CD74 Promotes Cyst Growth and Renal Fibrosis in Autosomal Dominant Polycystic Kidney Disease
Abstract
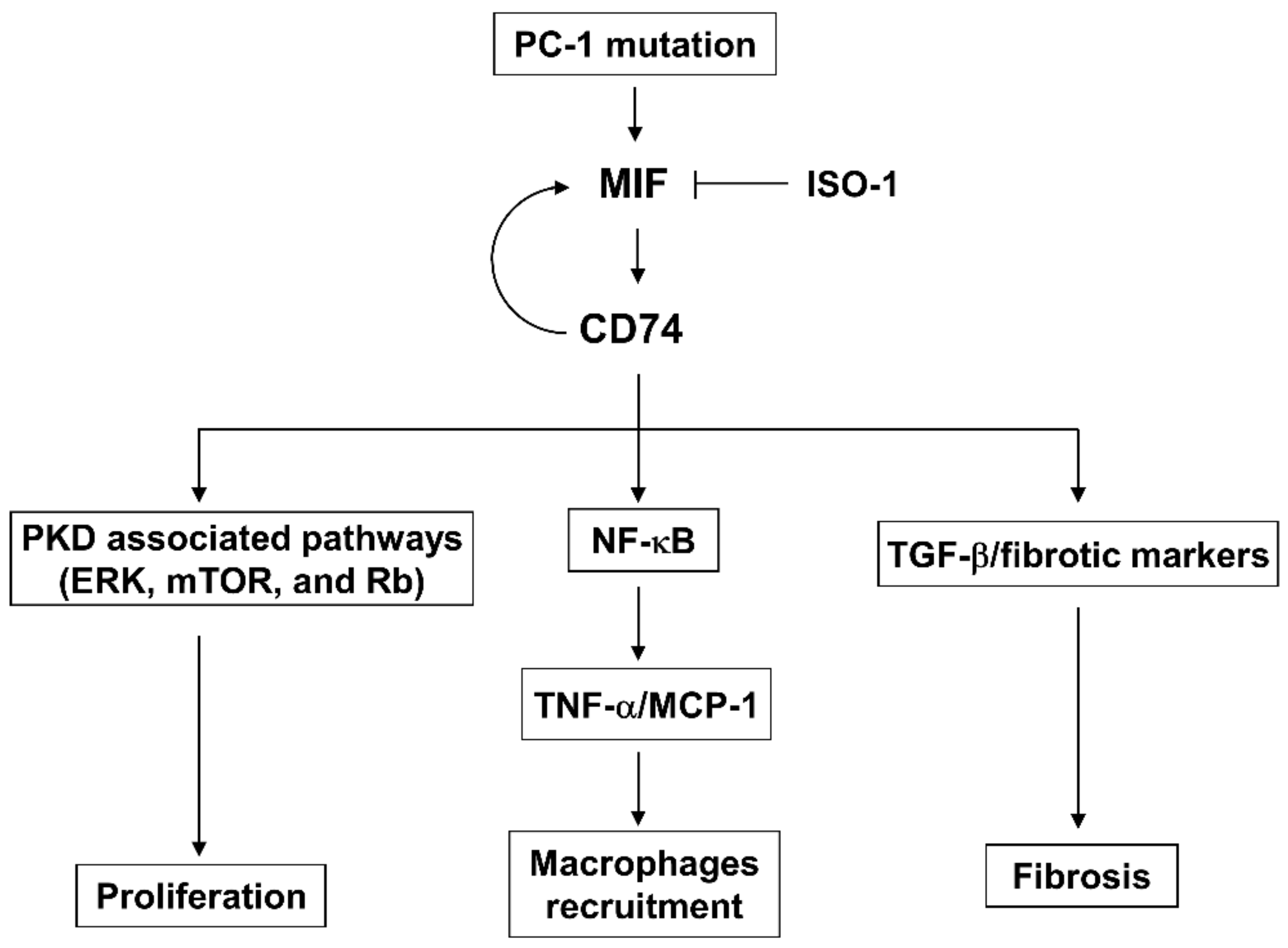
1. Introduction
2. Methods
2.1. Cell Cultures and Reagents
2.2. Western Blot Analysis
2.3. Immunohistochemistry and Immunofluorescence Staining
2.4. TUNEL Assay
2.5. Cystic Index
2.6. Quantitative Reverse-Transcription PCR (qRT-PCR)
2.7. MTS Assay
2.8. RNA Interference
2.9. Chromatin Immunoprecipiation Assay
2.10. Mouse Strains and Treatment
2.11. Statistics
3. Results
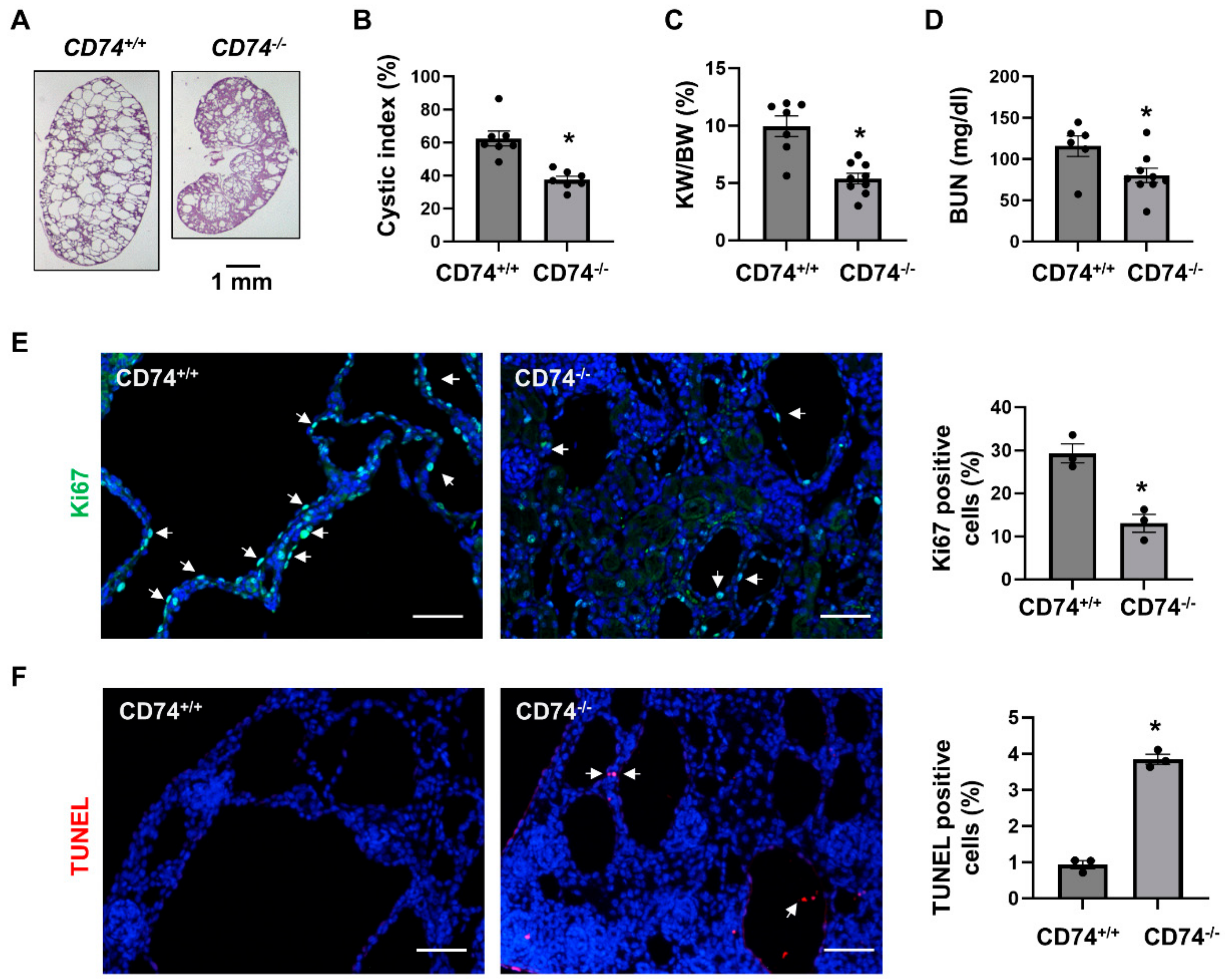
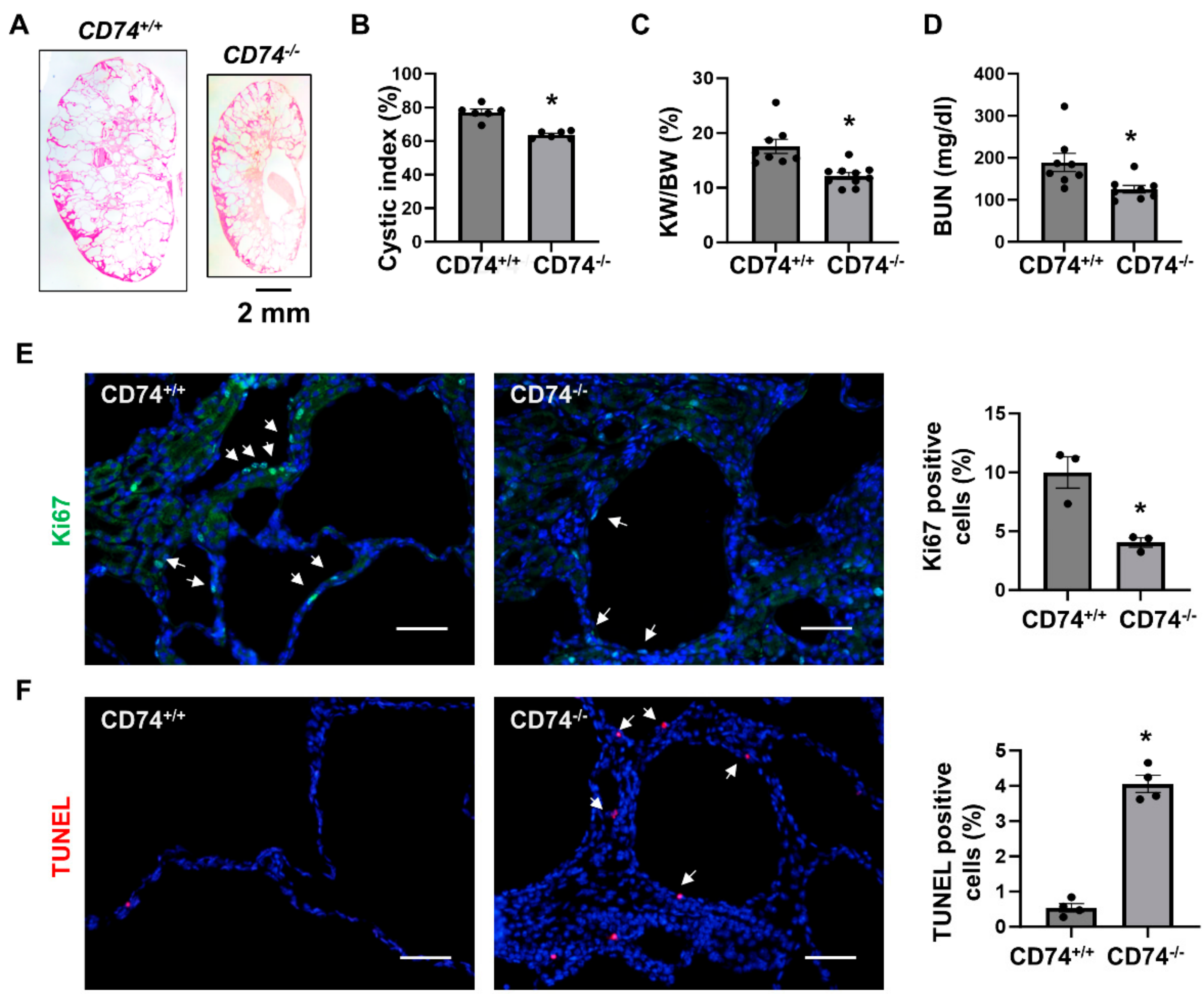
3.1. Double Knockout of CD74 and Pkd1 Delays Cyst Growth in Two Pkd1 Mutant Mouse Models
3.2. CD74 Promotes Pkd1 Mutant Renal Epithelial Cell Proliferation through the Activation of MAPK, mTOR and NF-κB Pathways
3.3. Deletion of CD74 Reduces the Recruitment of Macrophages and the Expression of Inflammatory Factors in Pkd1flox/flox:Ksp-Cre and Pkd1flox/flox:Pkhd1-Cre Kidneys
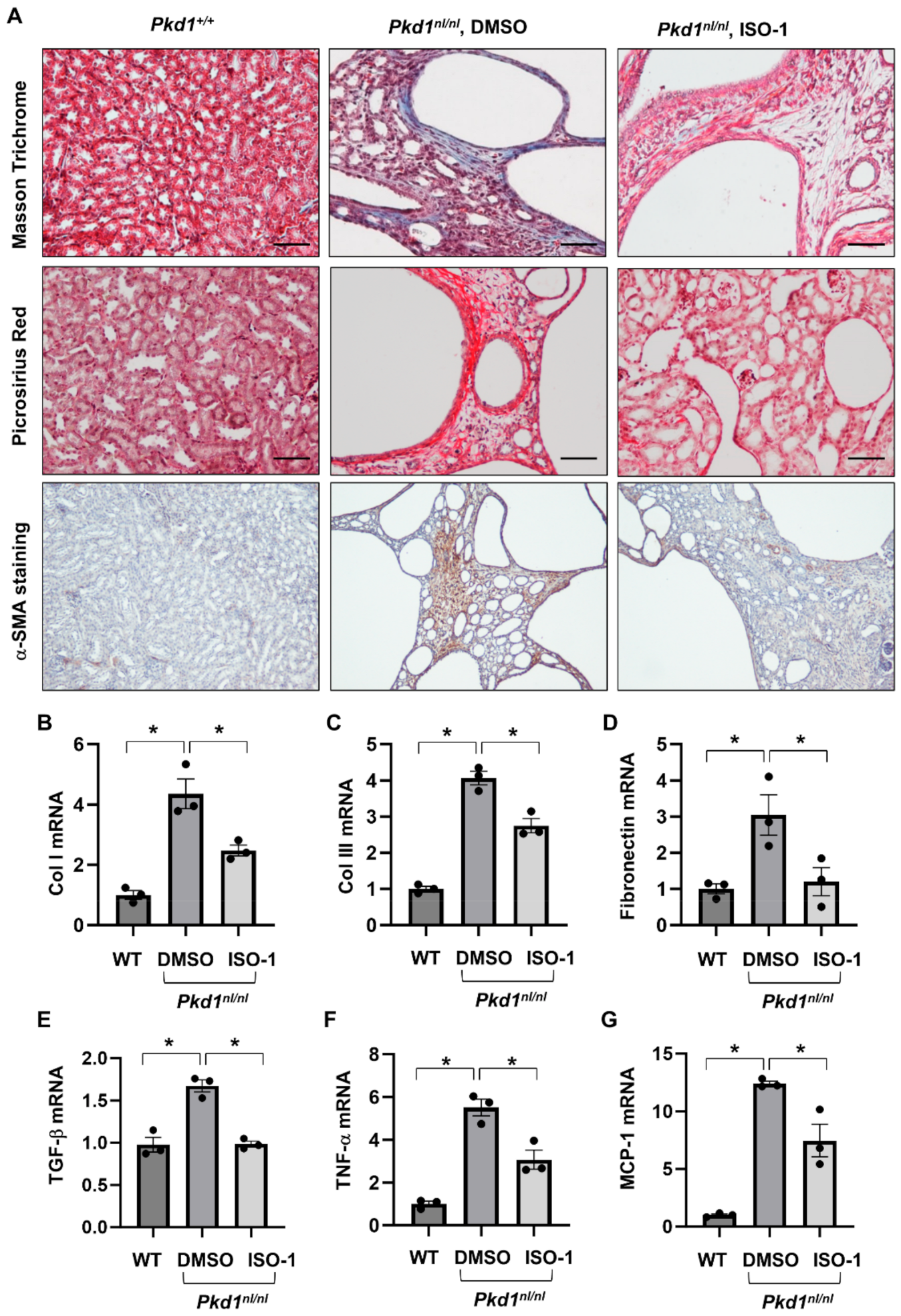
3.4. Upregulation of CD74 Promotes Renal Fibrosis in Pkd1flox/flox:Pkhd1-Cre Mice
3.5. Targeting MIF with Its Inhibitor ISO-1 Decrease Renal Fibrosis in Pkd1 Hypomorphic Mouse Kidneys
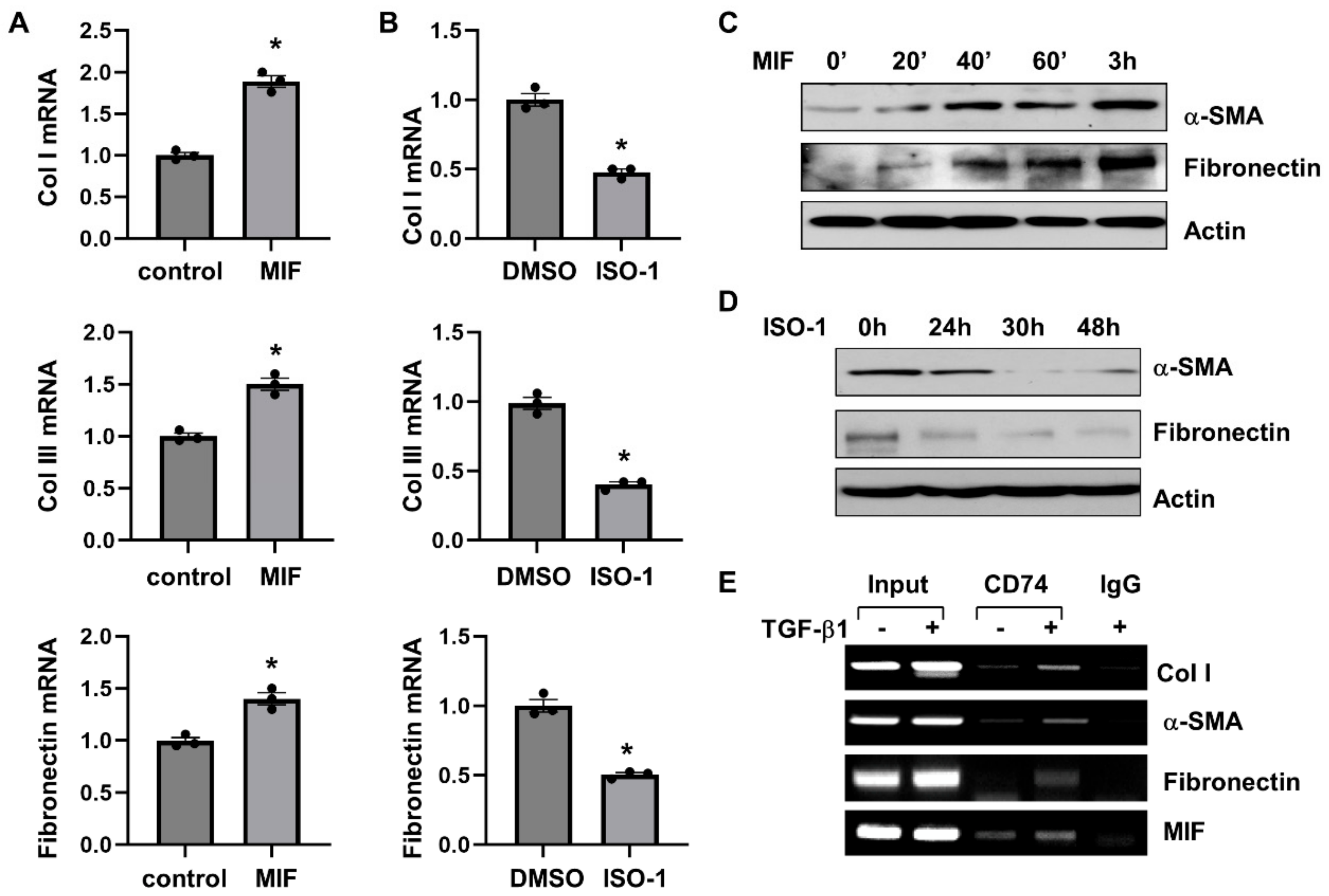
3.6. MIF-CD74 Signaling Activates Renal Fibroblasts
3.7. Targeting of MIF-CD74 Signaling with ISO-1 Blocked the Activation of Renal Fibroblasts Induced by TGF-β
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, P.C. Autosomal dominant polycystic kidney disease: Clues to pathogenesis. Hum. Mol. Genet. 1999, 8, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fan, L.X.; Sweeney, W.E., Jr.; Denu, J.M.; Avner, E.D.; Li, X. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J. Clin. Investig. 2013, 123, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.X.; Li, X.; Magenheimer, B.; Calvet, J.P.; Li, X. Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int. 2012, 81, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.X.; Zhou, X.; Sweeney, W.E., Jr.; Wallace, D.P.; Avner, E.D.; Grantham, J.J.; Li, X. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J. Am. Soc. Nephrol. JASN 2013, 24, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Magenheimer, B.S.; Xia, S.; Johnson, T.; Wallace, D.P.; Calvet, J.P.; Li, R. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat. Med. 2008, 14, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Swenson-Fields, K.I.; Vivian, C.J.; Salah, S.M.; Peda, J.D.; Davis, B.M.; van Rooijen, N.; Wallace, D.P.; Fields, T.A. Macrophages promote polycystic kidney disease progression. Kidney Int. 2013, 83, 855–864. [Google Scholar] [CrossRef]
- Karihaloo, A.; Koraishy, F.; Huen, S.C.; Lee, Y.; Merrick, D.; Caplan, M.J.; Somlo, S.; Cantley, L.G. Macrophages promote cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. JASN 2011, 22, 1809–1814. [Google Scholar] [CrossRef]
- Borghese, F.; Clanchy, F.I. CD74: An emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert Opin. Ther. Targets 2011, 15, 237–251. [Google Scholar] [CrossRef]
- Calandra, T.; Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef]
- Sanchez-Nino, M.D.; Sanz, A.B.; Ruiz-Andres, O.; Poveda, J.; Izquierdo, M.C.; Selgas, R.; Egido, J.; Ortiz, A. MIF, CD74 and other partners in kidney disease: Tales of a promiscuous couple. Cytokine Growth Factor Rev. 2013, 24, 23–40. [Google Scholar] [CrossRef]
- Sanchez-Nino, M.D.; Sanz, A.B.; Ihalmo, P.; Lassila, M.; Holthofer, H.; Mezzano, S.; Aros, C.; Groop, P.H.; Saleem, M.A.; Mathieson, P.W.; et al. The MIF receptor CD74 in diabetic podocyte injury. J. Am. Soc. Nephrol. JASN 2009, 20, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pagni, F.; Pieruzzi, F.; Zannella, S.; Di Giacomo, A.; Bovo, G.; Ferrario, F.; Torti, G.; Rivera, R.; Assi, E.; Viglione, F.; et al. Possible pathogenetic relationship between Fabry disease and renal cell carcinoma. Am. J. Nephrol. 2012, 36, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Sawyer, G.J.; Schofield, R.A.; Seymour, N.D.; Gustafsson, K.; Fabre, J.W. Discordant expression of major histocompatibility complex class II antigens and invariant chain in interstitial dendritic cells. Implications for self-tolerance and immunity. Transplantation 1997, 63, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Lin, C.Y.; Lin, W.C.; Tang, S.W.; Lai, M.K.; Lin, J.Y. Up-regulation of vascular endothelial growth factor-D expression in clear cell renal cell carcinoma by CD74: A critical role in cancer cell tumorigenesis. J. Immunol. 2008, 181, 6584–6594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, C.; Cheng, S.; Wang, X.; Li, W.; Charreyre, C.; Audonnet, J.C.; He, Q. Porcine CD74 is involved in the inflammatory response activated by nuclear factor kappa B during porcine circovirus type 2 (PCV-2) infection. Arch. Virol. 2013, 158, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Fan, L.X.; Yao, Y.; Swenson-Fields, K.I.; Gadjeva, M.; Wallace, D.P.; Peters, D.J.; Yu, A.; Grantham, J.J.; et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J. Clin. Investig. 2015, 125, 2399–2412. [Google Scholar] [CrossRef]
- Li, L.X.; Zhang, L.; Agborbesong, E.; Zhang, X.; Zhou, J.X.; Li, X. Cross talk between lysine methyltransferase Smyd2 and TGF-beta-Smad3 signaling promotes renal fibrosis in autosomal dominant polycystic kidney disease. Am. J. Physiol. Ren. Physiol. 2022, 323, F227–F242. [Google Scholar] [CrossRef]
- Schmidt, D.; Wilson, M.D.; Spyrou, C.; Brown, G.D.; Hadfield, J.; Odom, D.T. ChIP-seq: Using high-throughput sequencing to discover protein-DNA interactions. Methods 2009, 48, 240–248. [Google Scholar] [CrossRef]
- Shibazaki, S.; Yu, Z.; Nishio, S.; Tian, X.; Thomson, R.B.; Mitobe, M.; Louvi, A.; Velazquez, H.; Ishibe, S.; Cantley, L.G.; et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 2008, 17, 1505–1516. [Google Scholar] [CrossRef]
- Shao, X.; Somlo, S.; Igarashi, P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J. Am. Soc. Nephrol. 2002, 13, 1837–1846. [Google Scholar] [CrossRef]
- Patel, V.; Li, L.; Cobo-Stark, P.; Shao, X.; Somlo, S.; Lin, F.; Igarashi, P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 2008, 17, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Cassini, M.F.; Kakade, V.R.; Kurtz, E.; Sulkowski, P.; Glazer, P.; Torres, R.; Somlo, S.; Cantley, L.G. Mcp1 Promotes Macrophage-Dependent Cyst Expansion in Autosomal Dominant Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 2471–2481. [Google Scholar] [CrossRef]
- Song, C.J.; Zimmerman, K.A.; Henke, S.J.; Yoder, B.K. Inflammation and Fibrosis in Polycystic Kidney Disease. Results Probl. Cell Differ. 2017, 60, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Bulow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Hassane, S.; Leonhard, W.N.; van der Wal, A.; Hawinkels, L.J.; Lantinga-van Leeuwen, I.S.; ten Dijke, P.; Breuning, M.H.; de Heer, E.; Peters, D.J. Elevated TGFbeta-Smad signalling in experimental Pkd1 models and human patients with polycystic kidney disease. J. Pathol. 2010, 222, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.; Metz, C.N.; Peng, T.; Bucala, R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J. Biol. Chem. 1999, 274, 18100–18106. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.; Kapurniotu, A.; Fingerle-Rowson, G.; Roger, T.; Leng, L.; Thiele, M.; Calandra, T.; Bucala, R.; Bernhagen, J. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell. Signal. 2006, 18, 688–703. [Google Scholar] [CrossRef]
- Miller, E.J.; Li, J.; Leng, L.; McDonald, C.; Atsumi, T.; Bucala, R.; Young, L.H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 2008, 451, 578–582. [Google Scholar] [CrossRef]
- Brock, S.E.; Rendon, B.E.; Yaddanapudi, K.; Mitchell, R.A. Negative regulation of AMP-activated protein kinase (AMPK) activity by macrophage migration inhibitory factor (MIF) family members in non-small cell lung carcinomas. J. Biol. Chem. 2012, 287, 37917–37925. [Google Scholar] [CrossRef]
- Fingerle-Rowson, G.; Petrenko, O.; Metz, C.N.; Forsthuber, T.G.; Mitchell, R.; Huss, R.; Moll, U.; Muller, W.; Bucala, R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc. Natl. Acad. Sci. USA 2003, 100, 9354–9359. [Google Scholar] [CrossRef]
- Toh, M.L.; Aeberli, D.; Lacey, D.; Yang, Y.; Santos, L.L.; Clarkson, M.; Sharma, L.; Clyne, C.; Morand, E.F. Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J. Immunol. 2006, 177, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.L.; Morand, E.F.; McKeown, S.J.; Ralph, J.A.; Hall, P.; Yang, Y.H.; McColl, S.R.; Hickey, M.J. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J. Immunol. 2006, 177, 8072–8079. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.; Thiele, M.; Franz, J.; Dahl, E.; Speckgens, S.; Leng, L.; Fingerle-Rowson, G.; Bucala, R.; Luscher, B.; Bernhagen, J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene 2007, 26, 5046–5059. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, O.; Moll, U.M. Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol. Cell 2005, 17, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Takiar, V.; Nishio, S.; Seo-Mayer, P.; King, J.D., Jr.; Li, H.; Zhang, L.; Karihaloo, A.; Hallows, K.R.; Somlo, S.; Caplan, M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Shillingford, J.M.; Murcia, N.S.; Larson, C.H.; Low, S.H.; Hedgepeth, R.; Brown, N.; Flask, C.A.; Novick, A.C.; Goldfarb, D.A.; Kramer-Zucker, A.; et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5466–5471. [Google Scholar] [CrossRef]
- Shillingford, J.M.; Leamon, C.P.; Vlahov, I.R.; Weimbs, T. Folate-conjugated rapamycin slows progression of polycystic kidney disease. J. Am. Soc. Nephrol. 2012, 23, 1674–1681. [Google Scholar] [CrossRef]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Zheng, D.; Wolfe, M.; Cowley, B.D., Jr.; Wallace, D.P.; Yamaguchi, T.; Grantham, J.J. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. JASN 2003, 14, 2588–2595. [Google Scholar] [CrossRef]
- Gil-Yarom, N.; Radomir, L.; Sever, L.; Kramer, M.P.; Lewinsky, H.; Bornstein, C.; Blecher-Gonen, R.; Barnett-Itzhaki, Z.; Mirkin, V.; Friedlander, G.; et al. CD74 is a novel transcription regulator. Proc. Natl. Acad. Sci. USA 2017, 114, 562–567. [Google Scholar] [CrossRef]
- David, K.; Friedlander, G.; Pellegrino, B.; Radomir, L.; Lewinsky, H.; Leng, L.; Bucala, R.; Becker-Herman, S.; Shachar, I. CD74 as a regulator of transcription in normal B cells. Cell Rep. 2022, 41, 111572. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y. Role of macrophage migration inhibition factor in kidney disease. Nephron Exp. Nephrol. 2008, 109, e79–e83. [Google Scholar] [CrossRef] [PubMed]
- Hoi, A.Y.; Hickey, M.J.; Hall, P.; Yamana, J.; O’Sullivan, K.M.; Santos, L.L.; James, W.G.; Kitching, A.R.; Morand, E.F. Macrophage migration inhibitory factor deficiency attenuates macrophage recruitment, glomerulonephritis, and lethality in MRL/lpr mice. J. Immunol. 2006, 177, 5687–5696. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Y.; Yang, N.; Metz, C.; Mu, W.; Song, Q.; Nikolic-Paterson, D.J.; Bacher, M.; Bucala, R.; Atkins, R.C. TNF-alpha up-regulates renal MIF expression in rat crescentic glomerulonephritis. Mol. Med. 1997, 3, 136–144. [Google Scholar] [CrossRef]
- Sakata, Y.; Chancey, A.L.; Divakaran, V.G.; Sekiguchi, K.; Sivasubramanian, N.; Mann, D.L. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res. Cardiol. 2008, 103, 60–68. [Google Scholar] [CrossRef]
- Seiscento, M.; Vargas, F.S.; Antonangelo, L.; Acencio, M.M.; Bombarda, S.; Capelozzi, V.L.; Teixeira, L.R. Transforming growth factor beta-1 as a predictor of fibrosis in tuberculous pleurisy. Respirology 2007, 12, 660–663. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-beta and fibrosis. World J. Gastroenterol. 2007, 13, 3056–3062. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.X.; Cheng, A.S.; Chen, L.; Li, L.X.; Agborbesong, E.; Torres, V.E.; Harris, P.C.; Li, X. CD74 Promotes Cyst Growth and Renal Fibrosis in Autosomal Dominant Polycystic Kidney Disease. Cells 2024, 13, 489. https://doi.org/10.3390/cells13060489
Zhou JX, Cheng AS, Chen L, Li LX, Agborbesong E, Torres VE, Harris PC, Li X. CD74 Promotes Cyst Growth and Renal Fibrosis in Autosomal Dominant Polycystic Kidney Disease. Cells. 2024; 13(6):489. https://doi.org/10.3390/cells13060489
Chicago/Turabian StyleZhou, Julie Xia, Alice Shasha Cheng, Li Chen, Linda Xiaoyan Li, Ewud Agborbesong, Vicente E. Torres, Peter C. Harris, and Xiaogang Li. 2024. "CD74 Promotes Cyst Growth and Renal Fibrosis in Autosomal Dominant Polycystic Kidney Disease" Cells 13, no. 6: 489. https://doi.org/10.3390/cells13060489
APA StyleZhou, J. X., Cheng, A. S., Chen, L., Li, L. X., Agborbesong, E., Torres, V. E., Harris, P. C., & Li, X. (2024). CD74 Promotes Cyst Growth and Renal Fibrosis in Autosomal Dominant Polycystic Kidney Disease. Cells, 13(6), 489. https://doi.org/10.3390/cells13060489



