The Role of Cytokines in Cutaneous T Cell Lymphoma: A Focus on the State of the Art and Possible Therapeutic Targets
Abstract
1. Introduction
2. IL-1
IL-1 in MF/SS
3. IL-2
IL-2 in MF/SS
4. IL-3
IL-3 in MF/SS
5. IL-4
IL-4 in MF/SS
6. IL-5
IL-5 in MF/SS
7. IL-6
IL-6 in MF/SS
8. IL-7
IL-7 in MF/SS
9. IL-8
10. IL-9
IL-9 in MF/SS
11. IL-10
IL-10 in MF/SS
12. IL-11
IL-11 in MF/SS
13. IL-12
Il-12 in MF/SS
14. IL-13
IL-13 in MF/SS
15. IL-14
IL-14 and MF/SS
16. IL-15 and IL-17
IL-15 and IL-17 in MF/SS
17. IL-16
IL-16 and MF/SS
18. IL-18
IL-18 in MF/SS
19. IL-19, IL-20, IL-22, IL-24, and IL-26
IL-19, IL-20, IL-22, IL-24, and IL-26 in MF/SS
20. IL-21
IL-21 in MF/SS
21. IL-22
22. IL-23
IL-23 in MF/SS
23. IL-24
24. IL-25
IL-25 in MF/SS
25. IL-26
26. IL-27
IL-27 in MF/SS
27. IL-28 and IL-29
28. IL-30
IL-30 in MF/SS
29. IL-31 and IL-8
IL-31 and IL-8 in MF/SS
30. IL-32
IL-32 in MF/SS
31. IL-33
IL-33 in MF/SS
32. TNF-α
TNF-α in MF/SS
33. EGF
EGF in MF/SS
34. FGF
FGF in MF/SS
35. PDGR
PDGRα in MF/SS
36. Interferon Type I, Type II, and Type III
Interferon Type I, Type II, and Type III in MF/SS
37. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-Presenting Cell |
| CD | Cluster of Differentiation |
| CCL | C-C Motif Chemokine Ligand |
| CCR | C-C Motif Chemokine Receptor |
| CTCL | Cutaneous Cutaneous T cell lymphoma |
| CXCL | C-X-C Motif Chemokine Ligand |
| EGF | Epdermal Growth Gactors |
| FGF | Fibroblast Growth Factors |
| JAK | JAnus Kinase |
| GAB | GRB2-associated-binding protein |
| HDAC | Histone Deacetylases |
| HMGB | High Mobility Group Box protein |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IFN | Interferon |
| IL | InterLeukin |
| IP | Interferon-Inducible Protein |
| IT | ImmunoToxin |
| LDH | Lactate DeHydrogenase |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA7 | Melanoma Differentiation-Associated protein 7 |
| MF | Mycosis fungoides |
| nbUVB | narrow band Ultra Violet type B |
| NOS | Nitrogen Oxygen Species |
| PBMC | Peripheral Blood Mononuclear Cell |
| PI3Ks | Phosphoinositide 3-kinase inhibitors |
| PDGF | Platelet-Derived Growth Factors |
| PUVA | Psoralen and Ultra Violet type A |
| ROS | Reactive Oxygen Species |
| SATB1 | Special AT-rich sequence-Binding protein-1 |
| STAT | Signal Transducer and Activator of Transcription |
| SS | Sézary syndrome |
| TAM | Tumor-Associated Macrophage |
| TCGF | T cell growth factor |
| Tfh | T follicular helper cell |
| TGF | Transforming Growth Factor |
| TNF | Tumor Necrosis Factor |
| Th | (Lymphocyte) T helper (Cell) |
| T-Regs | (lymphocyte) T-regulatory cells |
| NK | Natural Killer (cell) |
| γc | Common gamma chain |
References
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Pileri, A.; Patrizi, A.; Agostinelli, C.; Neri, I.; Sabattini, E.; Bacci, F.; Piccaluga, P.P.; Pimpinelli, N.; Pileri, S.A. Primary cutaneous lymphomas: A reprisal. Semin. Diagn. Pathol. 2011, 28, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Criscione, V.D.; Weinstock, M.A. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch. Dermatol. 2007, 143, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Dobos, G.; de Masson, A.; Ram-Wolff, C.; Beylot-Barry, M.; Pham-Ledard, A.; Ortonne, N.; Ingen-Housz-Oro, S.; Battistella, M.; d’Incan, M.; Rouanet, J.; et al. Epidemiological changes in cutaneous lymphomas: An analysis of 8593 patients from the French Cutaneous Lymphoma Registry. Br. J. Dermatol. 2021, 184, 1059–1067. [Google Scholar] [CrossRef]
- Pileri, A.; Morsia, E.; Zengarini, C.; Torre, E.; Goteri, G.; Quaglino, P.; Pimpinelli, N.; Paulli, M.; A Pileri, S.; Zinzani, P.L.; et al. Epidemiology of cutaneous T-cell lymphomas: State of the art and a focus on the Italian Marche region. Eur. J. Dermatol. 2023, 33, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Kayishunge, D.; Ly, S.; Su, J.; Wong, H.K. Epidemiologic Trends of Cutaneous T-Cell Lymphoma in Arkansas Reveals Demographic Disparities. Cancers 2022, 14, 4329. [Google Scholar] [CrossRef]
- Miyashiro, D.; Sanches, J.A. Mycosis fungoides and Sézary syndrome: Clinical presentation, diagnosis, staging, and therapeutic management. Front. Oncol. 2023, 13, 1141108. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Duvic, M. Cutaneous T-Cell Lymphoma: Current and Emerging Therapies. Oncology 2023, 37, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wojewoda, K.; Gillstedt, M.; Englund, H.; Ali, S.; Lewerin, C.; Osmancevic, A. Diagnostic Outcomes and Treatment Modalities in Patients with Mycosis Fungoides in West Sweden—A Retrospective Register-Based Study. Cancers 2022, 14, 4661. [Google Scholar] [CrossRef]
- Pileri, A.; Guglielmo, A.; Grandi, V.; Violetti, S.A.; Fanoni, D.; Fava, P.; Agostinelli, C.; Berti, E.; Quaglino, P.; Pimpinelli, N. The Microenvironment’s Role in Mycosis Fungoides and Sézary Syndrome: From Progression to Therapeutic Implications. Cells 2021, 10, 2780. [Google Scholar] [CrossRef] [PubMed]
- Alberti-Violetti, S.; Sapienza, M.R.; Del Corvo, M.; Melle, F.; Motta, G.; Venegoni, L.; Cerroni, L.; Cota, C.; Pileri, A.; Berti, E.; et al. A Microenvironment-Related Nine-Gene Signature May Predict Survival in Mycosis Fungoides Patients at Diagnosis. Cells 2023, 12, 1944. [Google Scholar] [CrossRef] [PubMed]
- Dobos, G.; Lazaridou, I.; de Masson, A. Mycosis Fungoides and Sézary Syndrome: Microenvironment and Cancer Progression. Cancers 2023, 15, 746. [Google Scholar] [CrossRef]
- Johnson, V.E.; Vonderheid, E.C.; Hess, A.D.; Eischen, C.M.; McGirt, L.Y. Genetic Markers Associated with Progression in Early Mycosis Fungoides. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Wobser, M.; Roth, S.; Appenzeller, S.; Houben, R.; Schrama, D.; Goebeler, M.; Geissinger, E.; Rosenwald, A.; Maurus, K. Targeted Deep Sequencing of Mycosis Fungoides Reveals Intracellular Signaling Pathways Associated with Aggressiveness and Large Cell Transformation. Cancers 2021, 13, 5512. [Google Scholar] [CrossRef]
- McGirt, L.Y.; Jia, P.; Baerenwald, D.A.; Duszynski, R.J.; Dahlman, K.B.; Zic, J.A.; Zwerner, J.P.; Hucks, D.; Dave, U.; Zhao, Z.; et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood 2015, 126, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Shi, H.; Zhang, Y.; Sun, J.; Chen, H. Proteomic Screening and Verification of Biomarkers in Different Stages of Mycosis Fungoides: A pilot Study. Front. Cell Dev. Biol. 2021, 9, 747017. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Wautier, J.-L.; Wautier, M.-P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A Mini Review. Int. J. Mol. Sci. 2023, 24, 9647. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Schlaepfer, T.; Ignatova, D.; Chang, Y.-T.; Valaperti, A.; Amarov, B.; Blanchard, G.; Pehr, K.; Vonow-Eisenring, M.; Urosevic-Maiwald, M.; et al. Boost of innate immunity cytokines as biomarkers of response to extracorporeal photopheresis in patients with leukaemic cutaneous T-cell lymphoma. Br. J. Dermatol. 2023, 189, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, S.L.; Wolfe, J.T.; Fox, F.E.; DeNardo, B.J.; Macey, W.H.; Bromley, P.G.; Lessin, S.R.; Rook, A.H. Treatment of cutaneous T-cell lymphoma with extracorporeal photopheresis monotherapy and in combination with recombinant interferon alfa: A 10-year experience at a single institution. J. Am. Acad. Dermatol. 1996, 35, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Malek, T.R.; Bayer, A.L. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 2004, 4, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Brockdorff, J.L.; Gu, H.; Mustelin, T.; Kaltoft, K.; Geisler, C.; Röpke, C.; Ødum, N. Gab2 is phosphorylated on tyrosine upon interleukin-2/interleukin-15 stimulation in mycosis-fungoides-derived tumor T cells and associates inducibly with SHP-2 and Stat5a. Exp. Clin. Immunogenet. 2001, 18, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Shohat, M.; Hodak, E.; Sredni, B.; Shohat, B.; Sredni, D.; David, M. Cytokine profile of patients with mycosis fungoides and the immunomodulatory effect of AS101. Acta Derm. Venereol. 2001, 81, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Kozenitzky, L.; David, M.; Sredai, B.; Albeck, M.; Shohat, B. Immunomodulatory effects of AS101 on interleukin-2 production and T-lymphocyte function of lymphocytes treated with psoralens and ultraviolet A. Photodermatol. Photoimmunol. Photomed. 1992, 9, 24–28. [Google Scholar] [PubMed]
- Foss, F.M. Interleukin-2 Fusion Toxin: Targeted Therapy for Cutaneous T Cell Lymphoma. Ann. N. Y. Acad. Sci. 2001, 941, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, J.; Zhang, H.; Ramakrishna, R.; Mintzlaff, D.; Mathes, D.W.; Pomfret, E.A.; Lucia, M.S.; Gao, D.; Haverkos, B.M.; et al. CCR4-IL2 bispecific immunotoxin is more effective than brentuximab for targeted therapy of cutaneous T-cell lymphoma in a mouse CTCL model. FEBS Open Bio 2023, 13, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, V.; Toonstra, J.; Bihari, I.C.; Bruijnzeel-Koomen, C.A.; van Vloten, W.A.; Thepen, T. Interleukin 4 and interferon-gamma expression of the dermal infiltrate in patients with erythroderma and mycosis fungoides. An immuno-histochemical study. J. Cutan. Pathol. 2000, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Noben-Trauth, N.; Paul, W.E.; Sacks, D.L. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 1999, 162, 6132–6140. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Saulite, I.; Ignatova, D.; Chang, Y.-T.; Fassnacht, C.; Dimitriou, F.; Varypataki, E.; Anzengruber, F.; Nägeli, M.; Cozzio, A.; Dummer, R.; et al. Blockade of programmed cell death protein 1 (PD-1) in Sézary syndrome reduces Th2 phenotype of non-tumoral T lymphocytes but may enhance tumor proliferation. OncoImmunology 2020, 9, 1738797. [Google Scholar] [CrossRef]
- Ritlecitinib in CTCL. Available online: https://ctv.veeva.com/study/ritlecitinib-in-ctcl (accessed on 10 January 2024).
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Buffon, S.; Alberti Violetti, S.; Avallone, G.; Venegoni, L.; Marzano, A.V.; Mastorino, L.; Fava, P.; Ribero, S.; Quaglino, P.; Ortoncelli, M.; et al. Mycosis fungoides and Sézary syndrome following dupilumab treatment: Experience of two Italian tertiary care centres. Clin. Exp. Dermatol. 2023, 48, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Chatila, T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy 2020, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Nissen, M.H.; Gerwien, J.; Zocca, M.-B.; Rasmussen, H.M.; Nakajima, K.; Röpke, C.; Geisler, C.; Kaltoft, K.; Ødum, N. Spontaneous interleukin-5 production in cutaneous T-cell lymphoma lines is mediated by constitutively activated Stat3. Blood 2002, 99, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Katayama, I.; Nishioka, K. Expression of stem cell factor in the lesional skin of systemic sclerosis. Dermatology 1998, 197, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Whittaker, S.; Smith, N.; Vora, A.J.; Dumonde, D.C.; Brown, K.A. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin. Exp. Immunol. 1996, 105, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kadin, M.E.; Pavlov, I.Y.; Delgado, J.C.; Vonderheid, E.C. High soluble CD30, CD25, and IL-6 may identify patients with worse survival in CD30+ cutaneous lymphomas and early mycosis fungoides. J. Investig. Dermatol. 2012, 132, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Kadin, M.E. What Cytokines Can Tell Us About the Pathogenesis of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthetic Surg. J. 2019, 39, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, B.; Gleń, J.; Zabłotna, M.; Nowicki, R.J.; Sokołowska-Wojdyło, M. The polymorphisms of IL-6/STAT3 signaling pathway may contribute to cutaneous T-cell lymphomas susceptibility. Arch. Dermatol. Res. 2021, 313, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Asao, H.; Okuyama, C.; Kumaki, S.; Ishii, N.; Tsuchiya, S.; Foster, D.; Sugamura, K. Cutting Edge: The Common γ-Chain Is an Indispensable Subunit of the IL-21 Receptor Complex1. J. Immunol. 2001, 167, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Winer, H.; Rodrigues, G.O.L.; Hixon, J.A.; Aiello, F.B.; Hsu, T.C.; Wachter, B.T.; Li, W.; Durum, S.K. IL-7: Comprehensive review. Cytokine 2022, 160, 156049. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Haeussler, A.; Friedrich, M.; Siegling, A.; Olaizola-Horn, S.; Trefzer, U.; Volk, H.D.; Sterry, W. IL-7 mRNA is not overexpressed in mycosis fungoides and pleomorphic T-cell lymphoma and is likely to be an autocrine growth factor in vivo. Arch. Dermatol. Res. 1996, 289, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kimata, H.; Lindley, I. Detection of plasma interleukin-8 in atopic dermatitis. Arch. Dis. Child. 1994, 70, 119–122. [Google Scholar] [CrossRef]
- Abreu, M.; Miranda, M.; Castro, M.; Fernandes, I.; Cabral, R.; Santos, A.H.; Fonseca, S.; Rodrigues, J.; Leander, M.; Lau, C.; et al. IL-31 and IL-8 in Cutaneous T-Cell Lymphoma: Looking for Their Role in Itch. Adv. Hematol. 2021, 2021, e5582581. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Zuleta, W.G.; Sanchez, E. IL-9: Function, Sources, and Detection. Methods Mol. Biol. 2017, 1585, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Fredholm, S.; Cheng, A.; Mimitou, E.P.; Seffens, A.; Bar-Natan, M.; Sun, A.; Latkowski, J.-A.; Willerslew-Olsen, A.; Buus, T.B.; et al. Low SATB1 Expression Promotes IL-5 and IL-9 Expression in Sézary Syndrome. J. Investig. Dermatol. 2020, 140, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Vieyra-Garcia, P.A.; Wei, T.; Naym, D.G.; Fredholm, S.; Fink-Puches, R.; Cerroni, L.; Odum, N.; O’Malley, J.T.; Gniadecki, R.; Wolf, P. STAT3/5-Dependent IL9 Overexpression Contributes to Neoplastic Cell Survival in Mycosis Fungoides. Clin. Cancer Res. 2016, 22, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, B.; Sun, A.; Guo, F. Interleukin-10 Family Cytokines Immunobiology and Structure. Adv. Exp. Med. Biol. 2019, 1172, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.; Steinke, J.W.; Borish, L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J. Allergy Clin. Immunol. 2008, 121, 1108–1111. [Google Scholar] [CrossRef]
- Akatsuka, T.; Miyagaki, T.; Nakajima, R.; Kamijo, H.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Asano, Y.; Sugaya, M.; et al. Decreased IL-10-producing regulatory B cells in patients with advanced mycosis fungoides. Eur. J. Dermatol. EJD 2018, 28, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Tiffon, C.; Adams, J.; van der Fits, L.; Wen, S.; Townsend, P.; Ganesan, A.; Hodges, E.; Vermeer, M.; Packham, G. The histone deacetylase inhibitors vorinostat and romidepsin downmodulate IL-10 expression in cutaneous T-cell lymphoma cells. Br. J. Pharmacol. 2011, 162, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-P.; Poltoratsky, V.; Vancurova, I. Bortezomib Inhibits Expression of TGFβ1, IL-10, and CXCR4, Resulting in Decreased Survival and Migration of Cutaneous T Cell Lymphoma Cells. J. Immunol. 2015, 194, 2942–2953. [Google Scholar] [CrossRef]
- Fung, K.Y.; Louis, C.; Metcalfe, R.D.; Kosasih, C.C.; Wicks, I.P.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for IL-11 in inflammatory diseases. Cytokine 2022, 149, 155750. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Rook, A.H.; Kubin, M.; Cassin, M.; Vonderheid, E.C.; Vowels, B.R.; Wolfe, J.T.; Wolf, S.F.; Singh, A.; Trinchieri, G.; Lessin, S.R. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J. Immunol. 1995, 154, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Duvic, M.; Sherman, M.L.; Wood, G.S.; Kuzel, T.M.; Olsen, E.; Foss, F.; Laliberté, R.J.; Ryan, J.L.; Zonno, K.; Rook, A.H. A phase II open-label study of recombinant human interleukin-12 in patients with stage IA, IB, or IIA mycosis fungoides. J. Am. Acad. Dermatol. 2006, 55, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.M.; McIntire, J.J.; Berry, G.; McKenzie, A.N.; Donaldson, D.D.; DeKruyff, R.H.; Umetsu, D.T. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J. Immunol. 2001, 167, 4668–4675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Zhang, M.; Li, Z. IL-13 and IL-13Rα1 are overexpressed in extranodal natural killer/T cell lymphoma and mediate tumor cell proliferation. Biochem. Biophys. Res. Commun. 2018, 503, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Kemp, E.H.; Ajjan, R.A.; Metcalfe, R.A.; Watson, P.F.; Weetman, A.P. IL-14 and IL-16 are expressed in the thyroid of patients with either Graves’ disease or Hashimoto’s thyroiditis. Clin. Endocrinol. 2015, 83, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Liu, X.; Kasprzycka, M.; Witkiewicz, A.; Raghunath, P.N.; El-Salem, M.; Robertson, E.; Odum, N.; Wasik, M.A. IL-2- and IL-15-induced activation of the rapamycin-sensitive mTORC1 pathway in malignant CD4+ T lymphocytes. Blood 2008, 111, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Willerslev-Olsen, A.; Litvinov, I.V.; Fredholm, S.M.; Petersen, D.L.; Sibbesen, N.A.; Gniadecki, R.; Zhang, Q.; Bonefeld, C.M.; Wasik, M.A.; Geisler, C.; et al. IL-15 and IL-17F are differentially regulated and expressed in mycosis fungoides (MF). Cell Cycle 2014, 13, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Haeussler-Quade, A.; Gellrich, S.; Hanneken, S.; Hansen-Hagge, T.E.; Döcke, W.D.; Volk, H.D.; Sterry, W. IL-15 and IL-16 overexpression in cutaneous T-cell lymphomas: Stage-dependent increase in mycosis fungoides progression. Exp. Dermatol. 2000, 9, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Bae, S.-C. Histone deacetylase inhibitors: Molecular mechanisms of action and clinical trials as anti-cancer drugs. Am. J. Transl. Res. 2011, 3, 166–179. [Google Scholar] [PubMed]
- Mishra, A.; La Perle, K.; Kwiatkowski, S.; Sullivan, L.A.; Sams, G.H.; Johns, J.; Curphey, D.P.; Wen, J.; McConnell, K.; Qi, J.; et al. Mechanism, Consequences, and Therapeutic Targeting of Abnormal IL15 Signaling in Cutaneous T-cell Lymphoma. Cancer Discov. 2016, 6, 986–1005. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.; Tuzova, M.; Cruikshank, W.; Center, D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J. Cell Physiol. 2014, 229, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kołkowski, K.; Gleń, J.; Olszewska, B.; Zabłotna, M.; Nowicki, R.J.; Sokołowska-Wojdyło, M. Interleukin-17 Genes Polymorphisms are Significantly Associated with Cutaneous T-cell Lymphoma Susceptibility. Acta Derm. Venereol. 2022, 102, 2416. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Manfrere, K.C.G.; Torrealba, M.P.; Ferreira, F.M.; de Sousa, E.S.A.; Miyashiro, D.; Teixeira, F.M.E.; Custódio, R.W.A.; Nakaya, H.I.; Ramos, Y.A.L.; Sotto, M.N.; et al. Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome. Int. J. Mol. Sci. 2023, 24, 4674. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Frydas, S.; Kandere, K.; Madhappan, B.; Letourneau, R.; Christodoulou, S.; Boucher, W.; Riccioni, G.; Conti, P.; Theoharides, T.C. Interleukin-19 (IL-19) network revisited. Int. J. Immunopathol. Pharmacol. 2003, 16, 95–97. [Google Scholar] [CrossRef]
- Senda, N.; Miyagaki, T.; Kamijo, H.; Nakajima, R.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Asano, Y.; Sugaya, M.; et al. Increased HMGB1 levels in lesional skin and sera in patients with cutaneous T-cell lymphoma. Eur. J. Dermatol. 2018, 28, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; Wan, C.-K. IL-21 Signaling in Immunity. F1000Research 2016, 5, 224. [Google Scholar] [CrossRef]
- Aulasevich, N.; Haist, M.; Försch, S.; Weidenthaler-Barth, B.; Mailänder, V. The Role of the Immune Phenotype in Tumor Progression and Prognosis of Patients with Mycosis Fungoides: A Quantitative Immunohistology Whole Slide Approach. Cells 2022, 11, 3570. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.-C. Interleukin-23: A key cytokine in inflammatory diseases. Ann. Med. 2011, 43, 503–511. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, Z.; Shangguan, G.; Bie, Q.; Zhang, B. Biological Properties and the Role of IL-25 in Disease Pathogenesis. J. Immunol. Res. 2018, 2018, 6519465. [Google Scholar] [CrossRef] [PubMed]
- Kourko, O.; Seaver, K.; Odoardi, N.; Basta, S.; Gee, K. IL-27, IL-30, and IL-35: A Cytokine Triumvirate in Cancer. Front. Oncol. 2019, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Yoshimoto, T.; Yasuda, K.; Mizuguchi, J.; Nakanishi, K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: A novel therapeutic way for Th2-mediated allergic inflammation. J. Immunol. 2007, 179, 4415–4423. [Google Scholar] [CrossRef]
- Singer, E.M.; Shin, D.B.; Nattkemper, L.A.; Benoit, B.M.; Klein, R.S.; Didigu, C.A.; Loren, A.W.; Dentchev, T.; Wysocka, M.; Yosipovitch, G.; et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J. Investig. Dermatol. 2013, 133, 2783–2785. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Corcione, A.; Pistoia, V. The IL-31/IL-31 receptor axis: General features and role in tumor microenvironment. J. Leukoc. Biol. 2017, 102, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Gleń, J.; Rębała, K.; Kowalczyk, A.; Sobjanek, M.; Nowicki, R.; Ruckemann-Dziurdzińska, K.; Sokołowska-Wojdyło, M. Il-31 does not correlate to pruritus related to early stage cutaneous T-cell lymphomas but is involved in pathogenesis of the disease. Acta Derm. Venereol. 2015, 95, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.K.; Smith, N.P.; Essien, S.V.; Teague, J.E.; Vieyra-Garcia, P.; Gehad, A.; Zhan, Q.; Crouch, J.D.; Gerard, N.; Larocca, C.; et al. IL-32 Supports the Survival of Malignant T Cells in Cutaneous T-cell Lymphoma. J. Investig. Dermatol. 2022, 142, 2285–2288.e2. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Stolarski, B.; Kewin, P.; Murphy, G.; Corrigan, C.; Ying, S.; Pitman, N.; Mirchandani, A.; Rana, B.; Van Rooijen, N.; et al. IL-33 Amplifies the Polarization of Alternatively Activated Macrophages That Contribute to Airway Inflammation1. J. Immunol. 2009, 183, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Meephansan, J.; Tsuda, H.; Komine, M.; Tominaga, S.-I.; Ohtsuki, M. Regulation of IL-33 expression by IFN-γ and tumor necrosis factor-α in normal human epidermal keratinocytes. J. Investig. Dermatol. 2012, 132, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.F.; Riether, C.; Schürch, C.M.; Banz, Y.; Wasmer, M.-H.; Stuber, R.; Theocharides, A.P.; Li, X.; Xia, Y.; Saito, H.; et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J. Clin. Investig. 2015, 125, 2579–2591. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Rodriguez Perez, C.E.; Nie, W.; Edwards, R.A.; Sinnett-Smith, J.; Rozengurt, E. TNF-α induces upregulation of EGFR expression and signaling in human colonic myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G805–G814. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: A novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H.R.; Smith, M.P.; Francavilla, C. Fibroblast Growth Factor Receptors (FGFRs) and Noncanonical Partners in Cancer Signaling. Cells 2021, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, S.; Ishii, Y.; Hamashima, T.; Yamamoto, S.; Mori, H.; Fujimori, T.; Shen, J.; Inoue, R.; Nishizono, H.; Itoh, H.; et al. PDGFRα plays a crucial role in connective tissue remodeling. Sci. Rep. 2015, 5, 17948. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Vieyra-Garcia, P.; Benezeder, T.; Crouch, J.D.; Kim, I.R.; O’Malley, J.T.; Devlin, P.M.; Gehad, A.; Zhan, Q.; Gudjonsson, J.E.; et al. Phototherapy Restores Deficient Type I IFN Production and Enhances Antitumor Responses in Mycosis Fungoides. J. Investig. Dermatol. 2023, 144, 621–632.E1. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Uzé, G.; Monneron, D. IL-28 and IL-29: Newcomers to the interferon family. Biochimie 2007, 89, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Tensen, C.P.; Vermeer, M.H.; van der Stoop, P.M.; van Beek, P.; Scheper, R.J.; Boorsma, D.M.; Willemze, R. Epidermal Interferon-γ Inducible Protein-10 (IP-10) and Monokine Induced by γ-Interferon (Mig) but not IL-8 mRNA Expression is Associated with Epidermotropism in Cutaneous T Cell Lymphomas. J. Investig. Dermatol. 1998, 111, 222–226. [Google Scholar] [CrossRef] [PubMed]
- García-Vega, Y.; García-García, I.; Collazo-Caballero, S.E.; Santely-Pravia, E.E.; Cruz-Ramírez, A.; Tuero-Iglesias, A.D.; Alfonso-Alvarado, C.; Cabrera-Placeres, M.; Castro-Basart, N.; Duncan-Roberts, Y.; et al. Pharmacokinetic and pharmacodynamic characterization of a new formulation containing synergistic proportions of interferons alpha-2b and gamma (HeberPAG) in patients with mycosis fungoides: An open-label trial. BMC Pharmacol. Toxicol. 2012, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.; Tokura, Y.; Matsumoto, K.; Furukawa, F.; Takigawa, M. Tumour-specific cytotoxic T lymphocyte activity in Th2-type Sézary syndrome: Its enhancement by interferon-gamma (IFN-gamma) and IL-12 and fluctuations in association with disease activity. Clin. Exp. Immunol. 1998, 112, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Fu, S.; Zhang, J.; Liu, B.; Li, Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci. Rep. 2016, 6, 36107. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, F.; Smith, N.P.; Camp, R.D.; Bacon, K.B.; Black, A.K.; Greaves, M.W.; Gearing, A.J. Skin exudate levels of interleukin 6, interleukin i and other cytokines in mycosis fungoides. Br. J. Dermatol. 1990, 123, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Shiue, L.; Park, K.; Kollet, J.; Bijani, P.; Goswami, M.; Duvic, M.; Ni, X. Blood transcriptional profiling reveals IL-1 and integrin signaling pathways associated with clinical response to extracorporeal photopheresis in patients with leukemic cutaneous T-cell lymphoma. Oncotarget 2019, 10, 3183–3197. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Flower, R.; Han, P. Surface and intracellular interleukin-2 receptor expression on various resting and activated populations involved in cell-mediated immunity in human peripheral blood. Scand. J. Immunol. 2000, 51, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Refaeli, Y.; Van Parijs, L.; London, C.A.; Tschopp, J.; Abbas, A.K. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 1998, 8, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Rybojad, M.; Marolleau, J.P.; Flageul, B.; Baccard, M.; Brandely, M.; Morel, P.; Gisselbrecht, C. Successful interleukin-2 therapy of advanced cutaneous T-cell lymphoma. Br. J. Dermatol. 1992, 127, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Baccard, M.; Marolleau, J.P.; Rybojad, M. Middle-Term Evolution of Patients with Advanced Cutaneous T-Cell Lymphoma Treated with High-Dose Recombinant Interleukin-2. Arch. Dermatol. 1997, 133, 656. [Google Scholar] [CrossRef] [PubMed]
- Nagatani, T.; Kin, S.T.; Baba, N.; Miyamoto, H.; Nakajima, H.; Katoh, Y. A case of cutaneous T cell lymphoma treated with recombinant interleukin 2 (rIL-2). Acta Derm. Venereol. 1988, 68, 504–508. [Google Scholar]
- Querfeld, C.; Rosen, S.T.; Guitart, J.; Rademaker, A.; Foss, F.; Gupta, R.; Kuzel, T.M. Phase II trial of subcutaneous injections of human recombinant interleukin-2 for the treatment of mycosis fungoides and Sézary syndrome. J. Am. Acad. Dermatol. 2007, 56, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Figgitt, D.P.; Lamb, H.M.; Goa, K.L. Denileukin diftitox. Am. J. Clin. Dermatol. 2000, 1, 67–72; discussion 73. [Google Scholar] [CrossRef]
- Kaminetzky, D.; Hymes, K.B. Denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Biologics 2008, 2, 717–724. [Google Scholar] [PubMed]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the brain: A cytokine to remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Huanosta-Murillo, E.; Alcántara-Hernández, M.; Hernández-Rico, B.; Victoria-Acosta, G.; Miranda-Cruz, P.; Domínguez-Gómez, M.A.; Jurado-Santacruz, F.; Patiño-López, G.; Pérez-Koldenkova, V.; Palma-Guzmán, A.; et al. NLRP3 Regulates IL-4 Expression in TOX+ CD4+ T Cells of Cutaneous T Cell Lymphoma to Potentially Promote Disease Progression. Front. Immunol. 2021, 12, 668369. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.-B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Vowels, B.R.; Lessin, S.R.; Cassin, M.; Jaworsky, C.; Benoit, B.; Wolfe, J.T.; Rook, A.H. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J. Investig. Dermatol. 1994, 103, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, R.T.; Medeiros, L.J.; Warnke, R.A.; Wood, G.S. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J. Am. Acad. Dermatol. 1995, 32, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Economidou, J.; Psarra, A.; Kapsimali, V.; Mantzana, V.; Antoniou, C.; Limas, K.; Stratigos, A.; Stavrianeas, N.; Avgerinou, G.; et al. The relevance of peripheral blood T-helper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome. Br. J. Dermatol. 2003, 148, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Cordeiro, B.; Fredholm, S.; Ødum, N.; Zargham, H.; Huang, Y.; Zhou, Y.; Pehr, K.; Kupper, T.S.; Woetmann, A.; et al. Analysis of STAT4 expression in cutaneous T-cell lymphoma (CTCL) patients and patient-derived cell lines. Cell Cycle 2014, 13, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Vowels, B.R.; Cassin, M.; Vonderheid, E.C.; Rook, A.H. Aberrant cytokine production by Sezary syndrome patients: Cytokine secretion pattern resembles murine Th2 cells. J. Investig. Dermatol. 1992, 99, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Geskin, L.J.; Viragova, S.; Stolz, D.B.; Fuschiotti, P. Interleukin-13 is overexpressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood 2015, 125, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Kambayashi, Y.; Asano, M.; Watabe, A.; Aiba, S. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp. Dermatol. 2016, 25, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Hidaka, T.; Asano, M.; Aiba, S. Tumor-associated M2 macrophages in mycosis fungoides acquire immunomodulatory function by interferon alpha and interferon gamma. J. Dermatol. Sci. 2016, 83, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, E.; Hohaus, S.; Di Napoli, A.; Belmonte, B.; Cuccaro, A.; Cupelli, E.; Galli, E.; Rufini, V.; Tripodi, G.; Fraternali-Orcioni, G.; et al. Interleukin-31 and thymic stromal lymphopoietin expression in plasma and lymph node from Hodgkin lymphoma patients. Oncotarget 2017, 8, 85263–85275. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Pavel, A.B.; Diaz, A.; Zhang, N.; Del Duca, E.; Estrada, Y.; King, B.; Banerjee, A.; Banfield, C.; Cox, L.A.; et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J. Allergy Clin. Immunol. 2022, 149, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Guenova, E.; Watanabe, R.; Teague, J.E.; Desimone, J.A.; Jiang, Y.; Dowlatshahi, M.; Schlapbach, C.; Schaekel, K.; Rook, A.H.; Tawa, M.; et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin. Cancer Res. 2013, 19, 3755–3763. [Google Scholar] [CrossRef]
- Takahashi, N.; Sugaya, M.; Suga, H.; Oka, T.; Kawaguchi, M.; Miyagaki, T.; Fujita, H.; Sato, S. Thymic Stromal Chemokine TSLP Acts through Th2 Cytokine Production to Induce Cutaneous T-cell Lymphoma. Cancer Res. 2016, 76, 6241–6252. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Shibata, S.; Ohmatsu, H.; Fujita, H.; Tamaki, K. Serum interleukin-27 levels in patients with cutaneous T-cell lymphoma. Clin. Exp. Dermatol. 2010, 35, e143–e144. [Google Scholar] [CrossRef] [PubMed]
- Tuzova, M.; Richmond, J.; Wolpowitz, D.; Curiel-Lewandrowski, C.; Chaney, K.; Kupper, T.; Cruikshank, W. CCR4+T cell recruitment to the skin in mycosis fungoides: Potential contributions by thymic stromal lymphopoietin and interleukin-16. Leuk. Lymphoma 2015, 56, 440–449. [Google Scholar] [CrossRef]
- Stolearenco, V.; Namini, M.R.J.; Hasselager, S.S.; Gluud, M.; Buus, T.B.; Willerslev-Olsen, A.; Ødum, N.; Krejsgaard, T. Cellular Interactions and Inflammation in the Pathogenesis of Cutaneous T-Cell Lymphoma. Front. Cell Dev. Biol. 2020, 8, 851. [Google Scholar] [CrossRef]
- Jfri, A.; Smith, J.S.; Larocca, C. Diagnosis of mycosis fungoides or Sézary syndrome after dupilumab use: A systematic review. J. Am. Acad. Dermatol. 2023, 88, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ramachandran, V.; Weston, G.; Kim, R.H.; Latkowski, J.-A. Diagnosing mycosis fungoides after initiation of therapy with dupilumab: A case report and literature review. Int. J. Dermatol. 2023, 62, e500–e503. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M. Erythrodermic cutaneous T-cell lymphoma: How to differentiate this rare disease from atopic dermatitis. J. Dermatol. Sci. 2011, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, M. Immunoglobulin E in cutaneous lymphomas and in parapsoriasis en plaques (Brocq) (author’s transl). Dermatologica 1980, 161, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guilhou, J.J.; Clot, J.; Robinet-Lévy, M.; Meynadier, J. IgE and cellular immunity in cutaneous lymphomas (author’s transl). Ann. Dermatol. Venereol. 1977, 104, 533–537. [Google Scholar] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Suchin, K.R.; Cassin, M.; Gottleib, S.L.; Sood, S.; Cucchiara, A.J.; Vonderheid, E.C.; Rook, A.H. Increased interleukin 5 production in eosinophilic Sézary syndrome: Regulation by interferon alfa and interleukin 12. J. Am. Acad. Dermatol. 2001, 44, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Corless, C.L.; Duensing, A.; McGreevey, L.; Chen, C.-J.; Joseph, N.; Singer, S.; Griffith, D.J.; Haley, A.; Town, A.; et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003, 299, 708–710. [Google Scholar] [CrossRef]
- Karamova, A.E.; Verbenko, D.A.; Vorontsova, A.A.; Zhilova, M.B.; Nikonorov, A.A.; Gatiatulina, E.R.; Znamenskaya, L.F.; Kubanov, A.A. Effect of PUVA and NB-UVB Therapy on the Skin Cytokine Profile in Patients with Mycosis Fungoides. J. Oncol. 2022, 2022, 3149293. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, T.-X.; Deng, H.; Yang, X.-P.; Tang, Z.-H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Z.; Kamarashev, J.; Zhang, C.L.; Dummer, R.; Burg, G.; Döbbeling, U. Constitutive and interleukin-7- and interleukin-15-stimulated DNA binding of STAT and novel factors in cutaneous T cell lymphoma cells. J. Investig. Dermatol. 2001, 117, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Foss, F.M.; Koc, Y.; Stetler-Stevenson, M.A.; Nguyen, D.T.; O’Brien, M.C.; Turner, R.; Sausville, E.A. Costimulation of cutaneous T-cell lymphoma cells by interleukin-7 and interleukin-2: Potential autocrine or paracrine effectors in the Sézary syndrome. J. Clin. Oncol. 1994, 12, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Netchiporouk, E.; Litvinov, I.V.; Moreau, L.; Gilbert, M.; Sasseville, D.; Duvic, M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle 2014, 13, 3331–3335. [Google Scholar] [CrossRef] [PubMed]
- Merle-Béral, H.; Schmitt, C.; Mossalayi, D.; Bismuth, G.; Dalloul, A.; Mentz, F. Interleukin-7 and malignant T cells. Nouv. Rev. Fr. Hematol. 1993, 35, 231–232. [Google Scholar] [PubMed]
- Bagot, M.; Charue, D.; Boulland, M.L.; Gaulard, P.; Revuz, J.; Schmitt, C.; Wechsler, J. Interleukin-7 receptor expression in cutaneous T-cell lymphomas. Br. J. Dermatol. 1996, 135, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Sportès, C.; Gress, R.E. Interleukin-7 immunotherapy. Adv. Exp. Med. Biol. 2007, 601, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.A.; Broide, D.H. Insights into the biology of IL-9 in asthma. J. Allergy Clin. Immunol. 2022, 150, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, X.; Bai, Q.; Qin, C.; Mohamud, A.O.; Zhu, Z.; Ball, T.W.; Ruth, C.M.; Newcomer, D.R.; Herrick, E.J.; et al. IL-9 inhibits HTB-72 melanoma cell growth through upregulation of p21 and TRAIL. J. Surg. Oncol. 2015, 111, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Lu, Y. Targeting the IL-9 pathway in cancer immunotherapy. Hum. Vaccin. Immunother. 2020, 16, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.S.; Britton, G.; Hill, E.; Verhagen, J.; Burton, B.; Wraith, D. Regulation of Adaptive Immunity; The Role of Interleukin-10. Front. Immunol. 2013, 4, 129. [Google Scholar] [CrossRef]
- Howes, A.; Gabryšová, L.; O’Garra, A. Role of IL-10 and the IL-10 Receptor in Immune Responses. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-801238-3. [Google Scholar]
- Wu, X.; Hsu, D.K.; Wang, K.-H.; Huang, Y.; Mendoza, L.; Zhou, Y.; Hwang, S.T. IL-10 is overexpressed in human cutaneous T-cell lymphoma and is required for maximal tumor growth in a mouse model. Leuk. Lymphoma 2019, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Olszewska, B.; Roszkiewicz, J.; Glen, J.; Zabłotna, M.; Ługowska-Umer, H.; Nowicki, R.; Sokołowska-Wojdyło, M. The role of polymorphism of interleukin-2, -10, -13 and TNF-α genes in cutaneous T-cell lymphoma pathogenesis. Postep. Dermatol. Alergol. 2016, 33, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.; Hessenkemper, W.; Schaper, F. Systems biology of IL-6, IL-12 family cytokines. Cytokine Growth Factor. Rev. 2015, 26, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Glassman, C.R.; Mathiharan, Y.K.; Jude, K.M.; Su, L.; Panova, O.; Lupardus, P.J.; Spangler, J.B.; Ely, L.K.; Thomas, C.; Skiniotis, G.; et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 2021, 184, 983–999.e24. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef] [PubMed]
- Showe, L.C.; Fox, F.E.; Williams, D.; Au, K.; Niu, Z.; Rook, A.H. Depressed IL-12-mediated signal transduction in T cells from patients with Sézary syndrome is associated with the absence of IL-12 receptor beta 2 mRNA and highly reduced levels of STAT4. J. Immunol. 1999, 163, 4073–4079. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.S.; Spencer, R.K.; Johnson, C.E.; Elhage, K.G.; Jin, J.Q.; Hakimi, M.; Bhutani, T.; Liao, W. Risk of Cutaneous T Cell Lymphoma with Psoriasis Biologic Therapies. Dermatol. Ther. 2023, 14, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.E. The role of IL-13 and its receptor in allergy and inflammatory responses. J. Allergy Clin. Immunol. 1998, 102, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.F.; Noben-Trauth, N.; Donaldson, D.D.; Madden, K.B.; Morris, S.C.; Collins, M.; Finkelman, F.D. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 1998, 8, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Brombacher, F.; McGachy, H.A.; McKenzie, A.N.J.; Walker, W.; Carter, K.C. An essential role for IL-13 in maintaining a non-healing response following Leishmania mexicana infection. Eur. J. Immunol. 2002, 32, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Mohrs, M.; Ledermann, B.; Köhler, G.; Dorfmüller, A.; Gessner, A.; Brombacher, F. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 1999, 162, 7302–7308. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.; Ellis, R.; Wattie, J.; Donaldson, D.D.; Inman, M.D. Is interleukin-13 critical in maintaining airway hyperresponsiveness in allergen-challenged mice? Am. J. Respir. Crit. Care Med. 2004, 170, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Gabitass, R.F.; Annels, N.E.; Stocken, D.D.; Pandha, H.A.; Middleton, G.W. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 2011, 60, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and Their Receptors in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, H.; Zhang, H.; He, H.; Li, H.; Shen, Z.; Qin, J.; Qin, X.; Xu, J.; Sun, Y. Interleukin-13 receptor α2 is associated with poor prognosis in patients with gastric cancer after gastrectomy. Oncotarget 2016, 7, 49281–49288. [Google Scholar] [CrossRef] [PubMed]
- Skinnider, B.F.; Kapp, U.; Mak, T.W. The role of interleukin 13 in classical Hodgkin lymphoma. Leuk. Lymphoma 2002, 43, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Gluud, M.; Pallesen, E.M.H.; Buus, T.B.; Gjerdrum, L.M.R.; Lindahl, L.M.; Kamstrup, M.R.; Bzorek, M.; Danielsen, M.; Bech, R.; Monteiro, M.N.; et al. Malignant T cells induce skin barrier defects through cytokine-mediated JAK/STAT signaling in cutaneous T-cell lymphoma. Blood 2023, 141, 180–193. [Google Scholar] [CrossRef]
- Hashimoto, M.; Miyagaki, T.; Komaki, R.; Takeuchi, S.; Kadono, T. Development of Nodular Lesions after Dupilumab Therapy in Erythrodermic Mycosis Fungoides with Interleukin-13 Receptor alpha2 Expression. Acta Derm. Venereol. 2022, 102, adv00766. [Google Scholar] [CrossRef] [PubMed]
- Nogami, S.; Satoh, S.; Tanaka-Nakadate, S.; Yoshida, K.; Nakano, M.; Terano, A.; Shirataki, H. Identification and characterization of taxilin isoforms. Biochem. Biophys. Res. Commun. 2004, 319, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.; Tamayo, A.; Martin, B.; Niu, K.; Claypool, K.; Cabanillas, F.; Ambrus, J. Identification of B-cell growth factors (interleukin-14; high molecular weight-B-cell growth factors) in effusion fluids from patients with aggressive B-cell lymphomas. Blood 1995, 86, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bodine, B.G.; Bennion, B.G.; Leatham, E.; Jimenez, F.R.; Wright, A.J.; Jergensen, Z.R.; Erickson, C.J.; Jones, C.M.; Johnson, J.P.; Knapp, S.M.; et al. Conditionally induced RAGE expression by proximal airway epithelial cells in transgenic mice causes lung inflammation. Respir. Res. 2014, 15, 133. [Google Scholar] [CrossRef]
- Singh, A.R.; Peirce, S.K.; Joshi, S.; Durden, D.L. PTEN and PI-3 kinase inhibitors control LPS signaling and the lymphoproliferative response in the CD19+ B cell compartment. Exp. Cell Res. 2014, 327, 78–90. [Google Scholar] [CrossRef]
- Steel, J.C.; Waldmann, T.A.; Morris, J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012, 33, 35–41. [Google Scholar] [CrossRef]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef]
- Kopp, K.; Ralfkiaer, U.; Mette Gjerdrum, L.; Helvad, R.; Pedersen, I.; Litman, T.; Jønson, L.; Hagedorn, P.; Krejsgaard, T.; Gniadecki, R.; et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle 2013, 12, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Döbbeling, U.; Dummer, R.; Laine, E.; Potoczna, N.; Qin, J.Z.; Burg, G. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood 1998, 92, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Z.; Zhang, C.L.; Kamarashev, J.; Dummer, R.; Burg, G.; Döbbeling, U. Interleukin-7 and interleukin-15 regulate the expression of the bcl-2 and c-myb genes in cutaneous T-cell lymphoma cells. Blood 2001, 98, 2778–2783. [Google Scholar] [CrossRef]
- Leroy, S.; Dubois, S.; Tenaud, I.; Chebassier, N.; Godard, A.; Jacques, Y.; Dréno, B. Interleukin-15 expression in cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Br. J. Dermatol. 2001, 144, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M. The Role of Th17-Related Cytokines in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 1314. [Google Scholar] [CrossRef]
- Wysocka, M.; Benoit, B.M.; Newton, S.; Azzoni, L.; Montaner, L.J.; Rook, A.H. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood 2004, 104, 4142–4149. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, M.; Dawany, N.; Benoit, B.; Kossenkov, A.V.; Troxel, A.B.; Gelfand, J.M.; Sell, M.K.; Showe, L.C.; Rook, A.H. Synergistic enhancement of cellular immune responses by the novel Toll receptor 7/8 agonist 3M-007 and interferon-γ: Implications for therapy of cutaneous T-cell lymphoma. Leuk. Lymphoma 2011, 52, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Krejsgaard, T.; Litvinov, I.V.; Wang, Y.; Xia, L.; Willerslev-Olsen, A.; Koralov, S.B.; Kopp, K.L.; Bonefeld, C.M.; Wasik, M.A.; Geisler, C.; et al. Elucidating the role of interleukin-17F in cutaneous T-cell lymphoma. Blood 2013, 122, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Krejsgaard, T.; Vetter-Kauczok, C.S.; Woetmann, A.; Lovato, P.; Labuda, T.; Eriksen, K.W.; Zhang, Q.; Becker, J.C.; Ødum, N. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia 2006, 20, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Papathemeli, D.; Patsatsi, A.; Papanastassiou, D.; Koletsa, T.; Papathemelis, T.; Avgeros, C.; Pikou, O.; Lazaridou, E.; Georgiou, E. Protein and mRNA Expression Levels of Interleukin-17A, -17F and -22 in Blood and Skin Samples of Patients with Mycosis Fungoides. Acta Derm. -Venereol. 2020, 100, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.; Tuzova, M.; Parks, A.; Adams, N.; Martin, E.; Tawa, M.; Morrison, L.; Chaney, K.; Kupper, T.S.; Curiel-Lewandrowski, C.; et al. Interleukin-16 as a marker of Sézary syndrome onset and stage. J. Clin. Immunol. 2011, 31, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Wiltrout, R.H.; Young, H.A. IL-18 is a potent coinducer of IL-13 in NK and T cells: A new potential role for IL-18 in modulating the immune response. J. Immunol. 1999, 162, 5070–5077. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001, 19, 423–474. [Google Scholar] [CrossRef]
- Bostan, E.; Gokoz, O.; Atakan, N. The role of NLRP1 and NLRP3 inflammasomes in the etiopathogeneses of pityriasis lichenoides chronica and mycosis fungoides: An immunohistochemical study. Arch. Dermatol. Res. 2023, 315, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.-T.; Matsuo, Y.; Kuwamura, M.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Nakajima, H.; Karow, M.; Takeuchi, T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm. Bowel Dis. 2010, 16, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-C.; Hsu, Y.-H.; Li, H.-H.; Wang, Y.-C.; Hsieh, M.-Y.; Chen, W.-Y.; Hsing, C.-H.; Chang, M.-S. IL-20: Biological functions and clinical implications. J. Biomed. Sci. 2006, 13, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Hsing, C.-H.; Li, C.-F.; Chan, C.-H.; Chang, M.-C.; Yan, J.-J.; Chang, M.-S. Anti-IL-20 monoclonal antibody suppresses breast cancer progression and bone osteolysis in murine models. J. Immunol. 2012, 188, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Cho, S.-C.; Lee, E.-J.; Kim, S.; Lee, S.-B.; Lim, J.-H.; Choi, Y.H.; Kim, W.-J.; Moon, S.-K. Interleukin-20 promotes migration of bladder cancer cells through extracellular signal-regulated kinase (ERK)-mediated MMP-9 protein expression leading to nuclear factor (NF-κB) activation by inducing the up-regulation of p21(WAF1) protein expression. J. Biol. Chem. 2013, 288, 5539–5552. [Google Scholar] [CrossRef] [PubMed]
- Subtraction Hybridization Identifies a Novel Melanoma Differentiation Associated Gene, mda-7, Modulated during Human Melanoma Differentiation, Growth and Progression—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8545104/ (accessed on 9 January 2024).
- Whitaker, E.L.; Filippov, V.A.; Duerksen-Hughes, P.J. Interleukin 24: Mechanisms and therapeutic potential of an anti-cancer gene. Cytokine Growth Factor Rev. 2012, 23, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Fickenscher, H.; Bayry, J. IL-26: An Emerging Proinflammatory Member of the IL-10 Cytokine Family with Multifaceted Actions in Antiviral, Antimicrobial, and Autoimmune Responses. PLoS Pathog. 2016, 12, e1005624. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Leonard, W.J. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008, 26, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.-F.; Si, C.-Z.; Zhu, Y.-H.; Jin, Y.; Zhu, T.-T.; Liu, M.-Y.; Liu, G.-Y. CCL21/IL21-armed oncolytic adenovirus enhances antitumor activity against TERT-positive tumor cells. Virus Res. 2016, 220, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, B.; Kreher, S.; Anagnostopoulos, I.; Jöhrens, K.; Monteleone, G.; Jundt, F.; Stein, H.; Janz, M.; Dörken, B.; Mathas, S. Aberrant expression of the Th2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3α. Blood 2008, 112, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a drug target for autoimmune inflammatory diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 1995, 181, 381–386. [CrossRef] [PubMed]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 Protein Engages IL-12p40 to Form a Cytokine, IL-23, with Biological Activities Similar as Well as Distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Bardazzi, F.; Viviani, F.; Piraccini, B.M.; Lasagni, C.; Bigi, L.; Manfredini, M.; Pongetti, L.; Di Lernia, V.; Corazza, M.; Pepe, F. Tildrakizumab in Complex Psoriatic Patients: An Experience in Emilia-Romagna (Italy). J. Cutan. Med. Surg. 2023, 27, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Näslund-Koch, C.; Zachariae, C.; Skov, L. Tildrakizumab: An Evidence-Based Review of Its Use in the Treatment of Moderate-to-Severe Chronic Plaque Psoriasis. Ther. Clin. Risk Manag. 2020, 16, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M.; Miyagaki, T.; Ohmatsu, H.; Suga, H.; Kai, H.; Kamata, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; et al. Association of the numbers of CD163(+) cells in lesional skin and serum levels of soluble CD163 with disease progression of cutaneous T cell lymphoma. J. Dermatol. Sci. 2012, 68, 45–51. [Google Scholar] [CrossRef]
- Fahmy, L.M.; Schreidah, C.M.; Lapolla, B.A.; Magro, C.M.; Geskin, L.J. Mycosis fungoides diagnosed after exposure to risankizumab for psoriasis. JAAD Case Rep. 2023, 41, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Amitay-Laish, I.; Guenova, E.; Ortiz-Romero, P.L.; Vico-Alonso, C.; Rozati, S.; Geskin, L.J.; Nikolaou, V.; Papadavid, E.; Barzilai, A.; Pavlovsky, L.; et al. The Course of Mycosis Fungoides under Cytokine Pathway Blockers: A Multicentre Analysis of Real-life Clinical Data. Acta Derm. Venereol. 2020, 100, adv00277. [Google Scholar] [CrossRef] [PubMed]
- Gowhari Shabgah, A.; Amir, A.; Gardanova, Z.R.; Olegovna Zekiy, A.; Thangavelu, L.; Ebrahimi Nik, M.; Ahmadi, M.; Gholizadeh Navashenaq, J. Interleukin-25: New perspective and state-of-the-art in cancer prognosis and treatment approaches. Cancer Med. 2021, 10, 5191–5202. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Miyagaki, T.; Hirakawa, M.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Fujita, H.; Asano, Y.; Sugaya, M.; et al. Interleukin-25 is involved in cutaneous T-cell lymphoma progression by establishing a T helper 2-dominant microenvironment. Br. J. Dermatol. 2018, 178, 1373–1382. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Hisada, M.; Kamiya, S.; Fujita, K.; Belladonna, M.L.; Aoki, T.; Koyanagi, Y.; Mizuguchi, J.; Yoshimoto, T. Potent antitumor activity of interleukin-27. Cancer Res. 2004, 64, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, R.; Hixon, J.A.; Stauffer, J.K.; Jalah, R.; Brooks, A.D.; Khan, T.; Dai, R.-M.; Scheetz, L.; Lincoln, E.; Back, T.C.; et al. Immunologic and Therapeutic Synergy of IL-27 and IL-2: Enhancement of T Cell Sensitization, Tumor-Specific CTL Reactivity and Complete Regression of Disseminated Neuroblastoma Metastases in the Liver and Bone Marrow. J. Immunol. 2009, 182, 4328–4338. [Google Scholar] [CrossRef] [PubMed]
- Dibra, D.; Cutrera, J.; Xia, X.; Li, S. WSX1 Expression in Tumors Induces Immune Tolerance via Suppression of Effector Immune Cells. PLoS ONE 2011, 6, e19072. [Google Scholar] [CrossRef] [PubMed]
- Nattkemper, L.A.; Martinez-Escala, M.-E.; Gelman, A.B.; Singer, E.M.; Rook, A.H.; Guitart, J.; Yosipovitch, G. Cutaneous T-cell Lymphoma and Pruritus: The Expression of IL-31 and its Receptors in the Skin. Acta Derm. Venereol. 2016, 96, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef]
- Shi, Q.; Abbruzzese, J.L.; Huang, S.; Fidler, I.J.; Xiong, Q.; Xie, K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin. Cancer Res. 1999, 5, 3711–3721. [Google Scholar] [PubMed]
- Inoue, K.; Slaton, J.W.; Kim, S.J.; Perrotte, P.; Eve, B.Y.; Bar-Eli, M.; Radinsky, R.; Dinney, C.P. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000, 60, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Ohmatsu, H.; Sugaya, M.; Suga, H.; Morimura, S.; Miyagaki, T.; Kai, H.; Kagami, S.; Fujita, H.; Asano, Y.; Tada, Y.; et al. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm. Venereol. 2012, 92, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.; Werner, D.; Duschl, A.; Bohle, B.; Horejs-Hoeck, J. Human Th2 but not Th9 cells release IL-31 in a STAT6/NF-κB-dependent way. J. Immunol. 2014, 193, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, B.; Żawrocki, A.; Gleń, J.; Lakomy, J.; Karczewska, J.; Zabłotna, M.; Malek, M.; Jankau, J.; Lange, M.; Biernat, W.; et al. Interleukin-31 is overexpressed in skin and serum in cutaneous T-cell lymphomas but does not correlate to pruritus. Postep. Dermatol. Alergol. 2022, 39, 81–87. [Google Scholar] [CrossRef]
- Chechlinska, M.; Kowalewska, M.; Nowak, R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat. Rev. Cancer 2010, 10, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Sloot, Y.J.E.; Smit, J.W.; Joosten, L.A.B.; Netea-Maier, R.T. Insights into the role of IL-32 in cancer. Semin. Immunol. 2018, 38, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Dias, F.; Saar Gomes, R.; de Lima Silva, L.L.; dos Santos, J.C.; Joosten, L.A.B. Interleukin 32: A novel player in the control of infectious diseases. J. Leukoc. Biol. 2017, 101, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Xu, F.; Deng, C.; Nie, X.; Zhong, L.; Wu, Z.; Li, J.; Wu, X.; He, S.; Chen, Y. Fusobacterium nucleatum promotes the early occurrence of esophageal cancer through upregulation of IL-32/PRTN3 expression. Cancer Sci. 2023, 114, 2414–2428. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.-M.; An, Y.-H.; Wang, W.; Fan, Y.-G.; Yao, G.-L. IL-32 expression indicates unfavorable prognosis in patients with colon cancer. Oncol. Lett. 2019, 17, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- Nold-Petry, C.A.; Rudloff, I.; Baumer, Y.; Ruvo, M.; Marasco, D.; Botti, P.; Farkas, L.; Cho, S.X.; Zepp, J.A.; Azam, T.; et al. IL-32 promotes angiogenesis. J. Immunol. 2014, 192, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Chen, S.; Chen, Z.; Zhou, B.; Zheng, Y.; Shen, L. Interleukin 32 Promotes Foxp3+ Treg Cell Development and CD8+ T Cell Function in Human Esophageal Squamous Cell Carcinoma Microenvironment. Front. Cell Dev. Biol. 2021, 9, 704853. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, Y. Interleukin-32: Frenemy in cancer? BMB Rep. 2019, 52, 165–174. [Google Scholar] [CrossRef] [PubMed]
- van Kester, M.S.; Borg, M.K.; Zoutman, W.H.; Out-Luiting, J.J.; Jansen, P.M.; Dreef, E.J.; Vermeer, M.H.; van Doorn, R.; Willemze, R.; Tensen, C.P. A meta-analysis of gene expression data identifies a molecular signature characteristic for tumor-stage mycosis fungoides. J. Investig. Dermatol. 2012, 132, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Miyagaki, T.; Kawaguchi, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. The role of IL-32 in cutaneous T-cell lymphoma. J. Investig. Dermatol. 2014, 134, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Ohmatsu, H.; Humme, D.; Gulati, N.; Gonzalez, J.; Möbs, M.; Suárez-Fariñas, M.; Cardinale, I.; Mitsui, H.; Guttman-Yassky, E.; Sterry, W.; et al. IL32 is progressively expressed in mycosis fungoides independent of helper T-cell 2 and helper T-cell 9 polarization. Cancer Immunol. Res. 2014, 2, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R.; Katoh, N.; Kishimoto, S. Platelet activation in patients with psoriasis: Increased plasma levels of platelet-derived microparticles and soluble P-selectin. J. Am. Acad. Dermatol. 2010, 62, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Wasmer, M.-H.; Krebs, P. The Role of IL-33-Dependent Inflammation in the Tumor Microenvironment. Front. Immunol. 2016, 7, 682. [Google Scholar] [CrossRef] [PubMed]
- Rustowska-Rogowska, A.; Gleń, J.; Jarząbek, T.; Rogowski, W.; Rębała, K.; Zabłotna, M.; Czajkowska, K.; Nowicki, R.; Kowalczyk, A.; Sokołowska-Wojdyło, M. Interleukin-33 polymorphisms and serum concentrations in mycosis fungoides. Int. J. Dermatol. 2020, 59, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.-M.; Giles, F.J.; Duvic, M.; Kurzrock, R. Pilot study of etanercept in patients with relapsed cutaneous T-cell lymphomas. J. Am. Acad. Dermatol. 2004, 51, 200–204. [Google Scholar] [CrossRef]
- Tsimberidou, A.-M.; Alvarado, Y.; Giles, F.J. Evolving role of ribonucleoside reductase inhibitors in hematologic malignancies. Expert. Rev. Anticancer. Ther. 2002, 2, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Daliani, D.; Ulmer, R.A.; Jackow, C.; Pugh, W.; Gansbacher, B.; Cabanillas, F.; Duvic, M.; Sarris, A.H. Tumor necrosis factor-alpha and interferon-gamma, but not HTLV-I tax, are likely factors in the epidermotropism of cutaneous T-cell lymphoma via induction of interferon-inducible protein-10. Leuk. Lymphoma 1998, 29, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Giri, D.K.; Aggarwal, B.B. Constitutive Activation of NF-κB Causes Resistance to Apoptosis in Human Cutaneous T Cell Lymphoma HuT-78 Cells: Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J. Biol. Chem. 1998, 273, 14008–14014. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Escala, M.E.; Posligua, A.L.; Wickless, H.; Rutherford, A.; Sable, K.A.; Rubio-Gonzalez, B.; Zhou, X.A.; Kaplan, J.B.; Pro, B.; Choi, J.; et al. Progression of undiagnosed cutaneous lymphoma after anti-tumor necrosis factor-alpha therapy. J. Am. Acad. Dermatol. 2018, 78, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Partarrieu-Mejías, F.; Díaz-Corpas, T.; Pérez-Ferriols, A.; Alegre-de Miquel, V. Mycosis fungoides after treatment with tumor necrosis factor-alpha inhibitors for psoriasis: Progression or onset? Int. J. Dermatol. 2019, 58, e103–e105. [Google Scholar] [CrossRef] [PubMed]
- Amitay-Laish, I.; Davidovici, B.; Moyal, L.; Gurevich, M.; Akerman, L.; Hodak, E. Lack of detectable effect of narrow-band ultraviolet B on peripheral blood mononuclear cell cytokine expression in early-stage mycosis fungoides. Photodermatol. Photoimmunol. Photomed. 2018, 34, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Toyama, T.; Sumida, H.; Fujita, H.; Kogure, A.; Igarashi, A.; Sato, S. A Case of Mycosis Fungoides with Large Cell Transformation Associated with Infliximab Treatment. Acta Derm. -Venereol. 2014, 94, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.E.; Zwicker, J.; Curiel, C.; Kadin, M.E.; Falchuk, K.R.; Drews, R.; Kupper, T.S. Aggressive cutaneous T-cell lymphomas after TNFα blockade. J. Am. Acad. Dermatol. 2004, 51, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Lourari, S.; Prey, S.; Livideanu, C.; Jamard, B.; Lamant, L.; Cantagrel, A.; Paul, C. Cutaneous T-cell lymphoma following treatment of rheumatoid arthritis with tumour necrosis factor-α blocking agents: Two cases. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 967–968. [Google Scholar] [CrossRef]
- Boonstra, J.; Rijken, P.; Humbel, B.; Cremers, F.; Verkleij, A.; van Bergen en Henegouwen, P. The epidermal growth factor. Cell Biol. Int. 1995, 19, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.C.; Guillaud, L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor. Rev. 2004, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Catt, K.J.; Knecht, M. Transforming growth factor beta regulates the inhibitory actions of epidermal growth factor during granulosa cell differentiation. J. Biol. Chem. 1986, 261, 14167–14170. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.-Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Paraneoplastic Scleroderma in the Setting of CD30+ Large Cell Transformation of Mycosis Fungoides—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6357550/ (accessed on 9 January 2024).
- Krejsgaard, T.; Lindahl, L.M.; Mongan, N.P.; Wasik, M.A.; Litvinov, I.V.; Iversen, L.; Langhoff, E.; Woetmann, A.; Odum, N. Malignant inflammation in cutaneous T-cell lymphoma—A hostile takeover. Semin. Immunopathol. 2017, 39, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Lennartsson, J. Structural and functional properties of platelet-derived growth factor and stem cell factor receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009100. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, J.-B.; Montano-Almendras, C.P. Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis. Am. J. Blood Res. 2012, 2, 44–56. [Google Scholar] [PubMed]
- Belinsky, M.G.; Cai, K.Q.; Zhou, Y.; Luo, B.; Pei, J.; Rink, L.; von Mehren, M. Succinate dehydrogenase deficiency in a PDGFRA mutated GIST. BMC Cancer 2017, 17, 512. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.M.; Durgin, J.S.; Wysocka, M.; Rook, A.H. The Immunopathogenesis and Immunotherapy of Cutaneous T Cell Lymphoma: Part II, Current and Future Approaches. J. Am. Acad. Dermatol. 2021, 84, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.K.; Cassin, M.; Lessin, S.R.; Rook, A.H. Complete molecular remission during biologic response modifier therapy for Sézary syndrome is associated with enhanced helper T type 1 cytokine production and natural killer cell activity. J. Am. Acad. Dermatol. 2001, 45, 208–216. [Google Scholar] [CrossRef]
- Spaccarelli, N.; Rook, A.H. The Use of Interferons in the Treatment of Cutaneous T-Cell Lymphoma. Dermatol. Clin. 2015, 33, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Filipi, M.; Jack, S. Interferons in the Treatment of Multiple Sclerosis: A Clinical Efficacy, Safety, and Tolerability Update. Int. J. MS Care 2020, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Killestein, J.; Polman, C.H. Determinants of interferon β efficacy in patients with multiple sclerosis. Nat. Rev. Neurol. 2011, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Durbin, J.E. Contribution of type III interferons to antiviral immunity: Location, location, location. J. Biol. Chem. 2017, 292, 7295–7303. [Google Scholar] [CrossRef]
- Kuzel, T.M.; Roenigk, H.H.; Samuelson, E.; Herrmann, J.J.; Hurria, A.; Rademaker, A.W.; Rosen, S.T. Effectiveness of interferon alfa-2a combined with phototherapy for mycosis fungoides and the Sézary syndrome. J. Clin. Oncol. 1995, 13, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Mazza, P.; Gherlinzoni, F. Beta interferon in the treatment of mycosis fungoides. Haematologica 1988, 73, 547–548. [Google Scholar] [PubMed]
- Sarris, A.H.; Esgleyes-Ribot, T.; Crow, M.; Broxmeyer, H.E.; Karasawas, N.; Pugh, W.; Grossman, D.; Deisseroth, A.; Duvic, M. Cytokine Loops Involving Interferon-γ and IP-10, a Cytokine Chemotactic for CD4+ Lymphocytes: An Explanation for the Epidermotropism of Cutaneous T-Cell Lymphoma? Blood 1995, 86, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, K.; Haeussler, A.; Sterry, W.; Docke, W.; Volk, H. Interferon gamma and tumor necrosis factor alpha mRNA expression in mycosis fungoides progression [letter; comment]. Blood 1996, 88, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M.; Tokura, Y.; Hamada, T.; Tsuboi, R.; Moroi, Y.; Nakahara, T.; Amano, M.; Ishida, S.; Watanabe, D.; Tani, M.; et al. Phase II study of i.v. interferon-gamma in Japanese patients with mycosis fungoides. J. Dermatol. 2014, 41, 50–56. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, K.S.; Ubriani, R.; Newton, S.; Junkins-Hopkins, J.M.; Vittorio, C.C.; Kim, E.J.; Wysocka, M.; Rook, A.H. The addition of interferon gamma to oral bexarotene therapy with photopheresis for Sézary syndrome. Arch. Dermatol. 2005, 141, 1176–1178. [Google Scholar] [CrossRef]
- Gardner, J.M.; Introcaso, C.E.; Nasta, S.D.; Kim, E.J.; Vittorio, C.C.; Rook, A.H. A novel regimen of vorinostat with interferon gamma for refractory Sézary syndrome. J. Am. Acad. Dermatol. 2009, 61, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Shimauchi, T.; Sugita, K.; Nishio, D.; Isoda, H.; Abe, S.; Yamada, Y.; Hino, R.; Ogata, M.; Kabashima, K.; Tokura, Y. Alterations of serum Th1 and Th2 chemokines by combination therapy of interferon-gamma and narrowband UVB in patients with mycosis fungoides. J. Dermatol. Sci. 2008, 50, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Tittes, J.; Brunner, P.M.; Guenova, E. Mycosis fungoides and Sézary syndrome. J. Dtsch. Dermatol. Ges. 2021, 19, 1307–1334. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef]
- Pimpinelli, N.; Olsen, E.A.; Santucci, M.; Vonderheid, E.; Haeffner, A.C.; Stevens, S.; Burg, G.; Cerroni, L.; Dreno, B.; Glusac, E.; et al. Defining early mycosis fungoides. J. Am. Acad. Dermatol. 2005, 53, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Latzka, J. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2023. Eur. J. Cancer 2023, 195, 113343. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur J Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Prince, H.M.; Cowan, R.; Vermeer, M.; Papadavid, E.; Bagot, M.; Servitjie, O.; Berti, E.; Guenova, E.; Stadler, R.; et al. Treatment of early-stage mycosis fungoides: Results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br. J. Dermatol. 2021, 184, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Maule, M.; Prince, H.M.; Porcu, P.; Horwitz, S.; Duvic, M.; Talpur, R.; Vermeer, M.; Bagot, M.; Guitart, J.; et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: A multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann. Oncol. 2017, 28, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Gaydosik, A.M.; Stonesifer, C.J.; Tabib, T.; Lafyatis, R.; Geskin, L.J.; Fuschiotti, P. The mycosis fungoides cutaneous microenvironment shapes dysfunctional cell trafficking, antitumor immunity, matrix interactions, and angiogenesis. JCI Insight 2023, 8, 170015. [Google Scholar] [CrossRef] [PubMed]
- Kalliara, E.; Belfrage, E.; Gullberg, U.; Drott, K.; Ek, S. Spatially Guided and Single Cell Tools to Map the Microenvironment in Cutaneous T-Cell Lymphoma. Cancers 2023, 15, 2362. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Akilov, O.E. Targeting the CD47-SIRPα Axis: Present Therapies and the Future for Cutaneous T-cell Lymphoma. Cells 2022, 11, 3591. [Google Scholar] [CrossRef] [PubMed]
- Johanny, L.D.; Sokumbi, O.; Hobbs, M.M.; Jiang, L. Polarization of Macrophages in Granulomatous Cutaneous T Cell Lymphoma Granulomatous Mycosis Fungoides Microenvironment. Dermatopathology 2022, 9, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Kuttikrishnan, S.; Khan, A.Q.; Ahmad, F.; Alam, M.; Buddenkotte, J.; Ahmad, A.; Steinhoff, M.; Uddin, S. Molecular pathogenesis of Cutaneous T cell Lymphoma: Role of chemokines, cytokines, and dysregulated signaling pathways. Semin. Cancer Biol. 2022, 86, 382–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, S.; Tang, W.; Huang, Q.; Mei, Y.; Yang, H. Exosomes from tamoxifen-resistant breast cancer cells transmit drug resistance partly by delivering miR-9-5p. Cancer Cell Int. 2021, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Bresin, A.; Caprini, E.; Russo, G.; Narducci, M.G. Challenging Cutaneous T-Cell Lymphoma: What Animal Models Tell us So Far. J. Investig. Dermatol. 2022, 142, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- de Masson, A.; Darbord, D.; Dobos, G.; Boisson, M.; Roelens, M.; Ram-Wolff, C.; Cassius, C.; Le Buanec, H.; de la Grange, P.; Jouenne, F.; et al. Macrophage-derived CXCL9 and CXCL11, T-cell skin homing, and disease control in mogamulizumab-treated CTCL patients. Blood 2022, 139, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Barta, S.K.; Liu, N.; DerSarkissian, M.; Chang, R.; Ye, M.; Duh, M.S.; Surinach, A.; Fanale, M.; Yu, K.S. Real-World Treatment Patterns and Clinical Outcomes with Brentuximab Vedotin or Other Standard Therapies in Patients with Previously Treated Cutaneous T-Cell Lymphoma in the United States. Clin. Lymphoma Myeloma Leuk. 2023, 24, e21–e32.e4. [Google Scholar] [CrossRef] [PubMed]
- Stuver, R.; Geller, S. Advances in the treatment of mycoses fungoides and Sézary syndrome: A narrative update in skin-directed therapies and immune-based treatments. Front. Immunol. 2023, 14, 1284045. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vardhana, S.; Moskowitz, A.J.; Porcu, P.; Dogan, A.; Dubovsky, J.A.; Matasar, M.J.; Zhang, Z.; Younes, A.; Horwitz, S.M. Pilot trial of ibrutinib in patients with relapsed or refractory T-cell lymphoma. Blood Adv. 2018, 2, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Duvic, M. Treatment of Cutaneous T-Cell Lymphoma from a Dermatologist’s Perspective. Clin. Lymphoma 2000, 1, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Sibbesen, N.A.; Kopp, K.L.; Litvinov, I.V.; Jønson, L.; Willerslev-Olsen, A.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Krejsgaard, T.; Lindahl, L.M.; et al. Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget 2015, 6, 20555–20569. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, C.K.; Gluud, M.; Torres-Rusillo, S.; Boding, L.; Willerslev-Olsen, A.; Buus, T.B.; Nielsen, T.K.; Persson, J.L.; Bonefeld, C.M.; Geisler, C.; et al. JAK3 Is Expressed in the Nucleus of Malignant T Cells in Cutaneous T Cell Lymphoma (CTCL). Cancers 2021, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Kæstel, C.G.; Eriksen, K.W.; Woetmann, A.; Stokkedal, T.; Kaltoft, K.; Geisler, C.; Röpke, C.; Ødum, N. Inhibition of constitutively activated Stat3 correlates with altered Bcl-2/Bax expression and induction of apoptosis in mycosis fungoides tumor cells. Leukemia 1999, 13, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.E.; Romanelli, P.; Lev-Tov, H.; Kerdel, F. A case of erythrodermic mycosis fungoides responding to upadacitinib. JAAD Case Rep. 2022, 30, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Vardhana, S.; Ganesan, N.; Hancock, H.; et al. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood 2021, 138, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
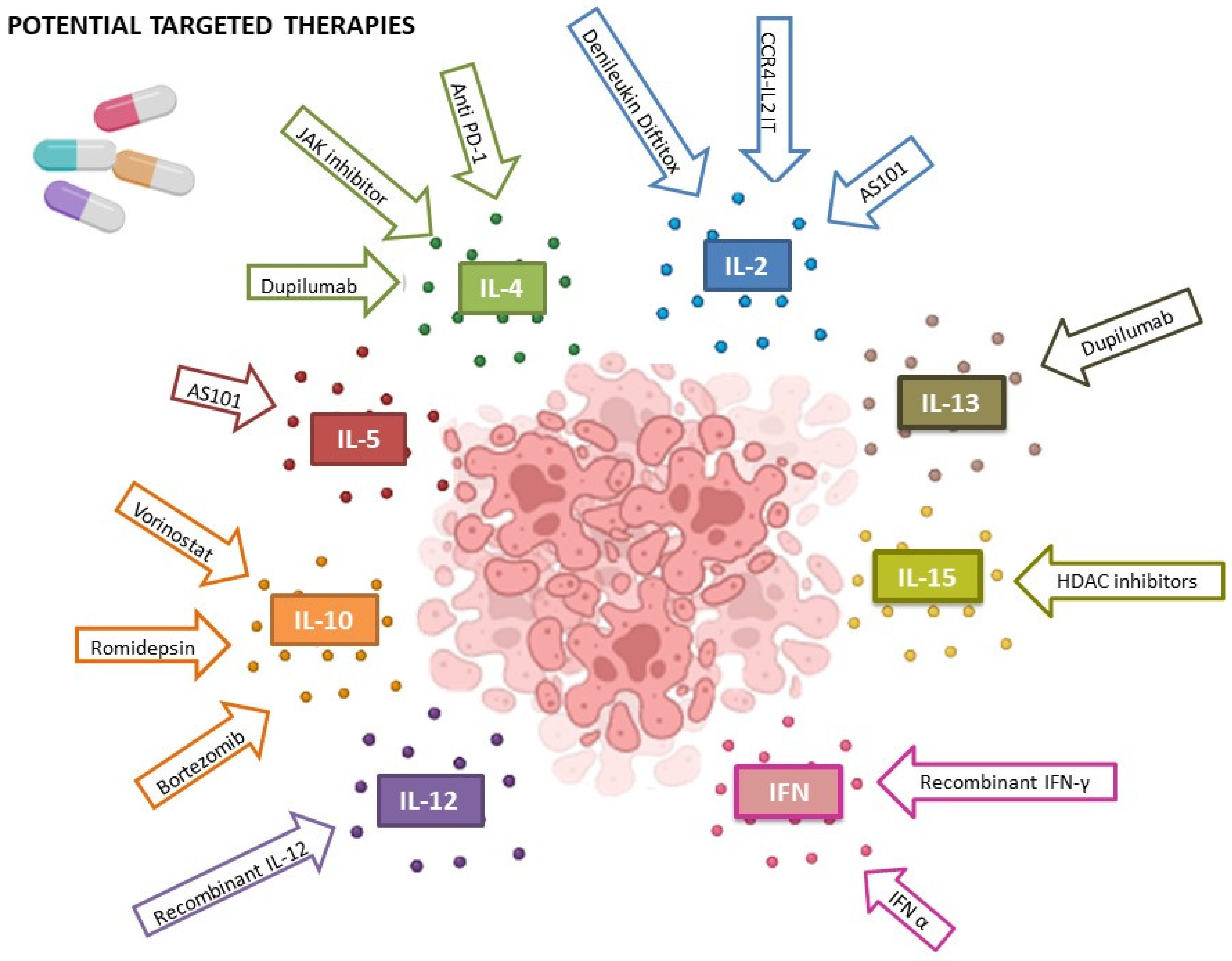
| Cytokine | Function | Role in MF/SS | Potential Targeted Therapies Evaluated |
|---|---|---|---|
| IL-1 | Pro-inflammatory [17]. | Elevated in treated patient; potential biomarker of photopheresis response [19,20]. | No studies available |
| IL-2 | Pro-inflammatory. Upregulate T cells and to increase the cytotoxicity of monocytes and natural killer (NK) [21]. | May have an CTCL suppressive action, but still controversial [22,23]. | AS101 inihbits IL2R and increases IL-2, suggesting an immunosuppressive role [24]. Denileukin Diftitox, which seems to reach a response of 36–40% of CTCLs in some studies [25]. CCR4-IL2 IT, in pre-clinical models seems to be promising in inducing CTCLs remission [26]. |
| IL-3 | Pluripotent and hematopoietic factor required for survival and proliferation of hematopoietic progenitor cells [27]. | Unknown. | No studies available. |
| IL-4 | Negatively modulates Th1 T cells; skews to a Th2 phenotype of naïve T cells [28]. | Contributes to immune evasion and tumor progression microenvironments (still debated) [29]. Can (with IL-33) induce IL-31 secretions, involved in itch pathogenesis [28,30]. | Anti PD-1 (Nivolumab) could reduce malignant Th2 cells, but it is controversial [31,32]. JAK inhibitor (Ritlecitinib) showed promising effect in reducing Th2 neoplastic cells (IIA trial ongoing) [33]. Dupilumab, by blocking IL-4 and Il-13, could induce an immune system against the tumor and blocking of Th2 cells’ proliferation, but is debated due to reported misdiagnosed MFs treated with dupilumab with a dramatic progression [34,35,36]. |
| IL-5 | Stimulates eosinophilic cascade [37]. | Related to erythroderma and elevated serum levels of IgE. Seems to be overexpressed by CTCL T cells [38]. | AS101 increases IFN-γ and a decreases Il-5, so to affect CTCL progression [24,39]. |
| IL-6 | Pro-inflammatory. Differentiates plasma cells and increases adhesion molecule production [40]. | Hyperxpressed in CTCL samples. Seems to be related to a higher risk of MF progression [41,42]. Il-6 polymorphism may be related to worse disease prognosis [43]. | No studies available. |
| IL-7 | Hemapoietic factor stimulates the development of lymphoid lineage [44]. | Linked to activation of CD8 + SS clone cells, but studies are not concordant [45,46]. | No studies available. |
| IL-8 | IL-8 is a chemotactic factor for neutrophils and other granulocytes. The oncogenic role is achieved by binding the IL-8 R localized on cancer cells and on microenvironment cells [47]. | Involved in pruritus and CTCL progression [48,49]. | No studies available. |
| IL-9 | Stimulates various hematopoietic cells’ proliferation and prevents immune cells’ apoptosis. Seems to be related to hematologic neoplasias [50]. | High levels have been linked in patients with SS [51] and MF [52]. | No studies available. |
| IL-10 | Anti-inflammatory. Prevention of autoimmune diseases. Can contribute to infection and tumor progression [53,54]. | Higher levels of IL-10 have been detected in MF/SS biopsies compared with normal skin [55,56]. | Vorinostat and romidepsin may exert their therapeutic action due to the downregulation of IL-10 RNA expression [56]. Bortezomib modulates cytokine expression in CTCL, acting on TGFβ1 and IL-10 down-regulation [57]. |
| IL-11 | Anti-inflammatory properties. Hematopoiesis, production of platelets from megakaryocytes and hemostasis [58]. | Unknown. | No studies available. |
| IL-12 | Activates NK cells and promotes the differentiation of Th1 cells [59]. | Reduces or suppresses IL-12 pathway in CTCL developement, especially in SS patients [60]. | Recombinant IL-12 may restore NK cells functions in CTCLs [61]. |
| IL-13 | Anti-inflammatory. Related to Th2 axis [62]. | IL-13 may act as an autocrine factor in lymphoma cell proliferation through IL-13Rα1 and IL-13-Rα2 signaling [63]. A higher expression of IL-13 and its receptors correlates with late stages, while in the early stages, the expression is low [63]. | Dupilumab, by blocking IL-4 and IL-13, could induce an immune system against the tumor and halt Th2 cell proliferation, but this is debated due to reported misdiagnosed MFs treated with dupilumab with a dramatic progression [34,35,36]. |
| IL-14 | Growth of B cells. Produced by T cells and certain malignant B cells [64]. | Unknown. | No studies available. |
| IL-15 | Enhances CD8 T cell cytotoxic activity, B cell differentiation, Ig synthesis, and DC maturation [65]. | This is implied in the recruitment of CD4+ memory T cells to the skin, induction of T cell proliferation, and inhibition of apoptotic cell death [65,66]. Linked to later stages of CTCL and assumed to promote disease progression [67]. | HDAC inhibitors [68] could halt Zeb1, which leads to an overexpression of IL-15 [69]. |
| IL-16 | Pro-inflammatory [70]. | Related to early MF stages [70]. | No studies available. |
| IL-17 | Enhances immune response against infectious agents. Upregulates pro-inflammatory cytokines, chemokines, metalloproteinases, and antimicrobial peptides [71]. | Low expression of IL-17 mRNA levels in MF/SS samples compared to healthy donors [66,72]. | No studies available. |
| IL-18 | Has a role in adaptive immunity. Induces IFN-γ production [73]. | Higher expression in all MF stages compared to control cases. Role in tumor escape in SS [74]. | No studies available. |
| IL-19 | Pro-inflammatory [75]. | IL-19 levels correlated positively with HMGB1, a protein associated with angiogenesis, Th2 polarization, and CTCL progression [76] | No studies available. |
| IL-20 | Immunosuppression [75]. | Unknown. | No studies available. |
| IL-21 | IL-21 enhances cytotoxicity and induces the production of IFN-γ and perforin by NK cells [77]. | Unknown. | No studies available. |
| IL-22 | Immunosuppression [54]. | IL-22 could play a role in establishing the tumor microenvironment in MF [78]. | No studies available. |
| IL-23 | Pro-inflammatory [79]. | Unknown. | No studies available. |
| IL-24 | Pro-inflammatory [54]. | Unknown. | No studies available. |
| IL-25 | Causes Th2 phenotype polarization and IL-4, IL-5, and IL-13 production. It inhibits TH1 and TH17 responses through inhibition of IL-12 and IL-23 [80]. | Higher expression in MF and SS epidermal keratinocytes, compared to controls. IL-25 levels in skin lesions related to disease progression and serum levels correlated with LDH levels [41]. IL-25 enhances IL-13 production by tumor shifting to a Th2 dominant microenvironment [80]. | No studies available. |
| IL-26 | Pro-inflammatory [54]. | Unknown. | No studies available. |
| IL-27 | IL-27 promotes Th1 immunity, IFN-γ production by NK and T cells, and inhibits Th2 response [81]. | IL-27 levels were higher in advanced stages compared to early stages or controls in MF [81,82]. | No studies available. |
| IL-28 | See IFN-γ. | Unknown. | No studies available. |
| IL-29 | See IFN-γ. | Unknown. | No studies available. |
| IL-30 | Pro-inflammatory [81]. | Unknown. | No studies available. |
| IL-31 | Produced by CD4+ Th2 cells, mast cells, and dendritic cells. It modulates fibroblasts and eosinophils. | Increased levels have been detected in both serum and skin lesions [83]. It seems to be related to pruritus, but is still debated [49,84,85]. | No studies available. |
| IL-32 | Immunosuppression and cancer progression [30]. | IL-32 is associated with MF development and progression [86]. | No studies available. |
| IL-33 | Causes M2 macrophages’ differentiation and induces maturation of dendritic cells [87]. Promotes Th1-mediated responses, including cell-mediated cytotoxicity [88]. | IL-33 might accelerate MF progression via a paracrine action in the tumor microenvironment [89,90]. | No studies available. |
| TNF-A | Pro-inflammatory [91]. | TNF–α has been implicated in the development of CTCL by the promotion of epidermotropism via the induction of interferon-inducible protein. TNF-a acts as an autocrine growth factor, enhancing its tumorigenic action and empowering the NF/KB pathway. | No studies available. |
| EGF | Epidermal stem cells’ proliferation and differentiation [92]. | Possible role due to its known effect in inhibiting IFN type 1, low levels of which are related to MF development [91]. | No studies available. |
| FGF | Mesenchimal stem cells’ proliferation, survival, migration, and differentiation [93]. | FGF’s role has been hypothesized in paraneoplastic scleroderma in MF [93]. | No studies available. |
| PDGFR | Varous cellular lines’ growth, proliferation, and differentiation [94]. | Unknown. | No studies available. |
| IFN [95,96] | Pro-inflammatory [97,98,99,100]. | The administration of IFN -α is a well-known efficient treatment in advanced-stage CTCLS [96,97,98,99,100,101]. Recombinant IFN-γ a in monotherapy or in association with other therapies, such as phototherapy, bexarotene, vorinostat, and ifn-α, did not provide clear-cut results due to the small sample size of the studies [96,97,98,99,100,101]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guglielmo, A.; Zengarini, C.; Agostinelli, C.; Motta, G.; Sabattini, E.; Pileri, A. The Role of Cytokines in Cutaneous T Cell Lymphoma: A Focus on the State of the Art and Possible Therapeutic Targets. Cells 2024, 13, 584. https://doi.org/10.3390/cells13070584
Guglielmo A, Zengarini C, Agostinelli C, Motta G, Sabattini E, Pileri A. The Role of Cytokines in Cutaneous T Cell Lymphoma: A Focus on the State of the Art and Possible Therapeutic Targets. Cells. 2024; 13(7):584. https://doi.org/10.3390/cells13070584
Chicago/Turabian StyleGuglielmo, Alba, Corrado Zengarini, Claudio Agostinelli, Giovanna Motta, Elena Sabattini, and Alessandro Pileri. 2024. "The Role of Cytokines in Cutaneous T Cell Lymphoma: A Focus on the State of the Art and Possible Therapeutic Targets" Cells 13, no. 7: 584. https://doi.org/10.3390/cells13070584
APA StyleGuglielmo, A., Zengarini, C., Agostinelli, C., Motta, G., Sabattini, E., & Pileri, A. (2024). The Role of Cytokines in Cutaneous T Cell Lymphoma: A Focus on the State of the Art and Possible Therapeutic Targets. Cells, 13(7), 584. https://doi.org/10.3390/cells13070584







