Induction of SUSD2 by STAT3 Activation Is Associated with Tumor Recurrence in HER2-Positive Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blots
2.3. Immunofluorescence Microscopy
2.4. Colony Formation Assays
2.5. Cell Cycle Analysis
2.6. Quantitative Reverse Transcription PCR (RT-qPCR)
2.7. Human Phospho-Kinase Antibody Array
2.8. Clinicopathological Characteristics of Breast Cancer Patients
2.9. Tissue Specimens
2.10. Immunohistochemistry (IHC)
2.11. SUSD2 Knockdown
2.12. IL-1B ELISA
2.13. Survival Analysis
2.14. Public Data Analysis
2.15. Statistical Analysis
3. Results
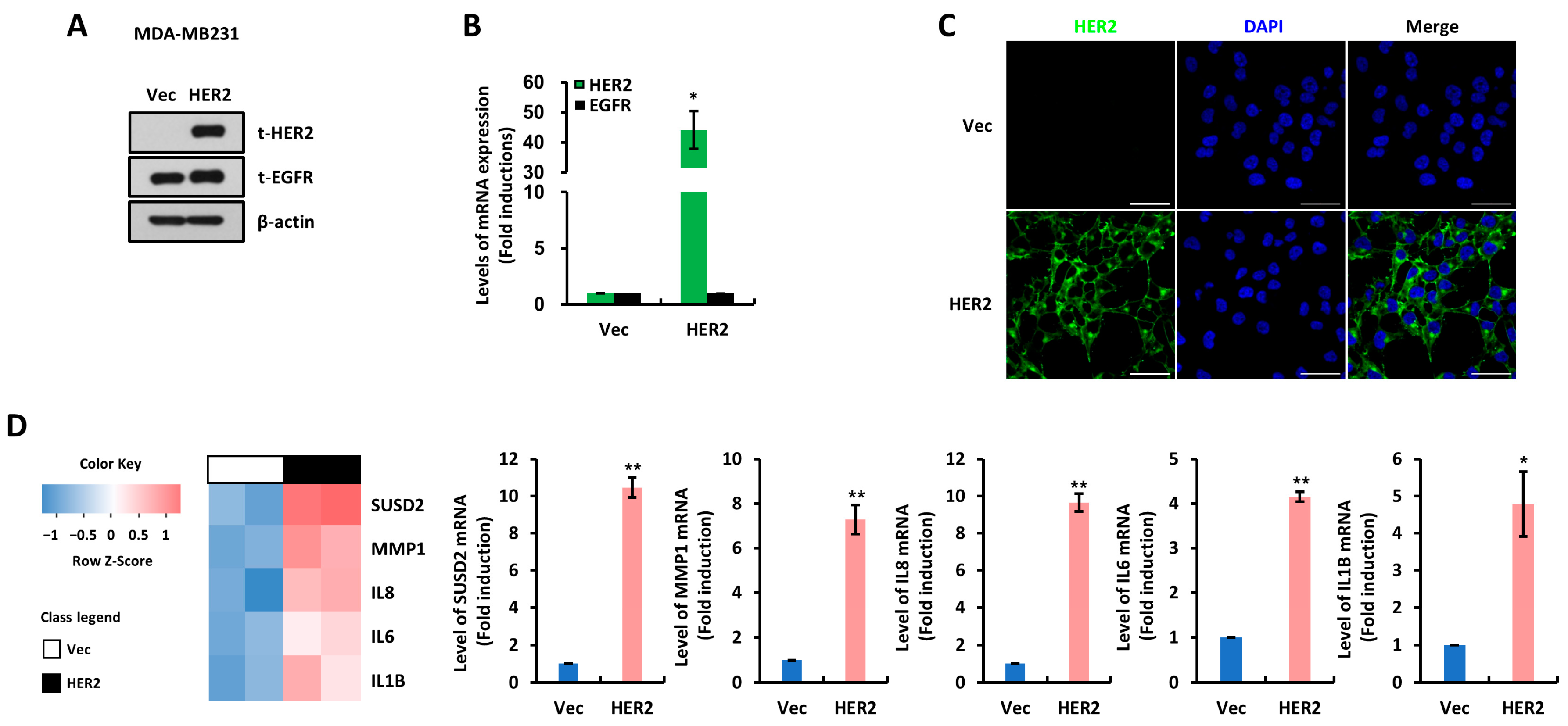
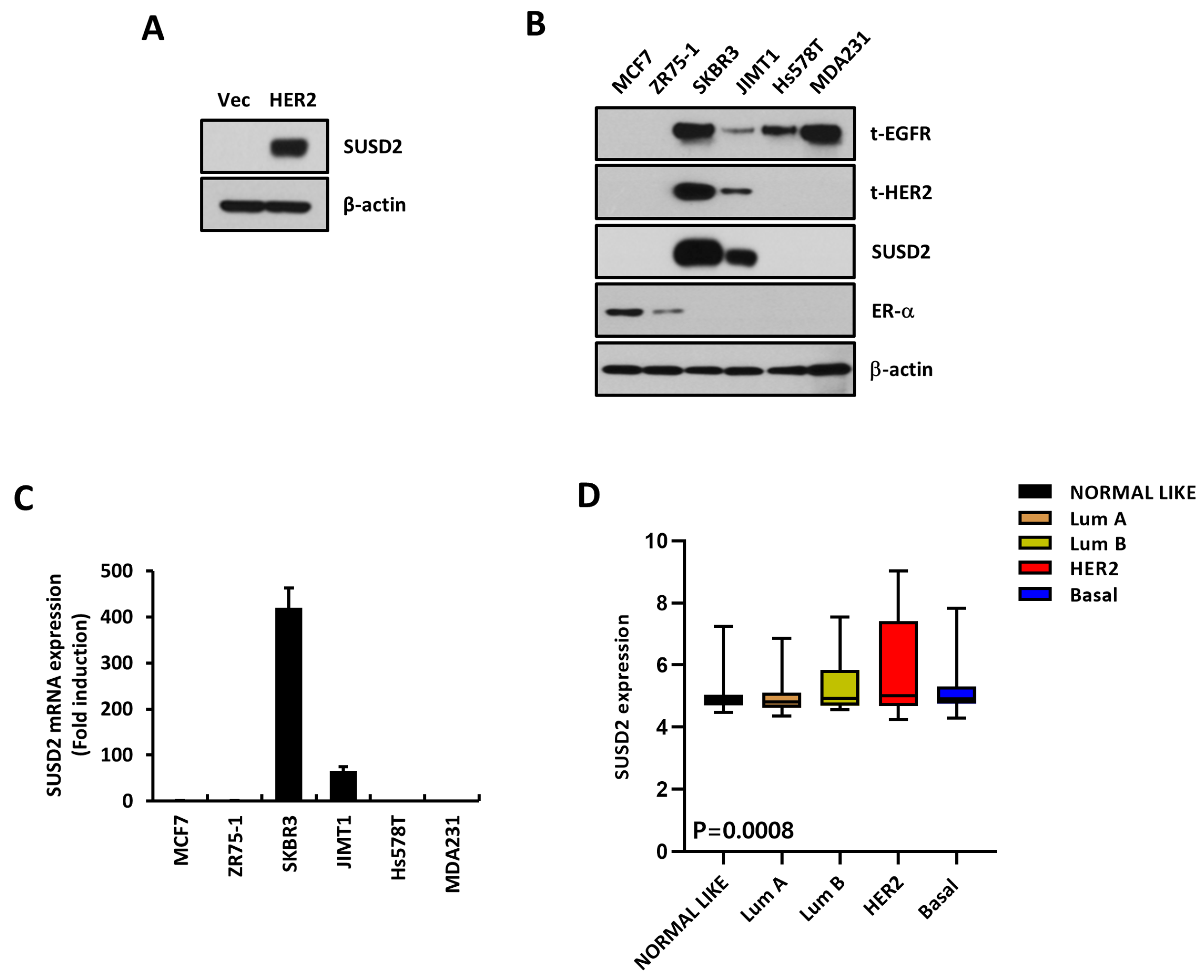
3.1. SUSD2 Is Increased by HER2 Overexpression
3.2. SUSD2 Expression Is Associated with Poor Prognosis of HER2+ Breast Cancer Patients
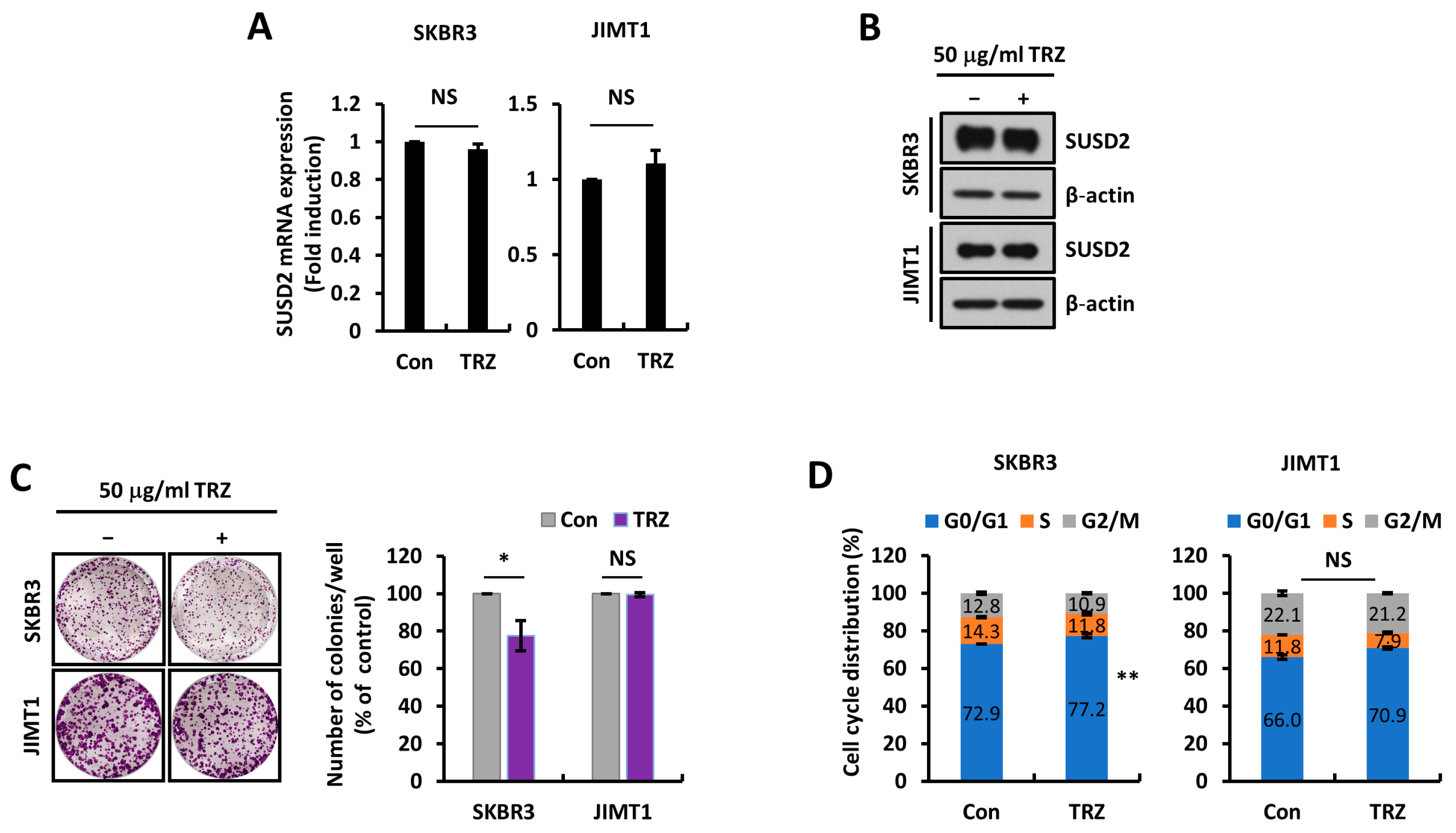
3.3. SUSD2 Expression Is Modulated by STAT3 Activity
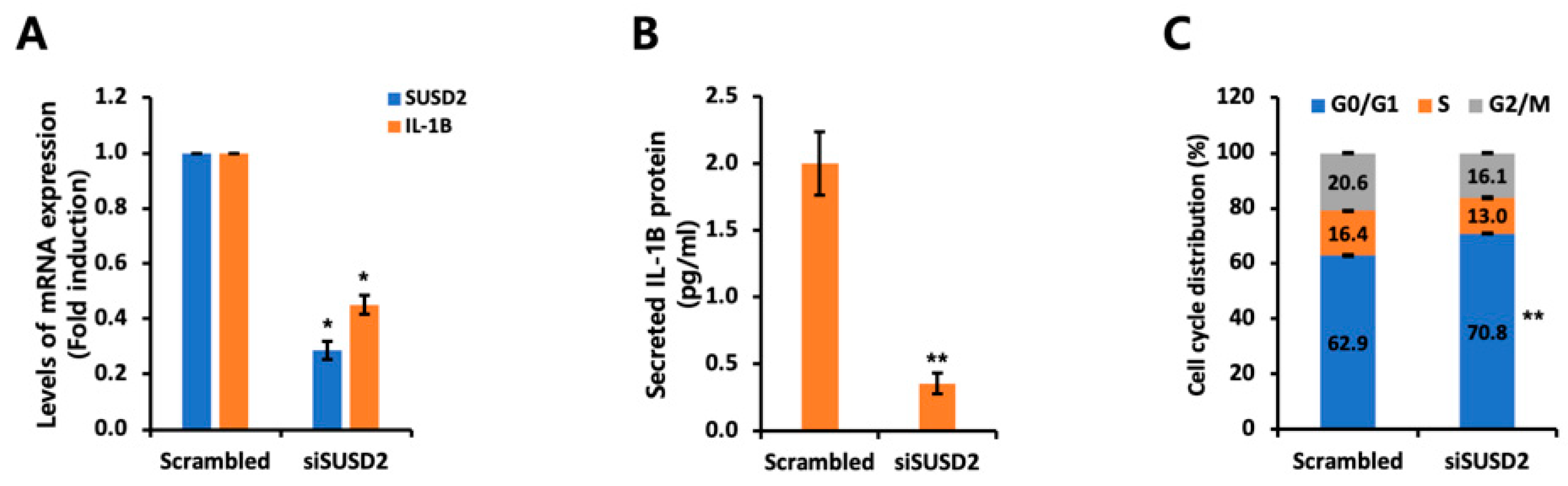
3.4. IL-1B Expression Is Regulated by SUSD2 Expression Levels
3.5. SUSD2 Expression Is Increased in EGFR+ HER2+ Breast Cancer Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arteaga, C.L.; Sliwkowski, M.X.; Osborne, C.K.; Perez, E.A.; Puglisi, F.; Gianni, L. Treatment of HER2-positive breast cancer: Current status and future perspectives. Nat. Rev. Clin. Oncol. 2011, 9, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.G.; Kulkarni, S. HER2+ breast cancer: Review of biologic relevance and optimal use of diagnostic tools. Am. J. Clin. Pathol. 2008, 129, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Milano, M.; Brambilla, M.; Mennitto, A.; Maggi, C.; Cona, M.S.; Prisciandaro, M.; Fabbroni, C.; Celio, L.; Mariani, G.; et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: Current knowledge, new research directions and therapeutic perspectives. Crit. Rev. Oncol./Hematol. 2019, 139, 53–66. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Kim, H.; Jeong, Y.; Yoon, S.Y.; Lo, E.; Kim, S.; Lee, J.E. Tumorigenicity of EGFR- and/or HER2-Positive Breast Cancers Is Mediated by Recruitment of Tumor-Associated Macrophages. Int. J. Mol. Sci. 2023, 24, 1443. [Google Scholar] [CrossRef]
- Cohen, B.D.; Kiener, P.A.; Green, J.M.; Foy, L.; Fell, H.P.; Zhang, K. The Relationship between Human Epidermal Growth-like Factor Receptor Expression and Cellular Transformation in NIH3T3 Cells. J. Biol. Chem. 1996, 271, 30897–30903. [Google Scholar] [CrossRef]
- Tsutsui, S.; Ohno, S.; Murakami, S.; Kataoka, A.; Kinoshita, J.; Hachitanda, Y. Prognostic value of the combination of epidermal growth factor receptor and c-erbB-2 in breast cancer. Surgery 2003, 133, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Nakanishi, K.; Yutsudo, M.; Inoue, H.; Li, Q.; Oka, K.; Yoshioka, N.; Hakura, A. Isolation of a novel gene down-regulated by v-src. FEBS Lett. 1996, 383, 21–25. [Google Scholar] [PubMed]
- Cai, C.; Shi, R.; Gao, Y.; Zeng, J.; Wei, M.; Wang, H.; Zheng, W.; Ma, W. Reduced expression of sushi domain containing 2 is associated with progression of non-small cell lung cancer. Oncol. Lett. 2015, 10, 3619–3624. [Google Scholar] [CrossRef]
- Liu, X.-R.; Cai, C.-X.; Luo, L.-M.; Zheng, W.-L.; Shi, R.; Zeng, J.; Xu, Y.-Q.; Wei, M.; Ma, W.-L. Decreased expression of Sushi Domain Containing 2 correlates to progressive features in patients with hepatocellular carcinoma. Cancer Cell Int. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Cheng, Y.; Zhang, H.; Liu, B.; Mo, X.; Li, T.; Li, L.; Cheng, X.; Zhang, L.; Ji, J.; et al. CSBF/C10orf99, a novel potential cytokine, inhibits colon cancer cell growth through inducing G1 arrest. Sci. Rep. 2014, 4, 6812. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.P.; Evans, R.L.; Egland, K.A. Multiple Functions of Sushi Domain Containing 2 (SUSD2) in Breast Tumorigenesis. Mol. Cancer Res. 2013, 11, 74–85. [Google Scholar] [CrossRef]
- Hultgren, E.M.; Patrick, M.E.; Evans, R.L.; Stoos, C.T.; Egland, K.A. SUSD2 promotes tumor-associated macrophage recruitment by increasing levels of MCP-1 in breast cancer. PLoS ONE 2017, 12, e0177089. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Jeong, Y.; You, D.; Yoon, S.Y.; Lo, E.; Nam, S.J.; Lee, J.E.; Kim, S.W. Suppression of Platelet-Derived Growth Factor Receptor-Alpha Overcomes Resistance to Trastuzumab through STAT3-Dependent IL-6 Reduction in HER2-Positive Breast Cancer Cells. Biomedicines 2023, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; You, D.; Jeong, Y.; Yu, J.; Kim, S.W.; Nam, S.J.; Lee, J.E. TP53 upregulates alpha-smooth muscle actin expression in tamoxifen-resistant breast cancer cells. Oncol. Rep. 2019, 41, 1075–1082. [Google Scholar] [PubMed]
- Choi, Y.-L.; Oh, E.; Park, S.; Kim, Y.; Park, Y.-H.; Song, K.; Cho, E.Y.; Hong, Y.-C.; Choi, J.S.; Lee, J.E.; et al. Triple-negative, basal-like, and quintuple-negative breast cancers: Better prediction model for survival. BMC Cancer 2010, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, S.H.; Park, S.Y.; Yoo, J.; Kim, S.K.; Kim, H.K. Identification of EGFR Mutations by Immunohistochemistry with EGFR Mutation–Specific Antibodies in Biopsy and Resection Specimens from Pulmonary Adenocarcinoma. Cancer Res. Treat. 2015, 47, 653–660. [Google Scholar] [CrossRef]
- Gyorffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Kitada, K.; Nakai, K.; Sarai, A. PrognoScan: A new database for meta-analysis of the prognostic value of genes. BMC Med. Genom. 2009, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, W.; Jiang, J. Prevention and treatment of cancer targeting chronic inflammation: Research progress, potential agents, clinical studies and mechanisms. Sci. China Life Sci. 2017, 60, 601–616. [Google Scholar] [CrossRef]
- Patel, A.; Unni, N.; Peng, Y. The Changing Paradigm for the Treatment of HER2-Positive Breast Cancer. Cancers 2020, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef]
- Garcia-Alvarez, A.; Papakonstantinou, A.; Oliveira, M. Brain Metastases in HER2-Positive Breast Cancer: Current and Novel Treatment Strategies. Cancers 2021, 13, 2927. [Google Scholar] [CrossRef]
- Abd El-Rehim, D.M.; Pinder, S.E.; Paish, C.E.; Bell, J.A.; Rampaul, R.S.; Blamey, R.W.; Robertson, J.F.R.; Nicholson, R.I.; Ellis, I.O. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br. J. Cancer 2004, 91, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Sheets, J.N.; Iwanicki, M.; Liu, J.F.; E Howitt, B.; Hirsch, M.S.; Gubbels, J.A.A.; Drapkin, R.; Egland, K.A. SUSD2 expression in high-grade serous ovarian cancer correlates with increased patient survival and defective mesothelial clearance. Oncogenesis 2016, 5, e264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, N.; Alowayed, N.; Singh, Y.; Cheng, A.; Lang, F.; Salker, M.S. Downregulation of endometrial mesenchymal marker SUSD2 causes cell senescence and cell death in endometrial carcinoma cells. PLoS ONE 2017, 12, e0183681. [Google Scholar] [CrossRef] [PubMed]
- Umeda, S.; Kanda, M.; Miwa, T.; Tanaka, H.; Tanaka, C.; Kobayashi, D.; Suenaga, M.; Hattori, N.; Hayashi, M.; Yamada, S.; et al. Expression of sushi domain containing two reflects the malignant potential of gastric cancer. Cancer Med. 2018, 7, 5194–5204. [Google Scholar] [CrossRef]
- Zhao, B.; Gong, W.; Ma, A.; Chen, J.; Velegraki, M.; Dong, H.; Liu, Z.; Wang, L.; Okimoto, T.; Jones, D.M.; et al. SUSD2 suppresses CD8+ T cell antitumor immunity by targeting IL-2 receptor signaling. Nat. Immunol. 2022, 23, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duan, Y.; Li, X.; Zhao, R.; Lan, B.; Zhang, X.; Wang, X.; Chen, H.; Feng, S.; Liu, Z.; et al. CBX8 Together with SET Facilitates Ovarian Carcinoma Growth and Metastasis by Suppressing the Transcription of SUSD2. Mol. Cancer Res. 2022, 20, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, X.; Wang, P.; Li, T.; Hu, F.; Liu, Q.; Yang, F.; Wang, J.; Xu, T.; Han, W. SUSD2 is frequently downregulated and functions as a tumor suppressor in RCC and lung cancer. Tumor Biol. 2016, 37, 9919–9930. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Miao, C.; Jin, C.; Qiu, C.; Li, Y.; Sun, X.; Gao, M.; Lu, N.; Kong, B. SUSD2 promotes cancer metastasis and confers cisplatin resistance in high grade serous ovarian cancer. Exp. Cell Res. 2018, 363, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.G.; Chen, M.H. TPM4 aggravates the malignant progression of hepatocellular carcinoma through negatively regulating SUSD2. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4756–4765. [Google Scholar]
- Sonnenblick, A.; Brohée, S.; Fumagalli, D.; Vincent, D.; Venet, D.; Ignatiadis, M.; Salgado, R.; Eynden, G.V.D.; Rothé, F.; Desmedt, C.; et al. Constitutive phosphorylated STAT3-associated gene signature is predictive for trastuzumab resistance in primary HER2-positive breast cancer. BMC Med. 2015, 13, 177. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Yazdanparast, R. Activation of STAT3/HIF-1alpha/Hes-1 axis promotes trastuzumab resistance in HER2-overexpressing breast cancer cells via down-regulation of PTEN. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Gao, M.; Fu, L.; Li, Y.; Quan, H.; Lou, L. STAT3 activation confers trastuzumab-emtansine (T-DM1) resistance in HER2-positive breast cancer. Cancer Sci. 2018, 109, 3305–3315. [Google Scholar] [CrossRef] [PubMed]



| Variables | All (n = 21) |
|---|---|
| Age group (years) | |
| >50 | 11 (52.4%) |
| ≤50 | 10 (47.6%) |
| Subtypes | |
| LumA | 6 (28.6%) |
| LumB | 2 (9.5%) |
| HER2 | 8 (38.1%) |
| TNBC | 5 (23.8%) |
| Estrogen receptor | |
| Negative | 15 (71.4%) |
| Positive | 6 (28.6%) |
| Progesterone receptor | |
| Negative | 17 (81.0%) |
| Positive | 4 (19.0%) |
| EGFR | |
| Negative | 0 (0%) |
| Positive | 21 (100%) |
| HER2 | |
| Negative | 11 (52.4%) |
| Positive | 10 (47.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Kim, H.; You, D.; Cho, S.Y.; Yoon, S.Y.; Kim, S.W.; Nam, S.J.; Lee, J.E.; Kim, S. Induction of SUSD2 by STAT3 Activation Is Associated with Tumor Recurrence in HER2-Positive Breast Cancer. Cells 2025, 14, 19. https://doi.org/10.3390/cells14010019
Jeong Y, Kim H, You D, Cho SY, Yoon SY, Kim SW, Nam SJ, Lee JE, Kim S. Induction of SUSD2 by STAT3 Activation Is Associated with Tumor Recurrence in HER2-Positive Breast Cancer. Cells. 2025; 14(1):19. https://doi.org/10.3390/cells14010019
Chicago/Turabian StyleJeong, Yisun, Hyungjoo Kim, Daeun You, Soo Youn Cho, Sun Young Yoon, Seok Won Kim, Seok Jin Nam, Jeong Eon Lee, and Sangmin Kim. 2025. "Induction of SUSD2 by STAT3 Activation Is Associated with Tumor Recurrence in HER2-Positive Breast Cancer" Cells 14, no. 1: 19. https://doi.org/10.3390/cells14010019
APA StyleJeong, Y., Kim, H., You, D., Cho, S. Y., Yoon, S. Y., Kim, S. W., Nam, S. J., Lee, J. E., & Kim, S. (2025). Induction of SUSD2 by STAT3 Activation Is Associated with Tumor Recurrence in HER2-Positive Breast Cancer. Cells, 14(1), 19. https://doi.org/10.3390/cells14010019






