HSP47 Increases the Expression of Type I Collagen in Fibroblasts through IRE1α Activation, XBP1 Splicing, and Nuclear Translocation of β-Catenin
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Acquisition of Mouse Skin Tissues
2.3. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
2.4. Preparation of Skin Tissue Extracts and Western Blot Analysis
2.5. Cell Culture
2.6. Construction of Lentiviral Transfer Vectors Harboring Human HSP47 and XBP1-s cDNAs
2.7. Production and Infection of Lentiviruses
2.8. Preparation of Conditioned Media and Cell Lysates and Western Blot Analysis
2.9. Production and Treatment of a Wnt3a-Containing Conditioned Medium
2.10. Dual-Luciferase Reporter Assay
3. Results
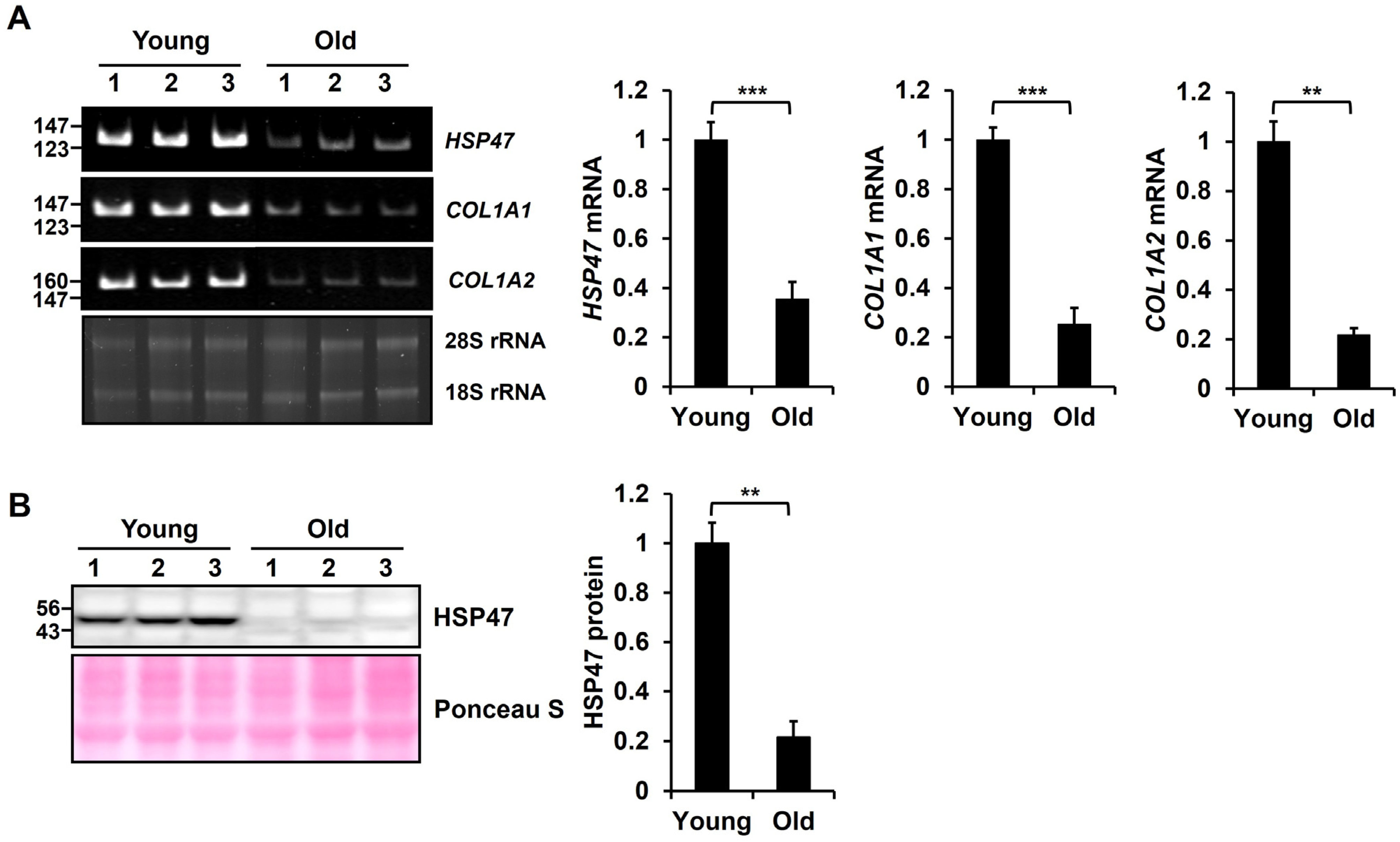
3.1. Expression of HSP47 Is Downregulated in Old Mouse Skin Tissues
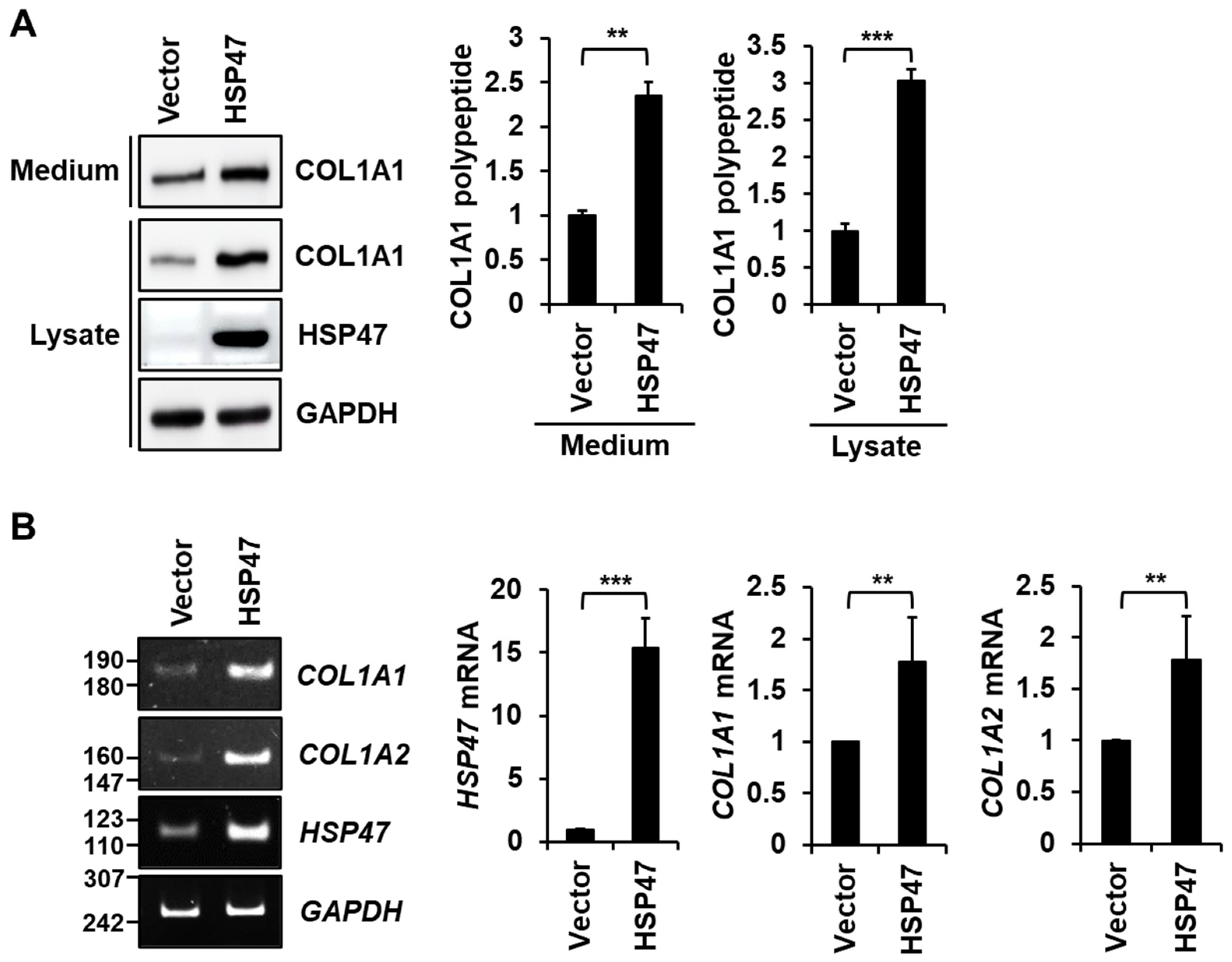
3.2. HSP47 Overexpression Enhances the Secretion and Expression of Type I Collagen in Human Fibroblasts
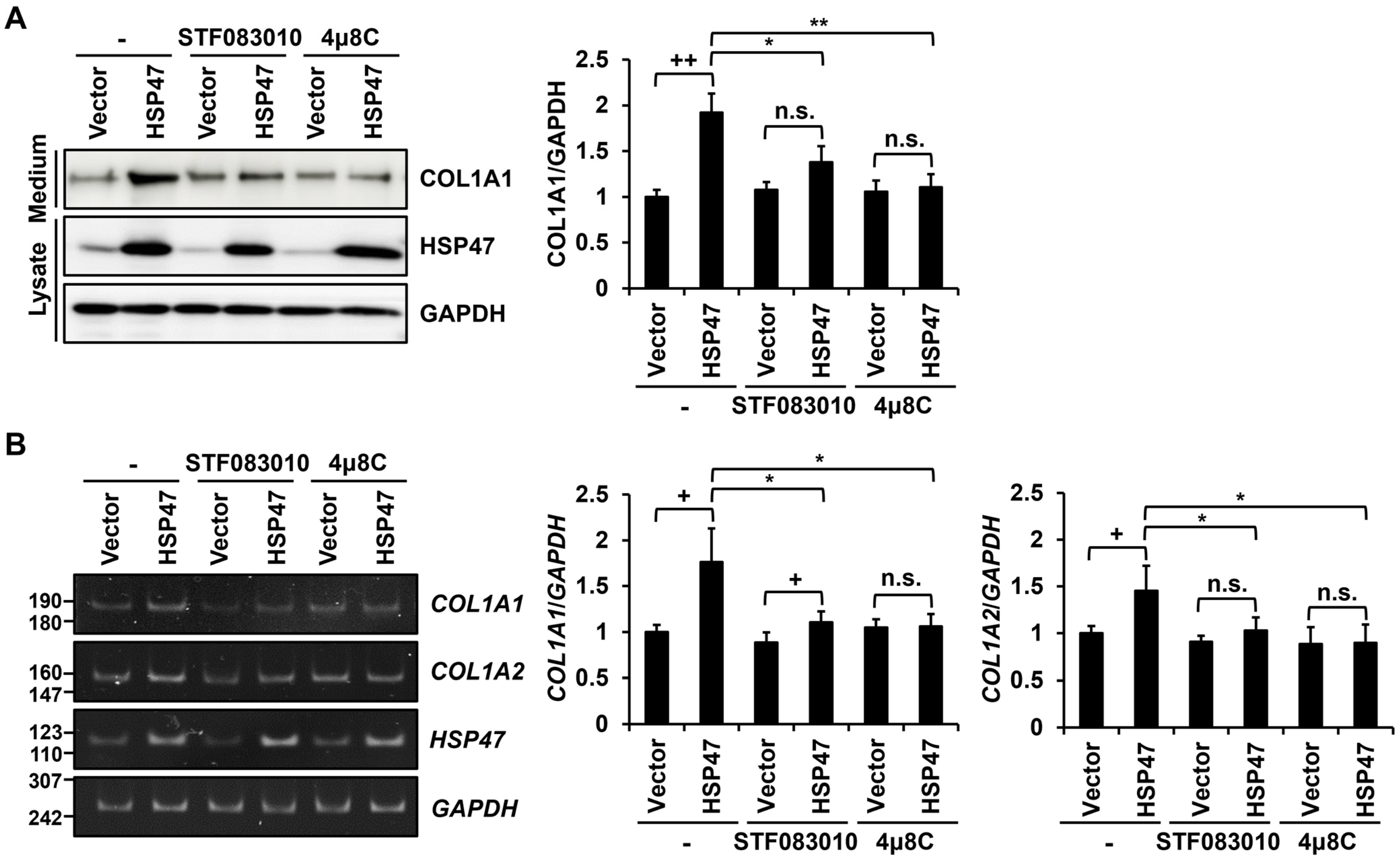
3.3. IRE1α Inhibitors Block the HSP47-Induced Increase in Type I Collagen Secretion and Expression in Human Fibroblasts
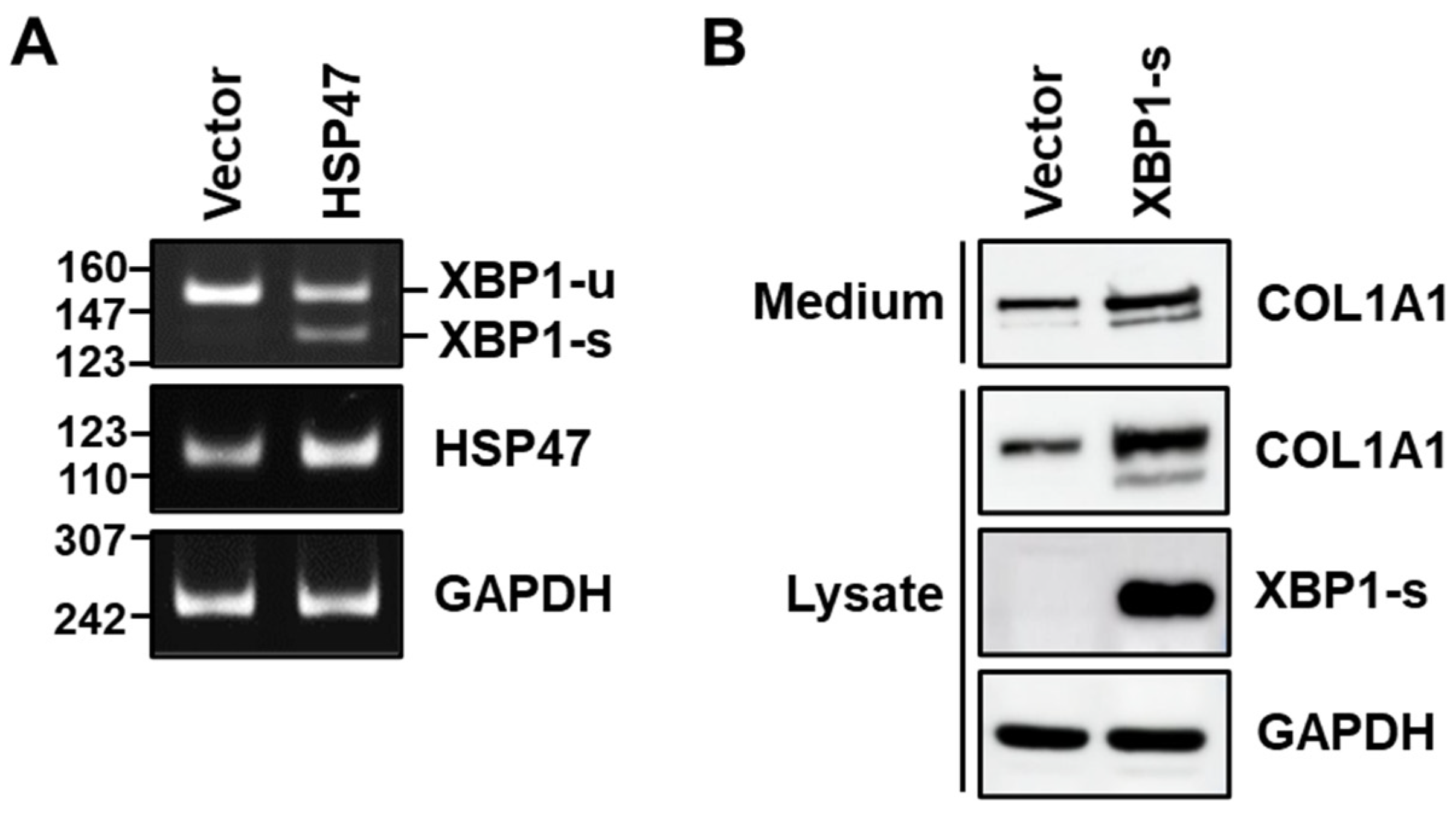
3.4. HSP47 Induces Splicing of XBP1 in Human Fibroblasts
3.5. Overexpression of HSP47 or XBP1-s Increases Nuclear Translocation of β-Catenin in Human Fibroblasts
3.6. Overexpression of HSP47 or XBP1-s, as well as Treatment with Wnt3a, Enhances TCF Reporter Activity
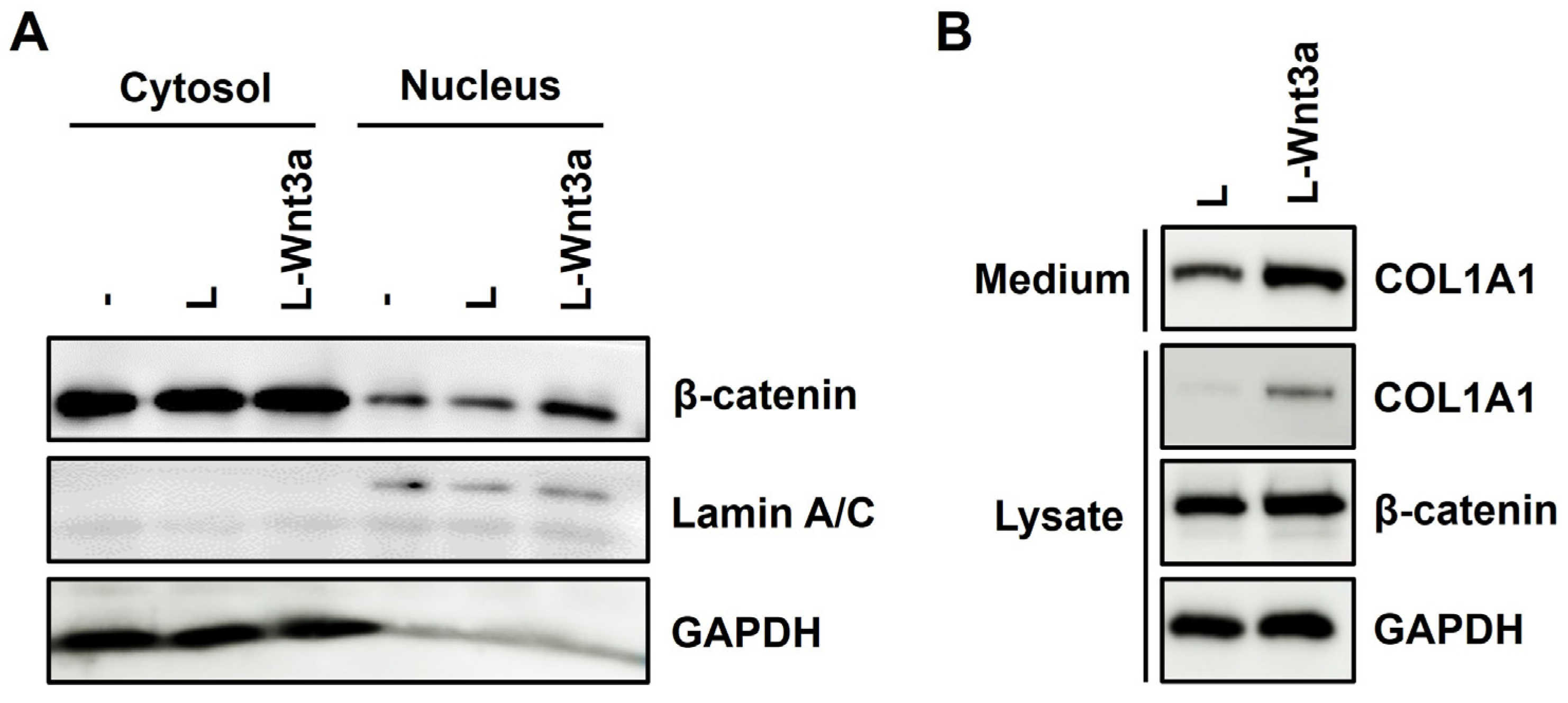
3.7. Treatment of Wnt3a Increases Nuclear Translocation of β-Catenin and Expression of Type I Collagen in Human Fibroblasts
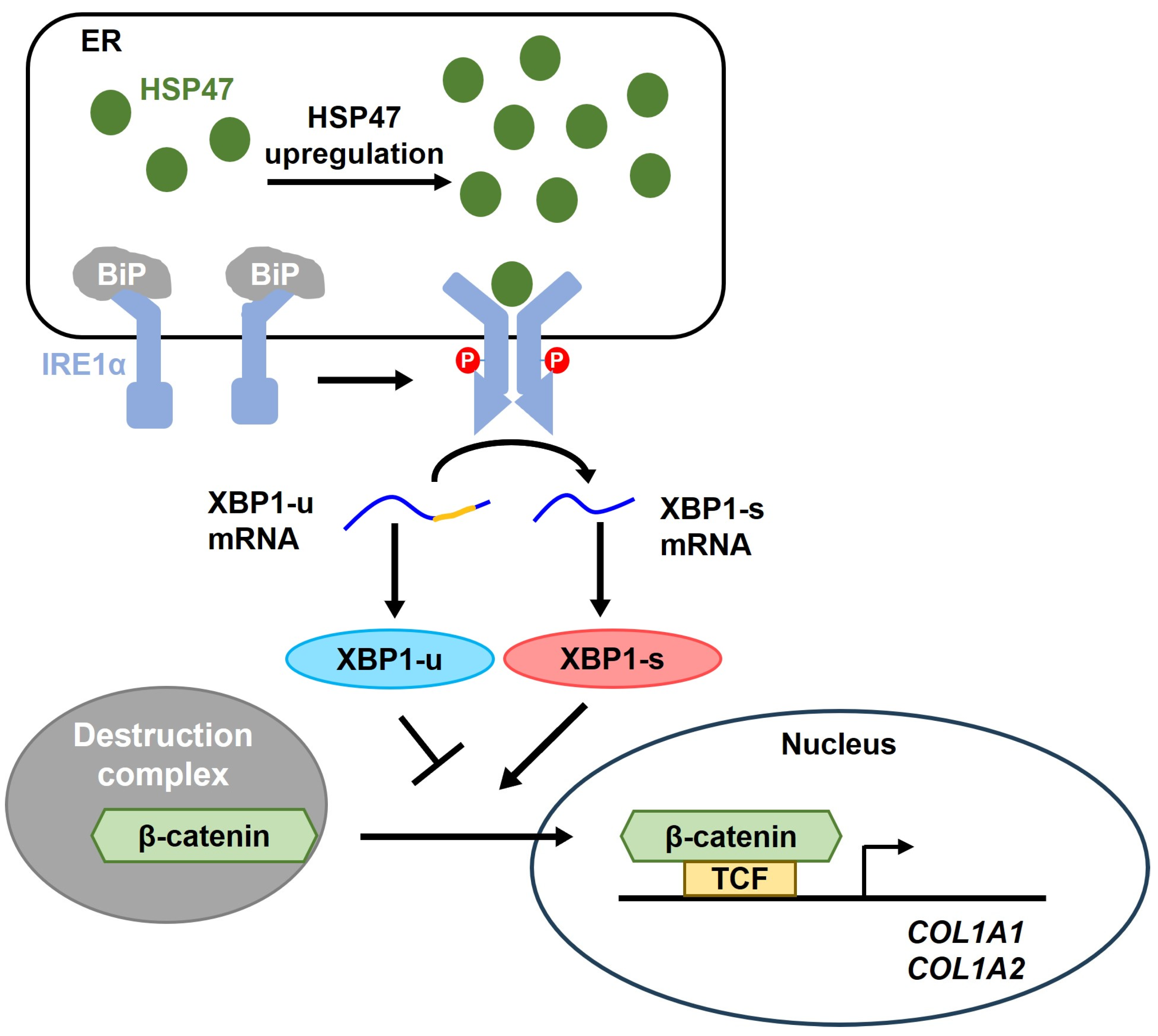
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of hydrolyzed collagen supplementation on skin aging: A systematic review and meta-analysis. Int. J. Dermatol. 2021, 60, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Binti Haji Idrus, R. Collagen Type I: A Versatile Biomaterial. Adv. Exp. Med. Biol. 2018, 1077, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Kisling, A.; Lust, R.M.; Katwa, L.C. What is the role of peptide fragments of collagen I and IV in health and disease? Life Sci. 2019, 228, 30–34. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J. Biol. Chem. 2019, 294, 2133–2141. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Kubota, H.; Adachi, E.; Nagai, N.; Marutani, T.; Hosokawa, N.; Nagata, K. Insufficient folding of type IV collagen and formation of abnormal basement membrane-like structure in embryoid bodies derived from Hsp47-null embryonic stem cells. Mol. Biol. Cell 2004, 15, 4467–4475. [Google Scholar] [CrossRef]
- Ishida, Y.; Kubota, H.; Yamamoto, A.; Kitamura, A.; Bächinger, H.P.; Nagata, K. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol. Biol. Cell 2006, 17, 2346–2355. [Google Scholar] [CrossRef]
- Ishida, Y.; Nagata, K. Chapter nine—Hsp47 as a Collagen-Specific Molecular Chaperone. In Methods in Enzymology; Whisstock, J.C., Bird, P.I., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 499, pp. 167–182. [Google Scholar]
- Ishida, Y.; Yamamoto, A.; Kitamura, A.; Lamandé, S.R.; Yoshimori, T.; Bateman, J.F.; Kubota, H.; Nagata, K. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell 2009, 20, 2744–2754. [Google Scholar] [CrossRef]
- Nagai, N.; Hosokawa, M.; Itohara, S.; Adachi, E.; Matsushita, T.; Hosokawa, N.; Nagata, K. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J. Cell Biol. 2000, 150, 1499–1506. [Google Scholar] [CrossRef]
- Sakamoto, N.; Okuno, D.; Tokito, T.; Yura, H.; Kido, T.; Ishimoto, H.; Tanaka, Y.; Mukae, H. HSP47: A Therapeutic Target in Pulmonary Fibrosis. Biomedicines 2023, 11, 2387. [Google Scholar] [CrossRef]
- Khan, E.S.; Sankaran, S.; Paez, J.I.; Muth, C.; Han, M.K.L.; Del Campo, A. Photoactivatable Hsp47: A Tool to Regulate Collagen Secretion and Assembly. Adv. Sci. 2019, 6, 1801982. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Arnold, S.M.; Miller, C.N.; Wu, J.; Li, J.; Gunnison, K.M.; Mori, K.; Sadighi Akha, A.A.; Raden, D.; Kaufman, R.J. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006, 4, e374. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Mizuno, T.; Koyama, Y.; Katayama, T.; Tohyama, M. The endoplasmic reticulum-resident chaperone heat shock protein 47 protects the Golgi apparatus from the effects of O-glycosylation inhibition. PLoS ONE 2013, 8, e69732. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M.M.U. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019, 26, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Siwecka, N.; Rozpędek-Kamińska, W.; Wawrzynkiewicz, A.; Pytel, D.; Diehl, J.A.; Majsterek, I. The Structure, Activation and Signaling of IRE1 and Its Role in Determining Cell Fate. Biomedicines 2021, 9, 156. [Google Scholar] [CrossRef]
- Heindryckx, F.; Binet, F.; Ponticos, M.; Rombouts, K.; Lau, J.; Kreuger, J.; Gerwins, P. Endoplasmic reticulum stress enhances fibrosis through IRE1α-mediated degradation of miR-150 and XBP-1 splicing. EMBO Mol. Med. 2016, 8, 729–744. [Google Scholar] [CrossRef]
- Joshi, A.; Newbatt, Y.; McAndrew, P.C.; Stubbs, M.; Burke, R.; Richards, M.W.; Bhatia, C.; Caldwell, J.J.; McHardy, T.; Collins, I.; et al. Molecular mechanisms of human IRE1 activation through dimerization and ligand binding. Oncotarget 2015, 6, 13019–13035. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Ryno, L.M.; Genereux, J.C.; Moresco, J.J.; Tu, P.G.; Wu, C.; Yates, J.R., 3rd; Su, A.I.; Kelly, J.W.; Wiseman, R.L. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013, 3, 1279–1292. [Google Scholar] [CrossRef]
- Lee, A.H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef]
- Tam, A.B.; Koong, A.C.; Niwa, M. Ire1 has distinct catalytic mechanisms for XBP1/HAC1 splicing and RIDD. Cell Rep. 2014, 9, 850–858. [Google Scholar] [CrossRef]
- Hollien, J.; Lin, J.H.; Li, H.; Stevens, N.; Walter, P.; Weissman, J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009, 186, 323–331. [Google Scholar] [CrossRef]
- Lamriben, L.; Hebert, D.N. Activating and Repressing IRE1α: The Hsp47 and BiP Tug of War. Mol. Cell 2018, 69, 159–160. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Duschl, J.; Steinbacher, P.; Salzmann, M.; Bischof, J.; Schuller, M.; Wimmer, H.; Peer, T.; Bauer, J.W.; Richter, K. Age-related changes in the composition of the cornified envelope in human skin. Exp. Dermatol. 2013, 22, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nagata, K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 2017, 62, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.D.; Kim, J.H.; Kwon, G.E.; Lee, S.T. Expression of Polyamine Oxidase in Fibroblasts Induces MMP-1 and Decreases the Integrity of Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 10487. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Song, M.J.; Hara, M.; Imanaka-Yoshida, K.; Lee, D.H.; Chung, J.H.; Lee, S.T. Effects of Tenascin C on the Integrity of Extracellular Matrix and Skin Aging. Int. J. Mol. Sci. 2020, 21, 8693. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Park, M.K.; Kim, J.H.; Oh, S.W.; Jang, J.Y.; Lee, H.; Lee, S.T. PTK7, a Catalytically Inactive Receptor Tyrosine Kinase, Increases Oncogenic Phenotypes in Xenograft Tumors of Esophageal Squamous Cell Carcinoma KYSE-30 Cells. Int. J. Mol. Sci. 2022, 23, 2391. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Oh, S.W.; Park, H.N.; Kim, J.H.; Lee, S.T. Knockdown of PTK7 Reduces the Oncogenic Potential of Breast Cancer Cells by Impeding Receptor Tyrosine Kinase Signaling. Int. J. Mol. Sci. 2023, 24, 2173. [Google Scholar] [CrossRef]
- Park, H.N.; Song, M.J.; Choi, Y.E.; Lee, D.H.; Chung, J.H.; Lee, S.T. LRG1 Promotes ECM Integrity by Activating the TGF-β Signaling Pathway in Fibroblasts. Int. J. Mol. Sci. 2023, 24, 2445. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.M.; Song, M.J.; Yoon, H.S.; Lee, D.H.; Chung, J.H.; Lee, S.T. SPARC Is Highly Expressed in Young Skin and Promotes Extracellular Matrix Integrity in Fibroblasts via the TGF-β Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12179. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Bose, P.; Leong-Quong, R.Y.; Fujita, D.J.; Riabowol, K. REAP: A two minute cell fractionation method. BMC Res. Notes 2010, 3, 294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, H.D.; Song, M.J.; Lee, D.H.; Chung, J.H.; Lee, S.T. SOD3 Suppresses the Expression of MMP-1 and Increases the Integrity of Extracellular Matrix in Fibroblasts. Antioxidants 2022, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, D.; Rojas-Rivera, D.; Rodríguez, D.A.; Groenendyk, J.; Köhler, A.; Lebeaupin, C.; Ito, S.; Urra, H.; Carreras-Sureda, A.; Hazari, Y.; et al. Interactome Screening Identifies the ER Luminal Chaperone Hsp47 as a Regulator of the Unfolded Protein Response Transducer IRE1α. Mol. Cell 2018, 69, 238–252.e237. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dai, R.; Wu, H.; Cai, Z.; Xie, N.; Zhang, X.; Shen, Y.; Gong, Z.; Jia, Y.; Yu, F.; et al. Unspliced XBP1 Counteracts β-Catenin to Inhibit Vascular Calcification. Circ. Res. 2022, 130, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xiao, Q.; Chen, M.; Margariti, A.; Martin, D.; Ivetic, A.; Xu, H.; Mason, J.; Wang, W.; Cockerill, G.; et al. Vascular endothelial cell growth-activated XBP1 splicing in endothelial cells is crucial for angiogenesis. Circulation 2013, 127, 1712–1722. [Google Scholar] [CrossRef]
- Sobel, K.; Tham, M.; Stark, H.J.; Stammer, H.; Prätzel-Wunder, S.; Bickenbach, J.R.; Boukamp, P. Wnt-3a-activated human fibroblasts promote human keratinocyte proliferation and matrix destruction. Int. J. Cancer 2015, 136, 2786–2798. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Calleja-Agius, J.; Brincat, M.; Borg, M. Skin connective tissue and ageing. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 727–740. [Google Scholar] [CrossRef]
- Rocnik, E.F.; van der Veer, E.; Cao, H.; Hegele, R.A.; Pickering, J.G. Functional linkage between the endoplasmic reticulum protein Hsp47 and procollagen expression in human vascular smooth muscle cells. J. Biol. Chem. 2002, 277, 38571–38578. [Google Scholar] [CrossRef]
- Guo, Q.; Tian, Q.; Tian, X.; Liu, T. Effect of Regulating the Expression of HSP47 on Collagen Metabolism in Scleral Fibroblasts. Curr. Eye Res. 2021, 46, 408–416. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, C.; Liu, H.; Tang, S. High glucose induces HSP47 expression and promotes the secretion of inflammatory factors through the IRE1α/XBP1/HIF-1α pathway in retinal Müller cells. Exp. Ther. Med. 2021, 22, 1411. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Roh, M.R.; Rajadurai, S.; Rajadurai, A.; Kumar, R.; Njauw, C.N.; Zheng, Z.; Tsao, H. Hypoxia and HIF-1α Regulate Collagen Production in Keloids. J. Investig. Dermatol. 2020, 140, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yu, P.B. A New Link in the Chain: Unspliced XBP1 in Wnt Signaling and Vascular Calcification. Circ. Res. 2022, 130, 230–233. [Google Scholar] [CrossRef]
- So, J.S. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Kang, T.I.; So, J.S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef]
- Lam, A.P.; Gottardi, C.J. β-catenin signaling: A novel mediator of fibrosis and potential therapeutic target. Curr. Opin. Rheumatol. 2011, 23, 562–567. [Google Scholar] [CrossRef]
- Nusse, R. Wnt signaling in disease and in development. Cell Res. 2005, 15, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ogata, K.; Yoshioka, H.; Shim, J.; Wassif, C.A.; Porter, F.D.; Iwata, J. Disruption of Dhcr7 and Insig1/2 in cholesterol metabolism causes defects in bone formation and homeostasis through primary cilium formation. Bone Res. 2020, 8, 1. [Google Scholar] [CrossRef]
- Roh, M.R.; Kumar, R.; Rajadurai, A.; Njauw, C.; Ryoo, U.H.; Chung, K.Y.; Tsao, H. Beta-catenin causes fibrotic changes in the extracellular matrix via upregulation of collagen I transcription. Br. J. Dermatol. 2017, 177, 312–315. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ham, S.Y.; Pyo, M.J.; Kang, M.; Kim, Y.-S.; Lee, D.H.; Chung, J.H.; Lee, S.-T. HSP47 Increases the Expression of Type I Collagen in Fibroblasts through IRE1α Activation, XBP1 Splicing, and Nuclear Translocation of β-Catenin. Cells 2024, 13, 527. https://doi.org/10.3390/cells13060527
Ham SY, Pyo MJ, Kang M, Kim Y-S, Lee DH, Chung JH, Lee S-T. HSP47 Increases the Expression of Type I Collagen in Fibroblasts through IRE1α Activation, XBP1 Splicing, and Nuclear Translocation of β-Catenin. Cells. 2024; 13(6):527. https://doi.org/10.3390/cells13060527
Chicago/Turabian StyleHam, So Young, Min Ju Pyo, Moonkyung Kang, Yeon-Soo Kim, Dong Hun Lee, Jin Ho Chung, and Seung-Taek Lee. 2024. "HSP47 Increases the Expression of Type I Collagen in Fibroblasts through IRE1α Activation, XBP1 Splicing, and Nuclear Translocation of β-Catenin" Cells 13, no. 6: 527. https://doi.org/10.3390/cells13060527
APA StyleHam, S. Y., Pyo, M. J., Kang, M., Kim, Y.-S., Lee, D. H., Chung, J. H., & Lee, S.-T. (2024). HSP47 Increases the Expression of Type I Collagen in Fibroblasts through IRE1α Activation, XBP1 Splicing, and Nuclear Translocation of β-Catenin. Cells, 13(6), 527. https://doi.org/10.3390/cells13060527





