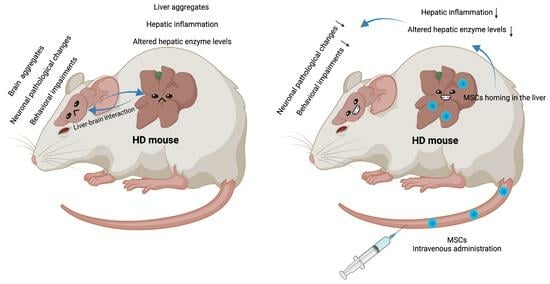
Intravenous MSC-Treatment Improves Impaired Brain Functions in the R6/2 Mouse Model of Huntington’s Disease via Recovered Hepatic Pathological Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Genotyping
2.2. Intravenous Injection
2.3. Rotarod Test
2.4. LabMaster
2.5. RNA Extraction and qPCR
2.6. Immunofluorescence Staining
2.7. Protein Extraction and Western Blot Analyses
2.8. Statistical Analyses
3. Results
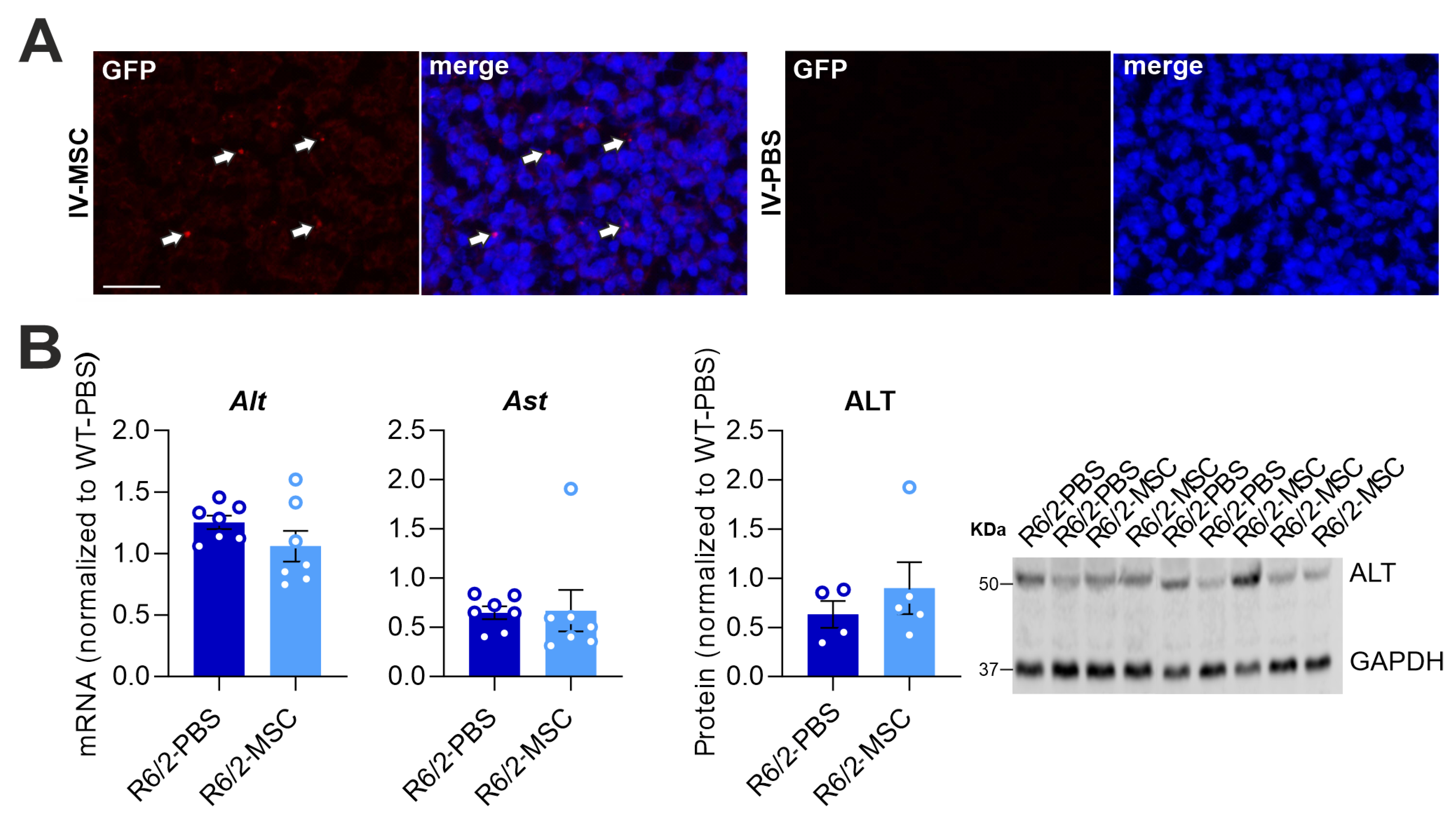
3.1. Pathological and Functional Changes in R6/2 Mice Liver
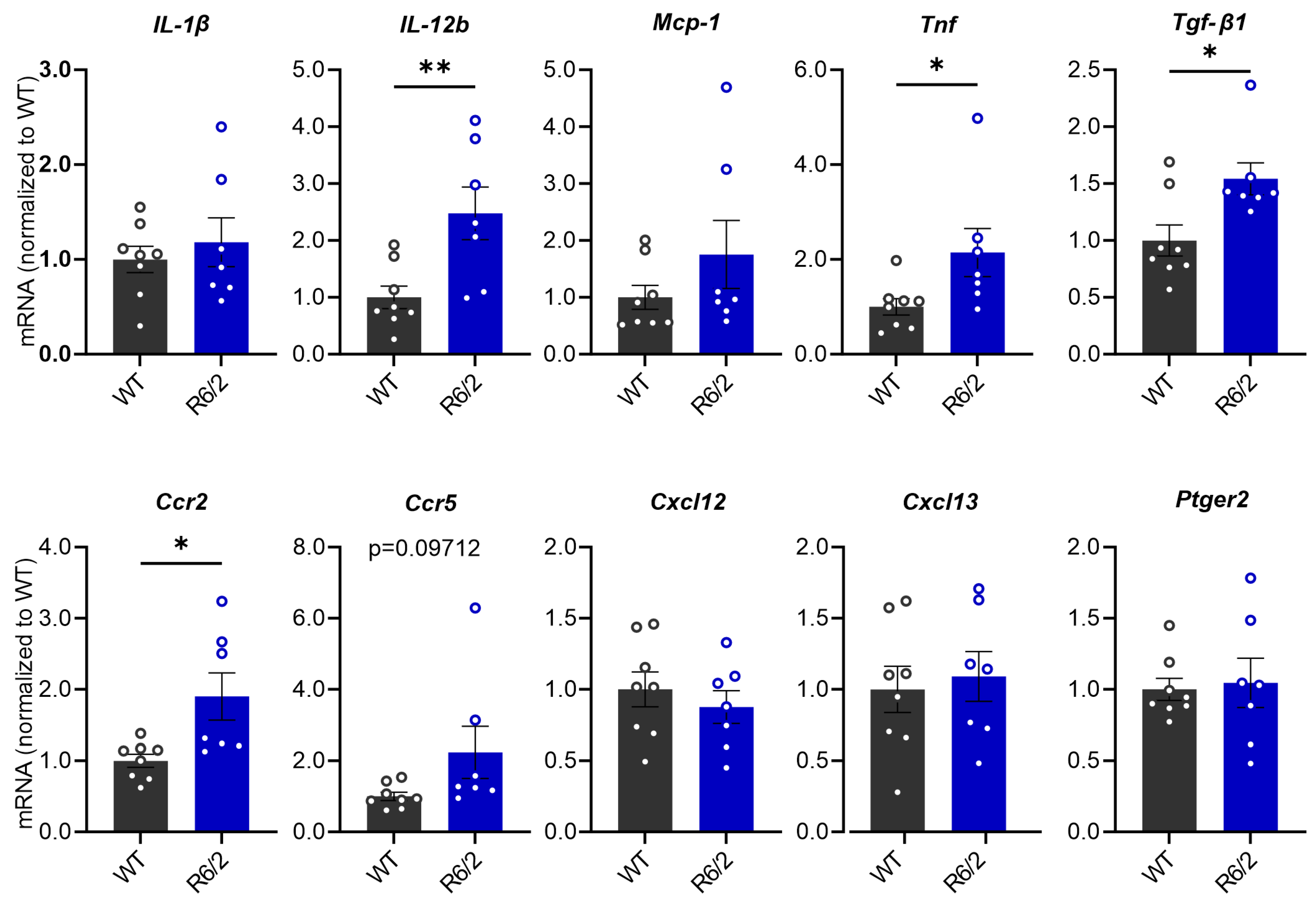
3.2. Elevated Inflammatory Markers in the Liver of R6/2 Mice
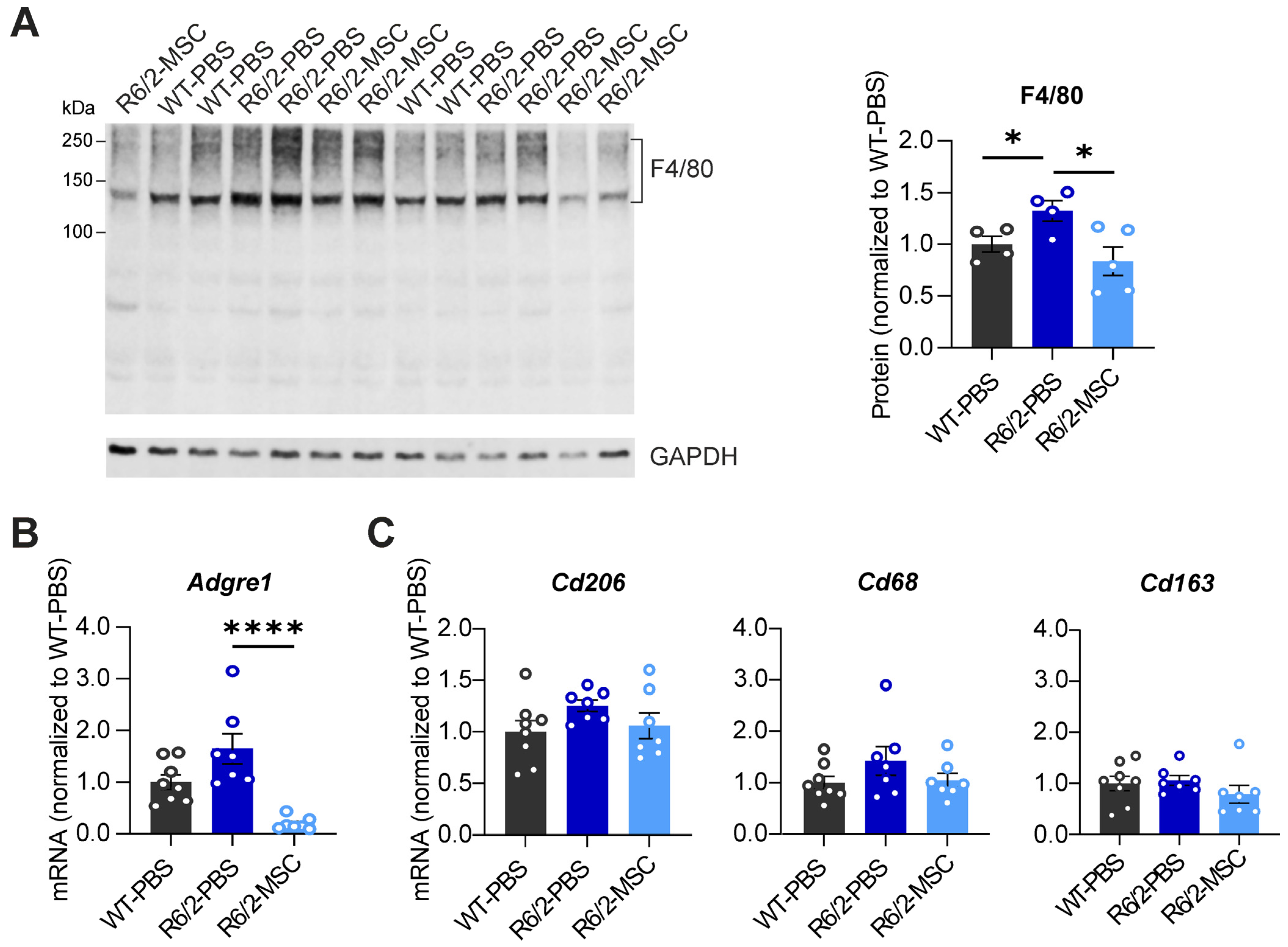
3.3. MSC Treatment Recovers Hepatic Enzyme Levels and Reduces Hepatic Inflammation in R6/2 Mice
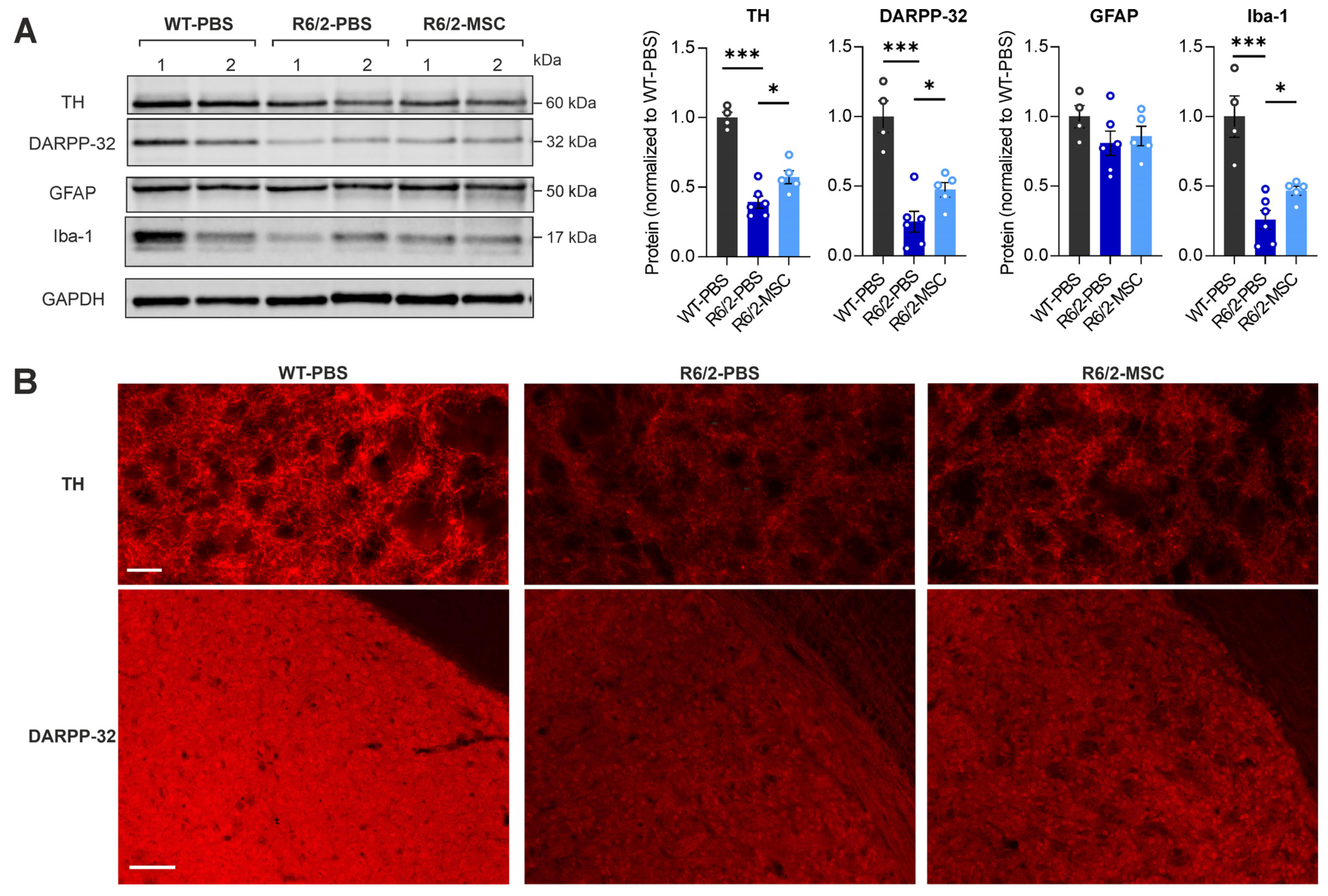
3.4. MSC Treatment Restores the Levels of Neuronal Markers and Microglia Markers in the Striatum of R6/2 Mice
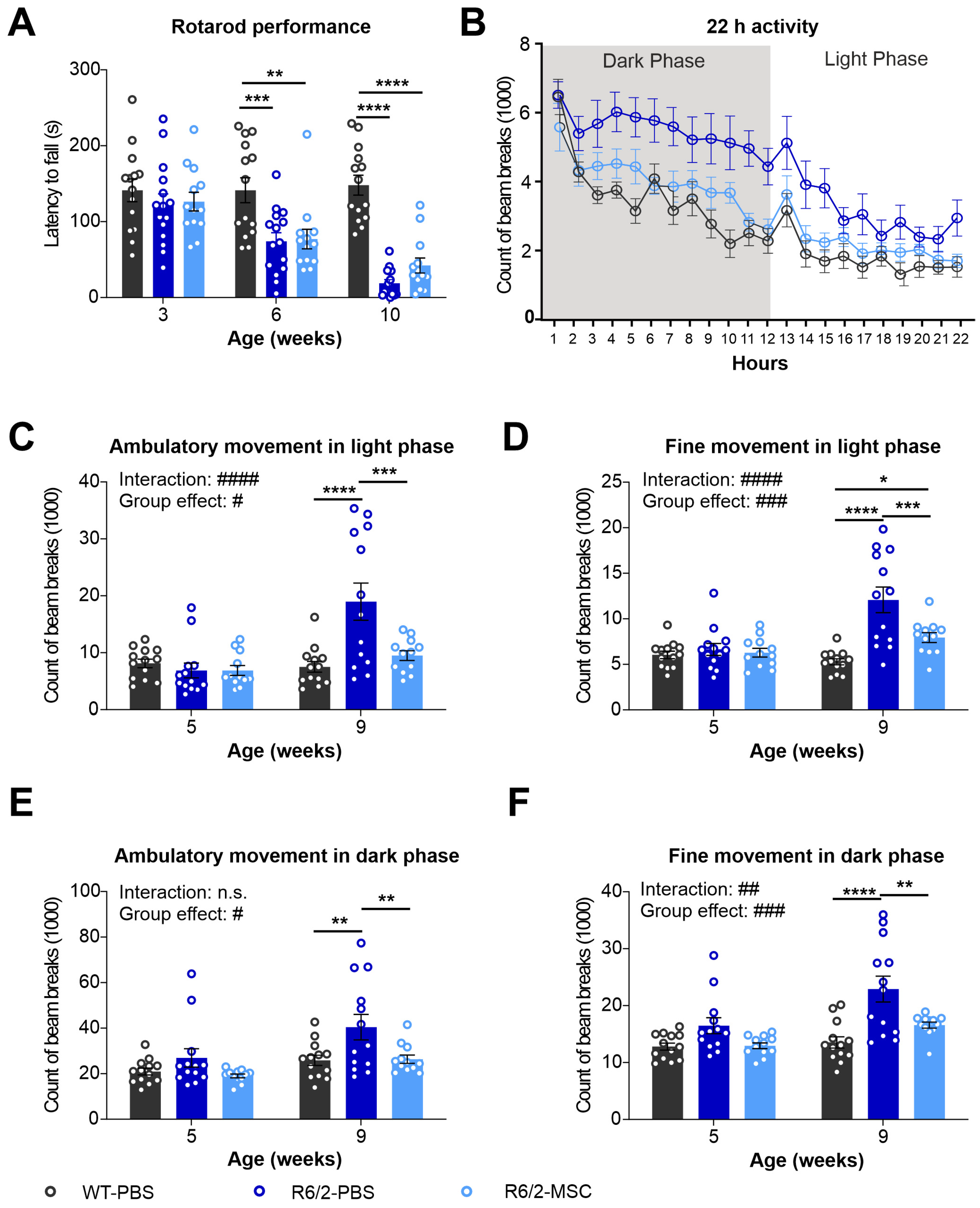
3.5. Altered Locomotor Activities in R6/2 Mice Were Improved after MSC Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albin, R.L.; Tagle, D.A. Genetics and molecular biology of Huntington’s disease. Trends Neurosci. 1995, 18, 11–14. [Google Scholar] [CrossRef]
- Herzog-Krzywoszanska, R.; Krzywoszanski, L. Sleep Disorders in Huntington’s Disease. Front. Psychiatry 2019, 10, 221. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Chuang, C.L.; Demontis, F. Systemic manifestation and contribution of peripheral tissues to Huntington’s disease pathogenesis. Ageing Res. Rev. 2021, 69, 101358. [Google Scholar] [CrossRef]
- Carroll, J.B.; Bates, G.P.; Steffan, J.; Saft, C.; Tabrizi, S.J. Treating the whole body in Huntington’s disease. Lancet Neurol. 2015, 14, 1135–1142. [Google Scholar] [CrossRef]
- Stuwe, S.H.; Goetze, O.; Lukas, C.; Klotz, P.; Hoffmann, R.; Banasch, M.; Orth, M.; Schmidt, W.E.; Gold, R.; Saft, C. Hepatic mitochondrial dysfunction in manifest and premanifest Huntington disease. Neurology 2013, 80, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Vinther-Jensen, T.; Nielsen, J.E.; Norremolle, A.; Hasholt, L.; Hjermind, L.E.; Josefsen, K. Liver function in Huntington’s disease assessed by blood biochemical analyses in a clinical setting. J. Neurol. Sci. 2016, 362, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Mendoza, J. Metabolic and reward feeding synchronises the rhythmic brain. Cell Tissue Res. 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B. pH after competitive rowing: The lower physiological range? Acta Physiol. Scand. 1999, 165, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.G.; Koroshetz, W.J.; Beal, M.F.; Rosen, B.R. Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology 1993, 43, 2689–2695. [Google Scholar] [CrossRef]
- Harms, L.; Meierkord, H.; Timm, G.; Pfeiffer, L.; Ludolph, A.C. Decreased N-acetyl-aspartate/choline ratio and increased lactate in the frontal lobe of patients with Huntington’s disease: A proton magnetic resonance spectroscopy study. J. Neurol. Neurosurg. Psychiatry 1997, 62, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Chen, H.M.; Lee, Y.H.; Chang, H.H.; Wu, Y.C.; Soong, B.W.; Chen, C.M.; Wu, Y.R.; Liu, C.S.; Niu, D.M.; et al. Dysregulation of C/EBPalpha by mutant Huntingtin causes the urea cycle deficiency in Huntington’s disease. Hum. Mol. Genet. 2007, 16, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, K.; Nielsen, S.M.; Campos, A.; Seifert, T.; Hasholt, L.; Nielsen, J.E.; Norremolle, A.; Skotte, N.H.; Secher, N.H.; Quistorff, B. Reduced gluconeogenesis and lactate clearance in Huntington’s disease. Neurobiol. Dis. 2010, 40, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Stuwe, S.H.; Goetze, O.; Banasch, M.; Klotz, P.; Lukas, C.; Tegenthoff, M.; Beste, C.; Orth, M.; Saft, C. Progressive hepatic mitochondrial dysfunction in premanifest Huntington’s disease. Mov. Disord. 2014, 29, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, P.; Zhao, H.; Ji, P.; Yang, Y.; Ma, J.; Zhao, X. Alterations of gut microbiota and its correlation with the liver metabolome in the process of ameliorating Parkinson’s disease with Buyang Huanwu decoction. J. Ethnopharmacol. 2024, 318, 116893. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola-Mani, R.; Islam, A.; Katare, D.P. Liver-Brain Axis in Sporadic Alzheimer’s Disease: Role of Ten Signature Genes in a Mouse Model. CNS Neurol. Disord. Drug Targets 2021, 20, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pike, J.R.; Hoogeveen, R.C.; Walker, K.A.; Raffield, L.M.; Selvin, E.; Avery, C.L.; Engel, S.M.; Mielke, M.M.; Garcia, T.; et al. Liver integrity and the risk of Alzheimer’s disease and related dementias. Alzheimer’s Dement. 2023, 1–10. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Role of Liver Growth Factor (LGF) in Parkinson’s Disease: Molecular Insights and Therapeutic Opportunities. Mol. Neurobiol. 2021, 58, 3031–3042. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Regmi, S.; Liu, D.D.; Shen, M.; Kevadiya, B.D.; Ganguly, A.; Primavera, R.; Chetty, S.; Yarani, R.; Thakor, A.S. Mesenchymal stromal cells for the treatment of Alzheimer’s disease: Strategies and limitations. Front. Mol. Neurosci. 2022, 15, 1011225. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, N.; Hong, H.; Qi, J.; Zhang, S.; Wang, J. Mesenchymal Stem Cells: Therapeutic Mechanisms for Stroke. Int. J. Mol. Sci. 2022, 23, 2550. [Google Scholar] [CrossRef]
- Shariati, A.; Nemati, R.; Sadeghipour, Y.; Yaghoubi, Y.; Baghbani, R.; Javidi, K.; Zamani, M.; Hassanzadeh, A. Mesenchymal stromal cells (MSCs) for neurodegenerative disease: A promising frontier. Eur. J. Cell Biol. 2020, 99, 151097. [Google Scholar] [CrossRef]
- Staff, N.P.; Jones, D.T.; Singer, W. Mesenchymal Stromal Cell Therapies for Neurodegenerative Diseases. Mayo Clin. Proc. 2019, 94, 892–905. [Google Scholar] [CrossRef]
- Dzyubenko, E.; Sardari, M.; Hermann, D.M. Editorial: The role of inflammation in neurodegenerative diseases. Front. Cell. Neurosci. 2023, 17, 1192514. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef]
- Mangiarini, L.; Sathasivam, K.; Seller, M.; Cozens, B.; Harper, A.; Hetherington, C.; Lawton, M.; Trottier, Y.; Lehrach, H.; Davies, S.W.; et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 1996, 87, 493–506. [Google Scholar] [CrossRef]
- Li, J.Y.; Popovic, N.; Brundin, P. The use of the R6 transgenic mouse models of Huntington’s disease in attempts to develop novel therapeutic strategies. NeuroRx 2005, 2, 447–464. [Google Scholar] [CrossRef]
- Stan, T.L.; Soylu-Kucharz, R.; Burleigh, S.; Prykhodko, O.; Cao, L.; Franke, N.; Sjogren, M.; Haikal, C.; Hallenius, F.; Bjorkqvist, M. Increased intestinal permeability and gut dysbiosis in the R6/2 mouse model of Huntington’s disease. Sci. Rep. 2020, 10, 18270. [Google Scholar] [CrossRef]
- Mihm, M.J.; Amann, D.M.; Schanbacher, B.L.; Altschuld, R.A.; Bauer, J.A.; Hoyt, K.R. Cardiac dysfunction in the R6/2 mouse model of Huntington’s disease. Neurobiol. Dis. 2007, 25, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Chern, Y.; Juo, C.G. The dysfunction of hepatic transcriptional factors in mice with Huntington’s Disease. Biochim. Biophys. Acta 2011, 1812, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Hasholt, L.; Norremolle, A.; Josefsen, K. Progressive Impairment of Lactate-based Gluconeogenesis in the Huntington’s Disease Mouse Model R6/2. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef]
- Sjogren, M.; Duarte, A.I.; McCourt, A.C.; Shcherbina, L.; Wierup, N.; Bjorkqvist, M. Ghrelin rescues skeletal muscle catabolic profile in the R6/2 mouse model of Huntington’s disease. Sci. Rep. 2017, 7, 13896. [Google Scholar] [CrossRef]
- Putra, A.; Rosdiana, I.; Darlan, D.M.; Alif, I.; Hayuningtyas, F.; Wijaya, I.; Aryanti, R.; Makarim, F.R.; Antari, A.D. Intravenous Administration is the Best Route of Mesenchymal Stem Cells Migration in Improving Liver Function Enzyme of Acute Liver Failure. Folia Med. 2020, 62, 52–58. [Google Scholar] [CrossRef]
- Danielyan, L.; Schafer, R.; Schulz, A.; Ladewig, T.; Lourhmati, A.; Buadze, M.; Schmitt, A.L.; Verleysdonk, S.; Kabisch, D.; Koeppen, K.; et al. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: The critical role of erythropoietin. Cell Death Differ. 2009, 16, 1599–1614. [Google Scholar] [CrossRef]
- Yu-Taeger, L.; Stricker-Shaver, J.; Arnold, K.; Bambynek-Dziuk, P.; Novati, A.; Singer, E.; Lourhmati, A.; Fabian, C.; Magg, J.; Riess, O.; et al. Intranasal Administration of Mesenchymal Stem Cells Ameliorates the Abnormal Dopamine Transmission System and Inflammatory Reaction in the R6/2 Mouse Model of Huntington Disease. Cells 2019, 8, 595. [Google Scholar] [CrossRef]
- Yu-Taeger, L.; Ott, T.; Bonsi, P.; Tomczak, C.; Wassouf, Z.; Martella, G.; Sciamanna, G.; Imbriani, P.; Ponterio, G.; Tassone, A.; et al. Impaired dopamine- and adenosine-mediated signaling and plasticity in a novel rodent model for DYT25 dystonia. Neurobiol. Dis. 2020, 134, 104634. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Luo, X.Y.; Meng, X.J.; Cao, D.C.; Wang, W.; Zhou, K.; Li, L.; Guo, M.; Wang, P. Transplantation of bone marrow mesenchymal stromal cells attenuates liver fibrosis in mice by regulating macrophage subtypes. Stem Cell Res. Ther. 2019, 10, 16. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press/Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Meade, C.A.; Deng, Y.P.; Fusco, F.R.; Del Mar, N.; Hersch, S.; Goldowitz, D.; Reiner, A. Cellular localization and development of neuronal intranuclear inclusions in striatal and cortical neurons in R6/2 transgenic mice. J. Comp. Neurol. 2002, 449, 241–269. [Google Scholar] [CrossRef]
- Reed, L.; Arlt, V.M.; Phillips, D.H. The role of cytochrome P450 enzymes in carcinogen activation and detoxication: An in vivo-in vitro paradox. Carcinogenesis 2018, 39, 851–859. [Google Scholar] [CrossRef]
- Yu-Taeger, L.; Novati, A.; Weber, J.J.; Singer-Mikosch, E.; Pabst, A.S.; Cheng, F.; Saft, C.; Koenig, J.; Ellrichmann, G.; Heikkinen, T.; et al. Evidences for Mutant Huntingtin Inducing Musculoskeletal and Brain Growth Impairments via Disturbing Testosterone Biosynthesis in Male Huntington Disease Animals. Cells 2022, 11, 3779. [Google Scholar] [CrossRef]
- Kreilaus, F.; Spiro, A.S.; McLean, C.A.; Garner, B.; Jenner, A.M. Evidence for altered cholesterol metabolism in Huntington’s disease post mortem brain tissue. Neuropathol. Appl. Neurobiol. 2016, 42, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Boussicault, L.; Alves, S.; Lamaziere, A.; Planques, A.; Heck, N.; Moumne, L.; Despres, G.; Bolte, S.; Hu, A.; Pages, C.; et al. CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain 2016, 139, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Cantle, J.P.; Chatzopoulou, D.; Wang, N.; Gao, F.; Al-Ramahi, I.; Lu, X.H.; Ramos, E.M.; El-Zein, K.; Zhao, Y.; et al. Integrated genomics and proteomics define huntingtin CAG length-dependent networks in mice. Nat. Neurosci. 2016, 19, 623–633. [Google Scholar] [CrossRef] [PubMed]
- El-Khateeb, E.; Achour, B.; Al-Majdoub, Z.M.; Barber, J.; Rostami-Hodjegan, A. Non-uniformity of Changes in Drug-Metabolizing Enzymes and Transporters in Liver Cirrhosis: Implications for Drug Dosage Adjustment. Mol. Pharm. 2021, 18, 3563–3577. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009, 37, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Woolsey, S.J.; Mansell, S.E.; Kim, R.B.; Tirona, R.G.; Beaton, M.D. CYP3A Activity and Expression in Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2015, 43, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Wu, Y.R.; Chen, Y.C.; Chen, C.M. Plasma inflammatory biomarkers for Huntington’s disease patients and mouse model. Brain Behav. Immun. 2015, 44, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fan, X.; Liu, Y.; Jie, P.; Mazhar, M.; Liu, Y.; Dechsupa, N.; Wang, L. Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2023, 19, 1214–1231. [Google Scholar] [CrossRef]
- Linares, G.R.; Chiu, C.T.; Scheuing, L.; Leng, Y.; Liao, H.M.; Maric, D.; Chuang, D.M. Preconditioning mesenchymal stem cells with the mood stabilizers lithium and valproic acid enhances therapeutic efficacy in a mouse model of Huntington’s disease. Exp. Neurol. 2016, 281, 81–92. [Google Scholar] [CrossRef]
- Rossignol, J.; Fink, K.D.; Crane, A.T.; Davis, K.K.; Bombard, M.C.; Clerc, S.; Bavar, A.M.; Lowrance, S.A.; Song, C.; Witte, S.; et al. Reductions in behavioral deficits and neuropathology in the R6/2 mouse model of Huntington’s disease following transplantation of bone-marrow-derived mesenchymal stem cells is dependent on passage number. Stem Cell Res. Ther. 2015, 6, 9. [Google Scholar] [CrossRef]
- Smatlikova, P.; Juhas, S.; Juhasova, J.; Suchy, T.; Hubalek Kalbacova, M.; Ellederova, Z.; Motlik, J.; Klima, J. Adipogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells in Pig Transgenic Model Expressing Human Mutant Huntingtin. J. Huntingt. Dis. 2019, 8, 33–51. [Google Scholar] [CrossRef]
- Drouin-Ouellet, J.; Sawiak, S.J.; Cisbani, G.; Lagace, M.; Kuan, W.L.; Saint-Pierre, M.; Dury, R.J.; Alata, W.; St-Amour, I.; Mason, S.L.; et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann. Neurol. 2015, 78, 160–177. [Google Scholar] [CrossRef]
- Del Tredici, K.; Duda, J.E. Peripheral Lewy body pathology in Parkinson’s disease and incidental Lewy body disease: Four cases. J. Neurol. Sci. 2011, 310, 100–106. [Google Scholar] [CrossRef]
- Huh, E.; Choi, J.G.; Lee, M.Y.; Kim, J.H.; Choi, Y.; Ju, I.G.; Eo, H.; Park, M.G.; Kim, D.H.; Park, H.J.; et al. Peripheral metabolic alterations associated with pathological manifestations of Parkinson’s disease in gut-brain axis-based mouse model. Front. Mol. Neurosci. 2023, 16, 1201073. [Google Scholar] [CrossRef] [PubMed]
- Morena, E.; Romano, C.; Marconi, M.; Diamant, S.; Buscarinu, M.C.; Bellucci, G.; Romano, S.; Scarabino, D.; Salvetti, M.; Ristori, G. Peripheral Biomarkers in Manifest and Premanifest Huntington’s Disease. Int. J. Mol. Sci. 2023, 24, 6051. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E. Alzheimer’s disease mechanisms in peripheral cells: Promises and challenges. Alzheimer’s Dement. 2019, 5, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, B.; Guo, J. Parkinson’s disease and gut microbiota: From clinical to mechanistic and therapeutic studies. Transl. Neurodegener. 2023, 12, 59. [Google Scholar]
- Kong, G.; Ellul, S.; Narayana, V.K.; Kanojia, K.; Ha, H.T.T.; Li, S.; Renoir, T.; Cao, K.L.; Hannan, A.J. An integrated metagenomics and metabolomics approach implicates the microbiota-gut-brain axis in the pathogenesis of Huntington’s disease. Neurobiol. Dis. 2021, 148, 105199. [Google Scholar] [CrossRef]
- Kong, G.; Cao, K.L.; Judd, L.M.; Li, S.; Renoir, T.; Hannan, A.J. Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington’s disease. Neurobiol. Dis. 2020, 135, 104268. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Solis-Urra, P.; Rodriguez-Rodriguez, F.; Olivares-Arancibia, J.; Navarro-Oliveros, M.; Abadia-Molina, F.; Alvarez-Mercado, A.I. The Gut Barrier, Intestinal Microbiota, and Liver Disease: Molecular Mechanisms and Strategies to Manage. Int. J. Mol. Sci. 2020, 21, 8351. [Google Scholar] [CrossRef]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Bjorkqvist, M.; Wild, E.J.; Thiele, J.; Silvestroni, A.; Andre, R.; Lahiri, N.; Raibon, E.; Lee, R.V.; Benn, C.L.; Soulet, D.; et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J. Exp. Med. 2008, 205, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.Y.; Chiu, F.L.; Chen, C.M.; Wu, Y.R.; Chen, H.M.; Chen, Y.C.; Kuo, H.C.; Chern, Y. Inhibition of soluble tumor necrosis factor is therapeutic in Huntington’s disease. Hum. Mol. Genet. 2014, 23, 4328–4344. [Google Scholar] [CrossRef] [PubMed]
- Pido-Lopez, J.; Tanudjojo, B.; Farag, S.; Bondulich, M.K.; Andre, R.; Tabrizi, S.J.; Bates, G.P. Inhibition of tumour necrosis factor alpha in the R6/2 mouse model of Huntington’s disease by etanercept treatment. Sci. Rep. 2019, 9, 7202. [Google Scholar] [CrossRef] [PubMed]
- Pido-Lopez, J.; Andre, R.; Benjamin, A.C.; Ali, N.; Farag, S.; Tabrizi, S.J.; Bates, G.P. In vivo neutralization of the protagonist role of macrophages during the chronic inflammatory stage of Huntington’s disease. Sci. Rep. 2018, 8, 11447. [Google Scholar] [CrossRef] [PubMed]
- Wild, E.; Magnusson, A.; Lahiri, N.; Krus, U.; Orth, M.; Tabrizi, S.J.; Bjorkqvist, M. Abnormal peripheral chemokine profile in Huntington’s disease. PLoS Curr. 2011, 3, RRN1231. [Google Scholar] [CrossRef]
- Ramadori, G.; Moriconi, F.; Malik, I.; Dudas, J. Physiology and pathophysiology of liver inflammation, damage and repair. J. Physiol. Pharmacol. 2008, 59 (Suppl. S1), 107–117. [Google Scholar] [PubMed]
- Karmen, A.; Wroblewski, F.; Ladue, J.S. Transaminase activity in human blood. J. Clin. Investig. 1955, 34, 126–131. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, G.; Swick, R.W. Metabolic implications of the distribution of the alanine aminotransferase isoenzymes. J. Biol. Chem. 1975, 250, 7961–7967. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pike, J.R.; Selvin, E.; Mosley, T.; Palta, P.; Sharrett, A.R.; Thomas, A.; Loehr, L.; Sidney Barritt, A.; Hoogeveen, R.C.; et al. Low Liver Enzymes and Risk of Dementia: The Atherosclerosis Risk in Communities (ARIC) Study. J. Alzheimer’s Dis. 2021, 79, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Lasman, N.; Shalom, M.; Turpashvili, N.; Goldhaber, G.; Lifshitz, Y.; Leibowitz, E.; Berger, G.; Saltzman-Shenhav, G.; Brom, A.; Cohen, D.; et al. Baseline low ALT activity is associated with increased long-term mortality after COPD exacerbations. BMC Pulm. Med. 2020, 20, 133. [Google Scholar] [CrossRef]
- Uliel, N.; Segal, G.; Perri, A.; Turpashvili, N.; Kassif Lerner, R.; Itelman, E. Low ALT, a marker of sarcopenia and frailty, is associated with shortened survival amongst myelodysplastic syndrome patients: A retrospective study. Medicine 2023, 102, e33659. [Google Scholar] [CrossRef]
- Laufer, M.; Perelman, M.; Sarfaty, M.; Itelman, E.; Segal, G. Low Alanine Aminotransferase, as a Marker of Sarcopenia and Frailty, Is Associated with Shorter Survival Among Prostate Cancer Patients and Survivors. A Retrospective Cohort Analysis of 4064 Patients. Eur. Urol. Open Sci. 2023, 55, 38–44. [Google Scholar] [CrossRef]
- Elinav, E.; Ackerman, Z.; Maaravi, Y.; Ben-Dov, I.Z.; Ein-Mor, E.; Stessman, J. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J. Am. Geriatr. Soc. 2006, 54, 1719–1724. [Google Scholar] [CrossRef]
- Gil, J.M.; Rego, A.C. The R6 lines of transgenic mice: A model for screening new therapies for Huntington’s disease. Brain Res. Rev. 2009, 59, 410–431. [Google Scholar] [CrossRef]
- Leoni, V.; Mariotti, C.; Tabrizi, S.J.; Valenza, M.; Wild, E.J.; Henley, S.M.; Hobbs, N.Z.; Mandelli, M.L.; Grisoli, M.; Bjorkhem, I.; et al. Plasma 24S-hydroxycholesterol and caudate MRI in pre-manifest and early Huntington’s disease. Brain 2008, 131, 2851–2859. [Google Scholar] [CrossRef]
- Benn, C.L.; Sun, T.; Sadri-Vakili, G.; McFarland, K.N.; DiRocco, D.P.; Yohrling, G.J.; Clark, T.W.; Bouzou, B.; Cha, J.H. Huntingtin modulates transcription, occupies gene promoters in vivo, and binds directly to DNA in a polyglutamine-dependent manner. J. Neurosci. 2008, 28, 10720–10733. [Google Scholar] [CrossRef]
- Bragg, R.M.; Coffey, S.R.; Cantle, J.P.; Hu, S.; Singh, S.; Legg, S.R.; McHugh, C.A.; Toor, A.; Zeitlin, S.O.; Kwak, S.; et al. Huntingtin loss in hepatocytes is associated with altered metabolism, adhesion, and liver zonation. Life Sci. Alliance 2023, 6, e202302098. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, A.; Chmielewska, N.; Maciejak, P.; Szyndler, J. Transcriptional Dysregulation in Huntington’s Disease: The Role in Pathogenesis and Potency for Pharmacological Targeting. Curr. Med. Chem. 2021, 28, 2783–2806. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wu, J.; Rosenblatt, M.; Dai, W.; Rodriguez, R.X.; Sui, J.; Qi, S.; Liang, Q.; Xu, B.; Meng, Q.; et al. Elevated C-reactive protein mediates the liver-brain axis: A preliminary study. EBioMedicine 2023, 93, 104679. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, C.; Swain, M.G. Liver-brain inflammation axis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G749–G761. [Google Scholar] [CrossRef] [PubMed]
- Kantor, S.; Szabo, L.; Varga, J.; Cuesta, M.; Morton, A.J. Progressive sleep and electroencephalogram changes in mice carrying the Huntington’s disease mutation. Brain 2013, 136, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.P.; Black, S.W.; Schwartz, M.D.; Wilk, A.J.; Chen, T.M.; Lincoln, W.U.; Liu, H.W.; Kilduff, T.S.; Morairty, S.R. Longitudinal analysis of the electroencephalogram and sleep phenotype in the R6/2 mouse model of Huntington’s disease. Brain 2013, 136, 2159–2172. [Google Scholar] [CrossRef] [PubMed]
- Mulroy, E.; Baschieri, F.; Magrinelli, F.; Latorre, A.; Cortelli, P.; Bhatia, K.P. Movement Disorders and Liver Disease. Mov. Disord. Clin. Pract. 2021, 8, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Plotogea, O.M.; Ilie, M.; Bungau, S.; Chiotoroiu, A.L.; Stanescu, A.M.A.; Diaconu, C.C. Comprehensive Overview of Sleep Disorders in Patients with Chronic Liver Disease. Brain Sci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Cauli, O.; Llansola, M.; Erceg, S.; Felipo, V. Hypolocomotion in rats with chronic liver failure is due to increased glutamate and activation of metabotropic glutamate receptors in substantia nigra. J. Hepatol. 2006, 45, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choung, J.S.; Kim, J.M.; Kim, H.; Kim, M. Distribution of Embryonic Stem Cell-Derived Mesenchymal Stem Cells after Intravenous Infusion in Hypoxic-Ischemic Encephalopathy. Life 2023, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Diaz, M.; Quinones-Vico, M.I.; Sanabria de la Torre, R.; Montero-Vilchez, T.; Sierra-Sanchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of Mesenchymal Stromal Cells after Administration in Animal Models and Humans: A Systematic Review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef]
- Liu, S.; Liu, F.; Zhou, Y.; Jin, B.; Sun, Q.; Guo, S. Immunosuppressive Property of MSCs Mediated by Cell Surface Receptors. Front. Immunol. 2020, 11, 1076. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Liu, B.; Shao, C.; Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 2022, 29, 1515–1530. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu-Taeger, L.; El-Ayoubi, A.; Qi, P.; Danielyan, L.; Nguyen, H.H.P. Intravenous MSC-Treatment Improves Impaired Brain Functions in the R6/2 Mouse Model of Huntington’s Disease via Recovered Hepatic Pathological Changes. Cells 2024, 13, 469. https://doi.org/10.3390/cells13060469
Yu-Taeger L, El-Ayoubi A, Qi P, Danielyan L, Nguyen HHP. Intravenous MSC-Treatment Improves Impaired Brain Functions in the R6/2 Mouse Model of Huntington’s Disease via Recovered Hepatic Pathological Changes. Cells. 2024; 13(6):469. https://doi.org/10.3390/cells13060469
Chicago/Turabian StyleYu-Taeger, Libo, Ali El-Ayoubi, Pengfei Qi, Lusine Danielyan, and Hoa Huu Phuc Nguyen. 2024. "Intravenous MSC-Treatment Improves Impaired Brain Functions in the R6/2 Mouse Model of Huntington’s Disease via Recovered Hepatic Pathological Changes" Cells 13, no. 6: 469. https://doi.org/10.3390/cells13060469
APA StyleYu-Taeger, L., El-Ayoubi, A., Qi, P., Danielyan, L., & Nguyen, H. H. P. (2024). Intravenous MSC-Treatment Improves Impaired Brain Functions in the R6/2 Mouse Model of Huntington’s Disease via Recovered Hepatic Pathological Changes. Cells, 13(6), 469. https://doi.org/10.3390/cells13060469