Strategies to Overcome Intrinsic and Acquired Resistance to Chemoradiotherapy in Head and Neck Cancer
Abstract
1. Introduction
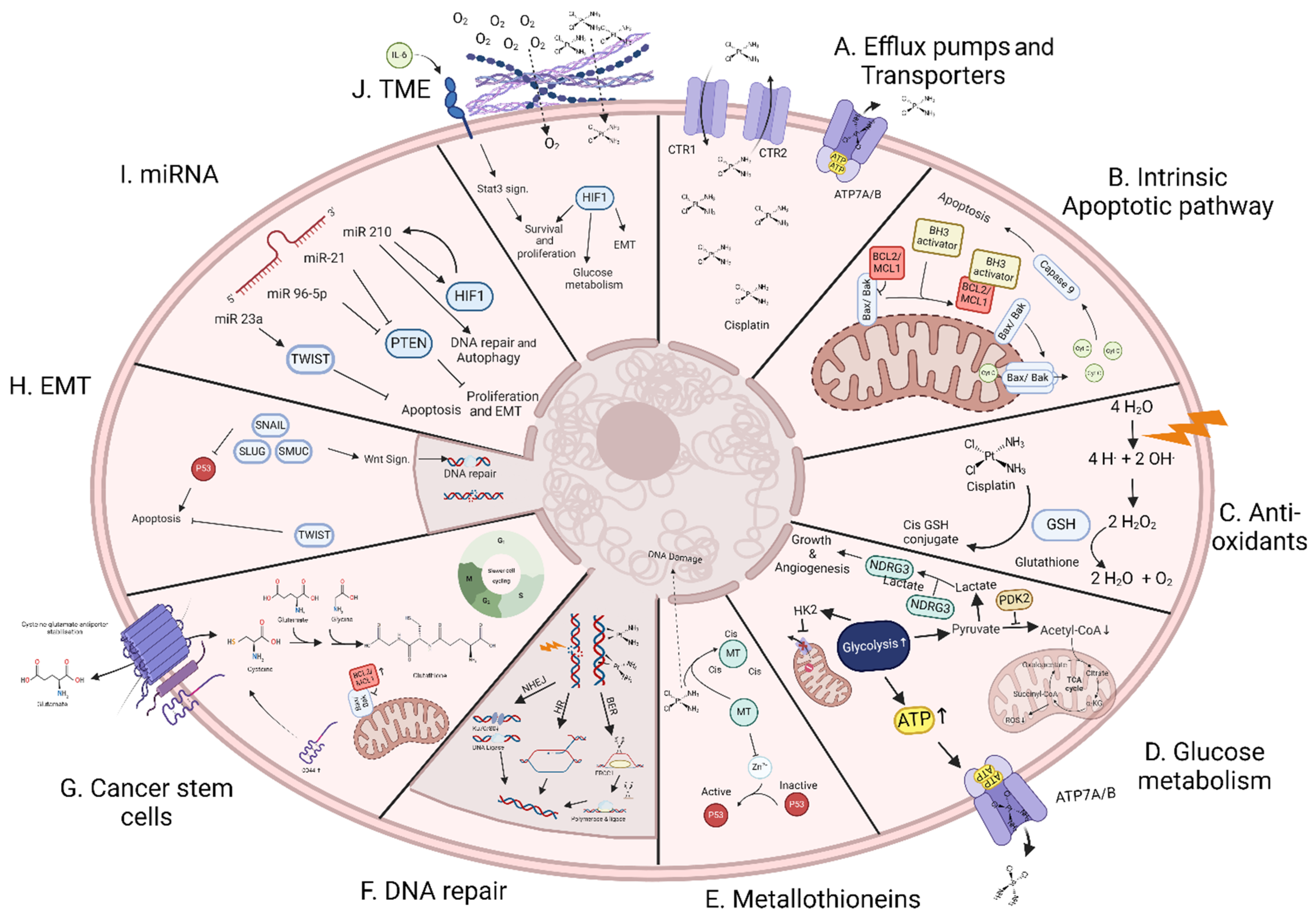
2. Mechanism of Resistance to Chemo- and/or Radiotherapy
2.1. Efflux Pumps and Transporters
2.2. Apoptotic Pathway
2.3. Antioxidant Defenses
2.4. Glucose Metabolism
2.5. Metallothioneins
2.6. DNA Damage Repair
2.7. Cancer Stem Cells
2.8. Epithelial-Mesenchymal Transition
2.9. Non-Coding RNA
2.10. Tumour Microenvironment
3. Recent Advances in Sensitizing HNSCC Cells to CRT
3.1. Targeting DNA Damage Response
3.2. Targeting Hypoxia
3.3. Targeting Immune Checkpoints
3.4. Targeting Autophagy Pathway
3.5. Targeting Apoptosis Pathway
3.6. Oxidative Stress
3.7. Others
4. Generating Acquired Resistance In Vitro
4.1. Acquired Resistance to Cisplatin
4.2. Acquired Resistance to Radiotherapy
4.3. Acquired Resistance to CRT
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leroy, R.; De Gendt, C.; Stordeur, S.; Schillemans, V.; Verleye, L.; Silversmit, G.; Van Eycken, E.; Savoye, I.; Grégoire, V.; Nuyts, S.; et al. Head and Neck Cancer in Belgium: Quality of Diagnostic Management and Variability Across Belgian Hospitals Between 2009 and 2014. Front. Oncol. 2019, 9, 1006. [Google Scholar] [CrossRef]
- Belgian Cancer Registry. Cancer Fact Sheet Head and Neck Cancer ICD10: C00-C14, C30-C32; Belgian Cancer Registry: Brussel, Belgium, 2021. [Google Scholar]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Ho, A.S.; Kraus, D.H.; Ganly, I.; Lee, N.Y.; Shah, J.P.; Morris, L.G.T. Decision making in the management of recurrent head and neck cancer. Head Neck 2014, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Duran, G.; Cruz, R.; Aguín, S.; Barros, F.; Giráldez, J.M.; Bernárdez, B.; Zarra, I.; López-López, R.; Carracedo, A.; Lamas, M.J. Predictive value of ERCC2, ABCC2 and MMP2 of response and long-term survival in locally advanced head and neck cancer patients treated with chemoradiotherapy. Cancer Chemother. Pharmacol. 2021, 88, 813–823. [Google Scholar] [CrossRef]
- Eljack, N.D.; Ma, H.-Y.M.; Drucker, J.; Shen, C.; Hambley, T.W.; New, E.J.; Friedrich, T.; Clarke, R.J. Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin. Metallomics 2014, 6, 2126–2133. [Google Scholar] [CrossRef]
- Kilari, D.; Guancial, E.; Kim, E.S. Role of copper transporters in platinum resistance. World J. Clin. Oncol. 2016, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Holzer, A.K.; Manorek, G.H.; Howell, S.B. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharmacol. 2006, 70, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Theile, D.; Ketabi-Kiyanvash, N.; Herold-Mende, C.; Dyckhoff, G.; Efferth, T.; Bertholet, V.; Haefeli, W.E.; Weiss, J. Evaluation of drug transporters’ significance for multidrug resistance in head and neck squamous cell carcinoma. Head & Neck 2011, 33, 959–968. [Google Scholar]
- Ughachukwu, P.; Unekwe, P. Efflux Pump-Mediated Resistance in Chemotherapy. Ann. Med. Health Sci. Res. 2012, 2, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Michaud, W.A.; Nichols, A.C.; Mroz, E.A.; Faquin, W.C.; Clark, J.R.; Begum, S.; Westra, W.H.; Wada, H.; Busse, P.M.; Ellisen, L.W.; et al. Bcl-2 Blocks Cisplatin-Induced Apoptosis and Predicts Poor Outcome Following Chemoradiation Treatment in Advanced Oropharyngeal Squamous Cell Carcinoma. Clin. Cancer Res. 2009, 15, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Filippou, A.; Pehkonen, H.; Karhemo, P.-R.; Väänänen, J.; Nieminen, A.I.; Klefström, J.; Grénman, R.; Mäkitie, A.A.; Joensuu, H.; Monni, O. ANO1 Expression Orchestrates p27Kip1/MCL1-Mediated Signaling in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 1170. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gao, J.; Guan, L.; Chen, X.; Gao, J.; Wang, K. Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-α signaling. Cell Death Dis. 2018, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, A.; Bill, A.; Brinkhaus, H.; Rothwell, C.; Clay, I.; Duss, S.; Rebhan, M.; Raman, P.; Guy, C.T.; Wetzel, K.; et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E1026–E1034. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Song, Y.; Gao, J.; Gao, J.; Wang, K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget 2016, 7, 78619–78630. [Google Scholar] [CrossRef]
- Tong, T.; Qin, X.; Jiang, Y.; Guo, H.; Wang, X.; Li, Y.; Xie, F.; Lu, H.; Zhai, P.; Ma, H.; et al. A novel CREB5/TOP1MT axis confers cisplatin resistance through inhibiting mitochondrial apoptosis in head and neck squamous cell carcinoma. BMC Med. 2022, 20, 231. [Google Scholar] [CrossRef]
- Fu, L.; Jin, Q.; Dong, Q.; Li, Q. AATF is Overexpressed in Human Head and Neck Squamous Cell Carcinoma and Regulates STAT3/Survivin Signaling. OncoTargets Ther. 2021, 14, 5237–5248. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Min, J.-N.; Park, E.-M.; Han, M.-Y.; Lee, Y.-S.; Lee, Y.J.; Park, Y.-M. Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. J. Cell. Physiol. 2000, 183, 100–107. [Google Scholar] [CrossRef]
- Dabrowiak, J.C.; Goodisman, J.; Souid, A.-K. Kinetic Study of the Reaction of Cisplatin with Thiols. Drug Metab. Dispos. Biol. Fate Chem. 2003, 30, 1378–1384. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-L.; Jang, H.; Kim, E.H.; Shin, D. Targeting of the Glutathione, Thioredoxin, and Nrf2 Antioxidant Systems in Head and Neck Cancer. Antioxid. Redox Signal. 2017, 27, 106–114. [Google Scholar] [CrossRef]
- Kriegs, M.; Kasten-Pisula, U.; Riepen, B.; Hoffer, K.; Struve, N.; Myllynen, L.; Braig, F.; Binder, M.; Rieckmann, T.; Grénman, R.; et al. Radiosensitization of HNSCC cells by EGFR inhibition depends on the induction of cell cycle arrests. Oncotarget 2016, 7, 45122–45133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Kuan, W.-H.; Chang, W.-L.; Kuo, I.-Y.; Liu, H.; Shieh, D.-B.; Liu, H.; Tan, B.; Wang, Y.-C. Dysregulation of SOX17/NRF2 axis confers chemoradiotherapy resistance and emerges as a novel therapeutic target in esophageal squamous cell carcinoma. J. Biomed. Sci. 2022, 29, 90. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Yoshida, R.; Kawahara, K.; Sakata, J.; Arita, H.; Nkashima, H.; Takahashi, N.; Hirayama, M.; Nagata, M.; Hirosue, A.; et al. The antioxidative stress regulator Nrf2 potentiates radioresistance of oral squamous cell carcinoma accompanied with metabolic modulation. Lab. Investig. 2022, 102, 896–907. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Mohd Omar, M.F.; Soong, R. The Warburg effect and drug resistance. Br. J. Pharmacol. 2016, 173, 970–979. [Google Scholar] [CrossRef]
- Luo, F.; Li, Y.; Yuan, F.; Zuo, J. Hexokinase II promotes the Warburg effect by phosphorylating alpha subunit of pyruvate dehydrogenase. Chin. J. Cancer Res. 2019, 31, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Ciscato, F.; Filadi, R.; Masgras, I.; Pizzi, M.; Marin, O.; Damiano, N.; Pizzo, P.; Gori, A.; Frezzato, F.; Chiara, F.; et al. Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca2+-dependent death of cancer cells. EMBO Rep. 2020, 21, e49117. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-C.; Huang, C.-H.; Hsieh, Y.-T.; Chen, T.-Y.; Cheng, L.-H.; Chen, C.-Y.; Liu, C.-J.; Chen, H.-M.; Lo, J.-F.; Chang, K.-W. Regulatory Role of Hexokinase 2 in Modulating Head and Neck Tumorigenesis. Front. Oncol. 2020, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Ren, Y.; Zheng, X.; Yang, S.; Lu, T.; Ji, H.; Hua, H.; Shan, K. Ginsenoside Rg3 and sorafenib combination therapy relieves the hepatocellular carcinomaprogression through regulating the HK2-mediated glycolysis and PI3K/Akt signaling pathway. Bioengineered 2022, 13, 13919–13928. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Sohn, H.A.; Park, Z.-Y.; Oh, S.; Kang, Y.K.; Lee, K.-M.; Kang, M.; Jang, Y.J.; Yang, S.-J.; Hong, Y.K.; et al. A Lactate-Induced Response to Hypoxia. Cell 2015, 161, 595–609. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Yan, Y.; Li, H. Pyruvate dehydrogenase kinases (PDKs): An overview toward clinical applications. Biosci. Rep. 2021, 41, BSR20204402. [Google Scholar] [CrossRef]
- Li, S.-J.; Guo, W.; Ren, G.-X.; Huang, G.; Chen, T.; Song, S.-L. Expression of Glut-1 in primary and recurrent head and neck squamous cell carcinomas, and compared with 2-[18F]fluoro-2-deoxy-D-glucose accumulation in positron emission tomography. Br. J. Oral Maxillofac. Surg. 2008, 46, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Cantuaria, G.; Fagotti, A.; Ferrandina, G.; Magalhaes, A.; Nadji, M.; Angioli, R.; Penalver, M.; Mancuso, S.; Scambia, G. GLUT-1 expression in ovarian carcinoma: Association with survival and response to chemotherapy. Cancer 2001, 92, 1144–1150. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Li, S.-J.; Liao, J.-X. Inhibition of Glucose Transporter 1 (GLUT1) Chemosensitized Head and Neck Cancer Cells to Cisplatin. Technol. Cancer Res. Treat. 2013, 12, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Jaggi, M.; Zhang, W.; Galich, A.; Du, C.; Sterrett, S.P.; Smith, L.M.; Balaji, K. Metallothioneins and resistance to cisplatin and radiation in prostate cancer. Urology 2006, 67, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, Y.; Hasegawa, Y.; Fukano, H.; Ogawa, T.; Namuba, M.; Mouri, K.; Fujimoto, Y.; Matsuura, H.; Takai, Y.; Mori, M. Metallothionein immunoreactivity in head and neck carcinomas; special reference to clinical behaviors and chemotherapy responses. Anticancer Res. 2000, 20, 257–264. [Google Scholar]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Chae, J.-W.; Kim, J.K.; Kim, H.J.; Chung, J.Y.; Kim, Y.-H. Inhibition of cisplatin-resistance by RNA interference targeting metallothionein using reducible oligo-peptoplex. J. Control. Release 2015, 215, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.L.; Stillman, M.J. Capturing platinum in cisplatin: Kinetic reactions with recombinant human apo-metallothionein 1a. Metallomics 2018, 10, 713–721. [Google Scholar] [CrossRef]
- Bouwman, P.; Jonkers, J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat. Rev. Cancer 2012, 12, 587–598. [Google Scholar] [CrossRef]
- Duan, M.; Ulibarri, J.; Liu, K.J.; Mao, P. Role of Nucleotide Excision Repair in Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 9248. [Google Scholar] [CrossRef]
- Albers; Köberle, B.; Ditz, C.; Kausch, I.; Wollenberg, B.; Ferris, R.L.; Albers, A.E. Metastases of squamous cell carcinoma of the head and neck show increased levels of nucleotide excision repair protein XPF in vivo that correlate with increased chemoresistance ex vivo. Int. J. Oncol. 2010, 36, 1277–1284. [Google Scholar] [CrossRef]
- Penninckx, S.; Pariset, E.; Cekanaviciute, E.; Costes, S.V. Quantification of radiation-induced DNA double strand break repair foci to evaluate and predict biological responses to ionizing radiation. NAR Cancer 2021, 3, zcab046. [Google Scholar] [CrossRef]
- Kadoch, C.; Williams, R.T.; Calarco, J.P.; Miller, E.L.; Weber, C.M.; Braun, S.M.G.; Pulice, J.L.; Chory, E.J.; Crabtree, G.R. Dynamics of BAF- Polycomb Complex Opposition on Heterochromatin in Normal and Oncogenic States. Nat. Genet. 2017, 49, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lin, F.-T.; Lin, W.-C. ACTL6A promotes repair of cisplatin-induced DNA damage, a new mechanism of platinum resistance in cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2015808118. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Shipony, Z.; Lin, S.G.; Kuo, A.; Xiong, X.; Loh, K.M.; Greenleaf, W.J.; Crabtree, G.R. Increased ACTL6A occupancy within mSWI/SNF chromatin remodelers drives human squamous cell carcinoma. Mol. Cell 2021, 81, 4964–4978.e8. [Google Scholar] [CrossRef]
- Velegzhaninov, I.O.; Belykh, E.S.; Rasova, E.E.; Pylina, Y.I.; Shadrin, D.M.; Klokov, D.Y. Radioresistance, DNA Damage and DNA Repair in Cells With Moderate Overexpression of RPA1. Front. Genet. 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Nickson, C.M.; Moori, P.; Carter, R.J.; Rubbi, C.P.; Parsons, J.L. Misregulation of DNA damage repair pathways in HPV-positive head and neck squamous cell carcinoma contributes to cellular radiosensitivity. Oncotarget 2017, 8, 29963–29975. [Google Scholar] [CrossRef]
- Ohkoshi, E.; Umemura, N. Induced overexpression of CD44 associated with resistance to apoptosis on DNA damage response in human head and neck squamous cell carcinoma cells. Int. J. Oncol. 2017, 50, 387–395. [Google Scholar] [CrossRef] [PubMed]
- van Neerven, S.M.; Tieken, M.; Vermeulen, L.; Bijlsma, M.F. Bidirectional interconversion of stem and non-stem cancer cell populations: A reassessment of theoretical models for tumor heterogeneity. Mol. Cell. Oncol. 2016, 3, e1098791. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug resistance and Cancer stem cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef]
- Januchowski, R.; Wojtowicz, K.; Zabel, M. The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomed. Pharmacother. Biomed. Pharmacother. 2013, 67, 669–680. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc− and Thereby Promotes Tumor Growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Avril, T.; Vauléon, E.; Chevet, E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 2017, 6, e373. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.-Y.; Yoon, H. Chemotherapy Resistance Explained through Endoplasmic Reticulum Stress-Dependent Signaling. Cancers 2019, 11, 338. [Google Scholar] [CrossRef]
- Amaresan, R.; Gopal, U. Cell surface GRP78: A potential mechanism of therapeutic resistant tumors. Cancer Cell Int. 2023, 23, 100. [Google Scholar] [CrossRef]
- Conner, C.; Lager, T.W.; Guldner, I.H.; Wu, M.-Z.; Hishida, Y.; Hishida, T.; Ruiz, S.; Yamasaki, A.E.; Gilson, R.C.; Belmonte, J.C.I.; et al. Cell surface GRP78 promotes stemness in normal and neoplastic cells. Sci. Rep. 2020, 10, 3474. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N.; Wu, C.; Prime, S.S. Heterogeneity of Cancer Stem Cells in Tumorigenesis, Metastasis, and Resistance to Antineoplastic Treatment of Head and Neck Tumours. Cells 2021, 10, 3068. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.-G.; Zheng, D.-L. Roles of the Wnt Signaling Pathway in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2021, 7, 590912. [Google Scholar] [CrossRef]
- Azzolin, L.; Zanconato, F.; Bresolin, S.; Forcato, M.; Basso, G.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Role of TAZ as mediator of Wnt signaling. Cell 2012, 151, 1443–1456. [Google Scholar] [CrossRef] [PubMed]
- Quayle, L.A.; Ottewell, P.D.; Holen, I. Chemotherapy resistance and stemness in mitotically quiescent human breast cancer cells identified by fluorescent dye retention. Clin. Exp. Metastasis 2018, 35, 831–846. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Ishii, H.; Takahashi, G.; Okamoto, A.; Moriyama, M.; Sakakura, K.; Masuyama, K. Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head Neck 2012, 34, 336–343. [Google Scholar] [CrossRef]
- Tagscherer, K.E.; Fassl, A.; Campos, B.; Farhadi, M.; Kraemer, A.; Böck, B.C.; Macher-Goeppinger, S.; Radlwimmer, B.; Wiestler, O.D.; Herold-Mende, C.; et al. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene 2008, 27, 6646–6656. [Google Scholar] [CrossRef]
- Signore, M.; Ricci-Vitiani, L.; De Maria, R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2013, 332, 374–382. [Google Scholar] [CrossRef]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.-I.; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, P.; Bissell, M.J.; Radisky, D.C. Epithelial-Mesenchymal Transition: General Principles and Pathological Relevance with Special Emphasis on the Role of Matrix Metalloproteinases. Cold Spring Harb. Perspect. Biol. 2012, 4, a011908. [Google Scholar] [CrossRef]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef]
- Lu, J.; Zhong, Y.; Chen, J.; Lin, X.; Lin, Z.; Wang, N.; Lin, S. Radiation Enhances the Epithelial–Mesenchymal Transition of A549 Cells via miR3591-5p/USP33/PPM1A. Cell. Physiol. Biochem. 2018, 50, 721–733. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Liang, S.; Liu, Q.; Wang, P.; Cai, L.; Wang, R. Radiation induces epithelial to mesenchymal transition via upregulation of PD-L1 in nasopharyngeal carcinoma cell. Transl. Cancer Res. 2021, 10, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Kurrey, N.K.; Jalgaonkar, S.P.; Joglekar, A.V.; Ghanate, A.D.; Chaskar, P.D.; Doiphode, R.Y.; Bapat, S.A. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 2009, 27, 2059–2068. [Google Scholar] [CrossRef]
- Nagarajan, D.; Melo, T.; Deng, Z.; Almeida, C.; Zhao, W. ERK/GSK3β/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition. Free Radic Biol Med 2012, 52, 983–992. [Google Scholar] [CrossRef]
- Coelho, B.P.; Fernandes, C.F.d.L.; Boccacino, J.M.; Souza, M.C.d.S.; Melo-Escobar, M.I.; Alves, R.N.; Prado, M.B.; Iglesia, R.P.; Cangiano, G.; Mazzaro, G.L.R.; et al. Multifaceted WNT Signaling at the Crossroads Between Epithelial-Mesenchymal Transition and Autophagy in Glioblastoma. Front. Oncol. 2020, 10, 597743. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Virshup, D.M. Wnt Signaling and Drug Resistance in Cancer. Mol Pharmacol 2020, 97, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Carrà, G.; Lingua, M.F.; Maffeo, B.; Taulli, R.; Morotti, A. P53 vs NF-κB: The role of nuclear factor-kappa B in the regulation of p53 activity and vice versa. Cell. Mol. Life Sci. 2020, 77, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Alshaimaa, A.; Maria, M.V.; Daniel, D.; Herbert, R.; Jozsef, D.; Ira-Ida, S. Epithelial-mesenchymal crosstalk induces radioresistance in HNSCC cells. Oncotarget 2018, 9, 3641–3652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinforma. 2019, 16, 20190027. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, H.; DU, Y.; Tan, P. miR-23a promotes cisplatin chemoresistance and protects against cisplatin-induced apoptosis in tongue squamous cell carcinoma cells through Twist. Oncol. Rep. 2015, 33, 942–950. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, W.; Hong, J.-Y.; Kang, D.; Park, S.; Suh, J.; You, D.; Park, Y.-Y.; Suh, N.; Hwang, J.J.; et al. miR-96-5p targets PTEN to mediate sunitinib resistance in clear cell renal cell carcinoma. Sci. Rep. 2022, 12, 3537. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, M.; Pulito, C.; Sacconi, A.; Donzelli, S.; D’andrea, M.; Manciocco, V.; Pellini, R.; Paci, P.; Sanguineti, G.; Strigari, L.; et al. miR-96-5p targets PTEN expression affecting radio-chemosensitivity of HNSCC cells. J. Exp. Clin. Cancer Res. CR 2019, 38, 141. [Google Scholar] [CrossRef]
- Sheng, S.; Su, W.; Mao, D.; Li, C.; Hu, X.; Deng, W.; Yao, Y.; Ji, Y. MicroRNA-21 induces cisplatin resistance in head and neck squamous cell carcinoma. PLoS ONE 2022, 17, e0267017. [Google Scholar] [CrossRef]
- Starzyńska, A.; Adamska, P.; Sejda, A.; Sakowicz-Burkiewicz, M.; Jan Adamski, Ł.; Marvaso, G.; Wychowański, P.; Jereczek-Fossa, B.A. Any Role of PIK3CA and PTEN Biomarkers in the Prognosis in Oral Squamous Cell Carcinoma? Life 2020, 10, 325. [Google Scholar] [CrossRef]
- Gee, H.E.; Camps, C.; Buffa, F.M.; Patiar, S.; Winter, S.C.; Betts, G.; Homer, J.; Corbridge, R.; Cox, G.; West, C.M.L.; et al. hsa-miR-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer 2010, 116, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Myers, K.A. The Role of Hypoxia-Induced miR-210 in Cancer Progression. Int. J. Mol. Sci. 2015, 16, 6353–6372. [Google Scholar] [CrossRef]
- You, G.-R.; Cheng, A.-J.; Shen, E.Y.-L.; Fan, K.-H.; Huang, Y.-F.; Huang, Y.-C.; Chang, K.-P.; Chang, J.T. MiR-630 Promotes Radioresistance by Induction of Anti-Apoptotic Effect via Nrf2-GPX2 Molecular Axis in Head-Neck Cancer. Cells 2023, 12, 2853. [Google Scholar] [CrossRef]
- Ni, J.; Zheng, H.; Huang, Z.; Hong, Y.; Ou, Y.; Tao, Y.; Wang, M.; Wang, Z.; Yang, Y.; Zhou, W. MicroRNA-197-3p acts as a prognostic marker and inhibits cell invasion in hepatocellular carcinoma. Oncol. Lett. 2018, 17, 2317–2327. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wu, Z.-Y.; Wang, G.-C.; Liu, K.; Niu, X.-B.; Gu, S.; Meng, J.-S. LINC00312 inhibits the migration and invasion of bladder cancer cells by targeting miR-197-3p. Tumor Biol. 2016, 37, 14553–14563. [Google Scholar] [CrossRef]
- Xie, W.; Shui, C.; Fang, X.; Peng, Y.; Qin, L. miR-197-3p reduces epithelial–mesenchymal transition by targeting ABCA7 in ovarian cancer cells. 3 Biotech 2020, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, Q.; Gong, J.; Jiang, W.; Chen, Y.; Zhou, Q.; Aldeen, A.; Wang, S.; Li, C.; Lv, W.; et al. Radiosensitizer EXO-miR-197-3p Inhibits Nasopharyngeal Carcinoma Progression and Radioresistance by Regulating the AKT/mTOR Axis and HSPA5-mediated Autophagy. Int. J. Biol. Sci. 2022, 18, 1878–1895. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours—Implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Martini, M.; Termini, J. Peroxy radical oxidation of thymidine. Chem. Res. Toxicol. 1997, 10, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.; Klein, C.; Zenke, F.T.; Abdollahi, A.; Debus, J.; Dokic, I.; Mairani, A. Modeling the Effect of Hypoxia and DNA Repair Inhibition on Cell Survival after Photon Irradiation. Int. J. Mol. Sci. 2019, 20, 6054. [Google Scholar] [CrossRef]
- Nisar, H.; González, P.M.S.; Brauny, M.; Labonté, F.M.; Schmitz, C.; Roggan, M.D.; Konda, B.; Hellweg, C.E. Hypoxia Changes Energy Metabolism and Growth Rate in Non-Small Cell Lung Cancer Cells. Cancers 2023, 15, 2472. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Al Mazeedi, M.A.M.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Ornatsky, O.I.; Siddiqui, I.; Straus, R.; Baranov, V.I.; Hedley, D.W. Biodistribution of cisplatin revealed by imaging mass cytometry identifies extensive collagen binding in tumor and normal tissues. Sci. Rep. 2016, 6, 36641. [Google Scholar] [CrossRef] [PubMed]
- Januchowski, R.; Świerczewska, M.; Sterzyńska, K.; Wojtowicz, K.; Nowicki, M.; Zabel, M. Increased Expression of Several Collagen Genes is Associated with Drug Resistance in Ovarian Cancer Cell Lines. J. Cancer 2016, 7, 1295–1310. [Google Scholar] [CrossRef]
- Kosmehl, H.; Berndt, A.; Strassburger, S.; Borsi, L.; Rousselle, P.; Mandel, U.; Hyckel, P.; Zardi, L.; Katenkamp, D. Distribution of laminin and fibronectin isoforms in oral mucosa and oral squamous cell carcinoma. Br. J. Cancer 1999, 81, 1071–1079. [Google Scholar] [CrossRef]
- Fukazawa, S.; Shinto, E.; Tsuda, H.; Ueno, H.; Shikina, A.; Kajiwara, Y.; Yamamoto, J.; Hase, K. Laminin β3 expression as a prognostic factor and a predictive marker of chemoresistance in colorectal cancer. Jpn. J. Clin. Oncol. 2015, 45, 533–540. [Google Scholar] [PubMed]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Radwanska, A.; Beghelli-de la Forest Divonne, S.; et al. Fibronectin-guided migration of carcinoma collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar] [CrossRef]
- Rintoul, R.C.; Sethi, T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Clin. Sci. Lond. Engl. 1979 2002, 102, 417–424. [Google Scholar] [CrossRef]
- Underwood, T.J.; Hayden, A.L.; Derouet, M.; Garcia, E.; Noble, F.; White, M.J.; Thirdborough, S.; Mead, A.; Clemons, N.; Mellone, M.; et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J. Pathol. 2015, 235, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Stanam, A.; Love-Homan, L.; Joseph, T.S.; Espinosa-Cotton, M.; Simons, A.L. Upregulated interleukin-6 expression contributes to erlotinib resistance in head and neck squamous cell carcinoma. Mol. Oncol. 2015, 9, 1371–1383. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Smith, H.L.; Southgate, H.; Tweddle, D.A.; Curtin, N.J. DNA damage checkpoint kinases in cancer. Expert Rev. Mol. Med. 2020, 22, e2. [Google Scholar] [CrossRef]
- Foote, K.M.; Nissink, J.W.M.; McGuire, T.; Turner, P.; Guichard, S.; Yates, J.W.T.; Lau, A.; Blades, K.; Heathcote, D.; Odedra, R.; et al. Discovery and Characterization of AZD6738, a Potent Inhibitor of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Kinase with Application as an Anticancer Agent. J. Med. Chem. 2018, 61, 9889–9907. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Oleinick, N.L.; Zhang, J. ATR/CHK1 inhibitors and cancer therapy. Radiother. Oncol. 2018, 126, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.C.; Lee, E.D.; Bhola, N.E.; Li, H.; Sogaard, K.K.; Bakkenist, C.J.; Grandis, J.R.; Johnson, D.E. ATR inhibition sensitizes HPV− and HPV+ head and neck squamous cell carcinoma to cisplatin. Oral Oncol. 2019, 95, 35–42. [Google Scholar] [CrossRef]
- Dillon, M.T.; Barker, H.E.; Pedersen, M.; Hafsi, H.; Bhide, S.A.; Newbold, K.L.; Nutting, C.M.; McLaughlin, M.; Harrington, K. Radiosensitization by the ATR Inhibitor AZD6738 through Generation of Acentric Micronuclei. Mol. Cancer Ther. 2017, 16, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Dok, R.; Glorieux, M.; Bamps, M.; Nuyts, S. Effect of ATR Inhibition in RT Response of HPV-Negative and HPV-Positive Head and Neck Cancers. Int. J. Mol. Sci. 2021, 22, 1504. [Google Scholar] [CrossRef]
- Jones, G.N.; Iyer, S.; Milo, M.; Lou, P.-J.; Nance, M.A.; Gomez-Roca, C.A.; Standifer, N.; Marco-Casanova, P.; Gill, S.; Surace, M.; et al. Abstract CT198: Immunomodulatory effects of the ATR inhibitor ceralasertib in a window of opportunity biomarker trial in patients with head and neck squamous cell carcinoma. Cancer Res. 2023, 83, CT198. [Google Scholar] [CrossRef]
- Vitti, E.T.; Kacperek, A.; Parsons, J.L. Targeting DNA Double-Strand Break Repair Enhances Radiosensitivity of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma to Photons and Protons. Cancers 2020, 12, 1490. [Google Scholar] [CrossRef]
- Pires, I.M.; Olcina, M.M.; Anbalagan, S.; Pollard, J.R.; Reaper, P.M.; A Charlton, P.; McKenna, W.G.; Hammond, E.M. Targeting radiation-resistant hypoxic tumour cells through ATR inhibition. Br. J. Cancer 2012, 107, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Faulhaber, E.-M.; Jost, T.; Symank, J.; Scheper, J.; Bürkel, F.; Fietkau, R.; Hecht, M.; Distel, L.V. Kinase Inhibitors of DNA-PK, ATM and ATR in Combination with Ionizing Radiation Can Increase Tumor Cell Death in HNSCC Cells While Sparing Normal Tissue Cells. Genes 2021, 12, 925. [Google Scholar] [CrossRef]
- Dobler, C.; Jost, T.; Hecht, M.; Fietkau, R.; Distel, L. Senescence Induction by Combined Ionizing Radiation and DNA Damage Response Inhibitors in Head and Neck Squamous Cell Carcinoma Cells. Cells 2020, 9, 2012. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Chen, Z.; Bruce, J.; Steuer, C.E.; Zandberg, D.P.; Riess, J.W.; Mitchell, D.; Davis, T.H.; Patel, M.; Kaur, V.; et al. 656MO Phase I study of M6620 (VX-970, berzosertib) in combination with cisplatin and XRT in patients with locally advanced head and neck squamous cell carcinoma. Annals of Oncology 2022, 33, S842. [Google Scholar] [CrossRef]
- Stockton, S.; Soares, H.P.; Dayyani, F.; Saeed, A.; Kim, E.S.; Jin, N.; Yacoub, G.H.; Whisenant, J.; Ayers, G.D.; Gore, S.; et al. A phase 2 single-arm study of berzosertib in combination with irinotecan in patients with progressive TP53 mutant gastric and gastro-esophageal junction cancer. J. Clin. Oncol. 2023, 41, 4044. [Google Scholar] [CrossRef]
- Koh, S.-B. The expanding role of WEE1. Cell. Signal. 2022, 94, 110310. [Google Scholar] [CrossRef]
- Osman, A.A.; Monroe, M.M.; Alves, M.V.O.; Patel, A.A.; Katsonis, P.; Fitzgerald, A.L.; Neskey, D.M.; Frederick, M.J.; Woo, S.H.; Caulin, C.; et al. Wee-1 Kinase Inhibition Overcomes Cisplatin Resistance Associated with High-Risk TP53 Mutations in Head and Neck Cancer through Mitotic Arrest Followed by Senescence. Mol. Cancer Ther. 2015, 14, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liao, J.; Lapidus, R.G.; Fan, X.; Mehra, R.; Cullen, K.J.; Dan, H. Targeting Wee1 kinase to suppress proliferation and survival of cisplatin-resistant head and neck squamous cell carcinoma. Cancer Chemother. Pharmacol. 2022, 89, 469–478. [Google Scholar] [CrossRef]
- Sarcar, B.; Kahali, S.; Prabhu, A.H.; Shumway, S.D.; Xu, Y.; Demuth, T.; Chinnaiyan, P. Targeting Radiation-Induced G2 Checkpoint Activation with the Wee-1 Inhibitor MK-1775 in Glioblastoma Cell Lines. Mol. Cancer Ther. 2011, 10, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Caretti, V.; Hiddingh, L.; Lagerweij, T.; Schellen, P.; Koken, P.W.; Hulleman, E.; van Vuurden, D.G.; Vandertop, W.P.; Kaspers, G.J.; Noske, D.P.; et al. WEE1 Kinase Inhibition Enhances the Radiation Response of Diffuse Intrinsic Pontine Gliomas. Mol. Cancer Ther. 2013, 12, 141–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Do, K.; Wilsker, D.; Ji, J.; Zlott, J.; Freshwater, T.; Kinders, R.J.; Collins, J.; Chen, A.P.; Doroshow, J.H.; Kummar, S. Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients With Refractory Solid Tumors. J. Clin. Oncol. 2015, 33, 3409–3415. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Good, J.; Kirkham, A.; Savage, J.; Mant, R.; Llewellyn, L.; Parish, J.; Spruce, R.; Forster, M.; Schipani, S.; et al. Phase I trial of WEE1 inhibition with chemotherapy and radiotherapy as adjuvant treatment, and a window of opportunity trial with cisplatin in patients with head and neck cancer: The WISTERIA trial protocol. BMJ Open 2020, 10, e033009. [Google Scholar] [CrossRef]
- Chera, B.S.; Sheth, S.H.; Patel, S.A.; Goldin, D.; Douglas, K.E.; Green, R.L.; Shen, C.J.; Gupta, G.P.; Moore, D.T.; Olson, J.E.G.; et al. Phase 1 trial of adavosertib (AZD1775) in combination with concurrent radiation and cisplatin for intermediate-risk and high-risk head and neck squamous cell carcinoma. Cancer 2021, 127, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Méndez, E.; Rodriguez, C.P.; Kao, M.C.; Raju, S.C.; Diab, A.; Harbison, R.A.; Konnick, E.Q.; Mugundu, G.M.; Santana-Davila, R.; Martins, R.; et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 2740–2748. [Google Scholar] [CrossRef]
- Zabludoff, S.D.; Deng, C.; Grondine, M.R.; Sheehy, A.M.; Ashwell, S.; Caleb, B.L.; Green, S.; Haye, H.R.; Horn, C.L.; Janetka, J.W.; et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol. Cancer Ther. 2008, 7, 2955–2966. [Google Scholar] [CrossRef]
- Gadhikar, M.A.; Sciuto, M.R.; Alves, M.V.O.; Pickering, C.R.; Osman, A.A.; Neskey, D.M.; Zhao, M.; Fitzgerald, A.L.; Myers, J.N.; Frederick, M.J. Chk1/2 Inhibition Overcomes the Cisplatin Resistance of Head and Neck Cancer Cells Secondary to the Loss of Functional p53. Mol. Cancer Ther. 2013, 12, 1860–1873. [Google Scholar] [CrossRef]
- Koniaras, K.; Cuddihy, A.R.; Christopoulos, H.; Hogg, A.; O'Connell, M.J. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene 2001, 20, 7453–7463. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Barker, H.E.; Kyula, J.; McLaughlin, M.; Dillon, M.T.; Schick, U.; Hafsi, H.; Thompson, A.; Khoo, V.; Harrington, K.; et al. An orally bioavailable Chk1 inhibitor, CCT244747, sensitizes bladder and head and neck cancer cell lines to radiation. Radiother. Oncol. 2017, 122, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Borst, G.R.; McLaughlin, M.; Kyula, J.N.; Neijenhuis, S.; Khan, A.; Good, J.; Zaidi, S.; Powell, N.G.; Meier, P.; Collins, I.; et al. Targeted Radiosensitization by the Chk1 Inhibitor SAR-020106. Int. J. Radiat. Oncol. 2013, 85, 1110–1118. [Google Scholar] [CrossRef]
- Zeng, L.; Nikolaev, A.; Xing, C.; Della Manna, D.L.; Yang, E.S. CHK1/2 Inhibitor Prexasertib Suppresses NOTCH Signaling and Enhances Cytotoxicity of Cisplatin and Radiation in Head and Neck Squamous Cell Carcinoma. Mol. Cancer Ther. 2020, 19, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Infante, J.; Janku, F.; Jones, S.; Nguyen, L.M.; Burris, H.; Naing, A.; Bauer, T.M.; Piha-Paul, S.; Johnson, F.M.; et al. Phase I Study of LY2606368, a Checkpoint Kinase 1 Inhibitor, in Patients With Advanced Cancer. J. Clin. Oncol. 2016, 34, 1764–1771. [Google Scholar] [CrossRef]
- Hong, D.S.; Moore, K.; Patel, M.; Grant, S.C.; Burris, H.A.; William, W.N.; Jones, S.; Meric-Bernstam, F.; Infante, J.; Golden, L.; et al. Evaluation of Prexasertib, a Checkpoint Kinase 1 Inhibitor, in a Phase Ib Study of Patients with Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.S.; Deutsch, E.; Mehmet, A.; Fayette, J.; Tao, Y.; Nabell, L.; Spencer, S.A.; Wang, X.A.; Spoljoric, E.A.; Zhang, W.; et al. A Phase 1b trial of prexasertib in combination with chemoradiation in patients with locally advanced head and neck squamous cell carcinoma. Radiother. Oncol. 2021, 157, 203–209. [Google Scholar] [CrossRef]
- Patel, M.R.; Hong, D.S.; Bendell, J.C.; Jones, S.F.; Hamilton, E.P.; Subbiah, V.; Karp, D.D.; Wang, J.S.-Z.; Aljumaily, R.; Hynes, S.; et al. A phase 1b dose-escalation study of prexasertib, a checkpoint kinase 1 (CHK1) inhibitor, in combination with cisplatin in patients with advanced cancer. J. Clin. Oncol. 2018, 36, 2579. [Google Scholar] [CrossRef]
- Moore, K.N.; Hong, D.S.; Patel, M.R.; Pant, S.; Ulahannan, S.V.; Jones, S.; Meric-Bernstam, F.; Wang, J.S.; Aljumaily, R.; Hamilton, E.P.; et al. A Phase 1b Trial of Prexasertib in Combination with Standard-of-Care Agents in Advanced or Metastatic Cancer. Target. Oncol. 2021, 16, 569–589. [Google Scholar] [CrossRef]
- Chiu, T.-J.; Chen, C.-H.; Chien, C.-Y.; Li, S.-H.; Tsai, H.-T.; Chen, Y.-J. High ERCC1 expression predicts cisplatin-based chemotherapy resistance and poor outcome in unresectable squamous cell carcinoma of head and neck in a betel-chewing area. J. Transl. Med. 2011, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Hannah, J.; Zhou, P. Regulation of DNA damage response pathways by the cullin-RING ubiquitin ligases. DNA Repair 2009, 8, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.M.; Carew, J.S.; Bauman, J.E.; Nawrocki, S.T. Targeting NEDDylation as a Novel Approach to Improve the Treatment of Head and Neck Cancer. Cancers 2021, 13, 3250. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.M.; Espitia, C.M.; Ooi, A.; Bauman, J.E.; Carew, J.S.; Nawrocki, S.T. Targeted CUL4A inhibition synergizes with cisplatin to yield long-term survival in models of head and neck squamous cell carcinoma through a DDB2-mediated mechanism. Cell Death Dis. 2022, 13, 350. [Google Scholar] [CrossRef]
- Spivak, G. Nucleotide excision repair in humans. DNA Repair 2015, 36, 13–18. [Google Scholar] [CrossRef]
- Vanderdys, V.; Allak, A.; Guessous, F.; Benamar, M.; Read, P.W.; Jameson, M.J.; Abbas, T. The Neddylation Inhibitor Pevonedistat (MLN4924) Suppresses and Radiosensitizes Head and Neck Squamous Carcinoma Cells and Tumors. Mol. Cancer Ther. 2018, 17, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Stavridi, E.S.; Halazonetis, T.D. Nbs1 moving up in the world. Nat. Cell Biol. 2005, 7, 648–650. [Google Scholar] [CrossRef]
- Beikzadeh, M.; Latham, M.P. The dynamic nature of the Mre11-Rad50 DNA break repair complex. Prog. Biophys. Mol. Biol. 2021, 163, 14–22. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Küster, B.; Mann, M.; Petrini JH, J.; Lange, T.D. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 2000, 25, 347–352. [Google Scholar] [CrossRef]
- Assenmacher, N.; Hopfner, K.-P. MRE11/RAD50/NBS1: Complex activities. Chromosoma 2004, 113, 157–166. [Google Scholar] [CrossRef]
- Abuzeid, W.M.; Jiang, X.; Shi, G.; Wang, H.; Paulson, D.; Araki, K.; Jungreis, D.; Carney, J.; O’malley, B.W.; Li, D. Molecular disruption of RAD50 sensitizes human tumor cells to cisplatin-based chemotherapy. J. Clin. Investig. 2009, 119, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Yamashita, T.; Reddy, N.; Wang, H.; Abuzeid, W.M.; Khan, K.; O'Malley, B.W.; Li, D. Molecular disruption of NBS1 with targeted gene delivery enhances chemosensitisation in head and neck cancer. Br. J. Cancer 2010, 103, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.G.; Li, D.; Suntharalingam, M.; Guo, C.; O’malley, B.W.; Carney, J.P. Radiosensitization of head/neck sqaumous cell carcinoma by adenovirus-mediated expression of the Nbs1 protein. Int. J. Radiat. Oncol. 2007, 67, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Ang, K.; Milas, L.; Hunter, N.; Fan, Z. The epidermal growth factor receptor mediates radioresistance. Int. J. Radiat. Oncol. 2003, 57, 246–254. [Google Scholar] [CrossRef]
- Ang, K.K.; Berkey, B.A.; Tu, X.; Zhang, H.-Z.; Katz, R.; Hammond, E.H.; Fu, K.K.; Milas, L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002, 62, 7350–7356. [Google Scholar]
- Matta, A.; Ralhan, R. Overview of current and future biologically based targeted therapies in head and neck squamous cell carcinoma. Head Neck Oncol. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Myllynen, L.; Rieckmann, T.; Dahm-Daphi, J.; Kasten-Pisula, U.; Petersen, C.; Dikomey, E.; Kriegs, M. In tumor cells regulation of DNA double strand break repair through EGF receptor involves both NHEJ and HR and is independent of p53 and K-Ras status. Radiother. Oncol. 2011, 101, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Kriegs, M.; Kasten-Pisula, U.; Rieckmann, T.; Holst, K.; Saker, J.; Dahm-Daphi, J.; Dikomey, E. The epidermal growth factor receptor modulates DNA double-strand break repair by regulating non-homologous end-joining. DNA Repair 2010, 9, 889–897. [Google Scholar] [CrossRef]
- Laban, S.; Steinmeister, L.; Gleißner, L.; Grob, T.J.; Grénman, R.; Petersen, C.; Gal, A.; Knecht, R.; Dikomey, E.; Kriegs, M. Sorafenib sensitizes head and neck squamous cell carcinoma cells to ionizing radiation. Radiother. Oncol. 2013, 109, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.K.; Moon, J.; Huang, C.H.; Guaglianone, P.P.; LeBlanc, M.; Wolf, G.T.; Urba, S.G. Phase II Evaluation of Sorafenib in Advanced and Metastatic Squamous Cell Carcinoma of the Head and Neck: Southwest Oncology Group Study S0420. J. Clin. Oncol. 2010, 28, 3330–3335. [Google Scholar] [CrossRef]
- Lalami, Y.; Garcia, C.; Flamen, P.; Ameye, L.; Paesmans, M.; Awada, A. Phase II trial evaluating the efficacy of sorafenib (BAY 43-9006) and correlating early fluorodeoxyglucose positron emission tomography–CT response to outcome in patients with recurrent and/or metastatic head and neck cancer. Head Neck 2016, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Suzuki, S.; Thomas, S.M.; Sen, M.; Leeman-Neill, R.J.; Chiosea, S.I.; Kuan, C.-T.; Bigner, D.D.; Gooding, W.E.; Lai, S.Y.; et al. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene 2010, 29, 5135–5145. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Mora, L.B.; Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 945–954. [Google Scholar]
- Real, P.J.; Sierra, A.; de Juan, A.; Segovia, J.C.; Lopez-Vega, J.M.; Fernandez-Luna, J.L. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene 2002, 21, 7611–7618. [Google Scholar] [CrossRef]
- Zhou, J.; Goh, B.-C.; Albert, D.H.; Chen, C.-S. ABT-869, a promising multi-targeted tyrosine kinase inhibitor: From bench to bedside. J. Hematol. Oncol. 2009, 2, 33. [Google Scholar] [CrossRef]
- Hsu, H.-W.; Gridley, D.S.; Kim, P.D.; Hu, S.; de Necochea-Campion, R.; Ferris, R.L.; Chen, C.-S.; Mirshahidi, S. Linifanib (ABT-869) enhances radiosensitivity of head and neck squamous cell carcinoma cells. Oral Oncol. 2013, 49, 591–597. [Google Scholar] [CrossRef]
- Wiechec, E.; Matic, N.; Ali, A.; Roberg, K. Hypoxia induces radioresistance, epithelial-mesenchymal transition, cancer stem cell-like phenotype and changes in genes possessing multiple biological functions in head and neck squamous cell carcinoma. Oncol. Rep. 2022, 47, 58. [Google Scholar] [CrossRef] [PubMed]
- Tonissi, F.; Lattanzio, L.; Astesana, V.; Cavicchioli, F.; Ghiglia, A.; Monteverde, M.; Vivenza, D.; Gianello, L.; Russi, E.; Merlano, M.; et al. Reoxygenation Reverses Hypoxia-related Radioresistance in Head and Neck Cancer Cell Lines. Anticancer Res. 2016, 36, 2211–2215. [Google Scholar]
- Sadri, N.; Zhang, P.J. Hypoxia-Inducible Factors: Mediators of Cancer Progression; Prognostic and Therapeutic Targets in Soft Tissue Sarcomas. Cancers 2013, 5, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Hassani, Z.M.; Nabiuni, M.; Parivar, K.; Abdirad, S.; Karimzadeh, L. Melittin inhibits the expression of key genes involved in tumor microenvironment formation by suppressing HIF-1α signaling in breast cancer cells. Med. Oncol. 2021, 38, 77. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, H.; Ge, Y.; Liu, J.; Cai, J.; Qin, Q.; Zhan, L.; Zhang, C.; Xu, L.; Liu, Z.; et al. Melittin enhances radiosensitivity of hypoxic head and neck squamous cell carcinoma by suppressing HIF-1α. Tumor Biol. 2014, 35, 10443–10448. [Google Scholar] [CrossRef]
- Yaromina, A.; Koi, L.; Schuitmaker, L.; van der Wiel, A.M.-M.A.; Dubois, L.J.; Krause, M.; Lambin, P. Overcoming radioresistance with the hypoxia-activated prodrug CP-506: A pre-clinical study of local tumour control probability. Radiother. Oncol. 2023, 186, 109738. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.-F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010, 12, R48. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Huang, D.; Ramsey, A.J.; Ig-Izevbekhai, K.; Zhang, K.; Lajud, S.A.; O’malley, B.W.; Li, D. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma. Br. J. Cancer 2020, 122, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; I Haddad, R.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.-H.; Lin, J.-C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Machiels, J.-P.H.; Licitra, L.F.; Tao, Y.; Yen, C.-J.; Rischin, D.; Waldron, J.; Burtness, B.; Gregoire, V.; Agarwala, S.S.; Yorio, J.; et al. Pembrolizumab plus chemoradiation vs chemoradiation alone for locally advanced head and neck squamous cell carcinoma: The phase 3 KEYNOTE-412 study. J. Clin. Oncol. 2018, 36, TPS6094. [Google Scholar] [CrossRef]
- Amaravadi, R.; Kimmelman, A.C.; White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016, 30, 1913–1930. [Google Scholar] [CrossRef]
- Runwal, G.; Stamatakou, E.; Siddiqi, F.H.; Puri, C.; Zhu, Y.; Rubinsztein, D.C. LC3-positive structures are prominent in autophagy-deficient cells. Sci. Rep. 2019, 9, 10147. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, W.; Zhu, L.; Yao, D.; Wang, C.; Song, Y.; Hu, S.; Liu, H.; Bai, Y.; Pan, Y.; et al. ANXA6 Contributes to Radioresistance by Promoting Autophagy via Inhibiting the PI3K/AKT/mTOR Signaling Pathway in Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2020, 8, 232. [Google Scholar] [CrossRef]
- Kumar, B.; Yadav, A.; Lang, J.C.; Cipolla, M.J.; Schmitt, A.C.; Arradaza, N.; Teknos, T.N.; Kumar, P. YM155 Reverses Cisplatin Resistance in Head and Neck Cancer by Decreasing Cytoplasmic Survivin Levels. Mol. Cancer Ther. 2012, 11, 1988–1998. [Google Scholar] [CrossRef]
- Tirrò, E.; Consoli, M.L.; Massimino, M.; Manzella, L.; Frasca, F.; Sciacca, L.; Vicari, L.; Stassi, G.; Messina, L.; Messina, A.; et al. Altered Expression of c-IAP1, Survivin, and Smac Contributes to Chemotherapy Resistance in Thyroid Cancer Cells. Cancer Res. 2006, 66, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kita, A.; Yamanaka, K.; Mori, M.; Amino, N.; Takeuchi, M.; Tominaga, F.; Hatakeyama, S.; Kinoyama, I.; Matsuhisa, A.; et al. YM155, a Novel Small-Molecule Survivin Suppressant, Induces Regression of Established Human Hormone-Refractory Prostate Tumor Xenografts. Cancer Res. 2007, 67, 8014–8021. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Mechanisms of Caspase Activation and Inhibition during Apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Tanimoto, T.; Tsuda, H.; Imazeki, N.; Ohno, Y.; Imoto, I.; Inazawa, J.; Matsubara, O. Nuclear expression of cIAP-1, an apoptosis inhibiting protein, predicts lymph node metastasis and poor patient prognosis in head and neck squamous cell carcinomas. Cancer Lett. 2005, 224, 141–151. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Blankenship, J.W.; Wayson, S.M.; Fedorova, A.V.; Kayagaki, N.; Garg, P.; Zobel, K.; Dynek, J.N.; Elliott, L.O.; Wallweber, H.J.A.; et al. IAP Antagonists Induce Autoubiquitination of c-IAPs, NF-κB Activation, and TNFα-Dependent Apoptosis. Cell 2007, 131, 669–681. [Google Scholar] [CrossRef]
- Vince, J.E.; Wong, W.W.-L.; Khan, N.; Feltham, R.; Chau, D.; Ahmed, A.U.; Benetatos, C.A.; Chunduru, S.K.; Condon, S.M.; McKinlay, M.; et al. IAP Antagonists Target cIAP1 to Induce TNFα-Dependent Apoptosis. Cell 2007, 131, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; McEachern, D.; Li, W.; Davis, M.A.; Li, H.; Morgan, M.A.; Bai, L.; Sebolt, J.T.; Sun, H.; Lawrence, T.S.; et al. Radiosensitization of Head and Neck Squamous Cell Carcinoma by a SMAC-Mimetic Compound, SM-164, Requires Activation of Caspases. Mol. Cancer Ther. 2011, 10, 658–669. [Google Scholar] [CrossRef]
- Gallo, O.; Chiarelli, I.; Boddi, V.; Bocciolini, C.; Bruschini, L.; Porfirio, B. Cumulative prognostic value ofp53 mutations and bcl-2 protein expression in head-and-neck cancer treated by radiotherapy. Int. J. Cancer 1999, 84, 573–579. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, X.-S.; Pointreau, Y.; Le Tourneau, C.; Sire, C.; Kaminsky, M.-C.; Coutte, A.; Alfonsi, M.; Calderon, B.; Boisselier, P.; et al. Extended follow-up of a phase 2 trial of xevinapant plus chemoradiotherapy in high-risk locally advanced squamous cell carcinoma of the head and neck: A randomised clinical trial. Eur. J. Cancer 2023, 183, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Daemen, A.; Hatzivassiliou, G.; Arnott, D.; Wilson, C.; Zhuang, G.; Gao, M.; Liu, P.; Boudreau, A.; Johnson, L.; et al. Metabolic and transcriptional profiling reveals pyruvate dehydrogenase kinase 4 as a mediator of epithelial-mesenchymal transition and drug resistance in tumor cells. Cancer Metab. 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Chien, C.-W.; Lin, S.-C.; Lee, C.-T.; Lin, B.-W.; Lee, J.-C.; Tsai, S.-J. Overexpression of Pyruvate Dehydrogenase Kinase 3 Increases Drug Resistance and Early Recurrence in Colon Cancer. Am. J. Pathol. 2011, 179, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, S.; Chang, S.S.; McFate, T.; Verma, A.; Califano, J.A. Mitochondrial Mutations Contribute to HIF1α Accumulation via Increased Reactive Oxygen Species and Up-regulated Pyruvate Dehydrogenease Kinase 2 in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2009, 15, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Sutendra, G.; Michelakis, E.D. Pyruvate dehydrogenase kinase as a novel therapeutic target in oncology. Front. Oncol. 2013, 3, 38. [Google Scholar] [CrossRef]
- Kankotia, S.; Stacpoole, P.W. Dichloroacetate and cancer: New home for an orphan drug? Biochim. Biophys. Acta BBA—Rev. Cancer 2014, 1846, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-L.; Park, J.Y.; Kim, E.H.; Jang, H.J.; Kwon, M. Activation of mitochondrial oxidation by PDK2 inhibition reverses cisplatin resistance in head and neck cancer. Cancer Lett. 2016, 371, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-M.; Manandhar, S.; Lee, H.-R.; Park, H.-M.; Kwak, M.-K. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: Implication to cancer cell resistance. Cancer Lett. 2008, 260, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Sun, Z.; Villeneuve, N.F.; Zhang, S.; Zhao, F.; Li, Y.; Chen, W.; Yi, X.; Zheng, W.; Wondrak, G.T.; et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008, 29, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, F.; Sun, Z.; Zhou, W.; Li, Z.-Y.; You, Q.-D.; Guo, Q.-L.; Hu, R. Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol. Carcinog. 2013, 52, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Wang, Y.; Zhong, Y.; Tang, J.; Zhang, J.; Li, Z.; Wang, Q.; Hu, R. Wogonin-enhanced reactive oxygen species-induced apoptosis and potentiated cytotoxic effects of chemotherapeutic agents by suppression Nrf2-mediated signaling in HepG2 cells. Free Radic. Res. 2014, 48, 607–621. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Yeh, W.-L.; Huang, S.M.; Tan, T.-W.; Lu, D.-Y. Wogonin Induces Reactive Oxygen Species Production and Cell Apoptosis in Human Glioma Cancer Cells. Int. J. Mol. Sci. 2012, 13, 9877–9892. [Google Scholar] [CrossRef]
- Kim, E.H.; Jang, H.; Shin, D.; Baek, S.H.; Roh, J.-L. Targeting Nrf2 with wogonin overcomes cisplatin resistance in head and neck cancer. Apoptosis 2016, 21, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, D.; Demuner, A.J.; Barbosa, L.C.; Csuk, R.; Heller, L. Hederagenin as a triterpene template for the development of new antitumor compounds. Eur. J. Med. Chem. 2015, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Baek, S.; Shin, D.; Lee, J.; Roh, J.-L. Hederagenin Induces Apoptosis in Cisplatin-Resistant Head and Neck Cancer Cells by Inhibiting the Nrf2-ARE Antioxidant Pathway. Oxid. Med. Cell. Longev. 2017, 2017, 5498908. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.-Z.; Qi, Q.; Tao, L.; Zhao, Q.; Mu, R.; Gu, H.-Y.; Wang, M.; Feng, X.; Guo, Q.-L. Macranthoside B, a hederagenin saponin extracted from Lonicera macranthoides and its anti-tumor activities in vitro and in vivo. Food Chem. Toxicol. 2009, 47, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Liu, Q.; Ho, I.H.; Wei, X.; Yin, T.; Zhan, Y.; Zhang, W.; Zhang, W.; Chen, B.; et al. Hederagenin potentiated cisplatin- and paclitaxel-mediated cytotoxicity by impairing autophagy in lung cancer cells. Cell Death Dis. 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Saga, R.; Ohuchi, K.; Kuwahara, Y.; Tomita, K.; Okumura, K.; Sato, T.; Fukumoto, M.; Tsuruga, E.; Hosokawa, Y. 4-Methylumebelliferone Enhances Radiosensitizing Effects of Radioresistant Oral Squamous Cell Carcinoma Cells via Hyaluronan Synthase 3 Suppression. Cells 2022, 11, 3780. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kar, M.; Roy, S.; Padhi, S.; Kumar, A.; Thakur, S.; Akhter, Y.; Gatto, G.; Banerjee, B. Inhibition of CD44 sensitizes cisplatin-resistance and affects Wnt/β-catenin signaling in HNSCC cells. Int. J. Biol. Macromol. 2020, 149, 501–512. [Google Scholar] [CrossRef]
- Yang, D.; Jia, Y.; Feng, L.; Ju, M.; Chen, K.; Dai, X.; Sun, M.; Yu, W.; Wang, H.; Fang, H. Efficacy and safety of the hyaluronic acid inhibitor Hymecromone for the treatment of COVID-19: Study protocol for a single-centre, randomized, controlled, Double-blind Clinical tria. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Malla, W.A.; Arora, R.; Khan, R.I.N.; Mahajan, S.; Tiwari, A.K. Apoptin as a Tumor-Specific Therapeutic Agent: Current Perspective on Mechanism of Action and Delivery Systems. Front. Cell Dev. Biol. 2020, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Schoop, R.A.L.; Verdegaal, E.M.E.; De Jong, R.J.B.; Noteborn, M.H.M. Apoptin Enhances Radiation-Induced Cell Death in Poorly Responding Head and Neck Squamous Cell Carcinoma Cells. Basic Clin. Pharmacol. Toxicol. 2010, 106, 130–134. [Google Scholar] [CrossRef]
- Bhat, A.H.; Ganguly, B.; Tiwari, A.K.; Das, A.K. Canine Parvovirus ns1 gene and Chicken Anemia vp3 gene induce partial oncolysis of Canine Transmissible Venereal Tumor. Sci. Rep. 2017, 7, 15419. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef] [PubMed]
- Khamis, A.; Gül, D.; Wandrey, M.; Lu, Q.; Knauer, S.K.; Reinhardt, C.; Strieth, S.; Hagemann, J.; Stauber, R.H. The Vitamin D Receptor–BIM Axis Overcomes Cisplatin Resistance in Head and Neck Cancer. Cancers 2022, 14, 5131. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, K.; Barsony, J. Retinoid X Receptor Dominates the Nuclear Import and Export of the Unliganded Vitamin D Receptor. Mol. Endocrinol. 2002, 16, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.; Zheng, Y.; Fournier, P.G.J.; Murthy, S.; John, S.; Schillo, S.; Dunstan, C.R.; Mohammad, K.S.; Zhou, H.; Seibel, M.J.; et al. The vitamin D receptor is involved in the regulation of human breast cancer cell growth via a ligand-independent function in cytoplasm. Oncotarget 2017, 8, 26687–26701. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.O.; Milan, T.M.; Bighetti-Trevisan, R.L.; Leopoldino, A.M.; de Almeida, L.O. Pharmacological inhibition of HDAC6 overcomes cisplatin chemoresistance by targeting cancer stem cells in oral squamous cell carcinoma. J. Oral Pathol. Med. 2022, 51, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, C.; Hassan, S.; Liu, X.; Song, F.; Chen, K.; Zhang, W.; Yang, J. Histone deacetylase 6 in cancer. J. Hematol. Oncol. 2018, 11, 111. [Google Scholar] [CrossRef]
- Wu, H.; Mu, X.; Liu, L.; Wu, H.; Hu, X.; Chen, L.; Liu, J.; Mu, Y.; Yuan, F.; Liu, W.; et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, N.; Zhang, Q.; Ding, G.; Yang, Z.; Zhang, Z. Serum microRNAs as potential new biomarkers for cisplatin resistance in gastric cancer patients. PeerJ 2020, 8, e8943. [Google Scholar] [CrossRef]
- Gosepath, E.M.; Eckstein, N.; Hamacher, A.; Servan, K.; von Jonquieres, G.; Lage, H.; Györffy, B.; Royer, H.D.; Kassack, M.U. Acquired cisplatin resistance in the head-neck cancer cell line Cal27 is associated with decreased DKK1 expression and can partially be reversed by overexpression of DKK1. Int. J. Cancer 2008, 123, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.P.; Gray, S.G.; Hoffmann, A.C.; Hilger, R.A.; Thomale, J.; O’flaherty, J.D.; Fennell, D.A.; Richard, D.; O’leary, J.J.; O’byrne, K.J. Generation and Characterisation of Cisplatin-Resistant Non-Small Cell Lung Cancer Cell Lines Displaying a Stem-Like Signature. PLoS ONE 2013, 8, e54193. [Google Scholar] [CrossRef]
- Siemer, S.; Bauer, T.A.; Scholz, P.; Breder, C.; Fenaroli, F.; Harms, G.; Dietrich, D.; Dietrich, J.; Rosenauer, C.; Barz, M.; et al. Targeting Cancer Chemotherapy Resistance by Precision Medicine-Driven Nanoparticle-Formulated Cisplatin. ACS Nano 2021, 15, 18541–18556. [Google Scholar] [CrossRef]
- Xiao, L.; Lan, X.; Shi, X.; Zhao, K.; Wang, D.; Wang, X.; Li, F.; Huang, H.; Liu, J. Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer. Cell Death Dis. 2017, 8, e2803. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ni, J.; Beretov, J.; Wasinger, V.C.; Wang, S.; Zhu, Y.; Graham, P.; Li, Y. Activation of the eIF2α/ATF4 axis drives triple-negative breast cancer radioresistance by promoting glutathione biosynthesis. Redox Biol. 2021, 43, 101993. [Google Scholar] [CrossRef]
- Fukuda, K.; Sakakura, C.; Miyagawa, K.; Kuriu, Y.; Kin, S.; Nakase, Y.; Hagiwara, A.; Mitsufuji, S.; Okazaki, Y.; Hayashizaki, Y.; et al. Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br. J. Cancer 2004, 91, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Zakelj, M.N.; Prevc, A.; Kranjc, S.; Cemazar, M.; Todorovic, V.; Savarin, M.; Scancar, J.; Kosjek, T.; Groselj, B.; Strojan, P.; et al. Electrochemotherapy of radioresistant head and neck squamous cell carcinoma cells and tumor xenografts. Oncol. Rep. 2019, 41, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wen, J.; Li, W.; Xue, J.; Zhang, Y.; Liu, H.; Han, J.; Ning, T.; Lu, Z. HSP90 promotes radioresistance of cervical cancer cells via reducing FBXO6-mediated CD147 polyubiquitination. Cancer Sci. 2022, 113, 1463–1474. [Google Scholar] [CrossRef]
- Wang, T.; Tamae, D.; LeBon, T.; Shively, J.E.; Yen, Y.; Li, J.J. The Role of Peroxiredoxin II in Radiation-Resistant MCF-7 Breast Cancer Cells. Cancer Res. 2005, 65, 10338–10346. [Google Scholar] [CrossRef]
- Liu, C.; Liao, K.; Gross, N.; Wang, Z.; Li, G.; Zuo, W.; Zhong, S.; Zhang, Z.; Zhang, H.; Yang, J.; et al. Homologous recombination enhances radioresistance in hypopharyngeal cancer cell line by targeting DNA damage response. Oral Oncol. 2020, 100, 104469. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Lee, M.-G.; Baek, J.; Kim, N.-Y.; Jang, H.; Kim, S. Acyl-CoA synthetase-4 mediates radioresistance of breast cancer cells by regulating FOXM1. Biochem. Pharmacol. 2021, 192, 114718. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Li, Y.; Lu, T.; Hu, G. Silencing Snail Reverses Epithelial-Mesenchymal Transition and Increases Radiosensitivity in Hypopharyngeal Carcinoma. OncoTargets Ther. 2020, 13, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Hagege, A.; Ambrosetti, D.; Boyer, J.; Bozec, A.; Doyen, J.; Chamorey, E.; He, X.; Bourget, I.; Rousset, J.; Saada, E.; et al. The Polo-like kinase 1 inhibitor onvansertib represents a relevant treatment for head and neck squamous cell carcinoma resistant to cisplatin and radiotherapy. Theranostics 2021, 11, 9571–9586. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Bakker, T.; Maes, A.; Dragan, T.; Martinive, P.; Penninckx, S.; Van Gestel, D. Strategies to Overcome Intrinsic and Acquired Resistance to Chemoradiotherapy in Head and Neck Cancer. Cells 2025, 14, 18. https://doi.org/10.3390/cells14010018
de Bakker T, Maes A, Dragan T, Martinive P, Penninckx S, Van Gestel D. Strategies to Overcome Intrinsic and Acquired Resistance to Chemoradiotherapy in Head and Neck Cancer. Cells. 2025; 14(1):18. https://doi.org/10.3390/cells14010018
Chicago/Turabian Stylede Bakker, Tycho, Anouk Maes, Tatiana Dragan, Philippe Martinive, Sébastien Penninckx, and Dirk Van Gestel. 2025. "Strategies to Overcome Intrinsic and Acquired Resistance to Chemoradiotherapy in Head and Neck Cancer" Cells 14, no. 1: 18. https://doi.org/10.3390/cells14010018
APA Stylede Bakker, T., Maes, A., Dragan, T., Martinive, P., Penninckx, S., & Van Gestel, D. (2025). Strategies to Overcome Intrinsic and Acquired Resistance to Chemoradiotherapy in Head and Neck Cancer. Cells, 14(1), 18. https://doi.org/10.3390/cells14010018






