Ryanodine Receptors in Islet Cell Function: Calcium Signaling, Hormone Secretion, and Diabetes
Abstract
1. Introduction
2. Effects of Different Agonists and Antagonists on RyRs
2.1. Caffeine
2.2. Ryanodine
2.2.1. Activation of RyRs by Ryanodine
2.2.2. Inhibition of RyRs by Ryanodine
2.3. 9-Methyl-7-bromoeudistomin D (MBED)
2.4. Thimerosal
2.5. Dantrolene
2.6. Other Agonists of RyRs
2.6.1. 4-Chloro-m-cresol (4-CmC) and 4-Chloro-3-ethylphenol (4-CEP)
2.6.2. Nitric Oxide (NO)
2.6.3. Arachidonic Acid
3. Role of RyRs in Mediating CICR in β-Cells
4. Magnitude of [Ca2+]i Increase Achieved Through RyR-Mediated CICR
5. Regulation of RyRs in β-Cells by Phosphorylation
5.1. CaMKII-Mediated Phosphorylation
5.2. Phosphorylation by PKA
6. FK506-Binding Protein 12.6 (FKBP12.6) and RyR2
7. Cyclic ADP-Ribose (cADPR) and RyRs of β-Cells
8. Role of RyRs in Mediating Insulin Secretion
9. Role of RyR-Mediated CICR in GLP-1-Induced Insulin Secretion
10. Link Between Glucose Metabolism and Activation of RyRs
11. Role of RyR-Mediated CICR in Regulating Somatostatin Secretion from δ-Cells
12. Role of RyR-Mediated CICR in Regulating Glucagon Secretion from α-Cells
13. Role of RyRs in Mediating Store-Operated Ca2+ Entry (SOCE)
14. Role of RyR-Mediated CICR in Regulating Electrical Activity of β-Cells
15. The Concept of “Leaky RyRs”
16. ER Stress and RyRs
- THADA mutations: A thyroid adenoma-associated (THADA) protein variant binds RyR2, inducing Ca2+ leakage that impairs insulin secretion and triggers ER stress-mediated apoptosis [140].
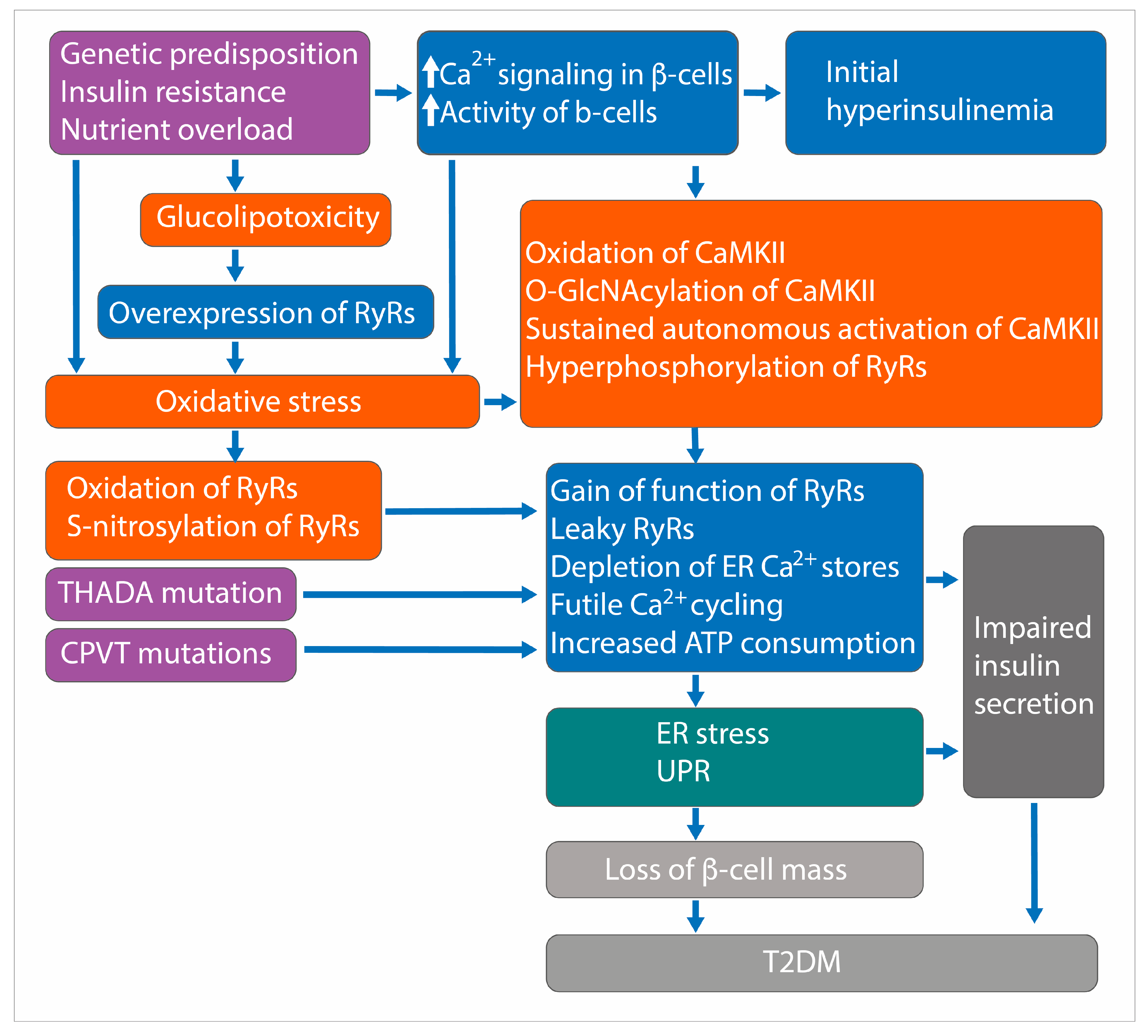
17. Role of RyRs in the Pathogenesis of T2DM
17.1. Leaky RyRs and Posttranslational Modifications
17.2. CaMKII-Mediated Phosphorylation of RyR2
17.3. Thyroid Adenoma Associated (THADA) and RyR2 Interaction
17.4. RYR2 Mutations and Glucose Intolerance
17.5. Other Evidence
18. RYRs and GWAS for T2DM
19. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-CEP | 4-Chloro-3-ethylphenol |
| 4-CmC | 4-Chloro-m-cresol |
| CaMKII | Ca2+-calmodulin-dependent protein kinase II |
| CICR | Ca2+-induced Ca2+ release |
| CPVT | Catecholaminergic polymorphic ventricular tachycardia |
| cADPR | Cyclic ADP-ribose |
| ER | Endoplasmic reticulum |
| FKBP12.6 | FK506 binding protein 12.6 |
| IP3 | Inositol 1,4,5-trisphosphate |
| IP3R | Inositol 1,4,5-trisphosphate receptor |
| IP3R3 | Inositol 1,4,5-trisphosphate receptor, type 3 |
| MBED9-Methyl-7-bromoeudistomin D | |
| NO | Nitric oxide |
| PDE | Phosphodiesterase |
| PKA | Protein kinase A |
| RyR | Ryanodine receptor |
| RyR1 | Type 1 ryanodine receptor |
| RyR2 | Type 2 ryanodine receptor |
| RyR3 | Type 3 ryanodine receptor |
| T2DM | Type 2 diabetes mellitus |
| THADA | Thyroid adenoma associated |
| UPR | Unfolded protein response |
| VGCC | Voltage-gated Ca2+ channel |
References
- Islam, M.S. Calcium Signaling: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Stimulus-Secretion Coupling in Beta-Cells: From Basic to Bedside. Adv. Exp. Med. Biol. 2020, 1131, 943–963. [Google Scholar] [CrossRef]
- Nordenskjold, F.; Andersson, B.; Islam, M.S. Expression of the Inositol 1,4,5-Trisphosphate Receptor and the Ryanodine Receptor Ca(2+)-Release Channels in the Beta-Cells and Alpha-Cells of the Human Islets of Langerhans. Adv. Exp. Med. Biol. 2020, 1131, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Bradford, P.G.; Laychock, S.G. Characterization of Inositol 1,4,5-Trisphosphate Receptor Isoform mRNA Expression and Regulation in Rat Pancreatic Islets, RINm5F Cells and betaHC9 Cells. J. Mol. Endocrinol. 1998, 21, 31–39. [Google Scholar] [CrossRef]
- Ríos, E. Calcium-Induced Release of Calcium in Muscle: 50 Years of Work and the Emerging Consensus. J. Gen. Physiol. 2018, 150, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Chepurny, O.G.; Rindler, M.J.; Collis, L.; Chepurny, Z.; Li, W.H.; Harbeck, M.; Roe, M.W.; Holz, G.G. A cAMP and Ca2+ Coincidence Detector in Support of Ca2+-Induced Ca2+ Release in Mouse Pancreatic Beta Cells. J. Physiol. 2005, 566, 173–188. [Google Scholar] [CrossRef]
- Dyachok, O.; Tufveson, G.; Gylfe, E. Ca2+-Induced Ca2+ Release by Activation of Inositol 1,4,5-Trisphosphate Receptors in Primary Pancreatic Beta-Cells. Cell Calcium 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Parys, J.B.; De Smedt, H. Inositol 1,4,5-Trisphosphate and Its Receptors. In Calcium Signaling; Islam, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 740, pp. 255–279. [Google Scholar]
- Marx, S.O.; Gaburjakova, J.; Gaburjakova, M.; Henrikson, C.; Ondrias, K.; Marks, A.R. Coupled Gating Between Cardiac Calcium Release Channels (Ryanodine Receptors). Circ. Res. 2001, 88, 1151–1158. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Watras, J.; Ehrlich, B.E. Bell-Shaped Calcium-Response Curves of Ins(1,4,5)P3-Gated and Calcium-Gated Channels from Endoplasmic-Reticulum of Cerebellum. Nature 1991, 351, 751–754. [Google Scholar] [CrossRef]
- Fill, M.; Copello, J.A. Ryanodine Receptor Calcium Release Channels. Physiol. Rev. 2002, 82, 893–922. [Google Scholar] [CrossRef]
- Islam, M.S.; Rorsman, P.; Berggren, P.O. Ca(2+)-Induced Ca2+ Release in Insulin-Secreting Cells. FEBS Lett. 1992, 296, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. The Ryanodine Receptor Calcium Channel of Beta-Cells: Molecular Regulation and Physiological Significance. Diabetes 2002, 51, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Cho, D.S.; Kim, J.Y.; Kim, B.J.; Lee, K.M.; Kim, S.H.; Kim, D.K.; Kim, S.H.; Park, H.S. Ca2+-Induced Ca2+ Release from Internal Stores in INS-1 Rat Insulinoma Cells. Korean J. Physiol. Pharmacol. 2011, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Nakagaki, I.; Kondo, H.; Hori, S. Involvement of the Ryanodine-Sensitive Ca2+ Store in GLP-1-Induced Ca2+ Oscillations in Insulin-Secreting Hit Cells. Pflug. Arch. 2002, 445, 342–351. [Google Scholar] [CrossRef]
- Kang, G.X.; Chepurny, O.G.; Holz, G.G. cAMP-Regulated Guanine Nucleotide Exchange Factor II (Epac2) Mediates Ca2+-Induced Ca2+ Release in INS-1 Pancreatic β-Cells. J. Physiol.-Lond. 2001, 536, 375–385. [Google Scholar] [CrossRef]
- Chen, T.H.; Lee, B.; Yang, C.; Hsu, W.H. Effects of Caffeine on Intracellular Calcium Release and Calcium Influx in a Clonal Beta-Cell Line RINm5F. Life Sci. 1996, 58, 983–990. [Google Scholar] [CrossRef]
- Willmott, N.J.; Galione, A.; Smith, P.A. Nitric-Oxide Induces Intracellular Ca2+ Mobilization and Increases Secretion of Incorporated 5-Hydroxytryptamine in Rat Pancreatic Beta-Cells. FEBS Lett. 1995, 371, 99–104. [Google Scholar] [CrossRef]
- Gamberucci, A.; Fulceri, R.; Pralong, W.; Bánhegyi, G.; Marcolongo, P.; Watkins, S.L.; Benedetti, A. Caffeine Releases a Glucose-Primed Endoplasmic Reticulum Ca2+ Pool in the Insulin Secreting Cell Line INS-1. FEBS Lett. 1999, 446, 309–312. [Google Scholar] [CrossRef]
- Graves, T.K.; Hinkle, P.M. Ca2+-induced Ca2+ Release in the Pancreatic Beta-Cell: Direct Evidence of Endoplasmic Reticulum Ca2+ Release. Endocrinology 2003, 144, 3565–3574. [Google Scholar] [CrossRef]
- Islam, M.S.; Kindmark, H.; Larsson, O.; Berggren, P.O. Thiol Oxidation by 2,2′-Dithiodipyridine Causes a Reversible Increase in Cytoplasmic Free Ca2+ Concentration in Pancreatic Beta-Cells. Role for Inositol 1,4,5-Trisphosphate-Sensitive Ca2+ Stores. Biochem. J. 1997, 321 Pt 2, 347–354. [Google Scholar] [CrossRef]
- Islam, M.S.; Larsson, O.; Nilsson, T.; Berggren, P.O. Effects of Caffeine on Cytoplasmic Free Ca2+ Concentration in Pancreatic Beta-Cells Are Mediated by Interaction with ATP-Sensitive K+ Channels and L-Type Voltage-Gated Ca2+ Channels but not the Ryanodine Receptor. Biochem. J. 1995, 306 Pt 3, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.E.; Gylfe, E. Caffeine Inhibits Cytoplasmic Ca2+ Oscillations Induced by by Carbachol and Guanosine 5′-O-(3-Thiotriphosphate) in Hyperpolarized Pancreatic Beta-Cells. Naunyn-Schmiedebergs Arch. Pharmacol. 1994, 349, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Theler, J.M.; Li, G.; Wollheim, C.B. Ca2+ Stores in Insulin-Secreting Cells—Lack of Efect of cADP Ribose. Cell Calcium 1994, 16, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Squires, P.E.; Hills, C.E.; Rogers, G.J.; Garland, P.; Farley, S.R.; Morgan, N.G. The Putative Imidazoline Receptor Agonist, Harmane, Promotes Intracellular Calcium Mobilisation in Pancreatic Beta-Cells. Eur. J. Pharmacol. 2004, 501, 31–39. [Google Scholar] [CrossRef]
- Herchuelz, A.; Lebrun, P. A Role for Na/Ca Exchange in the Pancreatic B-Cell—Studies with Thapsigargin and Caffeine. Biochem. Pharmacol. 1993, 45, 7–11. [Google Scholar] [CrossRef]
- Roe, M.W.; Lancaster, M.E.; Mertz, R.J.; Worley, J.F.; Dukes, I.D. Voltage-Dependent Intracellular Calcium Release from Mouse Islets Stimulated by Glucose. J. Biol. Chem. 1993, 268, 9953–9956. [Google Scholar] [CrossRef]
- Islam, M.S.; Leibiger, I.; Leibiger, B.; Rossi, D.; Sorrentino, V.; Ekstrom, T.J.; Westerblad, H.; Andrade, F.H.; Berggren, P.O. In Situ Activation of the Type 2 Ryanodine Receptor in Pancreatic Beta Cells Requires cAMP-Dependent Phosphorylation. Proc. Natl. Acad. Sci. USA 1998, 95, 6145–6150. [Google Scholar] [CrossRef]
- Ma, Z.; Bjorklund, A.; Islam, M.S. A TRPM4 Inhibitor 9-Phenanthrol Inhibits Glucose- and Glucagon-Like Peptide 1-Induced Insulin Secretion from Rat Islets of Langerhans. J. Diabetes Res. 2017, 2017, 5131785. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Beard, N.A.; Casarotto, M.G. Recent Advances in Understanding the Ryanodine Receptor Calcium Release Channels and Their Role in Calcium Signalling. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef]
- Sutko, J.L.; Airey, J.A.; Welch, W.; Ruest, L. The Pharmacology of Ryanodine and Related Compounds. Pharmacol. Rev. 1997, 49, 53–98. [Google Scholar] [CrossRef]
- Tanna, B.; Welch, W.; Ruest, L.; Sutko, J.L.; Williams, A.J. Excess Noise in Modified Conductance States Following the Interaction of Ryanoids with Cardiac Ryanodine Receptor Channels. FEBS Lett. 2002, 516, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Pessah, I.N.; Zimanyi, I. Characterization of Multiple [3H] Ryanodine Binding Sites on the Ca2+ Release Channel of Sarcoplasmic Reticulum from Skeletal and Cardiac-Muscle: Evidence for a Sequential Mechanism in Ryanodine Action. Mol. Pharmacol. 1991, 39, 679–689. [Google Scholar] [CrossRef]
- Postic, S.; Sarikas, S.; Pfabe, J.; Pohorec, V.; Krizancic Bombek, L.; Sluga, N.; Skelin Klemen, M.; Dolensek, J.; Korosak, D.; Stozer, A.; et al. High-Resolution Analysis of the Cytosolic Ca(2+) Events in Beta Cell Collectives In Situ. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E42–E55. [Google Scholar] [CrossRef] [PubMed]
- Llanos, P.; Contreras-Ferrat, A.; Barrientos, G.; Valencia, M.; Mears, D.; Hidalgo, C. Glucose-Dependent Insulin Secretion in Pancreatic Beta-Cell Islets from Male Rats Requires Ca2+ Release via ROS-Stimulated Ryanodine Receptors. PLoS ONE 2015, 10, e0129238. [Google Scholar] [CrossRef]
- Johnson, J.D.; Kuang, S.H.; Misler, S.; Polonsky, K.S. Ryanodine Receptors in Human Pancreatic Beta Cells: Localization and Effects on Insulin Secretion. FASEB J. 2004, 18, 878–880. [Google Scholar] [CrossRef]
- Fletcher, P.A.; Thompson, B.; Liu, C.T.; Bertram, R.; Satin, L.S.; Sherman, A.S. Ca2+Release or Ca2+Entry, That Is the Question: What Governs Ca2+Oscillations in Pancreatic β Cells? Am. J. Physiol.-Endocrinol. Metab. 2023, 324, E477–E487. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Grapengiesser, E. Pancreatic Beta-Cells from Obese-Hyperglycemic Mice are Characterized by Excessive Firing of Cytoplasmic Ca2+ Transients. Endocrine 2001, 15, 73–78. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Gustafsson, A.J.; Dzabic, M.; Pierro, C.; Tedeschi, P.; Sandgren, J.; Bari, M.R.; Nguyen, K.H.; Bianchi, M.; Rakonjac, M.; et al. Arachidonic Acid Is a Physiological Activator of the Ryanodine Receptor in Pancreatic Beta-Cells. Cell Calcium 2006, 39, 529–537. [Google Scholar] [CrossRef]
- Rosker, C.; Meur, G.; Taylor, E.J.A.; Taylor, C.W. Functional Ryanodine Receptors in the Plasma Membrane of RINm5F Pancreatic Beta-Cells. J. Biol. Chem. 2009, 284, 5186–5194. [Google Scholar] [CrossRef]
- Gromada, J.; Dissing, S.; Bokvist, K.; Renstrom, E.; Frokjaerjensen, J.; Wulff, B.S.; Rorsman, P. Glucagon-Like Peptide I Increases Cytoplasmic Calcium in Insulin-Secreting betaTC3-Cells by Enhancement of Intracellular Calcium Mobilization. Diabetes 1995, 44, 767–774. [Google Scholar] [CrossRef]
- Gustafsson, A.J.; Ingelman-Sundberg, H.; Dzabic, M.; Awasum, J.; Hoa, N.K.; Ostenson, C.G.; Pierro, C.; Tedeschi, P.; Woolcott, O.; Chiounan, S.; et al. Ryanodine Receptor-Operated Activation of TRP-Like Channels Can Trigger Critical Ca2+ Signaling Events in Pancreatic Beta-Cells. FASEB J. 2004, 18, 301–303. [Google Scholar] [CrossRef]
- Pessah, I.N.; Stambuk, R.A.; Casida, J.E. Ca2+-Activated Ryanodine Binding: Mechanisms of Sensitivity and and Intensity Modulation by Mg2+, Caffeine, and Adenine Nucleotides. Mol. Pharmacol. 1987, 31, 232–238. [Google Scholar] [CrossRef]
- Holz, G.G.; Leech, C.A.; Heller, R.S.; Castonguay, M.; Habener, J.F. cAMP-Dependent Mobilization of Intracellular Ca2+ Stores by Activation of Ryanodine Receptors in Pancreatic Beta-Cells. A Ca2+ Signaling System Stimulated by the Insulinotropic Hormone Glucagon-Like Peptide-1-(7-37). J. Biol. Chem. 1999, 274, 14147–14156. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, Z.; Yin, W.X.; Miao, L.; Zhou, Z.S.; Ji, G.J. Ryanodine Receptors Are Involved in Nuclear Calcium Oscillation in Primary Pancreatic Beta-Cells. Biochem. Biophys. Res. Commun. 2012, 423, 207–211. [Google Scholar] [CrossRef]
- Lemmens, R.; Larsson, O.; Berggren, P.O.; Islam, M.S. Ca2+-Induced Ca2+ Release from the Endoplasmic Reticulum Amplifies the Ca2+ Signal Mediated by Activation of Voltage-Gated L-Type Ca2+ Channels in Pancreatic Beta-Cells. J. Biol. Chem. 2001, 276, 9971–9977. [Google Scholar] [CrossRef]
- Diaz-Sylvester, P.L.; Porta, M.; Juettner, V.V.; Lv, Y.Z.; Fleischer, S.; Copello, J.A. Eudistomin D and Penaresin Derivatives as Modulators of Ryanodine Receptor Channels and Sarcoplasmic Reticulum Ca2+ ATPase in Striated Muscle. Mol. Pharmacol. 2014, 85, 564–575. [Google Scholar] [CrossRef]
- Bruton, J.D.; Lemmens, R.; Shi, C.L.; Persson-Sjögren, S.; Westerblad, H.; Ahmed, M.; Pyne, N.J.; Frame, M.; Furman, B.L.; Islam, M.S. Ryanodine Receptors of Pancreatic-Cells Mediate a Distinct Context-Dependent Signal for Insulin Secretion. FASEB J. 2002, 16, 301–303. [Google Scholar] [CrossRef]
- Abramson, J.J.; Zable, A.C.; Favero, T.G.; Salama, G. Thimerosal Interacts with the Ca2+ Release Channel Ryanodine Receptor from Skeletal Muscle Sarcoplasmic Reticulum. J. Biol. Chem. 1995, 270, 29644–29647. [Google Scholar] [CrossRef]
- Islam, M.S.; Larsson, O.; Berggren, P.O. Cyclic ADP-ribose in Beta Cells. Science 1993, 262, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Blondel, O.; Takeda, J.; Janssen, H.; Seino, S.; Bell, G.I. Sequence and Functional Characterization of a Third Inositol Trisphosphate Receptor Subtype, IP3R-3, Expressed in Pancreatic Islets, Kidney, Gastrointestinal Tract, and Other Tissues. J. Biol. Chem. 1993, 268, 11356–11363. [Google Scholar] [CrossRef]
- Khan, S.A.; Rossi, A.M.; Riley, A.M.; Potter, B.V.L.; Taylor, C.W. Subtype-Selective Regulation of IP3 Receptors by Thimerosal via Cysteine Residues within the 1P3-Binding Core and Suppressor Domain. Biochem. J. 2013, 451, 177–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sengupta, C.; Meyer, U.A.; Carafoli, E. Binding of Dantrolene Sodium to Muscle Intracellular Membranes. FEBS Lett. 1980, 117, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.X.; Herrmann, A.; Leon, J.; Jeyarajan, S.; Arunagiri, A.; Arvan, P.; Gilon, P.; Satin, L.S. ER Stress Increases Expression of Intracellular Calcium Channel RyR1 to Modify Ca(2+) Homeostasis in Pancreatic Beta Cells. J. Biol. Chem. 2023, 299, 105065. [Google Scholar] [CrossRef]
- Janjic, D.; Wollheim, C.B.; Sharp, G.W.G. Selective Inhibition of Glucose-Stimulated Insulin Release by Dantrolene. Am. J. Physiol. 1982, 243, E59–E67. [Google Scholar] [CrossRef]
- Park, J.H.; Shim, H.M.; Na, A.Y.; Bae, J.H.; Im, S.S.; Song, D.K. Orexin A Regulates Plasma Insulin and Leptin Levels in a Time-Dependent Manner Following a Glucose Load in Mice. Diabetologia 2015, 58, 1542–1550. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, S.; Yano, M.; Suetomi, T.; Ono, M.; Tateishi, H.; Mochizuki, M.; Xu, X.; Uchinoumi, H.; Okuda, S.; Yamamoto, T.; et al. Dantrolene, a Therapeutic Agent for Malignant Hyperthermia, Markedly Improves the Function of Failing Cardiomyocytes by Stabilizing Interdomain Interactions Within the Ryanodine Receptor. J. Am. Coll. Cardiol. 2009, 53, 1993–2005. [Google Scholar] [CrossRef]
- Gaburjakova, J.; Gaburjakova, M. Molecular Aspects Implicated in Dantrolene Selectivity with Respect to Ryanodine Receptor Isoforms. Int. J. Mol. Sci. 2023, 24, 5409. [Google Scholar] [CrossRef]
- Yu, G.; Zucchi, R.; Ronca-Testoni, S.; Ronca, G. Protection of Ischemic Rat Heart by Dantrolene, an Antagonist of the Sarcoplasmic Reticulum Calcium Release Channel. Basic Res. Cardiol. 2000, 95, 137–143. [Google Scholar] [CrossRef]
- Takasawa, S.; Kuroki, M.; Nata, K.; Noguchi, N.; Ikeda, T.; Yamauchi, A.; Ota, H.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Takahashi, I.; et al. A Novel Ryanodine Receptor Expressed in Pancreatic Islets by Alternative Splicing from Type 2 Ryanodine Receptor Gene. Biochem. Biophys. Res. Commun. 2010, 397, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Hadi-Alijanvand, H.; Sabbaghian, M.; Kiaei, M.; Khodagholi, F. Interaction of 2-APB, Dantrolene, and TDMT with IP3R and RyR Modulates ER Stress-Induced Programmed Cell Death I and II in Neuron-Like PC12 Cells: An Experimental and Computational Investigation. J. Biomol. Struct. Dyn. 2014, 32, 1211–1230. [Google Scholar] [CrossRef]
- MacMillan, D.; Chalmers, S.; Muir, T.C.; McCarron, J.G. IP3-Mediated Ca2+ Increases do not Involve the Ryanodine Receptor, but Ryanodine Receptor Antagonists Reduce IP3-Mediated Ca2+ Increases in Guinea-Pig Colonic Smooth Muscle Cells. J. Physiol. 2005, 569, 533–544. [Google Scholar] [CrossRef]
- Pian-Smith, M.C.; Wiedenkeller, D.E.; Sharp, G.W. Paradoxical Potentiation of Stimulated Insulin Release by Dantrolene in Rat Pancreatic Islets. Pancreas 1986, 1, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Murao, N.; Morikawa, R.; Seino, Y.; Shimomura, K.; Maejima, Y.; Yamada, Y.; Suzuki, A. β-Adrenergic Blockers Increase cAMP and Stimulate Insulin Secretion Through a PKA/RYR2/TRPM5 Pathway in Pancreatic β-Cells In Vitro. Pharmacol. Res. Perspect. 2025, 13, e70092. [Google Scholar] [CrossRef]
- HerrmannFrank, A.; Richter, M.; Sarkozi, S.; Mohr, U.; LehmannHorn, F. 4-Chloro-m-Cresol, a Potent and Specific Activator of the Skeletal Muscle Ryanodine Receptor. Biochim. Biophys. Acta-Gen. Subj. 1996, 1289, 31–40. [Google Scholar] [CrossRef]
- Jacobson, A.R.; Moe, S.T.; Allen, P.D.; Fessenden, J.D. Structural Determinants of 4-Chloro-m-Cresol Required for Activation of Ryanodine Receptor Type 1. Mol. Pharmacol. 2006, 70, 259–266. [Google Scholar] [CrossRef]
- Hosoi, E.; Nishizaki, C.; Gallagher, K.L.; Wyre, H.W.; Matsuo, Y.; Sei, Y. Expression of the Ryanodine Receptor Isoforms in Immune Cells. J. Immunol. 2001, 167, 4887–4894. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, G.L.; Daskoulidou, N.; Xu, S.Z. The Ryanodine Receptor Agonist 4-Chloro-3-Ethylphenol Blocks ORAI Store- Operated Channels. Br. J. Pharmacol. 2014, 171, 1250–1259. [Google Scholar] [CrossRef]
- Westerblad, H.; Andrade, F.H.; Islam, M.S. Effects of Ryanodine Receptor Agonist 4-Chloro-m-Cresol on Myoplasmic Free Ca2+ Concentration and Force of Contraction in Mouse Skeletal Muscle. Cell Calcium 1998, 24, 105–115. [Google Scholar] [CrossRef]
- Varadi, A.; Rutter, G.A. Dynamic Imaging of Endoplasmic Reticulum Ca2+ Concentration in Insulin-Secreting MIN6 Cells Using Recombinant Targeted Cameleons—Roles of Sarco(endo)plasmic Reticulum Ca2+-ATPase (SERCA)-2 and Ryanodine Receptors. Diabetes 2002, 51, S190–S201. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.J.; Pinton, P.; Varadi, A.; Tacchetti, C.; Ainscow, E.K.; Pozzan, T.; Rizzuto, R.; Rutter, G.A. Dense Core Secretory Vesicles Revealed as a Dynamic Ca2+ Store in Neuroendocrine Cells with a Vesicle-Associated Membrane Protein Aequorin Chimaera. J. Cell Biol. 2001, 155, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Eu, J.P.; Meissner, G.; Stamler, J.S. Activation of the Cardiac Calcium Release Channel (Ryanodine Receptor) by Poly-S-Nitrosylation. Science 1998, 279, 234–237. [Google Scholar] [CrossRef]
- Nakada, S.; Ishikawa, T.; Yamamoto, Y.; Kaneko, Y.; Nakayama, K. Constitutive Nitric Oxide Synthases in Rat Pancreatic Islets: Direct Imaging of Glucose-Induced Nitric Oxide Production in β-Cells. Pflug. Arch. 2003, 447, 305–311. [Google Scholar] [CrossRef]
- Nunemaker, C.S.; Buerk, D.G.; Zhang, M.; Satin, L.S. Glucose-Induced Release of Nitric Oxide from Mouse Pancreatic Islets as Detected with Nitric Oxide-Selective Glass Microelectrodes. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E907–E912. [Google Scholar] [CrossRef][Green Version]
- Dettbarn, C.; Palade, P. Arachidonic Acid-Induced Ca2+ Release from Isolated Sarcoplasmic Reticulum. Biochem. Pharmacol. 1993, 45, 1301–1309. [Google Scholar] [CrossRef]
- Muslikhov, E.R.; Sukhanova, I.F.; Avdonin, P.V. Arachidonic Acid Activates Release of Calcium Ions from Reticulum via Ryanodine Receptor Channels in C2C12 Skeletal Myotubes. Biochem.-Mosc. 2014, 79, 435–439. [Google Scholar] [CrossRef]
- Wolf, B.A.; Turk, J.; Sherman, W.R.; McDaniel, M.L. Intracellular Ca2+ Mobilization by Arachidonic Acid. Comparison with Myo-Inositol 1,4,5-Trisphosphate in Isolated Pancreatic Islets. J. Biol. Chem. 1986, 261, 3501–3511. [Google Scholar] [CrossRef]
- Yamamoto, W.R.; Bone, R.N.; Sohn, P.; Syed, F.; Reissaus, C.A.; Mosley, A.L.; Wijeratne, A.B.; True, J.D.; Tong, X.; Kono, T.; et al. Endoplasmic Reticulum Stress Alters Ryanodine Receptor Function in the Murine Pancreatic Cell. J. Biol. Chem. 2019, 294, 168–181. [Google Scholar] [CrossRef]
- Jones, P.P.; MacQuaide, N.; Louch, W.E. Dyadic Plasticity in Cardiomyocytes. Front. Physiol. 2018, 9, 1773. [Google Scholar] [CrossRef]
- Zhang, Q.; Bengtsson, M.; Partridge, C.; Salehi, A.; Braun, M.; Cox, R.; Eliasson, L.; Johnson, P.R.; Renstrom, E.; Schneider, T.; et al. R-Type Ca(2+)-Channel-Evoked CICR Regulates Glucose-Induced Somatostatin Secretion. Nat. Cell Biol. 2007, 9, 453–460. [Google Scholar] [CrossRef]
- Collier, M.L.; Ji, G.; Wang, Y.X.; Kotlikoff, M.I. Calcium-Induced Calcium Release in Smooth Muscle—Loose Coupling Between the Action Potential and Calcium Release. J. Gen. Physiol. 2000, 115, 653–662. [Google Scholar] [CrossRef]
- Jing, X.; Li, D.Q.; Olofsson, C.S.; Salehi, A.; Surve, V.V.; Caballero, J.; Ivarsson, R.; Lundquist, I.; Pereverzev, A.; Schneider, T.; et al. CaV2.3 Calcium Channels Control Second-Phase Insulin Release. J. Clin. Investig. 2005, 115, 146–154. [Google Scholar] [CrossRef]
- Islam, M.S. Calcium Signaling in the Islets. Adv. Exp. Med. Biol. 2010, 654, 235–259. [Google Scholar] [CrossRef]
- Liao, J.; Patel, D.; Zhao, Q.; Peng, R.; Guo, H.; Diwu, Z. A Novel Ca(2+) Indicator for Long-Term Tracking of Intracellular Calcium Flux. Biotechniques 2021, 70, 271–277. [Google Scholar] [CrossRef]
- Wan, Q.F.; Dong, Y.M.; Yang, H.; Lou, X.L.; Ding, J.P.; Xu, T. Protein Kinase Activation Increases Insulin Secretion by Sensitizing the Secretory Machinery to Ca2+. J. Gen. Physiol. 2004, 124, 653–662. [Google Scholar] [CrossRef]
- Rothberg, B.S.; Magleby, K.L. Gating Kinetics of Single Large-Conductance Ca2+-Activated K+ Channels in High Ca2+ Suggest a Two-Tiered Allosteric Gating Mechanism. J. Gen. Physiol. 1999, 114, 93–124. [Google Scholar] [CrossRef]
- Dixit, S.S.; Wang, T.N.; Manzano, E.J.Q.; Yoo, S.; Lee, J.; Chiang, D.Y.; Ryan, N.; Respress, J.L.; Yechoor, V.K.; Wehrens, X.H.T. Effects of CaMKII-Mediated Phosphorylation of Ryanodine Receptor Type 2 on Islet Calcium Handling, Insulin Secretion, and Glucose Tolerance. PLoS ONE 2013, 8, e58655. [Google Scholar] [CrossRef]
- Wehrens, X.H.; Lehnart, S.E.; Reiken, S.R.; Marks, A.R. Ca2+/Calmodulin-Dependent Protein Kinase II Phosphorylation Regulates the Cardiac Ryanodine Receptor. Circ. Res. 2004, 94, e61–e70. [Google Scholar] [CrossRef]
- Marx, S.O.; Reiken, S.; Hisamatsu, Y.; Jayaraman, T.; Burkhoff, D.; Rosemblit, N.; Marks, A.R. PKA Phosphorylation Dissociates FKBP12.6 from the Calcium Release Channel (Ryanodine Receptor): Defective Regulation in Failing Hearts. Cell 2000, 101, 365–376. [Google Scholar] [CrossRef]
- Dzhura, I.; Chepurny, O.G.; Kelley, G.G.; Leech, C.A.; Roe, M.W.; Dzhura, E.; Afshari, P.; Malik, S.; Rindler, M.J.; Xu, X.; et al. Epac2-Dependent Mobilization of Intracellular Ca2+ by Glucagon-Like Peptide-1 Receptor Agonist Exendin-4 is Disrupted in Beta-Cells of Phospholipase C-Epsilon Knockout Mice. J. Physiol.-Lond. 2010, 588, 4871–4889. [Google Scholar] [CrossRef] [PubMed]
- Hain, J.; Onoue, H.; Mayrleitner, M.; Fleischer, S.; Schindler, H. Phosphorylation Modulates the Function of the Calcium Release Channel of Sarcoplasmic Reticulum from Cardiac Muscle. J. Biol. Chem. 1995, 270, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, H.H.; Kaplan, J.H.; Ellis-Davies, G.C.; Lederer, W.J. Rapid Adaptation of Cardiac Ryanodine Receptors: Modulation by Mg2+ and Phosphorylation. Science 1995, 267, 1997–2000. [Google Scholar] [CrossRef]
- Noguchi, N.; Takasawa, S.; Nata, K.; Tohgo, A.; Kato, I.; Ikehata, F.; Yonekura, H.; Okamoto, H. Cyclic ADP-Ribose Binds to FK506-Binding Protein 12.6 to Release Ca2+ from Islet Microsomes. J. Biol. Chem. 1997, 272, 3133–3136. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.Z.; Wei, B.; Yin, W.X.; Xu, T.; Kotlikoff, M.I.; Ji, G.J. FKBP12.6-Knockout Mice Display Hyperinsulinemia and Resistance to High-Fat Diet-Induced Hyperglycemia. FASEB J. 2010, 24, 357–363. [Google Scholar] [CrossRef]
- Noguchi, N.; Yoshikawa, T.; Ikeda, T.; Takahashi, I.; Shervani, N.J.; Uruno, A.; Yamauchi, A.; Nata, K.; Takasawa, S.; Okamoto, H.; et al. FKBP12.6 Disruption Impairs Glucose-Induced Insulin Secretion. Biochem. Biophys. Res. Commun. 2008, 371, 735–740. [Google Scholar] [CrossRef]
- Santulli, G.; Pagano, G.; Sardu, C.; Xie, W.J.; Reiken, S.; D’Ascia, S.L.; Cannone, M.; Marziliano, N.; Trimarco, B.; Guise, T.A.; et al. Calcium Release Channel RyR2 Regulates Insulin Release and Glucose Homeostasis. J. Clin. Investig. 2015, 125, 1968–1978. [Google Scholar] [CrossRef]
- Meszaros, L.G.; Bak, J.; Chu, A. Cyclic ADP-Ribose as an Endogenous Regulator of the Non-Skeletal Type Ryanodine Receptor Ca2+ Channel. Nature 1993, 364, 76–79. [Google Scholar] [CrossRef]
- Venturi, E.; Pitt, S.; Galfre, E.; Sitsapesan, R. From Eggs to Hearts: What Is the Link Between Cyclic ADP-Ribose and Ryanodine Receptors? Cardiovasc. Ther. 2012, 30, 109–116. [Google Scholar] [CrossRef]
- Zhang, K.H.; Sun, W.; Huang, L.H.; Zhu, K.Y.; Pei, F.; Zhu, L.C.; Wang, Q.; Lu, Y.Y.; Zhang, H.M.; Jin, H.W.; et al. Identifying Glyceraldehyde 3-Phosphate Dehydrogenase as a Cyclic Adenosine Diphosphoribose Binding Protein by Photoaffinity Protein-Ligand Labeling Approach. J. Am. Chem. Soc. 2017, 139, 156–170. [Google Scholar] [CrossRef]
- Webb, D.L.; Islam, M.S.; Efanov, A.M.; Brown, G.; Kohler, M.; Larsson, O.; Berggren, P.O. Insulin Exocytosis and Glucose-Mediated Increase in Cytoplasmic Free Ca2+ Concentration in the Pancreatic Beta-Cell Are Independent of Cyclic ADP-Ribose. J. Biol. Chem. 1996, 271, 19074–19079. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Park, K.H.; Yim, C.Y.; Takasawa, S.; Okamoto, H.; Im, M.J.; Kim, U.H. Generation of Nicotinic Acid Adenine Dinucleotide Phosphate and Cyclic ADP-Ribose by Glucagon-Like Peptide-1 Evokes Ca2+ Signal That Is Essential for Insulin Secretion in Mouse Pancreatic Islets. Diabetes 2008, 57, 868–878. [Google Scholar] [CrossRef]
- Yaluri, N.; Modi, S.; Rodríguez, M.L.; Stancáková, A.; Kuusisto, J.; Kokkola, T.; Laakso, M. Simvastatin Impairs Insulin Secretion by Multiple Mechanisms in MIN6 Cells. PLoS ONE 2015, 10, e0142902. [Google Scholar] [CrossRef]
- Harvey, K.E.; LaVigne, E.K.; Dar, M.S.; Salyer, A.E.; Pratt, E.P.S.; Sample, P.A.; Aryal, U.; Gowher, H.; Hockerman, G.H. RyR2/IRBIT Regulates Insulin Gene Transcript, Insulin Content, and Secretion in the Insulinoma Cell Line INS-1. Sci. Rep. 2022, 12, 7713. [Google Scholar] [CrossRef]
- Makino, M.; Uchiyama, T.; Oshima, Y.; Daikoku, T.; Yamamoto, Y.; Okamoto, H.; Nakamura, S.; Takasawa, S. Alteration of Splice Type in Type 2 Ryanodine Receptor Causes Impaired Insulin Secretion in Mice. Diabetes 2024, 73, 176–183. [Google Scholar] [CrossRef]
- Meloni, A.R.; DeYoung, M.B.; Lowe, C.; Parkes, D.G. GLP-1 Receptor Activated Insulin Secretion from Pancreatic ß-Cells: Mechanism and Glucose Dependence. Diabetes Obes. Metab. 2013, 15, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Leech, C.A.; Dzhura, I.; Chepurny, O.G.; Kang, G.X.; Schwede, F.; Genieser, H.G.; Holz, G.G. Molecular Physiology of Glucagon-Like Peptide-1 Insulin Secretagogue Action in Pancreatic Beta Cells. Prog. Biophys. Mol. Biol. 2011, 107, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Xavier, G.D.; Holz, G.G.; Jouaville, L.S.; Thomas, A.P.; Rutter, G.A. Glucagon-Like Peptide-1 Mobilizes Intracellular Ca2+ and Stimulates Mitochondrial ATP Synthesis in Pancreatic MIN6 Beta-Cells. Biochem. J. 2003, 369, 287–299. [Google Scholar] [CrossRef]
- Holz, G.G. Epac: A New cAMP-Binding Protein in Support of Glucagon-Like Peptide-1 Receptor-Mediated Signal Transduction in the Pancreatic Beta-Cell. Diabetes 2004, 53, 5–13. [Google Scholar] [CrossRef]
- Marks, A.R. Calcium Cycling Proteins and Heart Failure: Mechanisms and Therapeutics. J. Clin. Investig. 2013, 123, 46–52. [Google Scholar] [CrossRef]
- Grapengiesser, E.; Gylfe, E.; Hellman, B. Glucose Sensing of Individual Pancreatic Beta-Cells Involves Transitions Between Steady-State and Oscillatory Cytoplasmic CA2+. Cell Calcium 1992, 13, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Laver, D.R. Ca2+ Stores Regulate Ryanodine Receptor Ca2+ Release Channels Via Luminal and Cytosolic Ca2+ Sites. Clin. Exp. Pharmacol. Physiol. 2007, 34, 889–896. [Google Scholar] [CrossRef]
- Kermode, H.; Williams, A.J.; Sitsapesan, R. The Interactions of ATP, ADP, and Inorganic Phosphate with the Sheep Cardiac Ryanodine Receptor. Biophys. J. 1998, 74, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Dukes, I.D.; Sreenan, S.; Roe, M.W.; Levisetti, M.; Zhou, Y.P.; Ostrega, D.; Bell, G.I.; Pontoglio, M.; Yaniv, M.; Philipson, L.; et al. Defective Pancreatic β-Cell Glycolytic Signaling in Hepatocyte Nuclear Factor-1α-Deficient Mice. J. Biol. Chem. 1998, 273, 24457–24464. [Google Scholar] [CrossRef] [PubMed]
- Laver, D.R. Regulation of the RyR Channel Gating by Ca(2+) and Mg(2+). Biophys. Rev. 2018, 10, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kermode, H.; Chan, W.M.; Williams, A.J.; Sitsapesan, R. Glycolytic Pathway Intermediates Activate Cardiac Ryanodine Receptors. FEBS Lett. 1998, 431, 59–62. [Google Scholar] [CrossRef]
- Takasawa, S.; Akiyama, T.; Nata, K.; Kuroki, M.; Tohgo, A.; Noguchi, N.; Kobayashi, S.; Kato, I.; Katada, T.; Okamoto, H. Cyclic ADP-Ribose and Inositol 1,4,5-Trisphosphate as Alternate Second Messengers for Intracellular Ca2+ Mobilization in Normal and Diabetic Beta-Cells. J. Biol. Chem. 1998, 273, 2497–2500. [Google Scholar] [CrossRef]
- Corkey, B.E.; Deeney, J.T.; Yaney, G.C.; Tornheim, K.; Prentki, M. The Role of Long-Chain Fatty acyl-CoA Esters in β-Cell Signal Transduction. J. Nutr. 2000, 130, 299S–304S. [Google Scholar] [CrossRef]
- Connelly, T.; Ahern, C.; Sukhareva, M.; Coronado, R. Removal of Mg2+ Inhibition of Cardiac Ryanodine Receptor by Palmitoyl Coenzyme A. FEBS Lett. 1994, 352, 285–290. [Google Scholar] [CrossRef]
- Denwood, G.; Tarasov, A.; Salehi, A.; Vergari, L.; Ramracheya, R.; Takahashi, H.; Nikolaev, V.O.; Seino, S.; Gribble, F.; Reimann, F.; et al. Glucose Stimulates Somatostatin Secretion in Pancreatic δ-cells by cAMP-Dependent Intracellular Ca2+ Release. J. Gen. Physiol. 2019, 151, 1094–1115. [Google Scholar] [CrossRef]
- Vergari, E.; Denwood, G.; Salehi, A.; Zhang, Q.; Adam, J.; Alrifaiy, A.; Asterholm, I.W.; Benrick, A.; Chibalina, M.V.; Eliasson, L.; et al. Somatostatin Secretion by Na+-Dependent Ca2+-Induced Ca2+ Release in Pancreatic Delta Cells. Nat. Metab. 2020, 2, 32–40. [Google Scholar] [CrossRef]
- Acreman, S.; Ma, J.F.; Denwood, G.; Gao, R.; Tarasov, A.; Rorsman, P.; Zhang, Q. The Endoplasmic Reticulum Plays a Key Role in a-Cell Intracellular Ca 2+ Dynamics and Glucose-Regulated Glucagon Secretion in Mouse Islets. Iscience 2024, 27, 109665. [Google Scholar] [CrossRef]
- Sabourin, J.; Allagnat, F. Store-Operated Ca2+ Entry: A Key Component of the Insulin Secretion Machinery. J. Mol. Endocrinol. 2016, 57, F35–F39. [Google Scholar] [CrossRef]
- Islam, M.S. Molecular Regulations and Functions of the Transient Receptor Potential Channels of the Islets of Langerhans and Insulinoma Cells. Cells 2020, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.E.; Tang, S.Q.; LaVigne, E.K.; Pratt, E.P.S.; Hockerman, G.H. RyR2 Regulates Store-Operated Ca2+ Entry, Phospholipase C Activity, and Electrical Excitability in the Insulinoma Cell Line INS-1. PLoS ONE 2023, 18, e0285316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.R.X.; Ren, J.H.; Vadrevu, S.; Raghavan, M.; Satin, L.S. ER Stress Increases Store-Operated Ca2+ Entry (SOCE) and Augments Basal Insulin Secretion in Pancreatic Beta Cells. J. Biol. Chem. 2020, 295, 5685–5700. [Google Scholar] [CrossRef]
- Song, S.S.; Ayon, R.J.; Yuan, J.X.J. Ryanodine Receptor-2: A Necessity for Gating Store-Operated Ca2+ Channels. Cardiovasc. Res. 2016, 111, 13–15. [Google Scholar] [CrossRef][Green Version]
- Kiselyov, K.I.; Shin, D.M.; Wang, Y.M.; Pessah, I.N.; Allen, P.D.; Muallem, S. Gating of Store-Operated Channels by Conformational Coupling To Ryanodine Receptors. Mol. Cell 2000, 6, 421–431. [Google Scholar] [CrossRef]
- Krishnan, K.; Ma, Z.; Bjorklund, A.; Islam, M.S. Role of Transient Receptor Potential Melastatin-Like Subtype 5 Channel in Insulin Secretion from Rat Beta-Cells. Pancreas 2014, 43, 597–604. [Google Scholar] [CrossRef]
- Henquin, J.C.; Meissner, H.P.; Schmeer, W. Cyclic Variations of Glucose-Induced Electrical Activity in Pancreatic B-Cells. Pflugers Arch. 1982, 393, 322–327. [Google Scholar] [CrossRef]
- Zhan, X.; Yang, L.; Yi, M.; Jia, Y. RyR Channels and Glucose-Regulated Pancreatic β-Cells. Eur. Biophys. J. Biophys. Lett. 2008, 37, 773–782. [Google Scholar] [CrossRef]
- Marks, A.R. Targeting Ryanodine Receptors to Treat Human Diseases. J. Clin. Investig. 2023, 133, e162891. [Google Scholar] [CrossRef]
- Wehrens, X.H.; Lehnart, S.E.; Huang, F.; Vest, J.A.; Reiken, S.R.; Mohler, P.J.; Sun, J.; Guatimosim, S.; Song, L.S.; Rosemblit, N.; et al. FKBP12.6 Deficiency and Defective Calcium Release Channel (Ryanodine Receptor) Function Linked to Exercise-Induced Sudden Cardiac Death. Cell 2003, 113, 829–840. [Google Scholar] [CrossRef]
- Yong, J.; Johnson, J.D.; Arvan, P.; Han, J.; Kaufman, R.J. Therapeutic Opportunities for Pancreatic β-Cell ER Stress in Diabetes Mellitus. Nat. Rev. Endocrinol. 2021, 17, 455–467. [Google Scholar] [CrossRef]
- Huang, C.J.; Lin, C.Y.; Haataja, L.; Gurlo, T.; Butler, A.E.; Rizza, R.A.; Butler, P.C. High Expression Rates of Human Islet Amyloid Polypeptide Induce Endoplasmic Reticulum Stress-Mediated β-Cell Apoptosis, a Characteristic of Humans with Type 2 but not Type 1 Diabetes. Diabetes 2007, 56, 2016–2027. [Google Scholar] [CrossRef]
- Hara, T.; Mahadevan, J.; Kanekura, K.; Hara, M.; Lu, S.M.; Urano, F. Calcium Efflux From the Endoplasmic Reticulum Leads to β-Cell Death. Endocrinology 2014, 155, 758–768. [Google Scholar] [CrossRef]
- Luciani, D.S.; Gwiazda, K.S.; Yang, T.L.B.; Kalynyak, T.B.; Bychkivska, Y.; Frey, M.H.Z.; Jeffrey, K.D.; Sampaio, A.V.; Underhill, T.M.; Johnson, J.D. Roles of IP3R and RyR Ca2+ Channels in Endoplasmic Reticulum Stress and beta-Cell Death. Diabetes 2009, 58, 422–432. [Google Scholar] [CrossRef]
- Johnson, J.D.; Han, Z.Q.; Otani, K.; Ye, H.G.; Zhang, Y.; Wu, H.; Horikawa, Y.; Misler, S.; Bell, G.I.; Polonsky, K.S. RyR2 and Calpain-10 Delineate a Novel Apoptosis Pathway in Pancreatic Islets. J. Biol. Chem. 2004, 279, 24794–24802. [Google Scholar] [CrossRef]
- Wang, J.; Takeuchi, T.; Tanaka, S.; Kubo, S.K.; Kayo, T.; Lu, D.H.; Takata, K.; Koizumi, A.; Izumi, T. A Mutation in the Insulin 2 Gene Induces Diabetes with Severe Pancreatic β-Cell Dysfunction in the Mody Mouse. J. Clin. Investig. 1999, 103, 27–37. [Google Scholar] [CrossRef]
- Bansal, V.; Boehm, B.O.; Darvasi, A. Identification of a Missense Variant in the WFS1 Gene that Causes a Mild form of Wolfram Syndrome and Is Associated with Risk for Type 2 Diabetes in Ashkenazi Jewish individuals. Diabetologia 2018, 61, 2180–2188. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Han, S.; Liu, C.C.; Zheng, Y.W.; Li, H.; Gao, F.; Bian, Y.H.; Liu, X.; Liu, H.B.; Hu, S.R.; et al. THADA Inhibition in Mice Protects Against Type 2 Diabetes Mellitus by Improving Pancreatic Beta-Cell Function and Preserving Beta-Cell Mass. Nat. Commun. 2023, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.A.; Desai, S.; Moitra, P.; Salis, S.; Agashe, S.; Battalwar, R.; Mehta, A.; Madan, J.; Kalita, S.; Udipi, S.A.; et al. Hyperinsulinemia: An Early Biomarker of Metabolic Dysfunction. Front. Clin. Diabetes Healthc. 2023, 4, 1159664. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Erickson, J.R.; Pereira, L.; Wang, L.; Han, G.; Ferguson, A.; Dao, K.; Copeland, R.J.; Despa, F.; Hart, G.W.; Ripplinger, C.M.; et al. Diabetic Hyperglycaemia Activates CaMKII and Arrhythmias by O-Linked Glycosylation. Nature 2013, 502, 372–376. [Google Scholar] [CrossRef]
- Simonis-Bik, A.M.; Nijpels, G.; van Haeften, T.W.; Houwing-Duistermaat, J.J.; Boomsma, D.I.; Reiling, E.; van Hove, E.C.; Diamant, M.; Kramer, M.H.; Heine, R.J.; et al. Gene Variants in the Novel Type 2 Diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B Affect Different Aspects of Pancreatic Beta-Cell Function. Diabetes 2010, 59, 293–301. [Google Scholar] [CrossRef]
- Bansal, V.; Winkelmann, B.R.; Dietrich, J.W.; Boehm, B.O. Whole-Exome Sequencing in Familial Type 2 Diabetes Identifies an Atypical Missense Variant in the RyR2 Gene. Front. Endocrinol. 2024, 15, 1258982. [Google Scholar] [CrossRef]
- Chen, J.; Spracklen, C.N.; Marenne, G.; Varshney, A.; Corbin, L.J.; Luan, J.; Willems, S.M.; Wu, Y.; Zhang, X.; Horikoshi, M. The Trans-Ancestral Genomic Architecture of Glycemic Traits. Nat. Genet. 2021, 53, 840–860. [Google Scholar] [CrossRef]
- Downie, C.G.; Dimos, S.F.; Bien, S.A.; Hu, Y.; Darst, B.F.; Polfus, L.M.; Wang, Y.; Wojcik, G.L.; Tao, R.; Raffield, L.M. Multi-Ethnic GWAS and Fine-Mapping of Glycaemic Traits Identify Novel Loci in the PAGE Study. Diabetologia 2022, 65, 477–489. [Google Scholar] [CrossRef]
- Gong, S.; Su, B.B.; Tovar, H.; Mao, C.; Gonzalez, V.; Liu, Y.; Lu, Y.; Wang, K.S.; Xu, C. Polymorphisms Within RYR3 Gene Are Associated With Risk and Age at Onset of Hypertension, Diabetes, and Alzheimer’s Disease. Am. J. Hypertens. 2018, 31, 818–826. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S. Ryanodine Receptors in Islet Cell Function: Calcium Signaling, Hormone Secretion, and Diabetes. Cells 2025, 14, 690. https://doi.org/10.3390/cells14100690
Islam MS. Ryanodine Receptors in Islet Cell Function: Calcium Signaling, Hormone Secretion, and Diabetes. Cells. 2025; 14(10):690. https://doi.org/10.3390/cells14100690
Chicago/Turabian StyleIslam, Md. Shahidul. 2025. "Ryanodine Receptors in Islet Cell Function: Calcium Signaling, Hormone Secretion, and Diabetes" Cells 14, no. 10: 690. https://doi.org/10.3390/cells14100690
APA StyleIslam, M. S. (2025). Ryanodine Receptors in Islet Cell Function: Calcium Signaling, Hormone Secretion, and Diabetes. Cells, 14(10), 690. https://doi.org/10.3390/cells14100690






