Effects of Cisplatin on the Radiation Response and DNA Damage Markers in Peripheral Blood Lymphocytes Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Peripheral Blood Mononuclear Cells
2.2. Irradiation
2.3. γH2AX and 53BP1 Focus Quantification
2.4. Cytokinesis Block Micronucleus (CBMN) Assay
2.5. Apoptosis (Annexin V-FITC/PI Staining)
2.6. Cell Cycle Measurement
2.7. Data and Statistical Analyses
3. Results
3.1. DSB Repair Foci
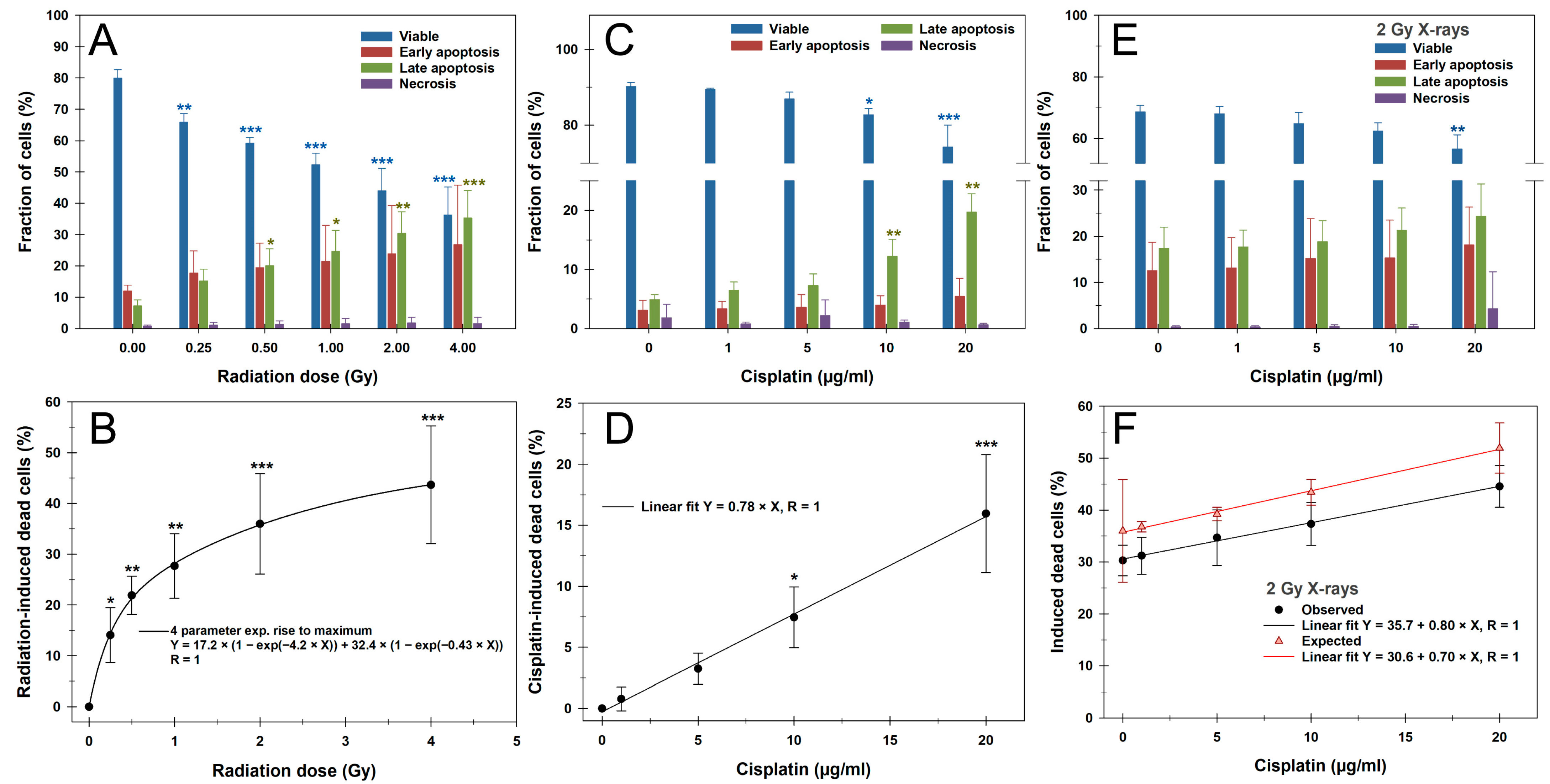
3.2. Apoptosis
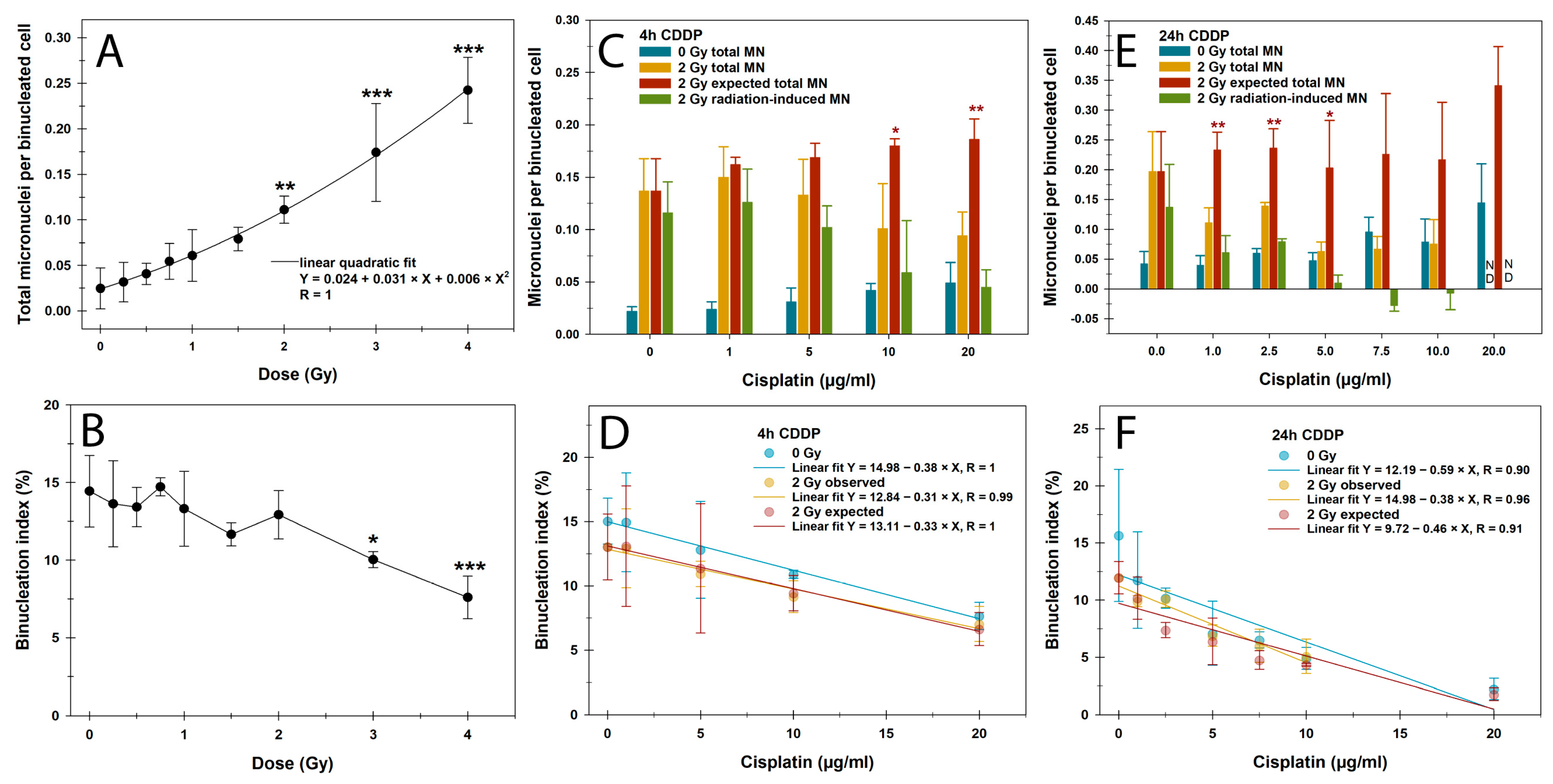
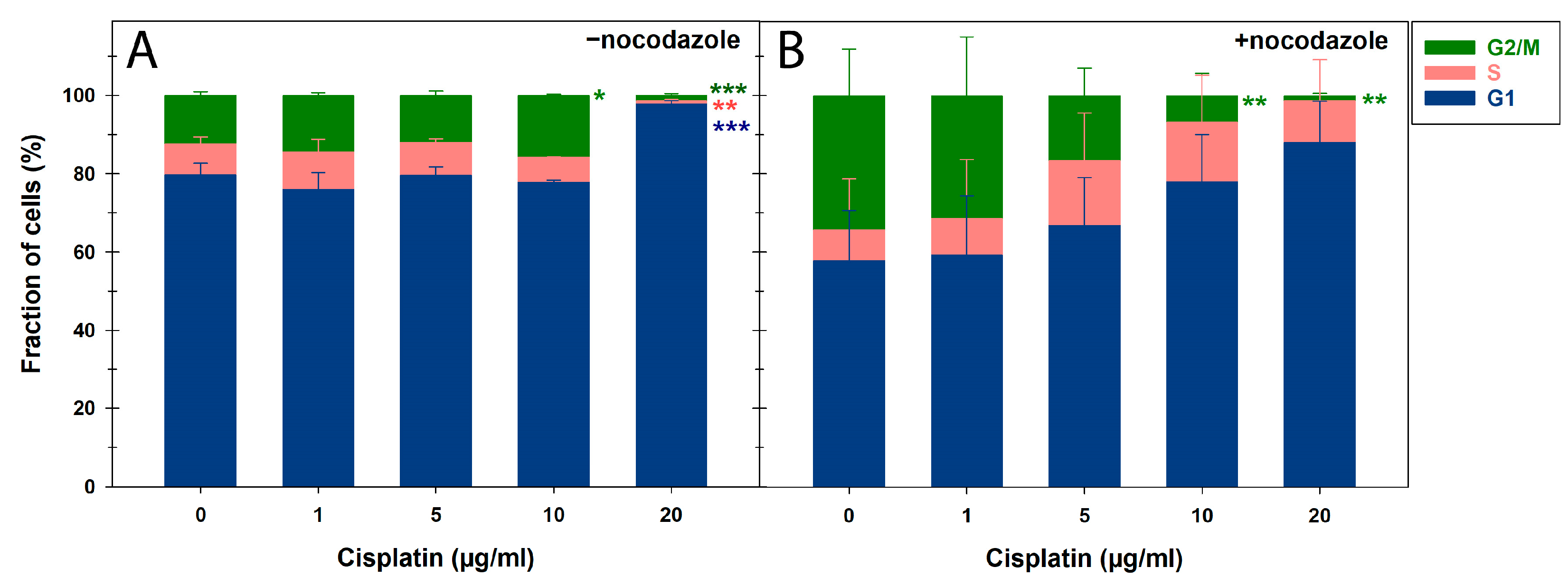
3.3. CBMN Assay and Cell Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IR | Ionizing radiation |
| PBMCs | Peripheral blood mononuclear cells |
| PBL | Peripheral blood lymphocyte |
| PI | Propidium iodide |
| CDDP | Cis-diamminedichloroplatinum II |
| CT | Chemotherapy |
| RT | Radiotherapy |
| CRT | Chemoradiotherapy |
| LA | Locally advanced |
| SSB | Single-strand break |
| DSB | Double-strand break |
| NHEJ | Non-homologous end joining |
| ICI | Immune checkpoint inhibitors |
| EDTA | Ethylenediaminetetraacetic acid |
| PBS | Phosphate-buffered saline |
| FCS | Fetal calf serum |
| RIF | Radiation-induced foci |
| BN | Binucleated |
| MN | Micronuclei |
| ND | Not determined |
References
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar] [PubMed]
- Steel, G.G.; Peckham, M.J. Exploitable mechanisms in combined radiotherapy-chemotherapy: The concept of additivity. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 85–91. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Salama, J.K.; Vokes, E.E. The concurrent chemoradiation paradigm—General principles. Nat. Clin. Pract. Oncol. 2007, 4, 86–100. [Google Scholar] [CrossRef]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef]
- Lemaire, M.A.; Schwartz, A.; Rahmouni, A.R.; Leng, M. Interstrand cross-links are preferentially formed at the d(GC) sites in the reaction between cis-diamminedichloroplatinum (II) and DNA. Proc. Natl. Acad. Sci. USA 1991, 88, 1982–1985. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Douple, E.B.; O’Hara, J.A.; Wang, H.J. Production of DNA double-strand breaks by interactions between carboplatin and radiation: A potential mechanism for radiopotentiation. Radiat. Res. 1995, 143, 309–315. [Google Scholar] [CrossRef]
- Sorenson, C.M.; Eastman, A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: Role of G2 arrest and DNA double-strand breaks. Cancer Res. 1988, 48, 4484–4488. [Google Scholar]
- Shinkai, T.; Saijo, N.; Eguchi, K.; Sasaki, Y.; Tamura, T.; Sakurai, M.; Suga, J.; Nakano, H.; Nakagawa, K.; Hong, W.S.; et al. Cytogenetic effect of carboplatin on human lymphocytes. Cancer Chemother. Pharmacol. 1988, 21, 203–207. [Google Scholar] [CrossRef]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 2005, 3, 277–285. [Google Scholar] [CrossRef]
- Sears, C.R.; Turchi, J.J. Complex cisplatin-double strand break (DSB) lesions directly impair cellular non-homologous end-joining (NHEJ) independent of downstream damage response (DDR) pathways. J. Biol. Chem. 2012, 287, 24263–24272. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, S.; Takahashi, J.; Suzuki, G.; Hideya, Y.; Yamada, K. Why Concurrent CDDP and Radiotherapy Has Synergistic Antitumor Effects: A Review of In Vitro Experimental and Clinical-Based Studies. Int. J. Mol. Sci. 2021, 22, 3140. [Google Scholar] [CrossRef]
- Kirwan, J.M.; Symonds, P.; Green, J.A.; Tierney, J.; Collingwood, M.; Williams, C.J. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother. Oncol. 2003, 68, 217–226. [Google Scholar] [CrossRef]
- Furuse, K.; Fukuoka, M.; Kawahara, M.; Nishikawa, H.; Takada, Y.; Kudoh, S.; Katagami, N.; Ariyoshi, Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J. Clin. Oncol. 1999, 17, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Trowell, O.A. The sensitivity of lymphocytes to ionising radiation. J. Pathol. Bacteriol. 1952, 64, 687–704. [Google Scholar] [CrossRef]
- Heylmann, D.; Ponath, V.; Kindler, T.; Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 2021, 11, 2478. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Ellsworth, S.; Campian, J.; Wild, A.T.; Herman, J.M.; Laheru, D.; Brock, M.; Balmanoukian, A.; Ye, X. Survival in Patients with Severe Lymphopenia Following Treatment with Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J. Natl. Compr. Cancer Netw. 2015, 13, 1225–1231. [Google Scholar] [CrossRef]
- Darragh, L.B.; Gadwa, J.; Pham, T.T.; Van Court, B.; Neupert, B.; Olimpo, N.A.; Nguyen, K.; Nguyen, D.; Knitz, M.W.; Hoen, M.; et al. Elective nodal irradiation mitigates local and systemic immunity generated by combination radiation and immunotherapy in head and neck tumors. Nat. Commun. 2022, 13, 7015. [Google Scholar] [CrossRef]
- Telarovic, I.; Yong, C.S.M.; Kurz, L.; Vetrugno, I.; Reichl, S.; Fernandez, A.S.; Cheng, H.W.; Winkler, R.; Guckenberger, M.; Kipar, A.; et al. Delayed tumor-draining lymph node irradiation preserves the efficacy of combined radiotherapy and immune checkpoint blockade in models of metastatic disease. Nat. Commun. 2024, 15, 5500. [Google Scholar] [CrossRef]
- Galluzzi, L.; Aryankalayil, M.J.; Coleman, C.N.; Formenti, S.C. Emerging evidence for adapting radiotherapy to immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Saddawi-Konefka, R.; O’Farrell, A.; Faraji, F.; Clubb, L.; Allevato, M.M.; Jensen, S.M.; Yung, B.S.; Wang, Z.; Wu, V.H.; Anang, N.A.; et al. Lymphatic-preserving treatment sequencing with immune checkpoint inhibition unleashes cDC1-dependent antitumor immunity in HNSCC. Nat. Commun. 2022, 13, 4298. [Google Scholar] [CrossRef]
- Rosenberg, A.J.; Juloori, A.; Jelinek, M.J.; Agrawal, N.; Cursio, J.F.; Cipriani, N.; Lingen, M.W.; Izumchenko, E.; Katipally, R.; Chin, J.; et al. Neoadjuvant Nivolumab Plus Chemotherapy Followed by Response-Stratified Chemoradiation Therapy in HPV-Negative Head and Neck Cancer: The DEPEND Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2025, e250081. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Ghasemzadeh, A.; Nirschl, T.R.; Theodros, D.; Kochel, C.M.; Francica, B.J.; Muroyama, Y.; Anders, R.A.; Sharabi, A.B.; Velarde, E.; et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin. Cancer 2018, 24, 5058–5071. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Machiels, J.P.; Tao, Y.; Licitra, L.; Burtness, B.; Tahara, M.; Rischin, D.; Alves, G.; Lima, I.P.F.; Hughes, B.G.M.; Pointreau, Y.; et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2024, 25, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Fayette, J.; Teixeira, M.; Prabhash, K.; Mesia, R.; Kawecki, A.; Dechaphunkul, A.; Dinis, J.; Guo, Y.; Masuda, M.; et al. Atezolizumab in High-Risk Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomized Clinical Trial. JAMA 2025, e251483. [Google Scholar] [CrossRef]
- Zahnreich, S.; Ebersberger, A.; Kaina, B.; Schmidberger, H. Biodosimetry Based on gamma-H2AX Quantification and Cytogenetics after Partial- and Total-Body Irradiation during Fractionated Radiotherapy. Radiat. Res. 2015, 183, 432–446. [Google Scholar] [CrossRef]
- Durante, M.; Yamada, S.; Ando, K.; Furusawa, Y.; Kawata, T.; Majima, H.; Nakano, T.; Tsujii, H. Measurements of the equivalent whole-body dose during radiation therapy by cytogenetic methods. Phys. Med. Biol. 1999, 44, 1289–1298. [Google Scholar] [CrossRef]
- Sak, A.; Grehl, S.; Erichsen, P.; Engelhard, M.; Grannass, A.; Levegrun, S.; Pottgen, C.; Groneberg, M.; Stuschke, M. gamma-H2AX foci formation in peripheral blood lymphocytes of tumor patients after local radiotherapy to different sites of the body: Dependence on the dose-distribution, irradiated site and time from start of treatment. Int. J. Radiat. Biol. 2007, 83, 639–652. [Google Scholar] [CrossRef]
- Rothkamm, K.; Beinke, C.; Romm, H.; Badie, C.; Balagurunathan, Y.; Barnard, S.; Bernard, N.; Boulay-Greene, H.; Brengues, M.; De Amicis, A.; et al. Comparison of established and emerging biodosimetry assays. Radiat. Res. 2013, 180, 111–119. [Google Scholar] [CrossRef]
- Fleckenstein, J.; Kühne, M.; Seegmüller, K.; Derschang, S.; Melchior, P.; Gräber, S.; Fricke, A.; Rübe, C.E.; Rübe, C. The impact of individual in vivo repair of DNA double-strand breaks on oral mucositis in adjuvant radiotherapy of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Sak, A.; Grehl, S.; Engelhard, M.; Wierlemann, A.; Kaelberlah, H.P.; Erichsen, P.; Pöttgen, C.; Groneberg, M.; Stuschke, M. Long-term in vivo effects of cisplatin on gamma-H2AX foci signaling in peripheral lymphocytes of tumor patients after irradiation. Clin. Cancer Res. 2009, 15, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Węgierek-Ciuk, A.; Lankoff, A.; Lisowska, H.; Kędzierawski, P.; Akuwudike, P.; Lundholm, L.; Wojcik, A. Cisplatin Reduces the Frequencies of Radiotherapy-Induced Micronuclei in Peripheral Blood Lymphocytes of Patients with Gynaecological Cancer: Possible Implications for the Risk of Second Malignant Neoplasms. Cells 2021, 10, 2709. [Google Scholar] [CrossRef] [PubMed]
- Zahnreich, S.; Ebersberger, A.; Karle, H.; Kaina, B.; Schmidberger, H. Quantification of Radiation Biomarkers in Leukocytes of Breast Cancer Patients Treated with Different Modalities of 3D-CRT or IMRT. Radiat. Res. 2016, 186, 508–519. [Google Scholar] [CrossRef]
- Zahnreich, S.; Rösler, H.P.; Schwanbeck, C.; Karle, H.; Schmidberger, H. Radiation-induced DNA double-strand breaks in peripheral leukocytes and therapeutic response of heel spur patients treated by orthovoltage X-rays or a linear accelerator. Strahlenther. Onkologie 2020, 196, 1116–1127. [Google Scholar] [CrossRef]
- Zahnreich, S.; Weber, B.; Rösch, G.; Schindler, D.; Schmidberger, H. Compromised repair of radiation-induced DNA double-strand breaks in Fanconi anemia fibroblasts in G2. DNA Repair 2020, 96, 102992. [Google Scholar] [CrossRef]
- Campian, J.L.; Sarai, G.; Ye, X.; Marur, S.; Grossman, S.A. Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck 2014, 36, 1747–1753. [Google Scholar] [CrossRef]
- Rajkumar, P.; Mathew, B.S.; Das, S.; Isaiah, R.; John, S.; Prabha, R.; Fleming, D.H. Cisplatin Concentrations in Long and Short Duration Infusion: Implications for the Optimal Time of Radiation Delivery. J. Clin. Diagn. Res. 2016, 10, Xc01–Xc04. [Google Scholar] [CrossRef]
- Gietema, J.A.; Meinardi, M.T.; Messerschmidt, J.; Gelevert, T.; Alt, F.; Uges, D.R.; Sleijfer, D.T. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000, 355, 1075–1076. [Google Scholar] [CrossRef]
- Trendowski, M.R.; El-Charif, O.; Ratain, M.J.; Monahan, P.; Mu, Z.; Wheeler, H.E.; Dinh, P.C., Jr.; Feldman, D.R.; Ardeshir-Rouhani-Fard, S.; Hamilton, R.J.; et al. Clinical and Genome-Wide Analysis of Serum Platinum Levels after Cisplatin-Based Chemotherapy. Clin. Cancer Res. 2019, 25, 5913–5924. [Google Scholar] [CrossRef] [PubMed]
- Turchi, J.J.; Henkels, K.M.; Zhou, Y. Cisplatin-DNA adducts inhibit translocation of the Ku subunits of DNA-PK. Nucleic Acids Res. 2000, 28, 4634–4641. [Google Scholar] [CrossRef]
- Diggle, C.P.; Bentley, J.; Knowles, M.A.; Kiltie, A.E. Inhibition of double-strand break non-homologous end-joining by cisplatin adducts in human cell extracts. Nucleic Acids Res. 2005, 33, 2531–2539. [Google Scholar] [CrossRef][Green Version]
- Wilson, G.D.; Bentzen, S.M.; Harari, P.M. Biologic basis for combining drugs with radiation. Semin. Radiat. Oncol. 2006, 16, 2–9. [Google Scholar] [CrossRef]
- Stiff, T.; O’Driscoll, M.; Rief, N.; Iwabuchi, K.; Löbrich, M.; Jeggo, P.A. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004, 64, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Dolling, J.A.; Boreham, D.R.; Brown, D.L.; Mitchel, R.E.; Raaphorst, G.P. Modulation of radiation-induced strand break repair by cisplatin in mammalian cells. Int. J. Radiat. Biol. 1998, 74, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Monjazeb, A.M.; Giobbie-Hurder, A.; Lako, A.; Thrash, E.M.; Brennick, R.C.; Kao, K.Z.; Manuszak, C.; Gentzler, R.D.; Tesfaye, A.; Jabbour, S.K.; et al. A Randomized Trial of Combined PD-L1 and CTLA-4 Inhibition with Targeted Low-Dose or Hypofractionated Radiation for Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2021, 27, 2470–2480. [Google Scholar] [CrossRef]
- Azab, B.; Alassaf, A.; Abu-Humdan, A.; Dardas, Z.; Almousa, H.; Alsalem, M.; Khabour, O.; Hammad, H.; Saleh, T.; Awidi, A. Genotoxicity of cisplatin and carboplatin in cultured human lymphocytes: A comparative study. Interdiscip. Toxicol. 2019, 12, 93–97. [Google Scholar] [CrossRef]
- Nobili, S.; Lavacchi, D.; Perrone, G.; Vicini, G.; Tassi, R.; Landini, I.; Grosso, A.; Roviello, G.; Mazzanti, R.; Santomaggio, C.; et al. Vinorelbine in Non-Small Cell Lung Cancer: Real-World Data From a Single-Institution Experience. Oncol. Res. 2020, 28, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Brix, N.; Samaga, D.; Belka, C.; Zitzelsberger, H.; Lauber, K. Analysis of clonogenic growth in vitro. Nat. Protoc. 2021, 16, 4963–4991. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahnreich, S.; Bhatti, A.; Ahmad, B.; Drabke, S.; Kaufmann, J.; Schmidberger, H. Effects of Cisplatin on the Radiation Response and DNA Damage Markers in Peripheral Blood Lymphocytes Ex Vivo. Cells 2025, 14, 682. https://doi.org/10.3390/cells14100682
Zahnreich S, Bhatti A, Ahmad B, Drabke S, Kaufmann J, Schmidberger H. Effects of Cisplatin on the Radiation Response and DNA Damage Markers in Peripheral Blood Lymphocytes Ex Vivo. Cells. 2025; 14(10):682. https://doi.org/10.3390/cells14100682
Chicago/Turabian StyleZahnreich, Sebastian, Aisha Bhatti, Barea Ahmad, Sophia Drabke, Justus Kaufmann, and Heinz Schmidberger. 2025. "Effects of Cisplatin on the Radiation Response and DNA Damage Markers in Peripheral Blood Lymphocytes Ex Vivo" Cells 14, no. 10: 682. https://doi.org/10.3390/cells14100682
APA StyleZahnreich, S., Bhatti, A., Ahmad, B., Drabke, S., Kaufmann, J., & Schmidberger, H. (2025). Effects of Cisplatin on the Radiation Response and DNA Damage Markers in Peripheral Blood Lymphocytes Ex Vivo. Cells, 14(10), 682. https://doi.org/10.3390/cells14100682






