Targeting Muscle Regeneration with Small Extracellular Vesicles from Adipose Tissue-Derived Stem Cells—A Review
Abstract
1. Introduction
2. Adipose Tissue-Derived Stem Cells
2.1. Overview of ATDSCs and Mechanistic Insights into Regenerative Medicine
2.2. ATDSCs’ Roles in Muscle Regeneration
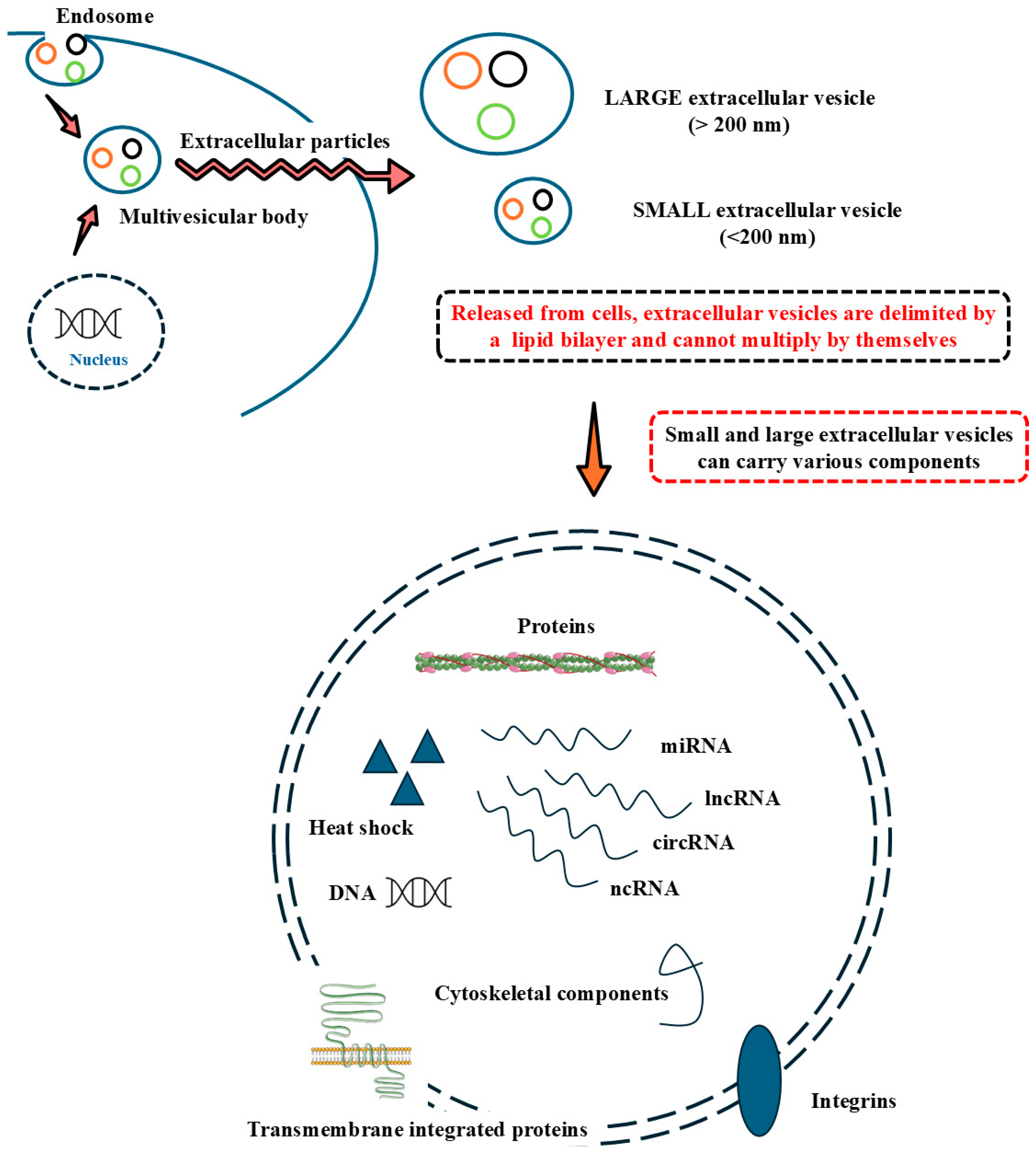
3. A Mechanistic Approach to Small Extracellular Vesicle Isolation, Characterization, and Mediated Communication
4. Small Extracellular Vesicles from ATDSCs in Muscle Targeting
4.1. Recent Advances in Delivery Methods to Target Muscles with Small Extracellular Vesicles
4.2. Mechanistic Insights: Molecular Pathways and Interaction with Muscle Cells
5. Clinical Implications: Potential Benefits for Sarcopenia and Muscle Loss
6. Conclusions and Implications for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef]
- Samuels, M.; Giamas, G. MISEV2023: Shaping the Future of EV Research by Enhancing Rigour, Reproducibility and Transparency. Cancer Gene Ther. 2024, 31, 649–651. [Google Scholar] [CrossRef]
- Miceli, R.T.; Chen, T.Y.; Nose, Y.; Tichkule, S.; Brown, B.; Fullard, J.F.; Saulsbury, M.D.; Heyliger, S.O.; Gnjatic, S.; Kyprianou, N.; et al. Extracellular vesicles, RNA sequencing, and bioinformatic analyses: Challenges, solutions, and recommendations. J. Extracell. Vesicles 2024, 13, e70005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, M.; Chen, Y. Minimal Information for Studies of Extracellular Vesicles (MISEV): Ten-Year Evolution (2014–2023). Pharmaceutics 2024, 16, 1394. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, D.; Raguraman, R.; Kim, D.; Ren, X.; Munshi, A.; Moore, K.; Sikavitsas, V.; Ramesh, R. Exosomes in diagnostic and therapeutic applications of ovarian cancer. J. Ovarian Res. 2024, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef]
- Upadhya, D.; Shetty, A.K. MISEV2023 provides an updated and key reference for researchers studying the basic biology and applications of extracellular vesicles. Stem Cells Transl. Med. 2024, 13, 848–850. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Understanding exosomes: Part 1—Characterization, quantification and isolation techniques. Periodontology 2000 2024, 94, 231–256. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Sun, X.; Sun, X.; Yang, G.; Shi, X. Exosome-mediated regulatory mechanisms in skeletal muscle: A narrative review. J. Zhejiang Univ. Sci. B 2023, 24, 1–14. [Google Scholar] [CrossRef]
- Wan, R.; Liu, S.; Feng, X.; Luo, W.; Zhang, H.; Wu, Y.; Chen, S.; Shang, X. The Revolution of exosomes: From biological functions to therapeutic applications in skeletal muscle diseases. J. Orthop. Translat. 2024, 45, 132–139. [Google Scholar] [CrossRef]
- Zanker, J.; Sim, M.; Anderson, K.; Balogun, S.; Brennan-Olsen, S.L.; Dent, E.; Duque, G.; Girgis, C.M.; Grossmann, M.; Hayes, A.; et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J. Cachexia Sarcopenia Muscle 2023, 14, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Kosmac, K.; Gonzalez-Freire, M.; McDermott, M.M.; White, S.H.; Walton, R.G.; Sufit, R.L.; Tian, L.; Li, L.; Kibbe, M.R.; Criqui, M.H.; et al. Correlations of Calf Muscle Macrophage Content With Muscle Properties and Walking Performance in Peripheral Artery Disease. J. Am. Heart Assoc. 2020, 9, e015929. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Careccia, G.; Mangiavini, L.; Cirillo, F. Regulation of Satellite Cells Functions during Skeletal Muscle Regeneration: A Critical Step in Physiological and Pathological Conditions. Int. J. Mol. Sci. 2023, 25, 512. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.W.; Kwak, D.; Liu, H.M.; Thompson, L.V. Age-induced oxidative stress: How does it influence skeletal muscle quantity and quality? J. Appl. Physiol. 2016, 121, 1047–1052. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Aslam, M.A.; Ma, E.B.; Huh, J.Y. Pathophysiology of sarcopenia: Genetic factors and their interplay with environmental factors. Metabolism 2023, 149, 155711. [Google Scholar] [CrossRef]
- Aldahhan, R.A.; Motawei, K.H.; Al-Hariri, M.T. Lipotoxicity-related sarcopenia: A review. J. Med. Life 2022, 15, 1334–1339. [Google Scholar] [CrossRef]
- Liu, Z.-j.; Zhu, C.-f. Causal relationship between insulin resistance and sarcopenia. Diabetol. Metab. Syndr. 2023, 15, 46. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.K.; Abbott, R.D. Adipose Tissue Paracrine-, Autocrine-, and Matrix-Dependent Signaling during the Development and Progression of Obesity. Cells 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; Copyright © 2000-2025; MDText.com, Inc.: Dartmouth, MA, USA, 2000. [Google Scholar]
- Sheptulina, A.F.; Antyukh, K.Y.; Kiselev, A.R.; Mitkovskaya, N.P.; Drapkina, O.M. Possible Mechanisms Linking Obesity, Steroidogenesis, and Skeletal Muscle Dysfunction. Life 2023, 13, 1415. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Wang, T. Searching for the link between inflammaging and sarcopenia. Ageing Res. Rev. 2022, 77, 101611. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Pescinini-Salzedas, L.M.; Minniti, G.; Laurindo, L.F.; Barbalho, S.M.; Vargas Sinatora, R.; Sloan, L.A.; Haber, R.S.A.; Araújo, A.C.; Quesada, K.; et al. Organokines, Sarcopenia, and Metabolic Repercussions: The Vicious Cycle and the Interplay with Exercise. Int. J. Mol. Sci. 2022, 23, 13452. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, D.; Hong, Z. Adipose tissue in older individuals: A contributing factor to sarcopenia. Metabolism 2024, 160, 155998. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, J.; Wu, G.; Wang, C.; Yang, Y.; Huang, T.; Wang, Y.; Yue, T.; Gao, Z.; Xie, H.; et al. Adipose progenitor cell-derived extracellular vesicles suppress macrophage M1 program to alleviate midlife obesity. Nat. Commun. 2025, 16, 2743. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wu, Y.; Wu, M. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front. Cell Dev. Biol. 2020, 8, 574223. [Google Scholar] [CrossRef]
- Porcu, C.; Dobrowolny, G.; Scicchitano, B.M. Exploring the Role of Extracellular Vesicles in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2024, 25, 5811. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Fang, Q.H.; Liu, M.L.; Lin, J.N. Current understanding of the role of Adipose-derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the distance between cells/tissues. Theranostics 2020, 10, 7422–7435. [Google Scholar] [CrossRef] [PubMed]
- Rome, S. Muscle and Adipose Tissue Communicate with Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 2161. [Google Scholar] [CrossRef]
- Penna, F.; Garcia-Castillo, L.; Costelli, P. Extracellular Vesicles and Exosomes in the Control of the Musculoskeletal Health. Curr. Osteoporos. Rep. 2024, 22, 257–265. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Romero-García, N.; Mas-Bargues, C.; Monleón, D.; Gordevicius, J.; Brooke, R.T.; Dromant, M.; Díaz, A.; Derevyanko, A.; Guío-Carrión, A.; et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci. Adv. 2022, 8, eabq2226. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced pluripotent stem cells (iPSCs): Molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Wei, L.; Wei, Z.Z.; Jiang, M.Q.; Mohamad, O.; Yu, S.P. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog. Neurobiol. 2017, 157, 49–78. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Zhu, Y.; Liu, Z.; Huang, L.; Zhao, H.; Zhou, Z.; Wu, Q. Anti-aging mechanism of different age donor-matched adipose-derived stem cells. Stem Cell Res. Ther. 2023, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Forcales, S.V. Potential of adipose-derived stem cells in muscular regenerative therapies. Front. Aging Neurosci. 2015, 7, 123. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Pisani, D.; Dechesne, C.A.; Turc-Carel, C.; Kurzenne, J.Y.; Wdziekonski, B.; Villageois, A.; Bagnis, C.; Breittmayer, J.P.; Groux, H.; et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 2005, 201, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Trevor, L.V.; Riches-Suman, K.; Mahajan, A.L.; Thornton, M.J. Adipose Tissue: A Source of Stem Cells with Potential for Regenerative Therapies for Wound Healing. J. Clin. Med. 2020, 9, 2161. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Durandt, C.; Dessels, C.; Da Silva, C.; Murdoch, C.; Pepper, M.S. The effect of early rounds of ex vivo expansion and cryopreservation on the adipogenic differentiation capacity of adipose-derived stromal/stem cells. Sci. Rep. 2019, 9, 15943. [Google Scholar] [CrossRef]
- Reumann, M.K.; Linnemann, C.; Aspera-Werz, R.H.; Arnold, S.; Held, M.; Seeliger, C.; Nussler, A.K.; Ehnert, S. Donor Site Location Is Critical for Proliferation, Stem Cell Capacity, and Osteogenic Differentiation of Adipose Mesenchymal Stem/Stromal Cells: Implications for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1868. [Google Scholar] [CrossRef]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, S.; Keshavarz, G.; Bozorgi, A.; Nazari, H.; Khazaei, M. Adipose tissue-derived stem cells: A comparative review on isolation, culture, and differentiation methods. Cell Tissue Bank. 2022, 23, 1–16. [Google Scholar] [CrossRef]
- Liu, N.; Wang, G.; Zhen, Y.; Shang, Y.; Nie, F.; Zhu, L.; Zhao, Z.; An, Y. Factors influencing myogenic differentiation of adipose-derived stem cells and their application in muscle regeneration. Chin. J. Plast. Reconstr. Surg. 2022, 4, 126–132. [Google Scholar] [CrossRef]
- Marcelle, C.; Stark, M.R.; Bronner-Fraser, M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development 1997, 124, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-E.; Hwang, M.; Kim, A.-Y.; Lee, E.-M.; Lee, E.-J.; Hwang, S.-K.; Kim, S.-Y.; Kim, H.-K.; Jeong, K.-S. MyoD overexpressed equine adipose-derived stem cells enhanced myogenic differentiation potential. Cell Transplant. 2016, 25, 2017–2026. [Google Scholar] [CrossRef]
- Sastourné-Arrey, Q.; Mathieu, M.; Contreras, X.; Monferran, S.; Bourlier, V.; Gil-Ortega, M.; Murphy, E.; Laurens, C.; Varin, A.; Guissard, C.; et al. Adipose tissue is a source of regenerative cells that augment the repair of skeletal muscle after injury. Nat. Commun. 2023, 14, 80. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Li, Y.; Cao, J.; Zhang, H.; Chen, M.; Wang, L.; Zhang, C. Long-term engraftment of myogenic progenitors from adipose-derived stem cells and muscle regeneration in dystrophic mice. Hum. Mol. Genet. 2015, 24, 6029–6040. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Wang, Y.X.; von Maltzahn, J.; Pasut, A.; Bentzinger, C.F.; Brun, C.E.; Rudnicki, M.A. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 2015, 21, 1455–1463. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, I.G.; Piao, S.; Jung, A.R.; Lee, J.Y.; Park, K.D.; Lee, J.Y. Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials 2013, 34, 6037–6045. [Google Scholar] [CrossRef]
- Vyas, K.S.; Kaufman, J.; Munavalli, G.S.; Robertson, K.; Behfar, A.; Wyles, S.P. Exosomes: The latest in regenerative aesthetics. Regen. Med. 2023, 18, 181–194. [Google Scholar] [CrossRef]
- Tienda-Vázquez, M.A.; Hanel, J.M.; Márquez-Arteaga, E.M.; Salgado-Álvarez, A.P.; Scheckhuber, C.Q.; Alanis-Gómez, J.R.; Espinoza-Silva, J.I.; Ramos-Kuri, M.; Hernández-Rosas, F.; Melchor-Martínez, E.M.; et al. Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells 2023, 12, 1625. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, N. A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef]
- González-Cubero, E.; González-Fernández, M.L.; Gutiérrez-Velasco, L.; Navarro-Ramírez, E.; Villar-Suárez, V. Isolation and characterization of exosomes from adipose tissue-derived mesenchymal stem cells. J. Anat. 2021, 238, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, T.; He, Z.; Cai, R.; Pang, W. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte 2021, 10, 587–604. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.P.; Schulz, A.; Morrison, H. The role of exosomes in intercellular and inter-organ communication of the peripheral nervous system. FEBS Lett. 2022, 596, 655–664. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Thakur, A.; Johnson, A.; Jacobs, E.; Zhang, K.; Chen, J.; Wei, Z.; Lian, Q.; Chen, H.J. Energy Sources for Exosome Communication in a Cancer Microenvironment. Cancers 2022, 14, 1698. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.; Chen, J.; Wang, C.; Sun, Y.; Yan, C.; Ren, S.; Xiong, H.; Xiang, K.; Zhang, M.; et al. Exosomal miR-125b-5p derived from adipose-derived mesenchymal stem cells enhance diabetic hindlimb ischemia repair via targeting alkaline ceramidase 2. J. Nanobiotechnol. 2023, 21, 189. [Google Scholar] [CrossRef]
- Mendhe, B.; Khan, M.B.; Dunwody, D.; El Baradie, K.B.Y.; Smith, K.; Zhi, W.; Sharma, A.; Lee, T.J.; Hamrick, M.W. Lyophilized Extracellular Vesicles from Adipose-Derived Stem Cells Increase Muscle Reperfusion but Degrade Muscle Structural Proteins in a Mouse Model of Hindlimb Ischemia-Reperfusion Injury. Cells 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Lu, L.; Pan, Z.; Fan, A.; Yin, F. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen. Med. 2021, 16, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes Isolated From Adipose-Derived Stem Cells: A New Cell-Free Approach to Prevent the Muscle Degeneration Associated With Torn Rotator Cuffs. Am. J. Sports Med. 2019, 47, 3247–3255. [Google Scholar] [CrossRef]
- Ni, J.; Li, H.; Zhou, Y.; Gu, B.; Xu, Y.; Fu, Q.; Peng, X.; Cao, N.; Fu, Q.; Jin, M.; et al. Therapeutic Potential of Human Adipose-Derived Stem Cell Exosomes in Stress Urinary Incontinence—An in Vitro and in Vivo Study. Cell Physiol. Biochem. 2018, 48, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Pham, P.V.; Vu, N.B. Exosomes from adipose-derived stem cells promote angiogenesis and reduce necrotic grade in hindlimb ischemia mouse models. Iran. J. Basic Med. Sci. 2023, 26, 429–437. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, J.; Cui, C.; Peng, Z.; Yang, S.; Lei, J.; Li, B.; Yang, X.; Qin, J.; Yin, M.; et al. Netrin1-Enriched Exosomes From Genetically Modified ADSCs as a Novel Treatment for Diabetic Limb Ischemia. Adv. Healthc. Mater. 2024, 14, e2403521. [Google Scholar] [CrossRef]
- Byun, S.E.; Sim, C.; Chung, Y.; Kim, H.K.; Park, S.; Kim, D.K.; Cho, S.; Lee, S. Skeletal Muscle Regeneration by the Exosomes of Adipose Tissue-Derived Mesenchymal Stem Cells. Curr. Issues Mol. Biol. 2021, 43, 1473–1488. [Google Scholar] [CrossRef]
- Koh, H.B.; Kim, H.J.; Kang, S.W.; Yoo, T.H. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics 2023, 15, 2042. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, A.; Wang, H.; Klein, J.D.; Tan, L.; Wang, Z.M.; Du, J.; Naqvi, N.; Liu, B.C.; Wang, X.H. miR-26a Limits Muscle Wasting and Cardiac Fibrosis through Exosome-Mediated microRNA Transfer in Chronic Kidney Disease. Theranostics 2019, 9, 1864–1877. [Google Scholar] [CrossRef]
- Qin, X.; He, J.; Wang, X.; Wang, J.; Yang, R.; Chen, X. The functions and clinical application potential of exosomes derived from mesenchymal stem cells on wound repair: A review of recent research advances. Front. Immunol. 2023, 14, 1256687. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, J.; Wei, H.; Hao, J.; Liu, W.; Wang, X. Ultrasound-Targeted Microbubble Destruction: Modulation in the Tumor Microenvironment and Application in Tumor Immunotherapy. Front. Immunol. 2022, 13, 937344. [Google Scholar] [CrossRef]
- Sridharan, B.; Lim, H.G. Exosomes and ultrasound: The future of theranostic applications. Mater. Today Bio 2023, 19, 100556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Jin, Y.; Wang; He, Q.; Liu, Z.; Ai, Q.; Lei, Y.; Li, Y.; Song, F.; et al. Alkaline ceramidase 2 is a novel direct target of p53 and induces autophagy and apoptosis through ROS generation. Sci. Rep. 2017, 7, 44573. [Google Scholar] [CrossRef]
- Mademtzoglou, D.; Relaix, F. From cyclins to CDKIs: Cell cycle regulation of skeletal muscle stem cell quiescence and activation. Exp. Cell Res. 2022, 420, 113275. [Google Scholar] [CrossRef]
- Arsic, N.; Zacchigna, S.; Zentilin, L.; Ramirez-Correa, G.; Pattarini, L.; Salvi, A.; Sinagra, G.; Giacca, M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther. 2004, 10, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, D.; Di Paolo, J.; Kumra, H.; Fois, G.; Candiani, G.; Reinhardt, D.P.; Mantovani, D. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials 2018, 180, 130–142. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.; Schmidt, M.; Li, L.; et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef]
- McArthur, S.; Juban, G.; Gobbetti, T.; Desgeorges, T.; Theret, M.; Gondin, J.; Toller-Kawahisa, J.E.; Reutelingsperger, C.P.; Chazaud, B.; Perretti, M.; et al. Annexin A1 drives macrophage skewing to accelerate muscle regeneration through AMPK activation. J. Clin. Investig. 2020, 130, 1156–1167. [Google Scholar] [CrossRef]
- Giordani, L.; Puri, P.L. Epigenetic control of skeletal muscle regeneration: Integrating genetic determinants and environmental changes. FEBS J. 2013, 280, 4014–4025. [Google Scholar] [CrossRef]
- Massenet, J.; Gardner, E.; Chazaud, B.; Dilworth, F.J. Epigenetic regulation of satellite cell fate during skeletal muscle regeneration. Skelet. Muscle 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Li, Y.L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 2023, 14, 1133355. [Google Scholar] [CrossRef]
- Briata, P.; Lin, W.J.; Giovarelli, M.; Pasero, M.; Chou, C.F.; Trabucchi, M.; Rosenfeld, M.G.; Chen, C.Y.; Gherzi, R. PI3K/AKT signaling determines a dynamic switch between distinct KSRP functions favoring skeletal myogenesis. Cell Death Differ. 2012, 19, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Moresi, V.; Adamo, S.; Berghella, L. The JAK/STAT Pathway in Skeletal Muscle Pathophysiology. Front. Physiol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Murphy, M.M.; Keefe, A.C.; Lawson, J.A.; Flygare, S.D.; Yandell, M.; Kardon, G. Transiently active Wnt/β-catenin signaling is not required but must be silenced for stem cell function during muscle regeneration. Stem Cell Rep. 2014, 3, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Latil, M.; Soave, S.; Polesskaya, A.; Chrétien, F.; Boucheix, C.; Rubinstein, E. Normal muscle regeneration requires tight control of muscle cell fusion by tetraspanins CD9 and CD81. Nat. Commun. 2013, 4, 1674. [Google Scholar] [CrossRef]
- Suzuki, T.; Mori, A.; Maeno, T.; Arimatsu, R.; Ichimura, E.; Nishi, Y.; Hisaeda, K.; Yamaya, Y.; Kobayashi, K.; Nakamura, M.; et al. Abundant Synthesis of Netrin-1 in Satellite Cell-Derived Myoblasts Isolated from EDL Rather Than Soleus Muscle Regulates Fast-Type Myotube Formation. Int. J. Mol. Sci. 2021, 22, 4499. [Google Scholar] [CrossRef]
- Konagaya, Y.; Takakura, K.; Sogabe, M.; Bisaria, A.; Liu, C.; Meyer, T.; Sehara-Fujisawa, A.; Matsuda, M.; Terai, K. Intravital imaging reveals cell cycle-dependent myogenic cell migration during muscle regeneration. Cell Cycle 2020, 19, 3167–3181. [Google Scholar] [CrossRef]
- Masuzawa, R.; Takahashi, K.; Takano, K.; Nishino, I.; Sakai, T.; Endo, T. DA-Raf and the MEK inhibitor trametinib reverse skeletal myocyte differentiation inhibition or muscle atrophy caused by myostatin and GDF11 through the non-Smad Ras-ERK pathway. J. Biochem. 2022, 171, 109–122. [Google Scholar] [CrossRef]
- Fujita, R.; Mizuno, S.; Sadahiro, T.; Hayashi, T.; Sugasawa, T.; Sugiyama, F.; Ono, Y.; Takahashi, S.; Ieda, M. Generation of a MyoD knock-in reporter mouse line to study muscle stem cell dynamics and heterogeneity. iScience 2023, 26, 106592. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S.; Hughes, S.M. Myogenin is an essential regulator of adult myofibre growth and muscle stem cell homeostasis. Elife 2020, 9, e60445. [Google Scholar] [CrossRef] [PubMed]
- Musarò, A.; Cusella De Angelis, M.G.; Germani, A.; Ciccarelli, C.; Molinaro, M.; Zani, B.M. Enhanced expression of myogenic regulatory genes in aging skeletal muscle. Exp. Cell Res. 1995, 221, 241–248. [Google Scholar] [CrossRef]
- Chinvattanachot, G.; Rivas, D.; Duque, G. Mechanisms of muscle cells alterations and regeneration decline during aging. Ageing Res. Rev. 2024, 102, 102589. [Google Scholar] [CrossRef]
- Millet, M.; Auroux, M.; Beaudart, C.; Demonceau, C.; Ladang, A.; Cavalier, E.; Reginster, J.Y.; Bruyère, O.; Chapurlat, R.; Rousseau, J.C. Association of circulating hsa-miRNAs with sarcopenia: The SarcoPhAge study. Aging Clin. Exp. Res. 2024, 36, 70. [Google Scholar] [CrossRef] [PubMed]
- Floriano, J.F.; Emanueli, C.; Vega, S.; Barbosa, A.M.P.; Oliveira, R.G.; Floriano, E.A.F.; Graeff, C.F.O.; Abbade, J.F.; Herculano, R.D.; Sobrevia, L.; et al. Pro-angiogenic approach for skeletal muscle regeneration. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130059. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C. Muscle weakness during aging: A deficiency state involving declining angiogenesis. Ageing Res. Rev. 2015, 23, 139–153. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W.; et al. Inflammation: Roles in Skeletal Muscle Atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.L.; Morton, A.B.; Segal, S.S. Angiogenesis precedes myogenesis during regeneration following biopsy injury of skeletal muscle. Skelet. Muscle 2023, 13, 3. [Google Scholar] [CrossRef]
- Marcotte-Chénard, A.; Oliveira, B.; Little, J.P.; Candow, D.G. Sarcopenia and type 2 diabetes: Pathophysiology and potential therapeutic lifestyle interventions. Diabetes Metab. Syndr. 2023, 17, 102835. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Dong, M.; Wen, S.; Zhou, L.; Yuan, X. The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy. Diabetes Metab. Syndr. Obes. 2023, 16, 1541–1554. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Cui, C.; Hu, H.; Zang, N.; Yang, M.; Yang, J.; Zou, Y.; Li, J.; Wang, L.; et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J. Cachexia Sarcopenia Muscle 2023, 14, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, Y.; Oishi, Y. Mechanisms of skeletal muscle-tendon development and regeneration/healing as potential therapeutic targets. Pharmacol. Ther. 2023, 243, 108357. [Google Scholar] [CrossRef]
- Canosa-Carro, L.; Bravo-Aguilar, M.; Abuín-Porras, V.; Almazán-Polo, J.; García-Pérez-de-Sevilla, G.; Rodríguez-Costa, I.; López-López, D.; Navarro-Flores, E.; Romero-Morales, C. Current understanding of the diagnosis and management of the tendinopathy: An update from the lab to the clinical practice. Disease-a-Month 2022, 68, 101314. [Google Scholar] [CrossRef] [PubMed]
- Yoneno, M.; Minegishi, Y.; Takahashi, H.; Takahata, K.; Miyamoto, H.; Usami, Y.; Kokubun, T. Muscle Contraction Is Essential for Tendon Healing and Muscle Function Recovery After Achilles Tendon Rupture and Surgical Repair. J. Orthop. Res. 2025, 43, 746–755. [Google Scholar] [CrossRef]
- Darrieutort-Laffite, C.; Blanchard, F.; Soslowsky, L.J.; Le Goff, B. Biology and physiology of tendon healing. Jt. Bone Spine 2024, 91, 105696. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef]
- Dhillon, R.J.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Das, S.; Gordián-Vélez, W.J.; Ledebur, H.C.; Mourkioti, F.; Rompolas, P.; Chen, H.I.; Serruya, M.D.; Cullen, D.K. Innervation: The missing link for biofabricated tissues and organs. NPJ Regen. Med. 2020, 5, 11. [Google Scholar] [CrossRef]
- Aswad, H.; Forterre, A.; Wiklander, O.P.; Vial, G.; Danty-Berger, E.; Jalabert, A.; Lamazière, A.; Meugnier, E.; Pesenti, S.; Ott, C.; et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia 2014, 57, 2155–2164. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Mellen, R.H.; Girotto, O.S.; Marques, E.B.; Laurindo, L.F.; Grippa, P.C.; Mendes, C.G.; Garcia, L.N.H.; Bechara, M.D.; Barbalho, S.M.; Sinatora, R.V.; et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines 2023, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Yamamoto, R.; Chung, R.; Vazquez, J.M.; Sheng, H.; Steinberg, P.L.; Ioannidis, N.M.; Sudmant, P.H. Tissue-specific impacts of aging and genetics on gene expression patterns in humans. Nat. Commun. 2022, 13, 5803. [Google Scholar] [CrossRef]

| Small EV Characteristics | Delivery Methods | Biological Functions | Mechanisms of Action | Small EV Biomarkers | Experimental Models | Ref. |
|---|---|---|---|---|---|---|
| miR-125 b-5p ATDSC-SEVs (159 ± 42.71 nm). |
|
|
|
|
| [71] |
| Lyophilized ATDSC-SEVs (169 nm). |
|
|
|
|
| [72] |
| ATDSC-SEVs (~30–150 nm). |
|
|
|
|
| [73] |
| ATDSC-SEVs (~50–150 nm). |
|
|
|
|
| [74] |
| ATDSC-SEVs (~30–150 nm). |
|
|
|
|
| [75] |
| ATDSC-SEVs. |
|
|
|
|
| [76] |
| Netrin1-enriched small EVs from ATDSC (~100–150 nm). |
|
|
|
|
| [77] |
| MSC-derived small EVs (118 nm). |
|
|
|
|
| [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; Lima, E.P.d.; Araújo, A.C.; Dogani Rodrigues, V.; Dias, J.A.; Barbosa Tavares Filho, M.; Zuccari, D.A.P.d.C.; Fornari Laurindo, L.; Miglino, M.A.; Chagas, E.F.B.; et al. Targeting Muscle Regeneration with Small Extracellular Vesicles from Adipose Tissue-Derived Stem Cells—A Review. Cells 2025, 14, 683. https://doi.org/10.3390/cells14100683
Laurindo LF, Lima EPd, Araújo AC, Dogani Rodrigues V, Dias JA, Barbosa Tavares Filho M, Zuccari DAPdC, Fornari Laurindo L, Miglino MA, Chagas EFB, et al. Targeting Muscle Regeneration with Small Extracellular Vesicles from Adipose Tissue-Derived Stem Cells—A Review. Cells. 2025; 14(10):683. https://doi.org/10.3390/cells14100683
Chicago/Turabian StyleLaurindo, Lucas Fornari, Enzo Pereira de Lima, Adriano Cressoni Araújo, Victória Dogani Rodrigues, Jefferson Aparecido Dias, Marcos Barbosa Tavares Filho, Debora Aparecida Pires de Campos Zuccari, Lívia Fornari Laurindo, Maria Angélica Miglino, Eduardo Federighi Baisi Chagas, and et al. 2025. "Targeting Muscle Regeneration with Small Extracellular Vesicles from Adipose Tissue-Derived Stem Cells—A Review" Cells 14, no. 10: 683. https://doi.org/10.3390/cells14100683
APA StyleLaurindo, L. F., Lima, E. P. d., Araújo, A. C., Dogani Rodrigues, V., Dias, J. A., Barbosa Tavares Filho, M., Zuccari, D. A. P. d. C., Fornari Laurindo, L., Miglino, M. A., Chagas, E. F. B., Gregório Mendes, C., Direito, R., Valenti, V. E., & Barbalho, S. M. (2025). Targeting Muscle Regeneration with Small Extracellular Vesicles from Adipose Tissue-Derived Stem Cells—A Review. Cells, 14(10), 683. https://doi.org/10.3390/cells14100683












