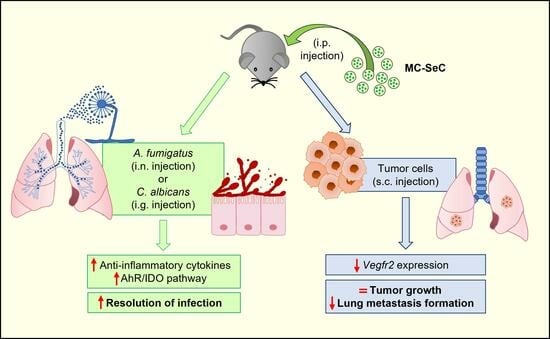
Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Animal Models of Tumor Growth and Fungal Infection
2.3. SeC Purification and Characterization
2.4. Preparation of the MC-SeCs
2.5. Histology and Immunohistochemistry
2.6. Morphological Quantification
2.7. Bronchoalveolar Lavage Fluid (BALF) Morphometry
2.8. Real-Time PCR
2.9. Statistical Analysis
2.10. Ethics Approval
3. Results
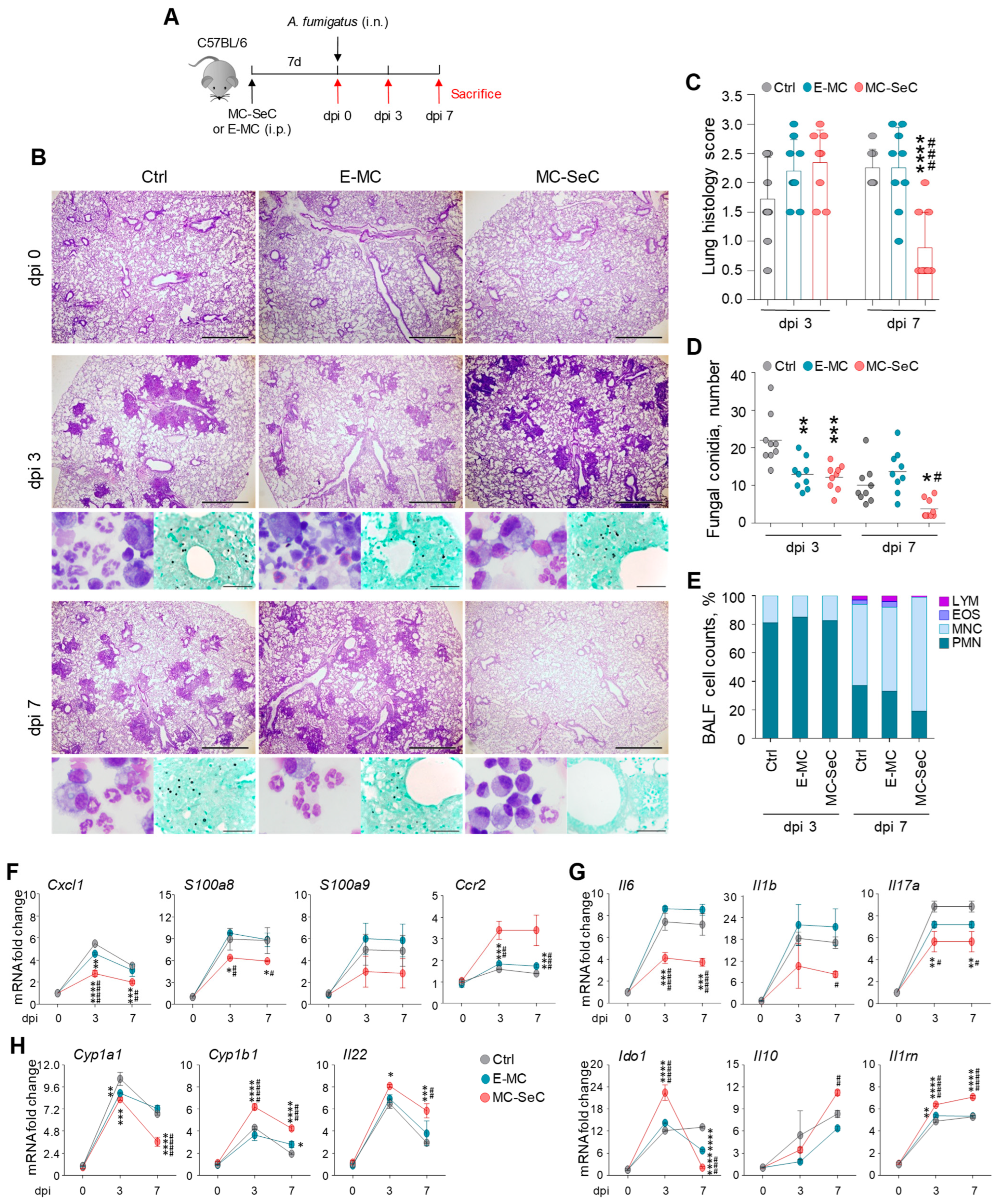
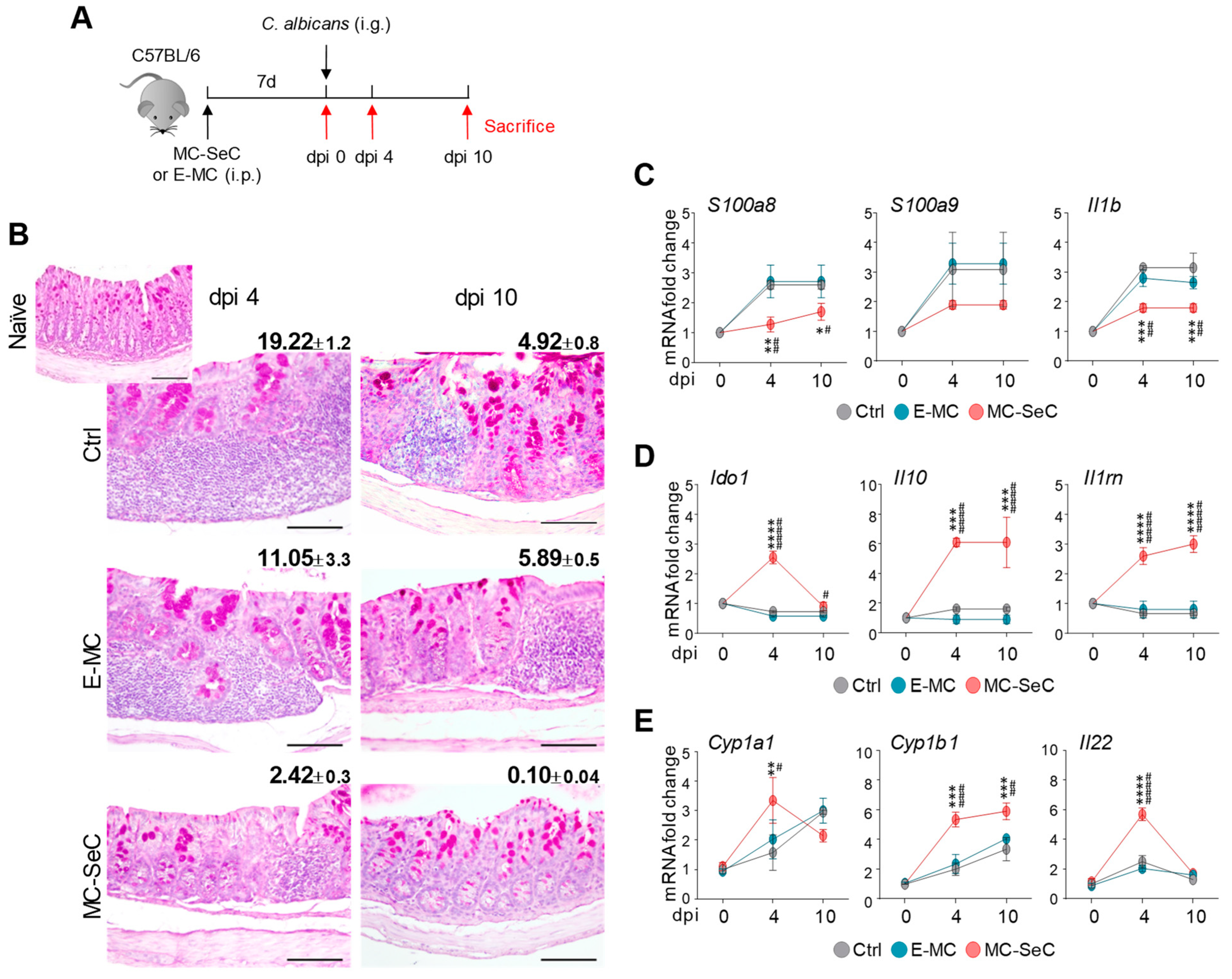
3.1. Grafted MC-SeCs Restrain Immunopathology in Infection
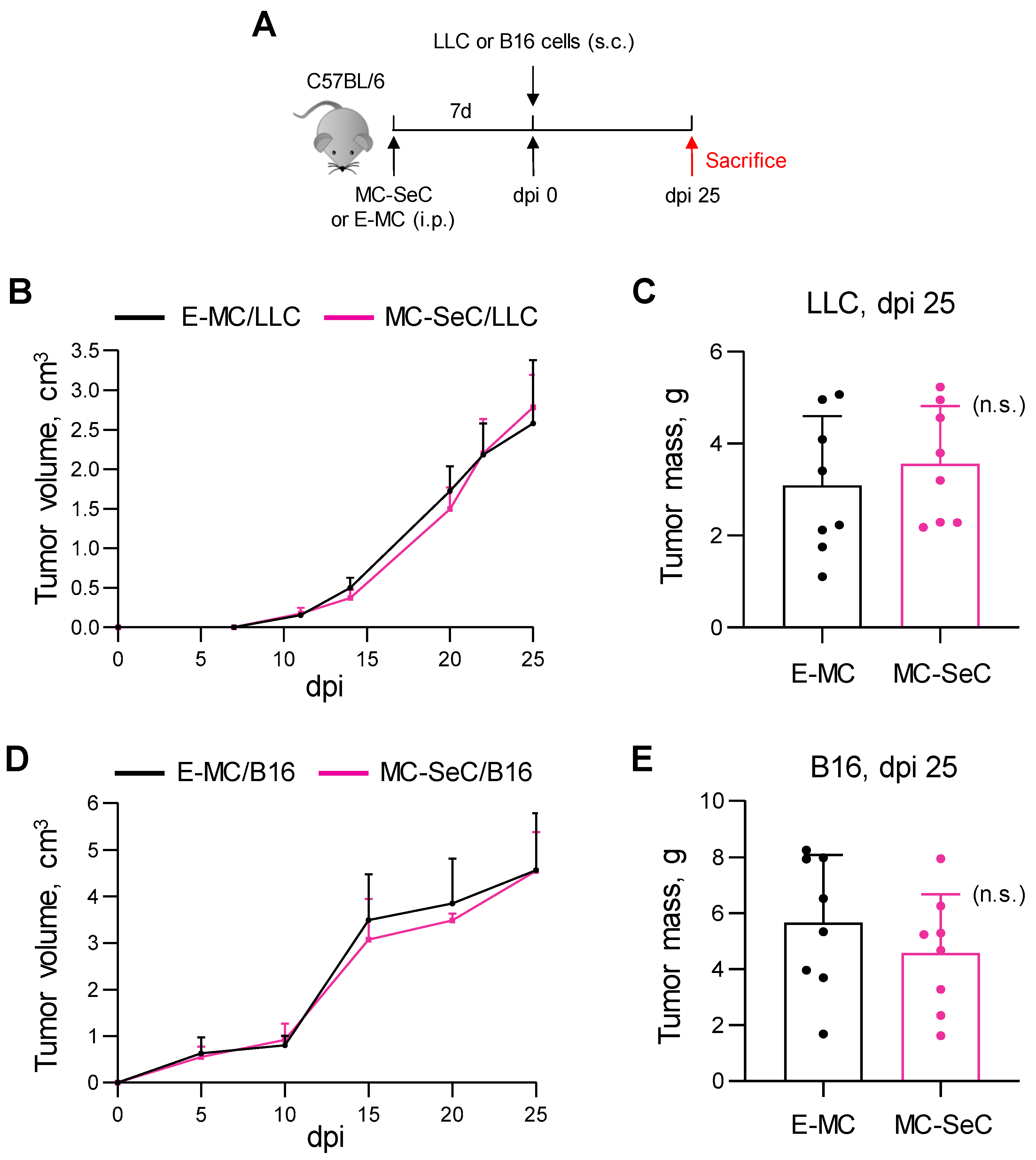
3.2. Grafted MC-SeCs Reduce Metastatic Cancer Spread
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mital, P.; Kaur, G.; Dufour, J.M. Immunoprotective Sertoli cells: Making allogeneic and xenogeneic trasplatantion feasible. Reproduction 2010, 139, 495–504. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells-immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef]
- Selawry, H.P.; Kotb, M.; Herrod, H.G.; Lu, Z.N. Production of a factor, or factors, suppressing IL-2 production and T cell proliferation by Sertoli cell-enriched preparations. A potential role for islet transplantation in an immunologically privileged site. Transplantation 1991, 52, 846–850. [Google Scholar] [CrossRef]
- De Cesaris, P.; Filippini, A.; Cervelli, C.; Riccioli, A.; Muci, S.; Starace, G.; Stefanini, M.; Ziparo, E. Immunosuppressive molecules produced by Sertoli cells cultured in vitro: Biological effects on lymphocytes. Biochem. Biophys. Res. Commun. 1992, 186, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Pinzon, W.; Korbutt, G.S.; Power, R.; Hooton, J.; Rajotte, R.V.; Rabinovitch, A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes 2000, 49, 1810–1818. [Google Scholar] [CrossRef]
- Campese, A.F.; Grazioli, P.; de Cesaris, P.; Riccioli, A.; Bellavia, D.; Pelullo, M.; Padula, F.; Noce, C.; Verkhovskaia, S.; Filippini, A.; et al. Mouse Sertoli cells sustain de novo generation of regulatory T cells by triggering the notch pathway through soluble JAGGED1. Biol. Reprod. 2014, 90, 53. [Google Scholar] [CrossRef]
- Chiappalupi, S.; Salvadori, L.; Luca, G.; Riuzzi, F.; Calafiore, R.; Donato, R.; Sorci, G. Do porcine Sertoli cells represent an opportunity for Duchenne muscular dystrophy? Cell. Prolif. 2019, 52, e12599. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Gotoh, M.; Dono, K.; Nishihara, M.; Grochowiecki, T.; Kimura, F.; Yoshida, T.; Ohta, Y.; Ota, H.; Ohzato, H.; et al. Protection of islet allografts transplanted together with Fas ligand expressing testicular allografts. Diabetologia 1998, 41, 315–321. [Google Scholar] [CrossRef]
- Wright, K.; Dziuk, R.; Mital, P.; Kaur, G.; Dufour, J.M. Xenotransplanted Pig Sertoli Cells Inhibit Both the Alternative and Classical Pathways of Complement-Mediated Cell Lysis While Pig Islets Are Killed. Cell Transplant. 2016, 25, 2027–2040. [Google Scholar] [CrossRef]
- Tokuda, N.; Kasahara, M.; Levy, R.B. Differential regulation and expression of major histocompatibility complex (MHC) and Ly-6 gene products on mouse testicular Leydig and Sertoli cell lines. J. Autoimmun. 1990, 3, 457–471. [Google Scholar] [CrossRef]
- Kaur, G.; Wright, K.; Mital, P.; Hibler, T.; Miranda, J.M.; Thompson, L.A.; Halley, K.; Dufour, J.M. Neonatal Pig Sertoli Cells Survive Xenotransplantation by Creating an Immune Modulatory Environment Involving CD4 and CD8 Regulatory T Cells. Cell Transplant. 2020, 29, 963689720947102. [Google Scholar] [CrossRef]
- Qu, N.; Ogawa, Y.; Kuramasu, M.; Nagahori, K.; Sakabe, K.; Itoh, M. Immunological microenvironment in the testis. Reprod. Med. Biol. 2019, 19, 24–31. [Google Scholar] [CrossRef]
- Luca, G.; Arato, I.; Sorci, G.; Cameron, D.F.; Hansen, B.C.; Baroni, T.; Donato, R.; White, D.G.J.; Calafiore, R. Sertoli cells for cell transplantation: Pre-clinical studies and future perspectives. Andrology 2018, 6, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.L.; Hibler, T.; Thompson, L.A.; Kaur, G.; Dufour, J.M. Therapeutic application of Sertoli cells for treatment of various diseases. Semin. Cell Dev. Biol. 2022, 121, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Chiappalupi, S.; Luca, G.; Mancuso, F.; Madaro, L.; Fallarino, F.; Nicoletti, C.; Calvitti, M.; Arato, I.; Falabella, G.; Salvadori, L.; et al. Intraperitoneal injection of microencapsulated Sertoli cells restores muscle morphology and performance in dystrophic mice. Biomaterials 2016, 75, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Chiappalupi, S.; Salvadori, L.; Mancuso, F.; Arato, I.; Calvitti, M.; Riuzzi, F.; Calafiore, R.; Luca, G.; Sorci, G. Microencapsulated Sertoli cells sustain myoblast proliferation without affecting the myogenic potential. In vitro data. Data Brief 2021, 40, 107744. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; Puccetti, M.; Pariano, M.; Renga, G.; Stincardini, C.; Ricci, M.; Giovagnoli, S.; Costantini, C.; Romani, L. Tryptophan as a Central Hub for Host/Microbial Symbiosis. Int. J. Tryptophan Res. 2020, 13, 1178646920919755. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Ballarò, R.; Costelli, P.; Penna, F. Animal models for cancer cachexia. Curr. Opin. Support. Palliat. Care. 2016, 10, 281–287. [Google Scholar] [CrossRef]
- Giavazzi, R.; Decio, A. Syngeneic murine metastasis models: B16 melanoma. Methods Mol. Biol. 2014, 1070, 131–140. [Google Scholar] [CrossRef]
- Ya, Z.; Hailemichael, Y.; Overwijk, W.; Restifo, N.P. Mouse model for pre-clinical study of human cancer immunotherapy. Curr. Protoc. Immunol. 2015, 108, 20.1.1–20.1.43. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2020, 21, 341–352, published correction appears in Nat. Rev. Mol. Cell. Biol. 2021, 22, 834. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Lian, L.; Li, X.L.; Xu, M.D.; Li, X.M.; Wu, M.Y.; Zhang, Y.; Tao, M.; Li, W.; Shen, X.M.; Zhou, C.; et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer 2019, 19, 183. [Google Scholar] [CrossRef]
- Ezekian, B.; Schroder, P.M.; Freischlag, K.; Yoon, J.; Kwun, J.; Knechtle, S.J. Contemporary Strategies and Barriers to Transplantation Tolerance. Transplantation 2018, 102, 1213–1222. [Google Scholar] [CrossRef]
- Marcen, R. Immunosuppressive drugs in kidney transplantation: Impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 2009, 69, 2227–2243. [Google Scholar] [CrossRef]
- Riccioli, A.; Starace, D.; Galli, R.; Fuso, A.; Scarpa, S.; Palombi, F.; De Cesaris, P.; Ziparo, E.; Filippini, A. Sertoli cells initiate testicular innate immune responses through TLR activation. J. Immunol. 2006, 177, 7122–7130. [Google Scholar] [CrossRef]
- Lakpour, M.R.; Koruji, M.; Shahverdi, A.; Aghajanpour, S.; Rajabian Naghandar, M.; Sadighi Gilani, M.A.; Sabbaghian, M.; Aflatoonian, R. The Expression of TLR2 and TLR3 in Sertoli Cells of Azoospermic Patients. Cell J. 2017, 19, 375–385. [Google Scholar] [CrossRef]
- Renga, G.; D’Onofrio, F.; Pariano, M.; Galarini, R.; Barola, C.; Stincardini, C.; Bellet, M.M.; Ellemunter, H.; Lass-Flörl, C.; Costantini, C.; et al. Bridging of host-microbiota tryptophan partitioning by the serotonin pathway in fungal pneumonia. Nat. Commun. 2023, 14, 5753. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, A.; Osada, Y.; Nakanishi, Y. Differences in the mode of phagocytosis of bacteria between macrophages and testicular Sertoli cells. Drug Discov. Ther. 2013, 7, 73–77. [Google Scholar]
- Washburn, R.L.; Hibler, T.; Kaur, G.; Dufour, J.M. Sertoli Cell Immune Regulation: A Double-Edged Sword. Front. Immunol. 2022, 13, 913502. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L., Jr.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A. Mechanisms by which IGF-I may promote cancer. Cancer Biol. Ther. 2003, 2, 630–635. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Qvist, G. John Hunter 1728–1793; William Heinemann Medical Books Ltd.: London, UK, 1981. [Google Scholar]
- Doyle, T.J.; Kaur, G.; Putrevu, S.M.; Dyson, E.L.; Dyson, M.; McCunniff, W.T.; Pasham, M.R.; Kim, K.H.; Dufour, J.M. Immunoprotective properties of primary Sertoli cells in mice: Potential functional pathways that confer immune privilege. Biol. Reprod. 2012, 86, 1–14. [Google Scholar] [CrossRef]
- Chamberlain, J.S.; Metzger, J.; Reyes, M.; Townsend, D.; Faulkner, J.A. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007, 21, 2195–2204. [Google Scholar] [CrossRef]
- Valdés-González, R.A.; Dorantes, L.M.; Garibay, G.N.; Bracho-Blanchet, E.; Mendez, A.J.; Dávila-Pérez, R.; Elliott, R.B.; Terán, L.; White, D.J. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: A 4-year study. Eur. J. Endocrinol. 2005, 153, 419–427. [Google Scholar] [CrossRef]
- Esquivel-Pérez, R.; Rodriguez-Ventura, A.L.; Dorantes, L.M.; Ramírez-González, B.; López-Santos, M.G.; Valdes-Gonzalez, R. Correlation between insulin requirements and anti-galactose antibodies in patients with type 1 diabetes transplanted with neonatal pig islets. Clin. Exp. Immunol. 2011, 165, 104–109. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappalupi, S.; Salvadori, L.; Borghi, M.; Mancuso, F.; Pariano, M.; Riuzzi, F.; Luca, G.; Romani, L.; Arato, I.; Sorci, G. Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer. Cells 2024, 13, 544. https://doi.org/10.3390/cells13060544
Chiappalupi S, Salvadori L, Borghi M, Mancuso F, Pariano M, Riuzzi F, Luca G, Romani L, Arato I, Sorci G. Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer. Cells. 2024; 13(6):544. https://doi.org/10.3390/cells13060544
Chicago/Turabian StyleChiappalupi, Sara, Laura Salvadori, Monica Borghi, Francesca Mancuso, Marilena Pariano, Francesca Riuzzi, Giovanni Luca, Luigina Romani, Iva Arato, and Guglielmo Sorci. 2024. "Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer" Cells 13, no. 6: 544. https://doi.org/10.3390/cells13060544
APA StyleChiappalupi, S., Salvadori, L., Borghi, M., Mancuso, F., Pariano, M., Riuzzi, F., Luca, G., Romani, L., Arato, I., & Sorci, G. (2024). Grafted Sertoli Cells Exert Immunomodulatory Non-Immunosuppressive Effects in Preclinical Models of Infection and Cancer. Cells, 13(6), 544. https://doi.org/10.3390/cells13060544