Cazzamalli, S.; Ziffels, B.; Widmayer, F.; Murer, P.; Pellegrini, G.; Pretto, F.; Wulhfard, S.; Neri, D. Enhanced Therapeutic Activity of Non-Internalizing Small-Molecule-Drug Conjugates Targeting Carbonic Anhydrase IX in Combination with Targeted Interleukin-2. Clin. Cancer Res. 2018, 24, 3656–3667. [Google Scholar] [CrossRef] [PubMed]
- Millul, J.; Krudewig, C.; Zana, A.; Dakhel Plaza, S.; Puca, E.; Villa, A.; Neri, D.; Cazzamalli, S. Immunotherapy with Immunocytokines and PD-1 Blockade Enhances the Anticancer Activity of Small Molecule-Drug Conjugates Targeting Carbonic Anhydrase IX. Mol. Cancer Ther. 2021, 20, 512–522. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic Anhydrase Inhibition and the Management of Hypoxic Tumors. Metabolites 2017, 7, 48. [Google Scholar] [CrossRef]
- Andreucci, E.; Peppicelli, S.; Carta, F.; Brisotto, G.; Biscontin, E.; Ruzzolini, J.; Bianchini, F.; Biagioni, A.; Supuran, C.T.; Calorini, L. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J. Mol. Med. 2017, 95, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Chia, S.; Bedard, P.L.; Chu, Q.; Lyle, M.; Tang, L.; Singh, M.; Zhang, Z.; Supuran, C.T.; Renouf, D.J.; et al. A Phase 1 Study of SLC-0111, a Novel Inhibitor of Carbonic Anhydrase IX, in Patients With Advanced Solid Tumors. Am. J. Clin. Oncol. 2020, 43, 484–490. [Google Scholar] [CrossRef]
- Pacchiano, F.; Carta, F.; McDonald, P.C.; Lou, Y.; Vullo, D.; Scozzafava, A.; Dedhar, S.; Supuran, C.T. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J. Med. Chem. 2011, 54, 1896–1902. [Google Scholar] [CrossRef]
- Wang, Y.; Buck, A.; Grimaud, M.; Culhane, A.C.; Kodangattil, S.; Razimbaud, C.; Bonal, D.M.; Nguyen, Q.-D.; Zhu, Z.; Wei, K.; et al. Anti-CAIX BBzeta CAR4/8 T cells exhibit superior efficacy in a ccRCC mouse model. Mol. Ther. Oncolytics 2022, 24, 385–399. [Google Scholar] [CrossRef]
- Lamers, C.H.; Sleijfer, S.; van Steenbergen, S.; van Elzakker, P.; van Krimpen, B.; Groot, C.; Vulto, A.; den Bakker, M.; Oosterwijk, E.; Debets, R.; et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol. Ther. 2013, 21, 904–912. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, L.; Wang, X.; Yuan, S.; Li, W.; Tian, W.; Chen, J.; Zhang, Q.; Zhang, Y.; Zhang, Q.; et al. Oncolytic adenovirus-mediated expression of decorin facilitates CAIX-targeting CAR-T therapy against renal cell carcinoma. Mol. Ther. Oncolytics 2022, 24, 14–25. [Google Scholar] [CrossRef]
- Sheff, J.G.; Kelly, J.F.; Robotham, A.; Sulea, T.; Malenfant, F.; L’Abbe, D.; Duchesne, M.; Pelletier, A.; Lefebvre, J.; Acel, A.; et al. Hydrogen-deuterium exchange mass spectrometry reveals three unique binding responses of mAbs directed to the catalytic domain of hCAIX. MAbs 2021, 13, 1997072. [Google Scholar] [CrossRef]
- Xun, Z.; Ding, X.; Zhang, Y.; Zhang, B.; Lai, S.; Zou, D.; Zheng, J.; Chen, G.; Su, B.; Han, L.; et al. Reconstruction of the tumor spatial microenvironment along the malignant-boundary-nonmalignant axis. Nat. Commun. 2023, 14, 933. [Google Scholar] [CrossRef]
- Moncada, R.; Barkley, D.; Wagner, F.; Chiodin, M.; Devlin, J.C.; Baron, M.; Hajdu, C.H.; Simeone, D.M.; Yanai, I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020, 38, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.V.; Moncada, R.; Weiss, J.M.; Yanai, I.; White, R.M. Spatially resolved transcriptomics reveals the architecture of the tumor-microenvironment interface. Nat. Commun. 2021, 12, 6278. [Google Scholar] [CrossRef] [PubMed]
- Hirz, T.; Mei, S.; Sarkar, H.; Kfoury, Y.; Wu, S.; Verhoeven, B.M.; Subtelny, A.O.; Zlatev, D.V.; Wszolek, M.W.; Salari, K.; et al. Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses. Nat. Commun. 2023, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- Stillebroer, A.B.; Zegers, C.M.L.; Boerman, O.C.; Oosterwijk, E.; Mulders, P.F.A.; O’Donoghue, J.A.; Visser, E.P.; Oyen, W.J. Dosimetric Analysis of 177Lu-cG250 Radioimmunotherapy in Renal Cell Carcinoma Patients: Correlation with Myelotoxicity and Pretherapeutic Absorbed Dose Predictions Based on 111In-cG250 Imaging. J. Nucl. Med. 2012, 53, 82–89. [Google Scholar] [CrossRef]
- Oosterwijk-Wakka, J.C.; de Weijert, M.C.A.; Franssen, G.M.; Kolev, D.R.; de Haan, T.A.F.J.; Boerman, O.C.; Mulders, P.F.; Oosterwijk, E. Combination of sunitinib and 177Lu-labeled antibody cG250 targeted radioimmunotherapy: A promising new therapeutic strategy for patients with advanced renal cell cancer. Neoplasia 2022, 32, 100826. [Google Scholar] [CrossRef]
- O’Connell, R.P.; Liaw, K.; Wellhausen, N.; Chuckran, C.A.; Bhojnagarwala, P.S.; Bordoloi, D.; Park, D.; Shupin, N.; Kulp, D.; June, C.H.; et al. Format-tuning of in vivo-launched bispecific T cell engager enhances efficacy against renal cell carcinoma. J. Immunother. Cancer 2024, 12, 8733. [Google Scholar] [CrossRef]
- Roll, W.; Müther, M.; Böning, G.; Delker, A.; Warneke, N.; Gildehaus, F.-J.; Schäfers, M.; Stummer, W.; Zeidler, R.; Reulen, H.-J.; et al. First clinical experience with fractionated intracavitary radioimmunotherapy using [177Lu]Lu-6A10-Fab fragments in patients with glioblastoma: A pilot study. EJNMMI Res. 2023, 13, 78. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Combs, J.; Singh, S.; Andring, J.; Wolff, A.; Tu, C.; Zhang, Z.; McKenna, R.; Frost, S.C. Inhibition of Carbonic Anhydrase Using SLC-149: Support for a Noncatalytic Function of CAIX in Breast Cancer. J. Med. Chem. 2021, 64, 1713–1724. [Google Scholar] [CrossRef]
- Hoffman, A.; Qadri, B.; Frant, J.; Katz, Y.; Bhusare, S.R.; Breuer, E.; Hadar, R.; Reich, R. Carbamoylphosphonate matrix metalloproteinase inhibitors 6: Cis-2-aminocyclohexylcarbamoylphosphonic acid, a novel orally active antimetastatic matrix metalloproteinase-2 selective inhibitor--synthesis and pharmacodynamic and pharmacokinetic analysis. J. Med. Chem. 2008, 51, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Chafe, S.C.; McDonald, P.C.; Saberi, S.; Nemirovsky, O.; Venkateswaran, G.; Burugu, S.; Gao, D.; Delaidelli, A.; Kyle, A.H.; Baker, J.H.E.; et al. Targeting Hypoxia-Induced Carbonic Anhydrase IX Enhances Immune-Checkpoint Blockade Locally and Systemically. Cancer Immunol. Res. 2019, 7, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.; Durivault, J.; Filippi, I.; Criscuoli, M.; Monaci, S.; Pouyssegur, J.; Naldini, A.; Carraro, F.; Parks, S. Carbonic anhydrase XII expression is linked to suppression of Sonic hedgehog ligand expression in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2019, 516, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef]
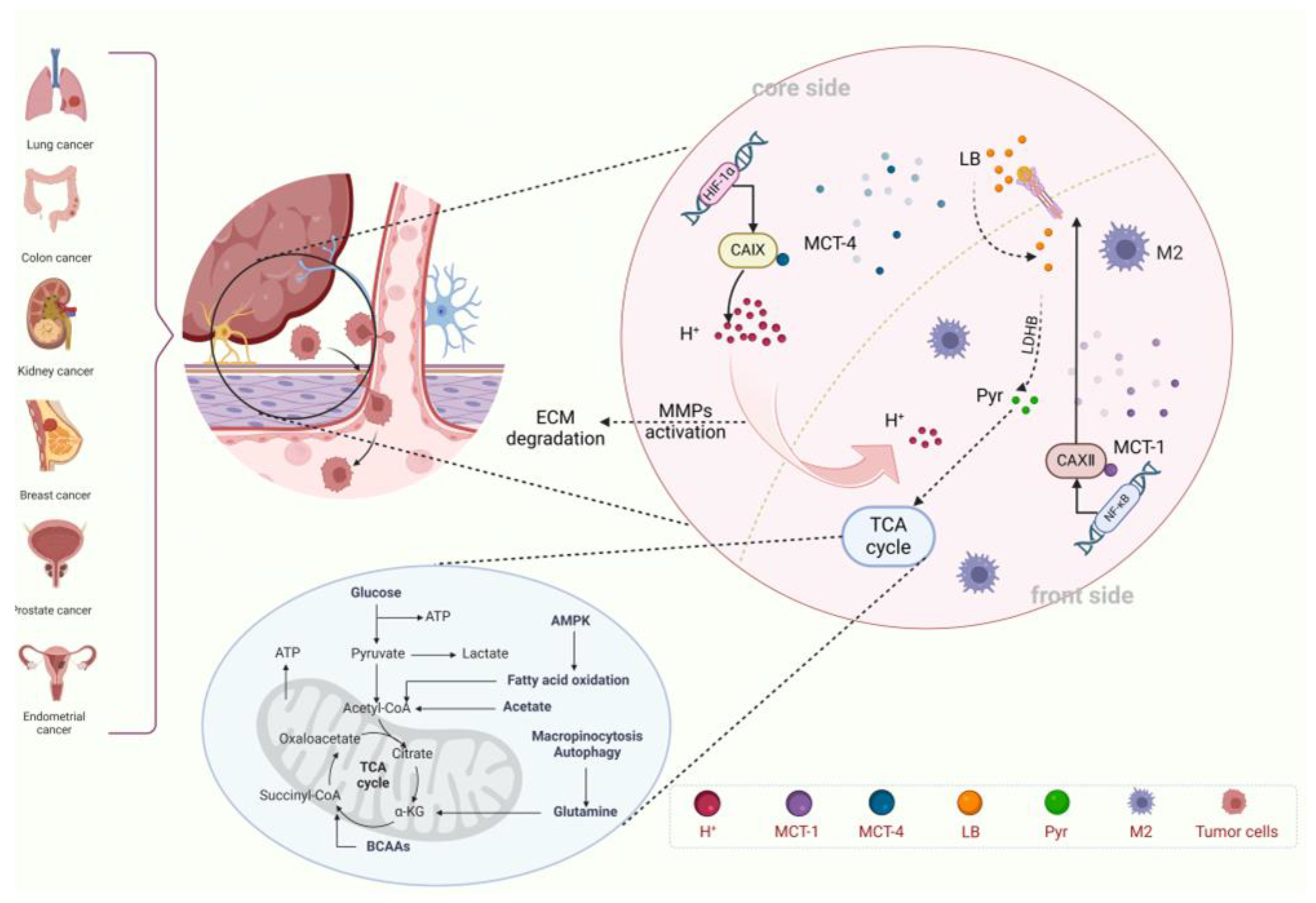
| Metabolic Compartments | Core Features | Key Targets | Intervention Strategies | Refs. |
|---|---|---|---|---|
| Tumor Core Zone | Hypoxia, High Expression of CA IX, Glycolysis | CA IX/MCT4/LDHA | CA IX Inhibitor + LDHA Inhibitor | [97] |
| Invasion Front Zone | Rich in Oxygen, High Expression of CA XII, OXPHOS | CA XII/MCT1/PDHK1 | MCT1 Inhibitor + PDHK1 Inhibitor | [87] |
| Metabolic Coupling Interface | Lactate Shuttle, Exosome Communication | Exosomal CA IX/ANXA2 | ANXA2 Antibody + Exosome Capture Agent | [22,36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Chen, R.; Zheng, X.; Luo, Y.; Yao, M.; Ke, F.; Guo, X.; Liu, X.; Liu, Q. Cooperative Role of Carbonic Anhydrase IX/XII in Driving Tumor Invasion and Metastasis: A Novel Targeted Therapeutic Strategy. Cells 2025, 14, 693. https://doi.org/10.3390/cells14100693
Yang H, Chen R, Zheng X, Luo Y, Yao M, Ke F, Guo X, Liu X, Liu Q. Cooperative Role of Carbonic Anhydrase IX/XII in Driving Tumor Invasion and Metastasis: A Novel Targeted Therapeutic Strategy. Cells. 2025; 14(10):693. https://doi.org/10.3390/cells14100693
Chicago/Turabian StyleYang, Hanyu, Rui Chen, Xiang Zheng, Yufan Luo, Mingxuan Yao, Famin Ke, Xiurong Guo, Xiaoyan Liu, and Qiuyu Liu. 2025. "Cooperative Role of Carbonic Anhydrase IX/XII in Driving Tumor Invasion and Metastasis: A Novel Targeted Therapeutic Strategy" Cells 14, no. 10: 693. https://doi.org/10.3390/cells14100693
APA StyleYang, H., Chen, R., Zheng, X., Luo, Y., Yao, M., Ke, F., Guo, X., Liu, X., & Liu, Q. (2025). Cooperative Role of Carbonic Anhydrase IX/XII in Driving Tumor Invasion and Metastasis: A Novel Targeted Therapeutic Strategy. Cells, 14(10), 693. https://doi.org/10.3390/cells14100693





