Abstract
Oxidative stress refers to the imbalance between the production of reactive oxygen species (ROS) and the endogenous antioxidant defense system. Its involvement in cell senescence, apoptosis, and series diseases has been demonstrated. Advances in carcinogenic research have revealed oxidative stress as a pivotal pathophysiological pathway in tumorigenesis and to be involved in lung cancer, glioma, hepatocellular carcinoma, leukemia, and so on. This review combs the effects of oxidative stress on tumorigenesis on each phase and cell fate determination, and three features are discussed. Oxidative stress takes part in the processes ranging from tumorigenesis to tumor death via series pathways and processes like mitochondrial stress, endoplasmic reticulum stress, and ferroptosis. It can affect cell fate by engaging in the complex relationships between senescence, death, and cancer. The influence of oxidative stress on tumorigenesis and progression is a multi-stage interlaced process that includes two aspects of promotion and inhibition, with mitochondria as the core of regulation. A deeper and more comprehensive understanding of the effects of oxidative stress on tumorigenesis is conducive to exploring more tumor therapies.
1. Introduction
Recently, the relationship between oxidative stress and cancer has become a hot issue. Oxidative stress is closely related to tumor development and can interfere with tumor cell fate through a complex regulatory network. Considering that a major challenge in cancer treatment lies in the diverse mechanisms of tumor development and evasion, targeting oxidative stress may be a comprehensive approach compared with some single efficacy targets. ROS, as one of the normal cellular metabolites, exists in various forms, such as radicals possessing a single unpaired reactive electron in the outermost orbital, including superoxide anion (O2•−), hydroxyl radical (OH•), carbonate radical anion (CO3•−), nitrogen dioxide (NO2•), alkoxyl/alkyl peroxyl (RO•/ROO•), etc., and non-radicals lacking unpaired electrons and characterized as two-electron oxidants, including hydrogen peroxide (H2O2), nitric oxide (NO), hypochlorous acid (HOCl), etc. [1,2]. With an intensive oxidative capacity, ROS can attack nucleic acids, proteins, and lipids, resulting in DNA damage, lipid peroxidation [3], and altering protein post-translational modification, represented by redox modification [4] and phosphorylation [5]. It is known that ROS originates from two main pathways: an endogenous pathway with mitochondria as the primary source, containing the endoplasmic reticulum, NADPH hydrogenase, and catalase, among which NOXs are considered as the central enzyme family for ROS production [6]; and an exogenous pathway including external factors such as radiation, chemotherapy, inflammatory factors, and air pollution [7]. The basic relationship between oxidative stress and cancer has been clearly demonstrated. Relatively high ROS levels can induce DNA mutations and pro-oncogenic signaling pathways to promote tumor formation, while excessive ROS levels can induce tumor cell death [8]. This implies that early tumor formation can be prevented by removing relatively high levels of ROS, or cancer cells can be killed explicitly by promoting the production of excessive levels of ROS in cancer cells. In this review, we discuss the crucial role of ROS-based oxidative stress in various aspects of tumorigenesis and progression. Based on its features and cell fate determination, some potential treatments are proposed.
2. Oxidative Stress and Tumorigenesis
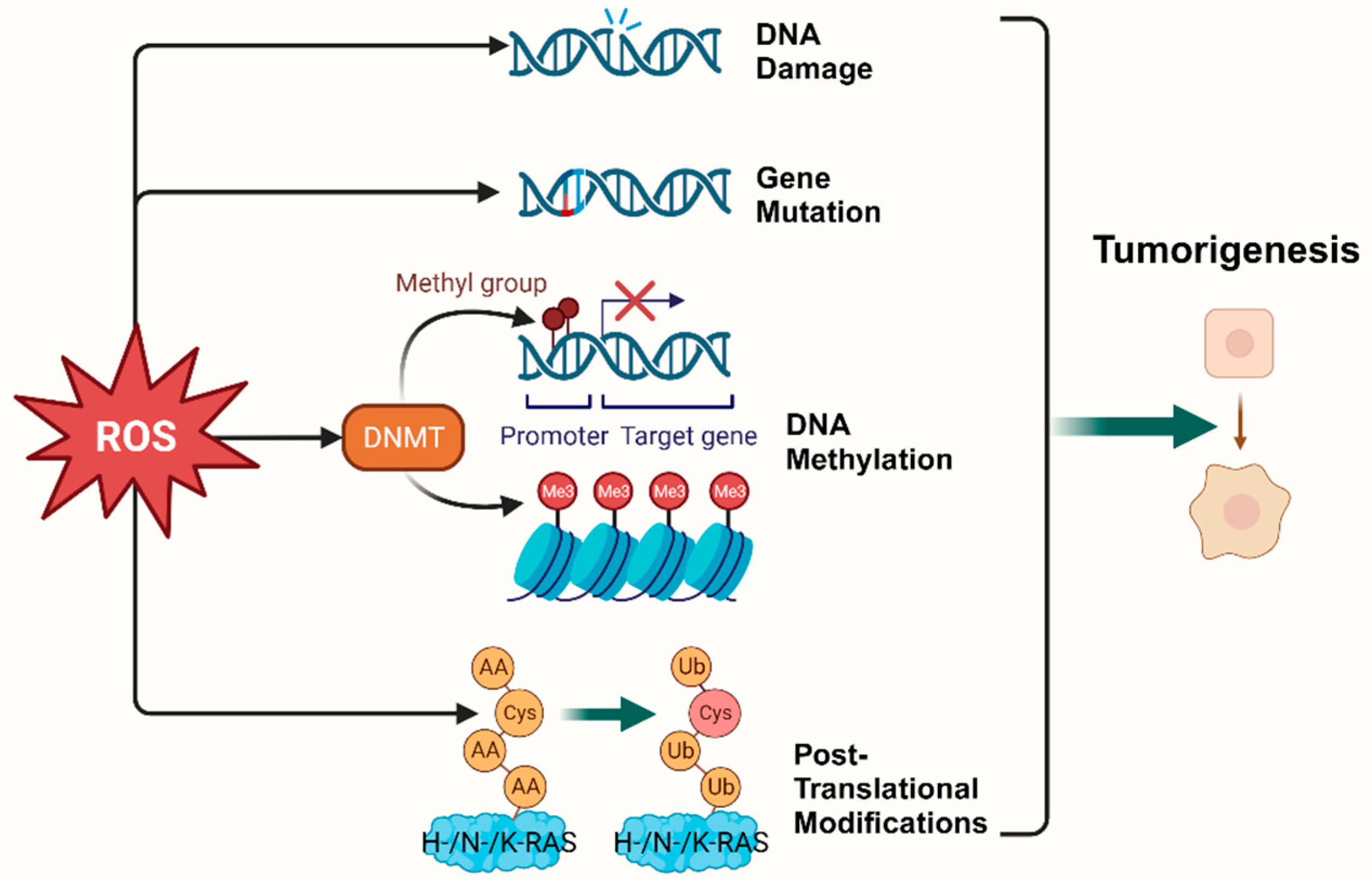
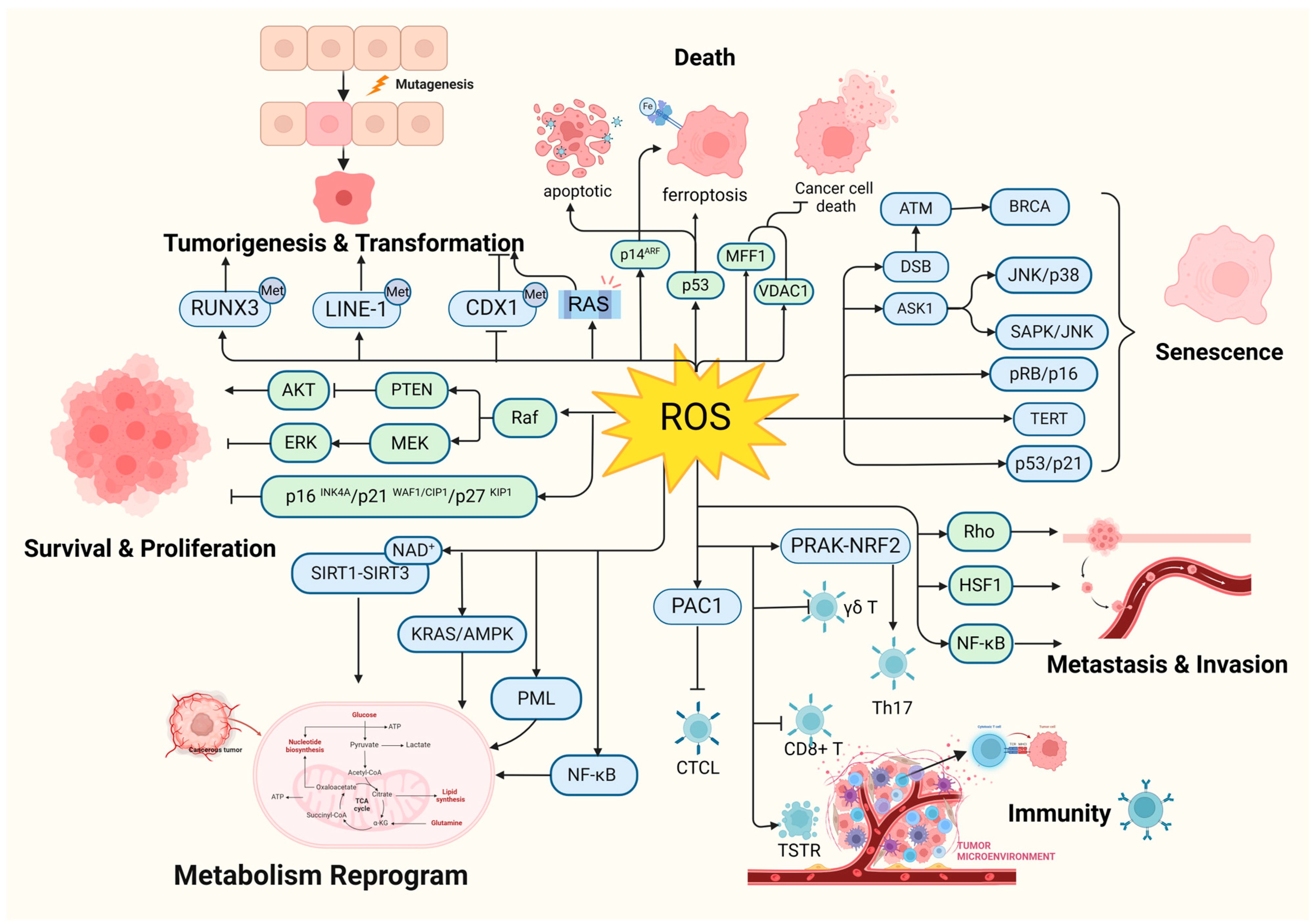
According to the hallmarks of cancer concluded by Douglas Hanahan and Robert Weinberg in 2000 and 2011, genome instability and mutation are enabling characteristics of cancer [9]. The mutability of cancer is achieved through internal factors such as spontaneous mutation accumulation and external factors such as the environment and radiation [10]. ROS, as cellular intermediates of both factors, will directly attack DNA, triggering various forms of DNA damage, such as DNA strand breaks, which affect the expression levels of key genes such as proto-oncogenes, oncogenes, and DNA damage repair-related genes, and promotes tumorigenesis [11,12]. In addition, it has been elaborated that ROS induces mutagenic break repair and the SOS response via damaging bases in DNA, then pausing the replisome and allowing the critical switch from high fidelity to error-prone DNA polymerases, which provoke more carcinogenic mutation [13,14]. It has been shown that ROS can affect tumorigenesis and transformation by oxidizing cysteine residues, which activates the three most common oncogenic switch genes in human cancers, HRAS, NRAS, and KRAS [15]. Mitochondrial ROS (mtROS) had a critical role in tumorigenesis through the ERK-MAPK signaling pathway in a mouse model of oncogenic Kras-mediated lung cancer [16]. Also, in Kras-driven mouse models of pancreatic cancer, ROS inhibition using NAC and MitoQ was found to significantly reduce the development and progression of precancerous lesions [17].
Epigenetic regulation is another vital mechanism altering expression of tumor-related genes [18]. It was found that ROS, as a catalyst for DNA methylation, is extensively involved in the regulation of aberrant hypermethylation and overall hypomethylation levels in the promoter region of tumor suppressor genes (TSG) such as CDX1 through upregulation of DNA methyltransferase (DNMT) expression or the formation of DNMT-containing complexes [19,20]. Simultaneously, ROS directly induced LINE-1 hypomethylation and RUNX3 promoter hypermethylation in bladder cancer cell lines, uroepithelial cell carcinogenesis [21], and hepatocellular carcinoma (HCC), making ROS-induced RUNX3 hypermethylation promising as a practical and valuable biomarker for diagnosis [22,23] (Figure 1).
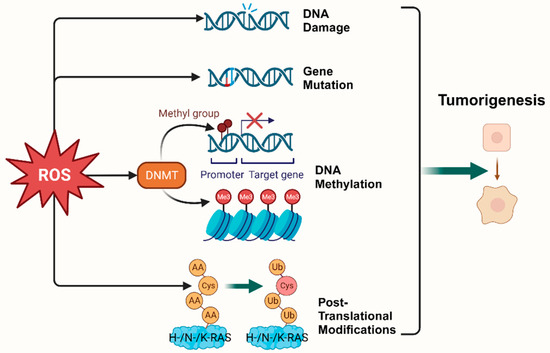
Figure 1.
Oxidative stress plays a pivotal role in tumorigenesis. (Created with BioRender.com).
3. Oxidative Stress and Tumor Metabolism
Tumor cell metabolic reprogramming is a crucial pathway in ROS-induced cancer cell development, and its primary role is to maintain tumor cell adaptive capacity [24]. As early as the 1920s, the German biochemist Warburg pointed out that tumor cells had higher ROS levels than normal cells because of increased aerobic glycolysis (Warburg effect) in cancer cells [8]. The inhibition of glycolysis directly leads to the death of colon tumorigenic cells (HCT116) and lymphoma cells (Raji) [25]. In addition to a preference for aerobic glycolysis and higher levels of ROS, tumor cell metabolic reprogramming is also characterized by enhanced lipid synthesis, abnormal amino acid metabolism, increased lactic acid production, and alteration of the antioxidant system. Cancer cells also possess more powerful antioxidant defenses in contrast to its multitudes of ROS [26]. Intensive ROS scavenging, including peroxiredoxin 1 (PRDX1) [27], SOD2 [28], CAT, GSH-PXs, thioredoxins (TRXs), and GSH, can be upregulated by the activation of TNFα, Nrf2, HIF1α, AMPK, and PGC1α, protecting cancer cells from damage and subsequent cell death [29,30]. Furthermore, NADPH, which is perceived as a pivot in the antioxidant system, with the capacity of renewing reduced glutathione (GSH) and thioredoxin (TRX), is drastically produced in cancer cells via fostering the pentose phosphate pathway, malic enzymes, one-carbon metabolism, etc. [31]. As research continues, mutual reinforcing between metabolic reprogramming and oxidative stress in cancer cells is gradually being uncovered. It has been suggested that PML, as an ROS sensor, is located in both the nucleus and MAM where it regulates the Warburg effect and metabolic reprogramming related to the division, differentiation, and chemical sensitivity of cancer cells [32,33]. Gemcitabine-induced ROS activates KRAS/AMPK signaling, inducing metabolic reprogramming, and enhances stem cell-like properties in pancreatic cancer [34]. Furthermore, sirtuin, including the SIRT1-SIRT3 axis, a kind of NAD+-consuming enzyme regarded as a stress responder, has been demonstrated to trigger metabolic reconstitution and affects the ROS level by deacetylating and activating metabolic enzymes and signaling molecules such as FOXO3a, PGC-1α, TFAM, Drp1, mTOR, and PINK1/Parkin [35,36,37]. In addition, ROS can activate NF-κB [38], NRF2 [38,39], and KHK-A [40] to reduce ROS production. Furthermore, ROS can directly regulate the function of metabolic enzymes through redox modification, which has been demonstrated in key redox-sensitive residues such as cysteine oxidation/S-sulfenylation/S-glutathionylation/S-nitrosylation and tyrosine nitration [4]. For example, oxidative stress contributes to pancreatic ductal adenocarcinoma via inhibiting the arginine methylation of malate dehydrogenase 1 (MDH1) [41].
4. Oxidative Stress and Tumor Cell Proliferation
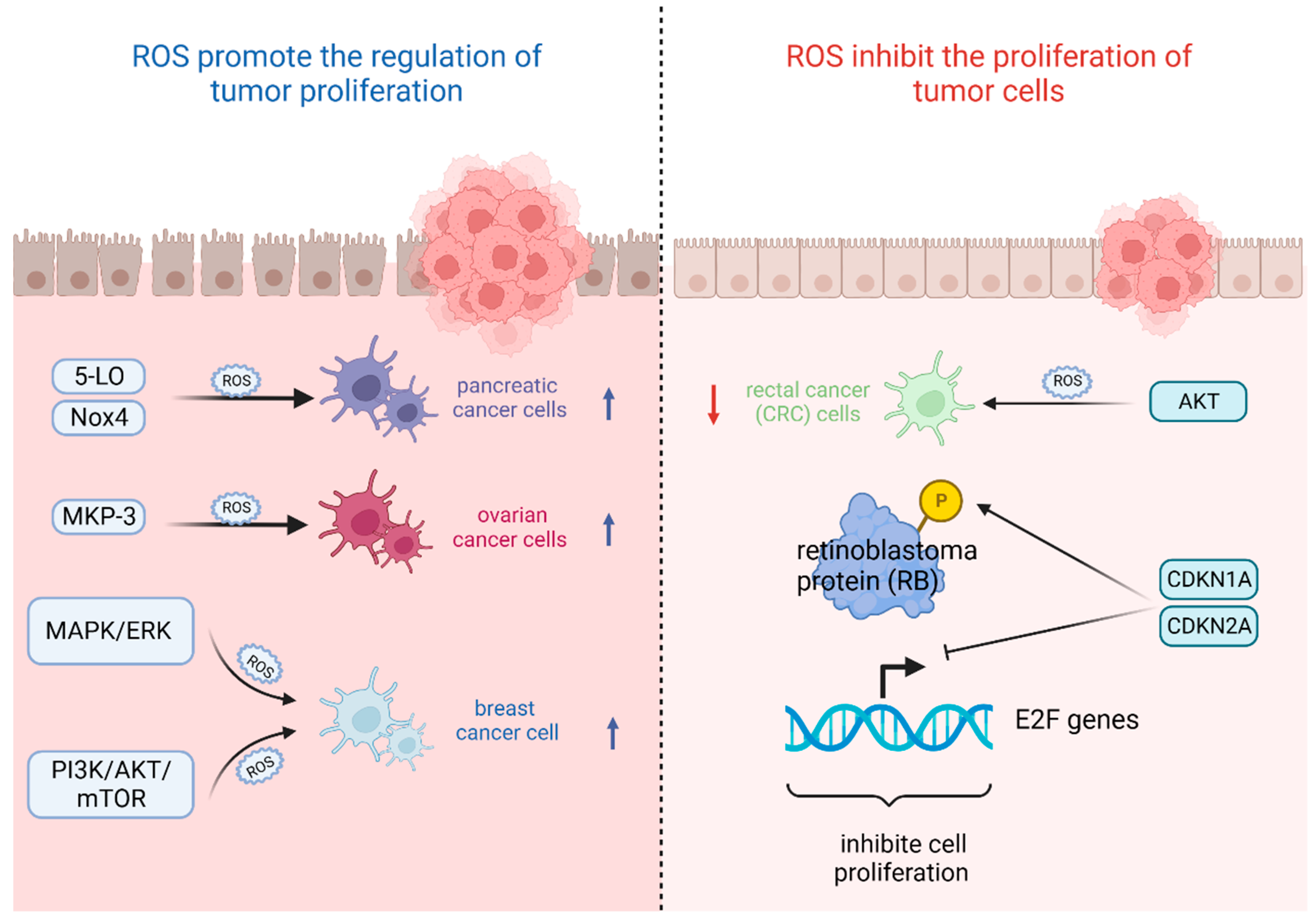
The most important feature of tumor cells is uncontrolled proliferation and growth, and oxidative stress based on ROS levels will directly affect their growth state [42]. That is, the ability of tumor cells to adapt to ROS is crucial for their proliferation [43]. On the one hand, ROS plays an important role in promoting the regulation of tumor proliferation. It was found that the survival rate of pancreatic cancer cells significantly increased after stimulation with high levels of ROS triggered by 5-lipoxygenase (5-LO) and NADPH oxidase 4 (Nox4) [44]. Also, oxidative stress-mediated mitogen-activated protein kinase phosphatase (MKP-3) deficiency is significantly correlated with enhanced tumorigenicity in ovarian cancer cells [45]. In contrast, ROS, as a secondary messenger molecule, can directly mediate the activation of PDGF [46], EGF [47], and MAPK [46] or lead to the inactivation of PTEN [48] to participate in the regulation of tumor cell proliferation [49]. For example, copper chaperones of superoxide dismutase promote breast cancer cell proliferation through ROS-mediated MAPK/ERK signaling [28]. Inactivation of PTEN due to H2O2 over-activates the PI3K/AKT/mTOR signaling pathway to promote breast cancer cell proliferation [50].
On the other hand, ROS can also inhibit the proliferation of tumor cells in several ways. It was found that high levels of ROS mediated AKT-dependent signaling pathways to effectively inhibit the proliferation of rectal cancer (CRC) cells [51]. In addition to this, high levels of ROS can also inhibit tumor growth through sustained activation of cell cycle inhibitory factors. It was noted that significant elevation of p27 KIP1 leads to quiescent cell cycle arrest in the G0 phase [52], whereas silencing p27 KIP1 significantly reverses this arrest and re-enters the cell cycle [53]. By contrast, the most frequently reported are p21 WAF1/CIP1 (CDKN1A) and p16 INK4A (CDKN2A), the accumulation of which first leads to the hypophosphorylation of retinoblastoma protein (RB) and then inhibits the trans-activation of E2F genes involved in nucleotide metabolism and DNA synthesis [54], leading to termination of the regular cell cycle and ultimately inhibiting cell proliferation [55] (Figure 2).
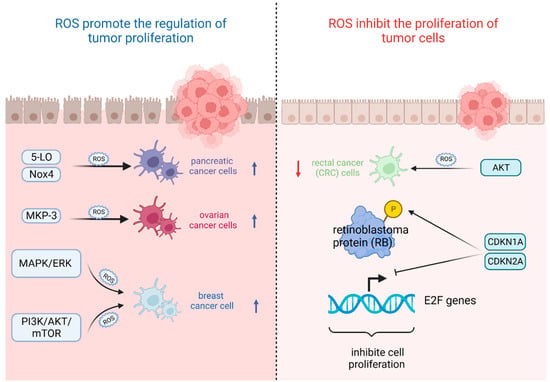
Figure 2.
Oxidative stress based on ROS levels will directly affect tumor cell growth state. (Created with BioRender.com).
5. Oxidative Stress and Tumor Immunity
Oxidative stress exerts a dual effect on tumor immunity. Oxidative stress not only plays a pivotal role in antitumor immune cell differentiation, maturation, and activation, but also imposes an inciting effect on tumor immune escape via evoking tumor-associated immune cells and wreaking havoc on the antitumor system.
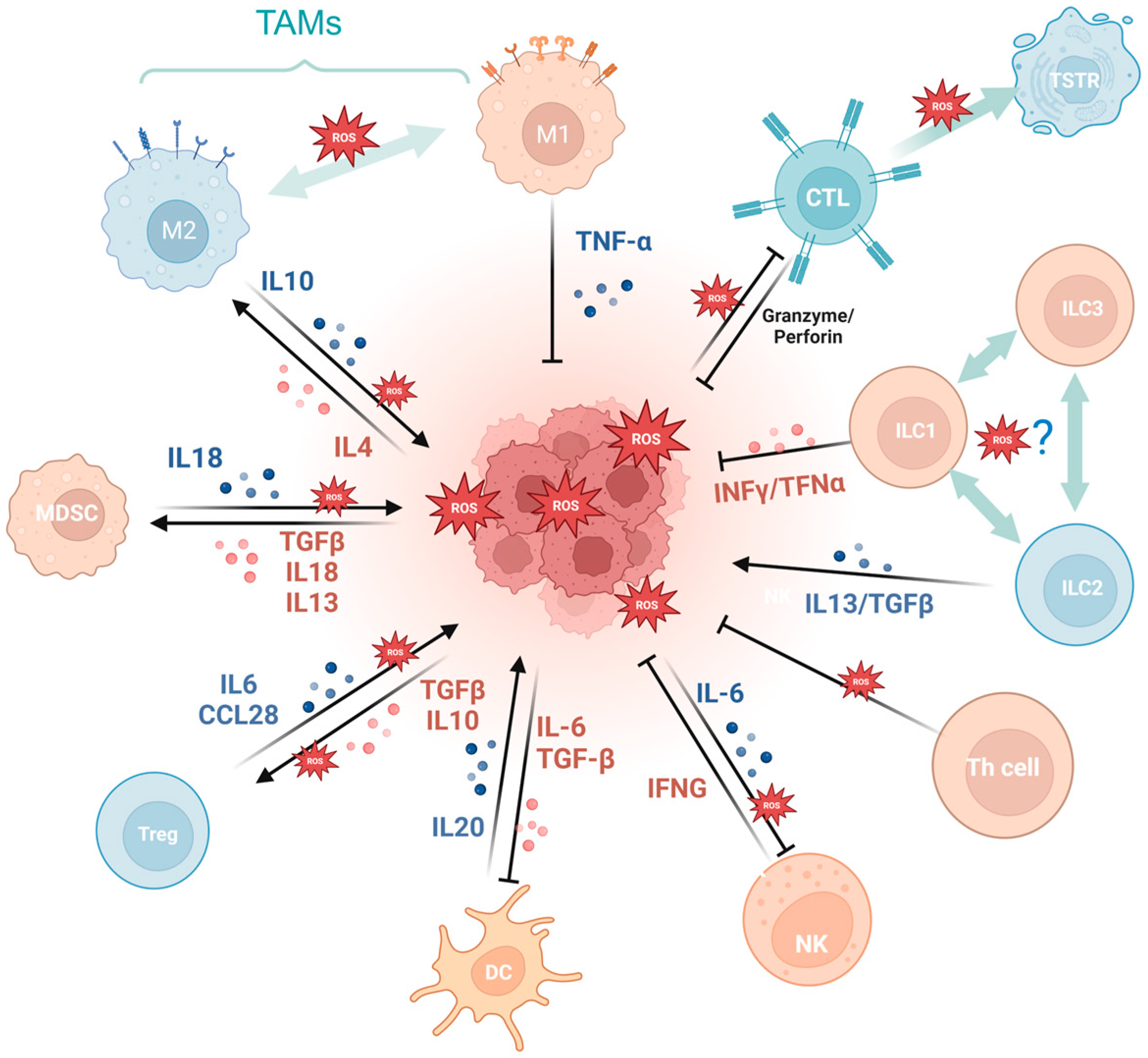
Firstly, a variety of immune cell-derived exosomes, represented by macrophage-derived exosomes, implement a tumoricidal effect by directly releasing ROS [56]. Furthermore, ROS indirectly induces an antitumor effect by boosting immune cells. For example, PRAK deficiency-induced ROS accumulation impairs the differentiation of Th17 cells and antitumor immunity though disrupting phosphorylation of STAT3 [57]. ROS spurs dendritic cell maturation by activating p38-MAPK and ERK1/2 [58]. In addition, ROS is involved in the activation of many immune cells, like CD8+ T cells [59,60], macrophagesTG [61], and dendritic cells [62]. NLRP3 inflammasome is a crucial component of the innate immune system mediated by ROS [63] and contributes to various cancers like gastrointestinal tract [64,65] and breast cancer [66], while it is also demonstrated to be tumor-promoting in gastric and skin cancer [67]. The cGAS/STING pathway, which has been reported to be ROS mediated, exerts a crucial dichotomous function in the antitumor process, including agitating type I IFN induction in DCs, prompting antitumor CD8+ T cell responses, maintaining CD8+ T cell stemness, etc. [62,68,69,70]. While in some tumor cells, enriched cGAS/STING signaling kindles a tumor-promoting function by activating NF-κB, TBK1, and IRF3 [71]. In addition, the aforementioned ROS-associated metabolic reprogramming also occurs in T cells, prompting CD4+T memory cell and CD8+T memory cell formation and survival [72,73].
Cancer development and immune escape cannot be separated from the interaction between tumor cells and the tumor microenvironment (TME), and the involvement of oxidative stress is essential in this process [74]. On the one hand, ROS takes part in activating and inhibiting the function of immune cell such as myeloid-derived suppressor cells (MDSCs) [75], regulatory T cells (Tregs) [76], and tumor-associated macrophages (TAMs) [77]. For example, SUMO-specific protease 3 (SENP3) accumulation triggered by ROS is involved in deubiquitinating modification of transcription factor BACH2 and its activity in maintaining Treg cell-mediated tumor immunosuppression [78]. In malignant melanoma, mitochondrial ROS produced by TAMs stimulates MAPK/ERK activity, which leads to the secretion of TNF-α and promotes tumor cell invasion [77]. On the other hand, the ROS-induced tumor-promoting microenvironment inhibits tumor-killing cells, such as cytotoxic T lymphocyte (CTL) [79] and CD8+ tumor-infiltrating lymphocytes [80]. For example, tumor-associated neutrophils produce O2• mediated by NOX2, inhibiting the expansion of γδ T cells, which promote tumor development by producing IL-17 [81]. Recent studies have indicated that the PRAK-NRF2 axis, which is associated with ROS, is essential for Th17 cells to maintain antitumor effects [57]. Phosphatase PAC1, as an oxidative stress responder [82] and a negative regulator of the immune system, specifically inhibits T lymphocyte defense and promotes tumor immune escape [83]. In addition, the potential of converting M2-TAMs into the immune-promoting M1 subtype has been identified as a promising approach to combat clinically challenging carcinomas [84]. It is ROS in the TME that promotes the polarization of M2 tumor-associated macrophages (TAMs) [85]. Furthermore, T cells in a cellular stress response state (TSTR) are predominantly observed in the TME, which contributes to immunotherapy resistance [86] (Figure 3).
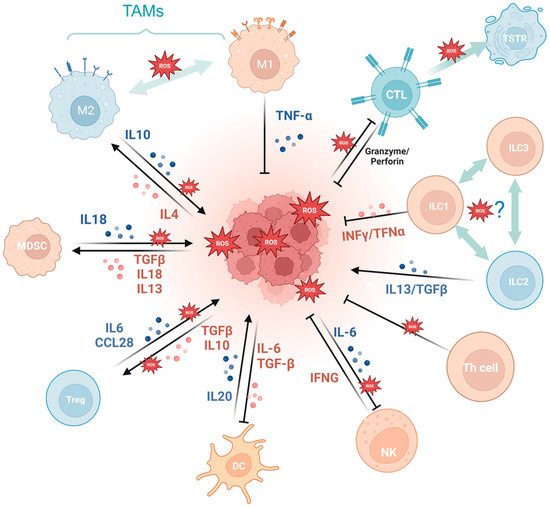
Figure 3.
Oxidative stress is involved in tumor immunity. MDSCs, myeloid-derived suppressor cells; Tregs, regulatory T cells; TAMs, tumor-associated macrophages; CTL, cytotoxic T lymphocyte; TSTR, T cell stress response state; ILC, innate lymphoid cell. (Created with BioRender.com).
6. Oxidative Stress and Tumor Metastasis
Increasing evidence suggests that higher levels of ROS are critical for promoting and maintaining malignant biological behaviors of cancer cells, such as their aggressive metastatic phenotype [87]. In order to achieve the malignant transformation of tumors, early-stage tumor cells usually use the epithelial–mesenchymal transition process (EMT) to invade neighboring stromal cells [88,89]. During this transformation process, ROS promotes tumor metastasis by inducing Rho family guanosine triphosphatase-dependent cytoskeletal rearrangements, promoting matrix metalloproteinase-dependent extracellular matrix protein degradation, and accelerating hypoxia-inducible factor-dependent angiogenesis [49]. Deacetylated SOD2 fosters mitochondrial antioxidant properties, thereby protecting cells from oxidative damage and inhibiting tumorigenesis [90]. However, in the process of tumor development, SOD2 is modified by acetylation, which instead increases mtROS to promote hypoxia signaling, thus promoting EMT in breast cancer cells [91]. Similarly, some factors regulated by redox, like HSF1 [92], NF-κB [93], and MMP [94], also promote metastasis. For example, high levels of ROS in tumor cells activate NF-κB, promoting transcription factor (Snail) expression, downregulating epithelial calmodulin (E-cadherin), and promoting the expression of neural calmodulin and waveform protein, which leads to disruption of cell–cell junctions and triggers the EMT process, stimulating tumor cell metastasis [95]. Furthermore, the downregulation of carnitine palmitoyltransferase 2 (CPT2) induces the ROS/NF-κB pathway in ovarian cancer to promote tumor growth and metastasis [96].
In addition, several studies have shown that ROS can also regulate protein hydrolases matrix metalloproteinases (MMP) [97] and serine proteases, leading to ECM [98] degradation and interfering with the invasive phenotype of tumor cells. For example, G6PD promotes ROS production and activates the MAPK signaling pathway in ccRCC cells, promoting MMP2 overexpression in ccRCC cells and clear cell renal cell carcinoma invasion [94]. In addition, AE-BCT inhibits MMP-9 activity by suppressing ROS-mediated NF-κB activation, thereby significantly reducing the metastatic activity of highly malignant cancer cells [99]. The effect of oxidative stress on EMT was also found to be variable. In stable non-small cell lung cancer cell lines (NSCLC), N-acetylcysteine (NAC) treatment can reduce ROS levels and inhibit EMT phenotypic transformation, which in turn restores the sensitivity of gefitinib-resistant NSCLC cells to gefitinib [100]. Also, in oral squamous cell carcinoma (OSCC) with aberrant expression of serine-threonine protein kinase A (AURKA), the knockdown of AURKA can increase ROS levels and inhibit EMT [101].
7. Oxidative Stress and the Relationship Between Aging and Tumors
Cellular senescence, i.e., irreversible arrest of proliferation, is composed of replicative senescence (RS) and stress-induced premature senescence (SIPS). It can lead to cancer development and age-related diseases [102,103]. Senescence was once thought to be the antithesis of tumorigenesis and progression as a universal barrier all tumor cells must overcome [104]. However, in recent years, more and more research has revealed that cellular senescence can also promote hyperplastic pathologies, including cancer [105]. Meanwhile, oxidative stress plays a critical role in the interaction between senescence and cancer, as cellular senescence responds to long-term cellular stress [104].
Oxidative stress directly inducing tumor aging has been well demonstrated. ROS, a product of oxidative stress, plays an important role in stress-induced premature senescence and contributes to the biochemical and molecular changes required for tumor formation, promotion, and progression [106]. For example, ROS triggers cellular DNA double-strand breaks and the associated ATM signaling pathway [107,108,109] or activates senescence-related signaling pathways such as P53/P21 [110], ASK1/JNK/p38 [111], and so on, leading to the inhibition of tumor cell proliferation and activation of antitumor immunity. As one of the hinges of the cellular stress response, mitochondria play a pivotal role in oxidative stress and stress-induced premature senescence. In colorectal cancer cells, artesunate treatment-induced mitochondrial dysfunction can drastically spur mitochondrial ROS generation, thereby promoting cell senescence [112], which has become a vital target of cancer therapy [113].
The role of cellular senescence in tumorigenesis and progression is many things. Senescence drives both aging and tumors, most likely by promoting chronic inflammation and the senescence-associated secretory phenotype (SASP) [105]. The SASP component consists of several chemokines and cytokines that activate immune surveillance and bring about innate and adaptive immune responses to clear senescent and proliferating tumor cells, and enhancing cancer senescence-induced tumor suppressive capacity can support tumor cell growth arrest [114,115,116]. On the contrary, in recent years, an auxo-action for tumorigenesis has been demonstrated in senescence. SASP promotes malignant phenotypes in nearby cells [105] and formats the tumor-permissive microenvironment [117]. In an indirect way, stress-induced immune cell senescence promotes tumor immune escape [83].
Oxidative stress is one of the reasons senescence is induced in SIPS and takes vital part in the processes of aging and cellular senescence. Apart from ROS oxidizing cysteine residues, activating the three most common oncogenic switch genes in humanity cancers [118], ROS possesses the capability to regulate DNMT activity, which is pivotal to the role of aging in tumors [20,119]. Moreover, ROS can activate various signaling pathways associated with cell senescence. For example, ROS-mediated activation of the ASK1 signalosome subsequently activates the p38 MAPK and SAPK/JNK pathways, which promotes senescence by oxidizing Trx [120]. TP53/CDKN1A (p21) [121], pRB/CDKN2A (p16) [122], and other decisive pathways of cell aging are affected by ROS directly or indirectly.
Normally, replicative senescence occurs accompanied by telomere attrition during a series of cell divisions [123]. Then, senescent cells are cleared via apoptosis or phagocytosis mediated by SASP and the innate immune system, such as NK cells [124,125]. However, erosion of telomeres can induce mitochondrial dysfunction and oxidative stress through the p53-PGC-1α-NRF-1 axis [126].
8. Oxidative Stress and the Relationship Between Death and Tumors
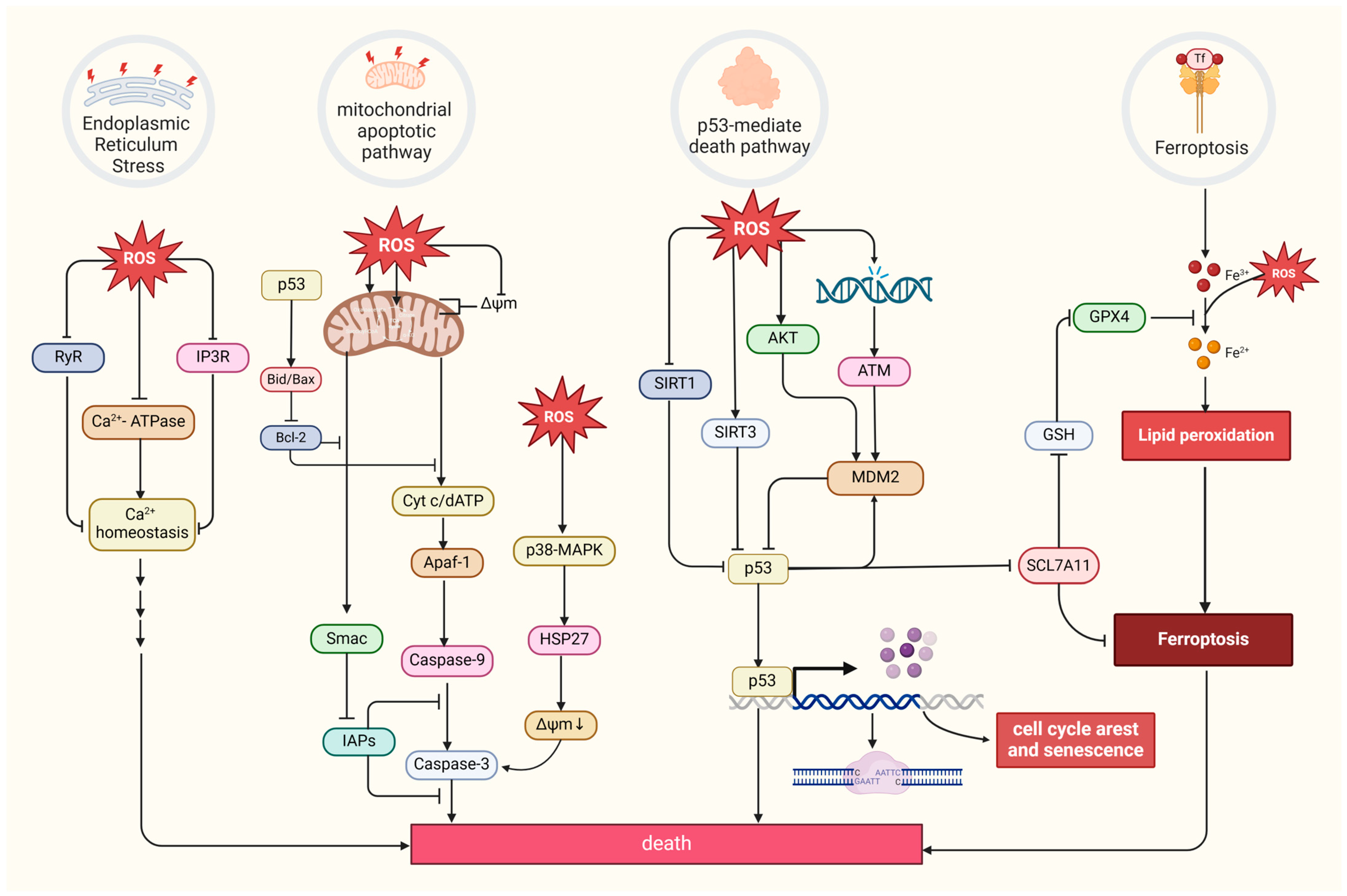
Excessive ROS levels can induce tumor cell death [8]. There are four primary forms of ROS-induced tumor cell death. The first is the mitochondrial apoptotic pathway. Oxidative stress stimulation directly affects mitochondrial membrane potential by disrupting mitochondrial inner membrane permeability [127], mediating pathways like bax/bcl-2-cyt-c [128] and p38 MAPK, and phosphorylating HSP27 [129], all of which promotes the release of cytochrome c (Cyt c) and activates caspase families, directly driving cancer cell apoptosis [130]. Notably, the mitochondrial permeability transition pore (MPTP) integrates ROS-induced cell apoptosis and calcium signaling through activation by both ROS and Ca2+, ensuing an influx of Ca2+ and release of Cyt c [131]. The second refers to the endoplasmic reticulum (ER) stress-mediated apoptosis pathway. Research suggests that ROS can cause disorder of Ca2+ homeostasis in the endoplasmic reticulum, via damaging RyR and IP3R gating and suppressing Ca2+-ATPase activity, thus inducing apoptosis in cancer cells such as prostate cancer cells [132,133]. The third refers to a form of iron-dependent cell death (ferroptosis) caused by the lethal accumulation of lipid ROS, which has recently been identified in various cancers [134,135,136]. Upregulated iron concentrations with elevated levels of ROS stimulate the expression of tumor suppressor p14ARF (CDKN2A) and activate p53, which ultimately inhibits NRF2 activity to promote the onset of ferroptosis [137]. The fourth refers to the p53-mediated apoptotic pathway, which has intricate crosstalk with other pathways. It has been demonstrated that p53 plays a synergistic role with pro-apoptotic factors such as the pro-apoptotic proteins p21cip1 and BAX, promoting the mitochondrial apoptotic pathway [138]. In addition, p53 can dually regulate ferroptosis via diverse pathways [139]. Most interesting, it has been suggested that p53 will transfer to the mitochondria to regulate mitochondrial membrane potential and trigger cellular apoptosis under oxidative stress [140,141], and several proteins proven to interact with p53 directly or indirectly also locate in the mitochondria under the state of oxidative stress, such as MDM2 [142], FOXO3a [143,144], ATM, c-Abl [145], Parkin [146], and TERT [144] (Figure 4).
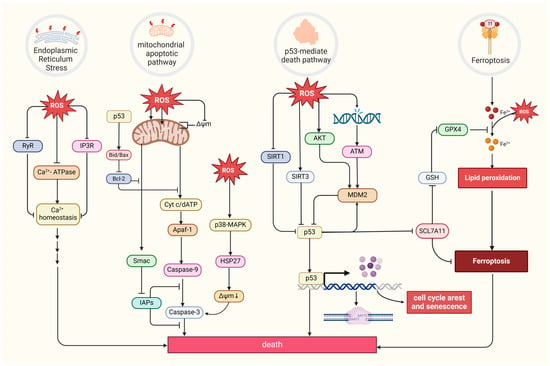
Figure 4.
Four primary forms of tumor cell death and regulation of oxidative stress. MDM2, a p53-specific E3 ubiquitin ligase, as a potential target for activating p53 function in cancer therapy, mediates p53 degradation while responding to oxidative stress via being phosphorylated by AKT, ATM, and c-Abl under the state of oxidative stress [145,147]. SIRT1 and SIRT3, as stress responders, deacetylate and inhibit p53, inducing apoptosis in HCC [148,149]. (Created with BioRender.com).
9. Oxidative Stress and Tumor Treatment
In theory, tumor therapies can be conducted via contradictory approaches, namely reducing oxidative stress to alleviate tumorigenesis [150], metabolic reprogramming [151], metastasis [152], immune escape [153], and transformation of tumor-promoting cells, such as CAFs [154], or bursting oxidative stress to induce aging and dying of tumor cells and tumor-associated cells. Traditional treatment focuses on directly stimulating ROS though radiotherapy; pro-oxidation chemotherapy drugs, including arsenic trioxide [155] and gemcitabine [156]; and drugs that non-specifically target the mitochondrial electron transport chain, such as atovaquone [157] and rotenone [158]. By contrast, with a capacity to scavenge ROS and interact with oxidative stress-related signaling pathways, polyphenols (such as quercetin [159] and curcumin [160]) are widely used in antitumor therapies to promote antioxidation [161]. Nowadays, with a more in-depth understanding of the mechanism of oxidative stress in tumorigenesis and progression, more and more therapies aim to regulate the metabolic processes of redox substances and target crucial signaling pathways. Regulating metabolic processes can be achieved by drugs that target key redox enzymes system. Drugs targeting GSH and the key enzymes taking part in the production and functioning of glutathione (γ-GCS, GSTs, and xCT) are assessed for their ability to disrupt self-protection responses to oxidative stress and drug efflux [162]. Great significance has been attached to blocking the oxidative stress-associated self-protective signaling pathways such as AMPK, NF-κB, Nrf2, c-Jun, and HIF-1α (Table 1). In addition, the antibody-drug-conjugate (ADC) strategy has become a darling of research and clinical treatmentby specifically and efficiently killing cancer cells. Oxidative stress can provide a promising vision for the linker design owing to its heterogeneous existence in tumor cells and their microenvironment. GSH has been reported to work, and other oxidative stress-relevant targets still need to be discovered [163,164].
In general, according to current clinical research and applications, use of pro-oxidants has developed into a relatively mature antitumor therapy with a long history and diverse roles, including prompting tumor cell senescence, damage, death, and regulating pivotal signaling [8,165]. Meanwhile, pro-oxidants tend to incite various side effects, such as nerve damage and bone marrow suppression [166] and may lead to ROS-induced drug resistance [167,168]. Antioxidants, on the contrary, are reckoned as possessing relatively less side effects when remedying cancer via regulating metabolism and alleviating OS-induced damage [169]. One of the most famous drugs is metformin, which has been demonstrated to reduce mtROS production and inhibit migration and invasion in breast cancer [170].
However, antitumor therapies based on oxidative stress sometimes contradict the expected effect. Inhibitors of EGFR and KRAS, both of which are associated with ROS [34,171], have been widely used in clinical studies with various antitumor effects. For example, adagrasib primarily targets NSCLC by alleviating intratumoral immunosuppression [172], MRTX1133 potently suppresses pancreatic cancer tumor growth via increasing cellular apoptosis and impeding proliferation [173], and lapatinib has been approved for the treatment of breast cancer [174]. While EGFR and KRAS inhibitors have also been demonstrated to slash oxidative stress [175], EGFR-TKI resistance promotes NSCLC by mediating ERRα re-expression, then detoxifying ROS [176]. As a vital member of the antioxidant system, Nrf2 is an ideal target of oxidative damage and cancer therapy. Meanwhile, hyperactivated Nrf2 presents pro-tumorigenic activity and is associated with a worse clinical prognosis [177]. It is also regarded as a marker of the cancer-associated fibroblast (CAFs) phenotype because of inciting the expression of genes characteristic for CAFs in skin fibroblasts, then deteriorating tumor development [178]. Clinical trials like SELECT and ATBC have indicated that tumor therapies based on antioxidative approaches such as dietary supplementation with vitamin E, beta carotene, and so on do not always achieve the expected goals [179,180] and sometimes promote tumor development instead [181]. According to a set of facts like oxidative stress exerting a dichotomous effect on tumor, the intratumoral heterogeneity [182], as well as the oxidative stress tolerance of cancer stem cells and drug-resistant sub-populations [183,184], the intricate interactions with tumor microenvironment members like CAFs, plus the cellular location of ROS where antioxidant elimination may also contribute to the observed failure due to inhomogenous distribution of ROS in the cell [185], we can draw the conclusion that practical effects on tumors are discrepant and largely determined by the multi-dimensional heterogeneity of tumors. On the other hand, excessive antioxidants may jeopardize normal physiological process relying on ROS, like immune killing. In a nutshell, the study of cancer therapy based on oxidative stress should comprehensively take into consideration multiple factors to achieve optimum therapy via precise targeting.
At present, clinical research still faces some problems, such as promoting mutations of some key genes that are undruggable [186] and plenty of first-line drugs target multiple kinases, causing non specificity and side effects. Finding more targets for the oxidative stress responses that protect cancer cells from death is necessary, while drugs specifically targeting key signaling molecules, even one of their downstream pathways, must also be developed. There is a bright prospect for much more precise treatment if drugs are developed to block the formation of a specific complex without affecting their individual functions. This reveals the intense need to understand the specific mechanisms of oxidative stress affecting tumors.

Table 1.
Current clinical applications of antitumor therapies based on oxidative stress. Drug names, properties, applicable cancer types, clinical research phase, and specific mechanisms are explained in detail in the table. Some drugs act on tumor-associated cells or the TME, and they are specifically described in the Function section. The information comes from references and the website ClinicalTrials.gov (https://clinicaltrials.gov/).
Table 1.
Current clinical applications of antitumor therapies based on oxidative stress. Drug names, properties, applicable cancer types, clinical research phase, and specific mechanisms are explained in detail in the table. Some drugs act on tumor-associated cells or the TME, and they are specifically described in the Function section. The information comes from references and the website ClinicalTrials.gov (https://clinicaltrials.gov/).
| Drug Name | Type | Clinical Phase | Specific Mechanism | Function | References |
|---|---|---|---|---|---|
| Arsenic trioxide | Chemically synthesized drug | In p53-mutated pediatric cancer, phase 2 | Including autophagy, apoptosis, necroptosis, and ferroptosis | Promote oxidative stress in tumor cells | [187] |
| Gemcitabine | Chemically synthesized drug | In biliary tract cancer, phase 3 trial | Inhibiting nuclear replication, promoting p-STAT3 binding to the promoters of Bmi1, Nanog, and Sox2 genes. | Promote oxidative stress in tumor cells | [156,188,189,190] |
| Elesclomol | Chemically synthesized drug | In ovarian, fallopian tube or primary peritoneal cancer, phase 2 | Promoting cupproposis and killing cancer cells | Promote oxidative stress in tumor cells | [191] |
| Rotenone | Natural active substance | In colon cancer | Inhibiting the PI3K/AKT/mTOR signaling pathway | Promote oxidative stress in tumor cells | [192] |
| Fucoidan | Natural active substance | In hepatocellular carcinoma, phase 2 | Boosting ROS and mitochondrial superoxide generation and draining ATP | Promote oxidative stress in tumor cells | [193] |
| 2-ME | Chemically synthesized drug | In patients with solid tumors, phase 1 | Inhibiting angiogenesisin, increasing CD3+ cell number and promoting tumour necrosis. | Promote oxidative stress in tumor cells | [194] |
| Naringenin | Natural active substance | In human tongue carcinoma CAL-27 cells | Inducing cell death via modulation of the Bid and Bcl-xl signaling pathways | Promote oxidative stress in tumor cells | [195] |
| BT-Br | Chemically synthesized drug | In castration-resistant prostate cancer DU145 cells | Binding to NADPH and inducing ferroptosis | Promote oxidative stress in tumor cells | [196] |
| Atovaquone | Chemically synthesized drug | In non-small cell lung carcinoma, early phase 1 | Inducing tumor cell apoptosis by elevating ROS levels | Promote oxidative stress in tumor cells | [197] |
| Metformin | Chemically synthesized drug | In advanced breast cancer, phase 2 | Increasing FOXO3a, p-FOXO3a, AMPK, p-AMPK, and MnSOD levels | Inhibit oxidative stress in tumor cells | [198] |
| Rapamycin | Chemically synthesized drug | In angiofibromas, phase 2 | Targeting mTOR, inhibits tumor proliferation | Inhibit oxidative stress in pre-cancerous cells | [199] |
| Pirfenidone | Chemically synthesized drug | In neurofibromatosis type 1 and progressive plexiform neurofibromas, phase 2 | Suppressing CAF activation | Inhibit oxidative stress in CAF cells | [200] |
| ME-143 | Chemically synthesized drug | In refractory solid tumors, phase 1 | Targeting NADPH oxidase, blocking ROS production | Inhibit oxidative stress in tumor cells | [201] |
| Carboplatin | Chemically synthesized drug | In locally advanced triple negative breast cancer, phase 2 | Facilitating early and durable CAR T cell infiltration | Promote oxidative stress in TME | [202] |
| Apatinib | Chemically synthesized drug | In metastatic colorectal cancer, phase 2 | Alleviating hypoxia, increasing infiltration of CD8+ T cells, reducing recruitment of TAMs | Promote oxidative stress in TME | [153,203] |
| Propofol | Chemically synthesized drug | In pediatric tumor, phase 4 | Inducing oxidative stress and apoptosis | Promote oxidative stress in tumor cells | [204] |
| Doxorubicin | Chemically synthesized drug | In advanced solid tumors, phase 1 | Perturbing mitochondrial structure and function in tumor cells | Promote oxidative stress in tumor cells | [205] |
| Sunitinib | Chemically synthesized drug | In advanced solid tumors, phase 1 | Alleviating the tumor hypoxia, improving pericyte coverage on endothelial cells | Promote oxidative stress in TME | [206] |
| Salidroside | Natural active substance | In human gastric cancer cell line | Downregulating Src-associated signaling pathway and HSP70 expression | Inhibit oxidative stress in tumor cells | [207] |
| Lipoxin A4 | Natural active substance | In pancreatic cancer cells | Suppressing the ROS/ERK/MMPs pathway | Inhibit oxidative stress in tumor cells | [208] |
| Lobaplatin | Chemically synthesized drug | In human gastric carcinoma cell line BGC-823 | Decreasing mitochondrial membrane potential | Promote oxidative stress in tumor cells | [209] |
| Quercetin | Natural active substance | In metastatic breast cancer, phase 1 | Inhibiting signaling pathways, including MAPK/ERK1/2, JAK/STAT, AMPKα1/ASK1/p38, etc. and inducing cell cycle arrest | Inhibit oxidative stress in tumor cells | [159] |
| Curcumin | Natural active substance | In advanced pancreatic cancer, phase 2 | Promoting apoptosis through inhibiting NF-κB | Inhibit oxidative stress in tumor cells | [160] |
| α-T-K | Chemically synthesized drug | In clinical immunotherapy of sensitized anti-PD-1 | Reprogramming M2 macrophages, elevating the curative effect of PD-1 antibody | Inhibit oxidative stress in TME | [85] |
| Artesunate | Natural active substance | In hepatocellular carcinoma, phase 1 | Promoting the accumulation of intracellular lipid peroxides to induce cancer cell ferroptosis | Promote oxidative stress in tumor cells | [113] |
| MRTX1133 | Chemically synthesized drug | In advanced non-small cell lung cancer with KRAS G12D mutation, phase 3 | Inhibiting KRAS G12D mutation, eliminating ROS, and alleviating intratumoral immunosuppression | Promote oxidative stress in tumor cells | [173,210] |
| Lapatinib | Chemically synthesized drug | In advanced or metastatic breast cancer, phase 1 | Inhibiting EGFR and apoptotic pathways | Promote oxidative stress in tumor cells | [174,211] |
10. Conclusions
After reviewing the above information, it can be concluded that the effects of oxidative stress on tumorigenesis and development have three characteristics.
First, tumor development is a multi-stage dynamic process involving at least three defined phases: initiation, promotion, and progression. Oxidative stress is directly involved in all phases of the process and plays a key role (Figure 5). Currently, the biggest obstacle to cancer treatment is the diversity of mechanisms of tumor induction and escape, that is to say, one target gene, pathway, or process is insufficient and a comprehensive target is necessary. Oxidative stress touches the spot.
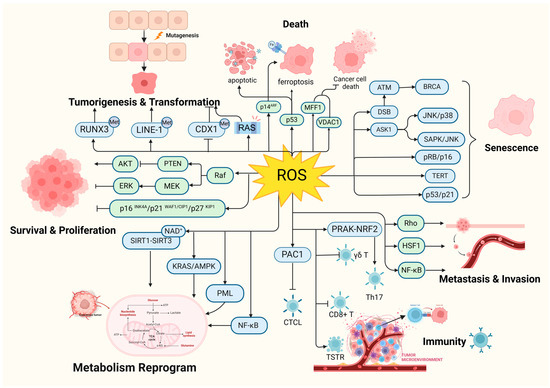
Figure 5.
Oxidative stress is involved in all phases of tumorigenesis. RAS: HRAS, NRAS, and KRAS; CTCL: cutaneous T-cell lymphoma; γδ T: γδ T cells. (Created with BioRender.com).
Second, the processes by which oxidative stress acts on tumor development are crosslinked. The different processes described above are not independent but connected with each other. For instance, tumoral metabolic reprogramming has a close connection with proliferation [212], in which ROS plays a pivotal role via regulating both of them. Furthermore, oxidative stress can affect cellular aging and the composition of the senescence-associated secretory phenotype (SASP) depends on different stimuli, such as pro-inflammatory factors and chemokines, which are associated with inflammatory responses [213], further affecting immunity and cancer. Moreover, oxidative stress is connected with other stresses, such as hypoxia and metabolic stress. It has been observed that different stressors and their responses are crossed. As abscisic acid plays a crucial role in cross-adaptation in plants, ROS could be the hinge of stressors, and some key factors such as FOXO3a and FOXO3a respond to different types of stress through differential modulation [143]. ROS and HIF-1α, which respond to hypoxia inhibition, attenuate each other’s expression [214]. HIF-1a has a comprehensive effect in cancer [215]. Metabolic stress can trigger ROS signaling via the AMPK and AKT pathways [216], and AMPK is closely correlated to carcinogenesis and cancer drug resistance [217].
Last but not least, mitochondria play a crucial role in the impact. (1) As mentioned earlier, mitochondria are the main source and control center of ROS, with both the central enzyme family for ROS production and the majority ROS scavenging systems, such as SOD [218], GSH [219], and MnSOD [220], located there. (2) The target processes of tumor cell metabolic reprogramming, such as the tricarboxylic acid cycle and electron transport chain, are carried out in mitochondria. (3) Signaling molecules associated with tumorigenesis, such as STAT3 [132], TERT [221], PML [222], p53 [223], and HSP60 [213], can also be found in mitochondria. (4) Mitochondria are connected to other subcellular organelles involved in regulating tumorigenesis, such as the nucleus [224] and endoplasmic reticulum (ER). (5) Mitochondria are closely related to the aging of tumor cells. Mitochondrial dysfunction is one of the hallmarks of cell senescence [225] and arouses the mitochondrial dysfunction-associated senescent response (MiDAS) [226]. (6) Mitochondria play a key role in apoptosis and ferroptosis of tumor cells. Mitochondria are the executors of the intrinsic pathway of apoptosis, with apoptosis-related proteins such as the Bcl-2 family proteins and Cyt C [130]. Besides controlling ROS levels, mitochondria also contain large amounts of irons and possess the ability to take up iron. Mitochondrial ferritin is closely associated with oxidative stress and ferroptosis [86,215]. (7) Mitochondria are involved in tumor immunity. It has been demonstrated that the mass, quantity, and morphology of mitochondria can directly regulate immune [227].
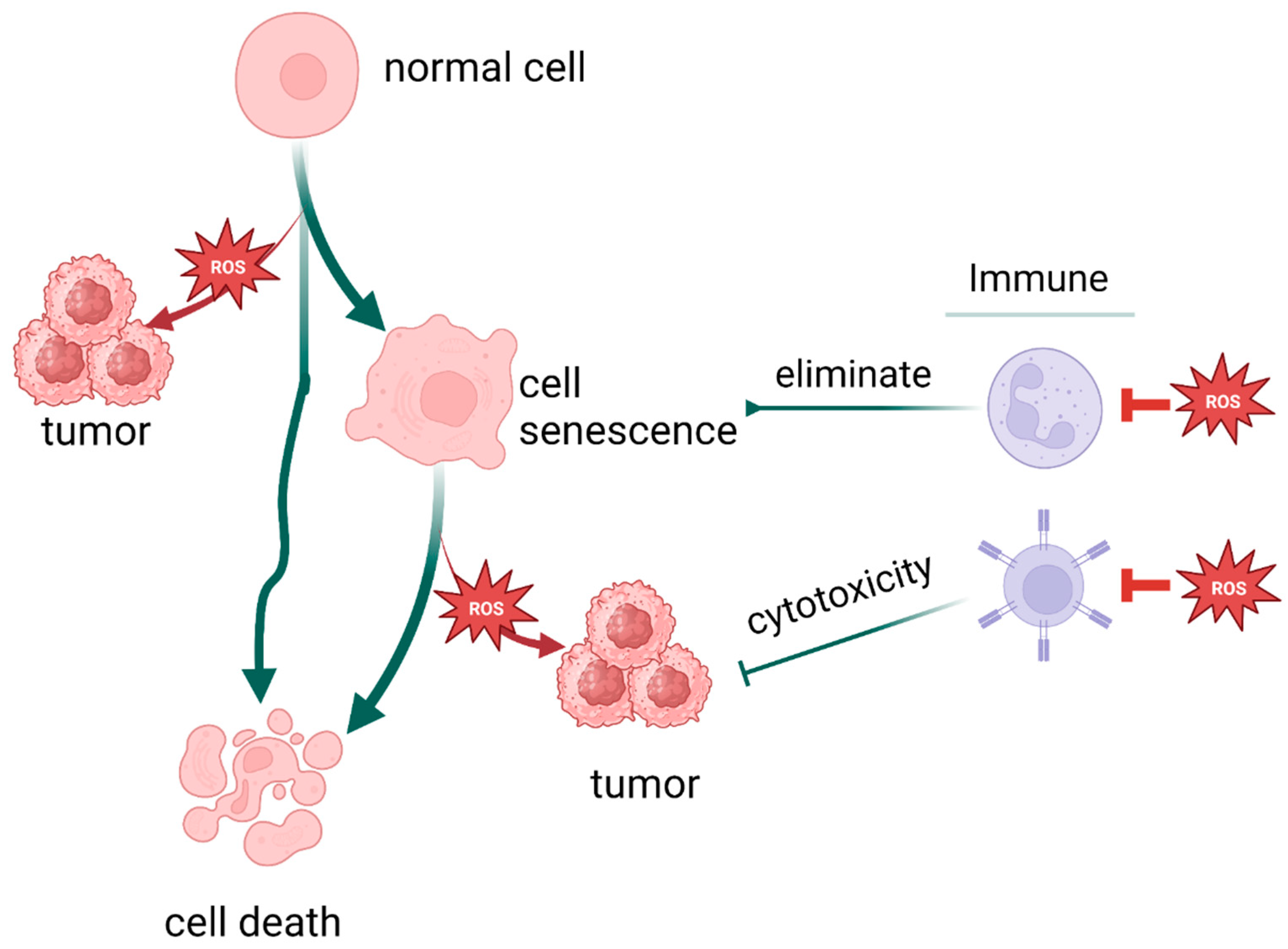
Notably, not only is cancer connected with oxidative stress, but a variety of stresses and stress responses, including cellular senescence and cell death, are related to it. There are more factors and pathways than expected involved in cellular senescence, including but not limited to sirtuin, TERT [226], and PML [228]. As illustrated earlier, oxidative stress promotes death escape by way of inducing gene mutation, setting survival signals on, and suppressing immune system function, eventually achieving immortality. Therefore, oxidative stress could be the intersection of these process and play a pivotal role in cell fate determination (Figure 6).
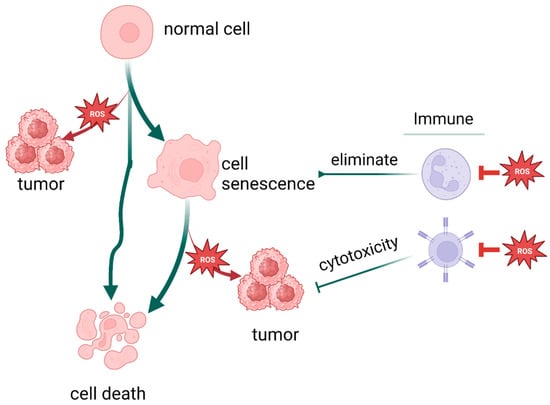
Figure 6.
Oxidative stress plays a pivotal role in cell fate determination. (Created with BioRender.com).
Author Contributions
All authors substantially contributed to this work. Conceptualization: C.W., K.L. and Z.D.; writing—original draft preparation: K.L., Z.D., C.L., X.D. and J.L.; writing—review and editing, K.L., Z.D., C.L., X.D. and J.L.; visualization: Z.D. and C.L.; project administration, C.W., K.L. and Z.D.; funding acquisition: C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (no. 81660024), and the Natural Science Foundation of Inner Mongolia Autonomous Region (No. 2020MS08096) to C.W.
Acknowledgments
We want to convey great appreciation to colleagues who contributed their constructive work in this rapidly expanding field.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Abbreviations | Full Name | Abbreviations | Full Name |
| ROS | reactive oxygen species | PAC1 | phosphatase of activated cells 1 |
| NF-κB | nuclear factor -κB | EMT | epithelial-mesenchymal transition |
| Nrf2 | NF-E2-related factor 2 | HSF1 | heat shock transcription factor 1 |
| PRAK | p38 regulated/activated protein kinase | E-cadherin | epithelial calmodulin |
| ERK | extracellular regulated kinase | CPT2 | carnitine palmitoyltransferase 2 |
| MAPK | mitogen-activated protein kinase | MMP | matrix metalloproteinases |
| PI3K | phosphatidylinositol 3-kinase | ccRCC | clear cell renal cell carcinoma |
| AKT | protein kinase B | NAC | N-acetylcysteine |
| mTOR | mammalian target of rapamycin | OSCC | oral squamous cell carcinoma |
| NOX | NADPH oxidase | AURKA | Aurora kinase A |
| mtROS | mitochondrial ROS | ASK | apoptosis stimulating kinase |
| NAC | N-acetylcysteine | SAPK | stress activated protein kinase |
| TSG | tumor suppressor gene | JNK | c-JunNH2 terminal kinase |
| DNMT | DNA methyltransferase | D-gal | D-galactose |
| CDX1 | caudal type homeobox-1 | AR | aldose reductase |
| LINE-1 | long-interspersed nuclear element-1 | IR | ionizing radiation |
| RUNX3 | runt-related transcription factor 3 | ATM | ataxia telangiectasia mutated |
| CRC | rectal cancer | SASP | senescent-associatedsecretory phenotype |
| RB | retinoblastoma | TSG | tumor suppressor gene |
| HCC | hepatocellular carcinoma | TERT | telomerase reverse transcriptase |
| LKB1 | Live kinase B1 | Trx | thioredoxin |
| MKP | mitogen-activated protein kinase phosphatase | Cytc | cytochrome c |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 | ER | endoplasmic reticulum |
| PML | promyelocytic leukemia | FINs | ferroptosis inducers |
| SIRT1 | sirtuin 1 | ICIs | immune checkpoint inhibitor |
| IDH2 | isocitrate dehydrogenase 2 | mtHSP70 | mitochondrial HSP70 |
| SOD2 | superoxide dismutase 2 | MFF1 | mitochondrial fission factor 1 |
| FOXO3a | Forkhead box O3 | VDAC1 | voltage-dependent anion channel 1 |
| PGC | peroxisome proliferators-activated receptor γ coactivator | ALDH | aldehyde dehydrogenase |
| TFAM | mitochondrial transcription factor A | STAT3 | Signal Transducer and Activator of Transcription 3 |
| Drp | dynamin-related protein | JAK | Janus kinase |
| PINK | PTEN induced putative kinase | NSCLC | non-small cell lung cancer cells |
| KHK-A | protein kinase activity of fructokinase A | RIOS | oxidative stress damage |
| MDA | malondialdehyde | POLRMT | mitochondrial RNA polymerase |
| TME | tumor microenvironment | AMPK | AMP-activated protein kinase |
| MDSCs | myeloid-derived suppressor cells | MDM2 | Murine double minute 2 |
| Tregs | regulatory T cells | CTL | cytotoxic T lymphocyte |
| TAMs | tumor-associated macrophages | TSTR | T cells in a cellular stress response |
| IL | interleukin |
References
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive Oxygen and Nitrogen Species-Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ren, J.; Lu, J.; Li, P.; Zhang, W.; Wang, H.; Tang, B. Elucidating the Relationship between ROS and Protein Phosphorylation through In Situ Fluorescence Imaging in the Pneumonia Mice. Anal. Chem. 2021, 93, 10907–10915. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat. Rev. Cancer 2012, 12, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Garg, M.; Braunstein, G.; Koeffler, H.P. LAMC2 as a therapeutic target for cancers. Expert. Opin. Ther. Targets 2014, 18, 979–982. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef]
- Moore, J.M.; Correa, R.; Rosenberg, S.M.; Hastings, P.J. Persistent damaged bases in DNA allow mutagenic break repair in Escherichia coli. PLoS Genet. 2017, 13, e1006733. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Crisafulli, G.; Sogari, A.; Reilly, N.M.; Arena, S.; Lamba, S.; Bartolini, A.; Amodio, V.; Magrì, A.; Novara, L.; et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019, 366, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; De Simone, G.; Ascenzi, P. Cysteine-based regulation of redox-sensitive Ras small GTPases. Redox Biol. 2019, 26, 101282. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Döppler, H.; DelGiorno, K.E.; Zhang, L.; Leitges, M.; Crawford, H.C.; Murphy, M.P.; Storz, P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep. 2016, 14, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sawalha, A.H. The Role of Oxidative Stress in Epigenetic Changes Underlying Autoimmunity. Antioxid. Redox Signal. 2022, 36, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.A.; Kim, K.C.; Na, S.Y.; Chang, W.Y.; Kim, G.Y.; Kim, H.S.; Hyun, J.W. Oxidative stress causes epigenetic alteration of CDX1 expression in colorectal cancer cells. Gene 2013, 524, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ni, X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets 2015, 16, 13–19. [Google Scholar] [CrossRef]
- Wongpaiboonwattana, W.; Tosukhowong, P.; Dissayabutra, T.; Mutirangura, A.; Boonla, C. Oxidative stress induces hypomethylation of LINE-1 and hypermethylation of the RUNX3 promoter in a bladder cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 3773–3778. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Z.; Zou, Z.; Xiao, G.; Luo, G.; Yang, H. Clinicopathological significance of RUNX3 gene hypermethylation in hepatocellular carcinoma. Tumour Biol. 2014, 35, 10333–10340. [Google Scholar] [CrossRef]
- Poungpairoj, P.; Whongsiri, P.; Suwannasin, S.; Khlaiphuengsin, A.; Tangkijvanich, P.; Boonla, C. Increased Oxidative Stress and RUNX3 Hypermethylation in Patients with Hepatitis B Virus-Associated Hepatocellular Carcinoma (HCC) and Induction of RUNX3 Hypermethylation by Reactive Oxygen Species in HCC Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5343–5348. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Bonanni, B. Relationship Between Metabolic Reprogramming and Mitochondrial Activity in Cancer Cells. Understanding the Anticancer Effect of Metformin and Its Clinical Implications. Anticancer Res. 2015, 35, 5789–5796. [Google Scholar] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Ruan, Y.; Che, X.; Feng, W. Dual role of PRDX1 in redox-regulation and tumorigenesis: Past and future. Free. Radic. Biol. Med. 2024, 210, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zhao, M.; Liu, B.; Han, X.; Li, Y.; Wang, W.; Zhang, Q.; Lv, P.; Xing, L.; Shen, H.; et al. TNF-α-mediated upregulation of SOD-2 contributes to cell proliferation and cisplatin resistance in esophageal squamous cell carcinoma. Oncol. Rep. 2019, 42, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Huang, Y.; Wu, J.; Wang, Y.; Chen, A.; Guo, Q.; Zhang, Y.; Zhang, S.; Wang, L.; et al. An oncolytic system produces oxygen selectively in pancreatic tumor cells to alleviate hypoxia and improve immune activation. Pharmacol. Res. 2024, 199, 107053. [Google Scholar] [CrossRef] [PubMed]
- Zakic, T.; Pekovic-Vaughan, V.; Cvoro, A.; Korac, A.; Jankovic, A.; Korac, B. Redox and metabolic reprogramming in breast cancer and cancer-associated adipose tissue. FEBS Lett. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Ju, H.Q.; Lin, J.F.; Tian, T.; Xie, D.; Xu, R.H. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Niwa-Kawakita, M.; Ferhi, O.; Soilihi, H.; Le Bras, M.; Lallemand-Breitenbach, V.; de Thé, H. PML is a ROS sensor activating p53 upon oxidative stress. J. Exp. Med. 2017, 214, 3197–3206. [Google Scholar] [CrossRef]
- Niwa-Kawakita, M.; Wu, H.C.; Thé, H.; Lallemand-Breitenbach, V. PML nuclear bodies, membrane-less domains acting as ROS sensors? Semin. Cell Dev. Biol. 2018, 80, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, S.; Li, H.; Duan, Q.; Zhang, Z.; Shen, Q.; Wang, C.; Yin, T. ROS/KRAS/AMPK Signaling Contributes to Gemcitabine-Induced Stem-Like Cell Properties in Pancreatic Cancer. Mol. Ther. Oncolytics 2019, 14, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Shen, L. Mitochondrial proteins in heart failure: The role of deacetylation by SIRT3. Pharmacol. Res. 2021, 172, 105802. [Google Scholar] [CrossRef]
- Kitamura, H.; Takeda, H.; Motohashi, H. Genetic, metabolic and immunological features of cancers with NRF2 addiction. FEBS Lett. 2022, 596, 1981–1993. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, X.; Shao, F.; Lv, G.; Lv, H.; Lee, J.H.; Qian, X.; Wang, Z.; Xia, Y.; Du, L.; et al. The protein kinase activity of fructokinase A specifies the antioxidant responses of tumor cells by phosphorylating p62. Sci. Adv. 2019, 5, eaav4570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Zhou, W.; Wang, J.; Huang, X.; Zuo, Y.; Wang, T.S.; Gao, X.; Xu, Y.Y.; Zou, S.W.; Liu, Y.B.; et al. Arginine Methylation of MDH1 by CARM1 Inhibits Glutamine Metabolism and Suppresses Pancreatic Cancer. Mol. Cell 2016, 64, 673–687. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Du, W.; Jiang, P.; Mancuso, A.; Stonestrom, A.; Brewer, M.D.; Minn, A.J.; Mak, T.W.; Wu, M.; Yang, X. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat. Cell Biol. 2013, 15, 991–1000. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Hong, P.; Vaquero, E.C.; Lee, J.K.; Fischer, L.; Friess, H.; Buchler, M.W.; Lerch, M.M.; Pandol, S.J.; Gukovskaya, A.S. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1137–G1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chi, Y.; Gao, K.; Zhang, X.; Yao, J. p53 protein-mediated up-regulation of MAP kinase phosphatase 3 (MKP-3) contributes to the establishment of the cellular senescent phenotype through dephosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2). J. Biol. Chem. 2015, 290, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Shen, Z.; Yang, Q.; Sui, F.; Pu, J.; Ma, J.; Ma, S.; Yao, D.; Ji, M.; Hou, P. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics 2019, 9, 4461–4473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, T.; Nie, G.; Hu, R.; Pi, S.; Wei, Z.; Wang, C.; Xing, C.; Hu, G. Cadmium and molybdenum co-induce pyroptosis via ROS/PTEN/PI3K/AKT axis in duck renal tubular epithelial cells. Environ. Pollut. 2021, 272, 116403. [Google Scholar] [CrossRef] [PubMed]
- Pani, G.; Galeotti, T.; Chiarugi, P. Metastasis: Cancer cell’s escape from oxidative stress. Cancer Metastasis Rev. 2010, 29, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 Controls Breast Cancer Cell Migration by Regulating Hydrogen Peroxide Transport and Its Downstream Cell Signaling. Mol. Cell Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hu, J.; Lv, Y.; Bai, B.; Shan, L.; Chen, K.; Dai, S.; Zhu, H. Pyrvinium pamoate inhibits cell proliferation through ROS-mediated AKT-dependent signaling pathway in colorectal cancer. Med. Oncol. 2021, 38, 21. [Google Scholar] [CrossRef]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.; Flanagan, W.M.; Nourse, J.; Roberts, J.M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 1996, 272, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998, 9, 585–593. [Google Scholar] [PubMed]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Shin, S.; Baek, M.C.; Yea, K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: Current advances and therapeutic applications. Exp. Mol. Med. 2024, 56, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Y.; Gao, Y.; Ju, Y.; Zhao, Y.; Wu, Z.; Gao, S.; Zhang, B.; Pang, X.; Zhang, Y.; et al. The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc. Natl. Acad. Sci. USA 2023, 120, e2212613120. [Google Scholar] [CrossRef] [PubMed]
- Paardekooper, L.M.; Vos, W.; van den Bogaart, G. Oxygen in the tumor microenvironment: Effects on dendritic cell function. Oncotarget 2019, 10, 883–896. [Google Scholar] [CrossRef]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef]
- Bao, Y.; Li, G.; Li, S.; Zhang, H.; Wu, X.; Yan, R.; Wang, Z.; Guo, C.; Jin, Y. Multifunctional Tumor-Targeting Carbon Dots for Tumor Microenvironment Activated Ferroptosis and Immunotherapy in Cancer Treatment. ACS Appl. Mater. Interfaces 2023, 15, 56834–56845. [Google Scholar] [CrossRef]
- Hu, X.; Ding, S.; Lu, G.; Lin, Z.; Liao, L.; Xiao, W.; Ding, Y.; Zhang, Y.; Wang, Z.; Gong, W.; et al. Apolipoprotein C-III itself stimulates the Syk/cPLA2-induced inflammasome activation of macrophage to boost anti-tumor activity of CD8+ T cell. Cancer Immunol. Immunother. CII 2023, 72, 4123–4144. [Google Scholar] [CrossRef]
- Hu, Z.; Teng, X.L.; Zhang, T.; Yu, X.; Ding, R.; Yi, J.; Deng, L.; Wang, Z.; Zou, Q. SENP3 senses oxidative stress to facilitate STING-dependent dendritic cell antitumor function. Mol. Cell 2021, 81, 940–952.e945. [Google Scholar] [CrossRef] [PubMed]
- Akbal, A.; Dernst, A.; Lovotti, M.; Mangan, M.S.J.; McManus, R.M.; Latz, E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell. Mol. Immunol. 2022, 19, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Liu, L.; Fan, Z. Mechanisms and effects of NLRP3 in digestive cancers. Cell Death Discov. 2024, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Arrè, V.; Scialpi, R.; Centonze, M.; Giannelli, G.; Scavo, M.P.; Negro, R. The ‘speck’-tacular oversight of the NLRP3-pyroptosis pathway on gastrointestinal inflammatory diseases and tumorigenesis. J. Biomed. Sci. 2023, 30, 90. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.S.; Costantini, S.; de Lima, V.C.C.; de Andrade, V.P.; Rialland, M.; Cedric, R.; Budillon, A.; Magalhães, K.G. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J. Biomed. Sci. 2021, 28, 26. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer 2019, 19, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Vatner, R.E.; Janssen, E.M. STING, DCs and the link between innate and adaptive tumor immunity. Mol. Immunol. 2019, 110, 13–23. [Google Scholar] [CrossRef]
- Cao, L.; Tian, H.; Fang, M.; Xu, Z.; Tang, D.; Chen, J.; Yin, J.; Xiao, H.; Shang, K.; Han, H.; et al. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials 2022, 290, 121856. [Google Scholar] [CrossRef]
- Li, W.; Lu, L.; Lu, J.; Wang, X.; Yang, C.; Jin, J.; Wu, L.; Hong, X.; Li, F.; Cao, D.; et al. cGAS-STING-mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci. Transl. Med. 2020, 12, eaay9013. [Google Scholar] [CrossRef]
- Gan, Y.; Li, X.; Han, S.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. The cGAS/STING Pathway: A Novel Target for Cancer Therapy. Front. Immunol. 2021, 12, 795401. [Google Scholar] [CrossRef]
- Krueger, P.D.; Osum, K.C.; Jenkins, M.K. CD4+ Memory T-Cell Formation during Type 1 Immune Responses. Cold Spring Harb. Perspect. Biol. 2021, 13, a038141. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D. The metabolic spectrum of memory T cells. Immunol. Cell Biol. 2019, 97, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef]
- OuYang, L.Y.; Wu, X.J.; Ye, S.B.; Zhang, R.X.; Li, Z.L.; Liao, W.; Pan, Z.Z.; Zheng, L.M.; Zhang, X.S.; Wang, Z.; et al. Tumor-induced myeloid-derived suppressor cells promote tumor progression through oxidative metabolism in human colorectal cancer. J. Transl. Med. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Kraaij, M.D.; Savage, N.D.; van der Kooij, S.W.; Koekkoek, K.; Wang, J.; van den Berg, J.M.; Ottenhoff, T.H.; Kuijpers, T.W.; Holmdahl, R.; van Kooten, C.; et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2010, 107, 17686–17691. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zheng, W.; Liu, J.; Zhang, Y.; Qin, H.; Wu, H.; Xue, B.; Lu, Y.; Shen, P. Oxidative stress in malignant melanoma enhances tumor necrosis factor-α secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid. Redox Signal. 2013, 19, 1337–1355. [Google Scholar] [CrossRef]
- Yu, X.; Lao, Y.; Teng, X.L.; Li, S.; Zhou, Y.; Wang, F.; Guo, X.; Deng, S.; Chang, Y.; Wu, X.; et al. SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat. Commun. 2018, 9, 3157. [Google Scholar] [CrossRef]
- Balwit, J.M.; Kalinski, P.; Sondak, V.K.; Coulie, P.G.; Jaffee, E.M.; Gajewski, T.F.; Marincola, F.M. Review of the 25th annual scientific meeting of the International Society for Biological Therapy of Cancer. J. Transl. Med. 2011, 9, 60. [Google Scholar] [CrossRef]
- Notarangelo, G.; Spinelli, J.B.; Perez, E.M.; Baker, G.J.; Kurmi, K.; Elia, I.; Stopka, S.A.; Baquer, G.; Lin, J.R.; Golby, A.J.; et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 2022, 377, 1519–1529. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ibrahim, K.E.; Kundu, S.; Attia, S.M.; Alanazi, W.A.; AlSharari, S.D. Inhibition of spleen tyrosine kinase signaling protects against acute lung injury through blockade of NADPH oxidase and IL-17A in neutrophils and γδ T cells respectively in mice. Int. Immunopharmacol. 2019, 68, 39–47. [Google Scholar] [CrossRef]
- Wu, J.J.; Fan, H.J.; Fan, Q.X.; Zhang, M.Z. Roles and mechanisms of E2F-1 and PAC1 in signaling oxidative stress-induced apoptosis of Saos-2 cells. Zhonghua Yi Xue Za Zhi 2012, 92, 1219–1221. [Google Scholar]
- Dan, L.; Liu, L.; Sun, Y.; Song, J.; Yin, Q.; Zhang, G.; Qi, F.; Hu, Z.; Yang, Z.; Zhou, Z.; et al. The phosphatase PAC1 acts as a T cell suppressor and attenuates host antitumor immunity. Nat. Immunol. 2020, 21, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, X.; Zhang, J.; Lu, Y.; Shi, Y.; Zhu, C.; Liu, Y.; Qin, B.; Luo, Z.; Du, Y.; et al. Dual Inhibition of Endoplasmic Reticulum Stress and Oxidation Stress Manipulates the Polarization of Macrophages under Hypoxia to Sensitize Immunotherapy. ACS Nano 2021, 15, 14522–14534. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Dai, E.; Li, Y.; Han, G.; Pei, G.; Ingram, D.R.; Thakkar, K.; Qin, J.J.; Dang, M.; Le, X.; et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 2023, 29, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Muthuramalingam, K.; Cho, M. Redox Regulation of NOX Isoforms on FAK((Y397))/SRC((Y416)) Phosphorylation Driven Epithelial-to-Mesenchymal Transition in Malignant Cervical Epithelial Cells. Cells 2020, 9, 1555. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Patel, R.P. SOD2 acetylation and deacetylation: Another tale of Jekyll and Hyde in cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 23376–23378. [Google Scholar] [CrossRef]
- He, C.; Danes, J.M.; Hart, P.C.; Zhu, Y.; Huang, Y.; de Abreu, A.L.; O’Brien, J.; Mathison, A.J.; Tang, B.; Frasor, J.M.; et al. SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 23534–23541. [Google Scholar] [CrossRef]
- Xi, C.; Hu, Y.; Buckhaults, P.; Moskophidis, D.; Mivechi, N.F. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J. Biol. Chem. 2012, 287, 35646–35657. [Google Scholar] [CrossRef]
- Li, W.; Cao, L.; Han, L.; Xu, Q.; Ma, Q. Superoxide dismutase promotes the epithelial-mesenchymal transition of pancreatic cancer cells via activation of the H2O2/ERK/NF-κB axis. Int. J. Oncol. 2015, 46, 2613–2620. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Q.; Yang, Z.; Ni, Y.; Agbana, Y.L.; Bai, H.; Yi, Z.; Yi, X.; Kuang, Y.; Zhu, Y. G6PD facilitates clear cell renal cell carcinoma invasion by enhancing MMP2 expression through ROS-MAPK axis pathway. Int. J. Oncol. 2020, 57, 197–212. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox regulation in tumor cell epithelial-mesenchymal transition: Molecular basis and therapeutic strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Vlachostergios, P.J. Loss of tumor suppressive properties of lipid metabolism enzyme CPT2 in ovarian carcinoma: Comment on “CPT2 down-regulation promotes tumor growth and metastasis through inducing ROS/NFκB pathway in ovarian cancer” by Zhang et al. Transl. Oncol. 2021, 14, 101067. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Corsini, E.; Kouretas, D.; Tsatsakis, A.; Tzanakakis, G. ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem. Toxicol. 2013, 61, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, S.W.; Kim, J.R. Reactive oxygen species regulate urokinase plasminogen activator expression and cell invasion via mitogen-activated protein kinase pathways after treatment with hepatocyte growth factor in stomach cancer cells. J. Exp. Clin. Cancer Res. 2009, 28, 73. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Im, M.; Yim, N.H.; Jung, Y.P.; Ma, J.Y. Aqueous extract of Bambusae Caulis in Taeniam inhibits PMA-induced tumor cell invasion and pulmonary metastasis: Suppression of NF-κB activation through ROS signaling. PLoS ONE 2013, 8, e78061. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.H.; Hu, J.; Shi, M.; Zhang, L.; Chen, H. Combined treatment with N-acetylcysteine and gefitinib overcomes drug resistance to gefitinib in NSCLC cell line. Cancer Med. 2020, 9, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Dong, H.; Wang, Q. AURKA contributes to the progression of oral squamous cell carcinoma (OSCC) through modulating epithelial-to-mesenchymal transition (EMT) and apoptosis via the regulation of ROS. Biochem. Biophys. Res. Commun. 2018, 507, 83–90. [Google Scholar]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Aging, cellular senescence, and cancer. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free. Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Li, Z.; Xiong, J.; Geng, Z.; Wei, W.; Zhang, Y.; Wu, G.; Zhuang, T.; Tian, X.; Liu, Z.; et al. LARP7 ameliorates cellular senescence and aging by allosterically enhancing SIRT1 deacetylase activity. Cell Rep. 2021, 37, 110038. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Si, F.; Bagley, D.; Ma, F.; Zhang, Y.; Tao, Y.; Shaw, E.; Peng, G. Blockades of effector T cell senescence and exhaustion synergistically enhance antitumor immunity and immunotherapy. J. Immunother. Cancer 2022, 10, e005020. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Kriesen, S.; Neels, O.; Hildebrandt, G.; Manda, K. Investigation of epothilone B-induced cell death mechanisms in human epithelial cancer cells -in consideration of combined treatment with ionizing radiation. Cancer Investig. 2015, 33, 213–224. [Google Scholar] [CrossRef]
- Vaddavalli, P.L.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in cancer and aging. Trends Genet. 2022, 38, 598–612. [Google Scholar] [CrossRef]
- Ichijo, H.; Nishida, E.; Irie, K.; ten Dijke, P.; Saitoh, M.; Moriguchi, T.; Takagi, M.; Matsumoto, K.; Miyazono, K.; Gotoh, Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997, 275, 90–94. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, S.; Zhuang, X.; Chen, Y.; Lu, L.; Wang, Y.; Qi, X.; Feng, Q.; Huang, Q.; Du, B.; et al. Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy. Cells 2022, 11, 2472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, X.; Ren, M.; Wang, Z. Ferroptosis: A New Research Direction of Artemisinin and Its Derivatives in Anti-Cancer Treatment. Am. J. Chin. Med. 2024, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Mausset-Bonnefont, A.L.; Jorgensen, C.; Louis-Plence, P.; Brondello, J.M. Cellular senescence impact on immune cell fate and function. Aging Cell 2016, 15, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Prieur, A.; Peeper, D.S. Cellular senescence in vivo: A barrier to tumorigenesis. Curr. Opin. Cell Biol. 2008, 20, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, M.; Zanoni, M.; Pirini, F.; Tumedei, M.M.; Ravaioli, S.; Rapposelli, I.G.; Frassineti, G.L.; Bravaccini, S. Pancreatic Cancer and Cellular Senescence: Tumor Microenvironment under the Spotlight. Int. J. Mol. Sci. 2021, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968–983. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Verma, A.; Marderstein, A.R.; White, J.; Bhinder, B.; Garcia Medina, J.S.; Elemento, O. Pan-cancer analysis reveals molecular patterns associated with age. Cell Rep. 2021, 37, 110100. [Google Scholar] [CrossRef]
- Papaconstantinou, J. The Role of Signaling Pathways of Inflammation and Oxidative Stress in Development of Senescence and Aging Phenotypes in Cardiovascular Disease. Cells 2019, 8, 1383. [Google Scholar] [CrossRef]
- Macip, S.; Igarashi, M.; Fang, L.; Chen, A.; Pan, Z.Q.; Lee, S.W.; Aaronson, S.A. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. Embo J. 2002, 21, 2180–2188. [Google Scholar] [CrossRef]
- Lv, F.; Li, N.; Kong, M.; Wu, J.; Fan, Z.; Miao, D.; Xu, Y.; Ye, Q.; Wang, Y. CDKN2a/p16 Antagonizes Hepatic Stellate Cell Activation and Liver Fibrosis by Modulating ROS Levels. Front. Cell Dev. Biol. 2020, 8, 176. [Google Scholar] [CrossRef]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef]
- Schank, M.; Zhao, J.; Wang, L.; Li, Z.; Cao, D.; Nguyen, L.N.; Dang, X.; Khanal, S.; Nguyen, L.N.T.; Thakuri, B.K.C.; et al. Telomeric injury by KML001 in human T cells induces mitochondrial dysfunction through the p53-PGC-1α pathway. Cell Death Dis. 2020, 11, 1030. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by β-Caryophyllene: A Focus on the Nervous System. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef]
- AlBasher, G.; AlKahtane, A.A.; Alarifi, S.; Ali, D.; Alessia, M.S.; Almeer, R.S.; Abdel-Daim, M.M.; Al-Sultan, N.K.; Al-Qahtani, A.A.; Ali, H.; et al. Methotrexate-induced apoptosis in human ovarian adenocarcinoma SKOV-3 cells via ROS-mediated bax/bcl-2-cyt-c release cascading. Onco Targets Ther. 2019, 12, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cui, L.; Ye, J.; Yang, G.; Lu, G.; Fang, X.; Zeng, Z.; Zhou, J. Dioscin facilitates ROS-induced apoptosis via the p38-MAPK/HSP27-mediated pathways in lung squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J. Cytochrome c in cancer therapy and prognosis. Biosci. Rep. 2022, 42, BSR20222171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jing, S.; Liu, S.; Shen, X.; Cai, L.; Zhu, C.; Zhao, Y.; Pang, M. Double-activation of mitochondrial permeability transition pore opening via calcium overload and reactive oxygen species for cancer therapy. J. Nanobiotechnol. 2022, 20, 188. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. Biomed. Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.Y.; Yu, S.N.; Park, S.G.; Yu, H.S.; Seo, Y.K.; Ahn, S.C. Monensin Induces PC-3 Prostate Cancer Cell Apoptosis via ROS Production and Ca2+ Homeostasis Disruption. Anticancer Res. 2016, 36, 5835–5843. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.M.; Seo, S.U.; Min, K.J.; Im, S.S.; Nam, J.O.; Chang, J.S.; Kim, S.; Park, J.W.; Kwon, T.K. Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells. Int. J. Mol. Sci. 2018, 19, 1309. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.W.; Sviderskiy, V.O.; Terzi, E.M.; Papagiannakopoulos, T.; Moreira, A.L.; Adams, S.; Sabatini, D.M.; Birsoy, K.; Possemato, R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 2017, 551, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Lin, B.; Zhou, M.; Wu, L.; Zheng, T. Role of ferroptosis in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tavana, O.; Chu, B.; Erber, L.; Chen, Y.; Baer, R.; Gu, W. NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol. Cell 2017, 68, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Punganuru, S.R.; Madala, H.R.; Arutla, V.; Srivenugopal, K.S. Selective killing of human breast cancer cells by the styryl lactone (R)-goniothalamin is mediated by glutathione conjugation, induction of oxidative stress and marked reactivation of the R175H mutant p53 protein. Carcinogenesis 2018, 39, 1399–1410. [Google Scholar] [CrossRef]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free. Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Kaul, S.C.; Aida, S.; Yaguchi, T.; Kaur, K.; Wadhwa, R. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J. Biol. Chem. 2005, 280, 39373–39379. [Google Scholar] [CrossRef]
- Trinh, D.L.; Elwi, A.N.; Kim, S.W. Direct interaction between p53 and Tid1 proteins affects p53 mitochondrial localization and apoptosis. Oncotarget 2010, 1, 396–404. [Google Scholar] [CrossRef]
- Arena, G.; Cissé, M.Y.; Pyrdziak, S.; Chatre, L.; Riscal, R.; Fuentes, M.; Arnold, J.J.; Kastner, M.; Gayte, L.; Bertrand-Gaday, C.; et al. Mitochondrial MDM2 Regulates Respiratory Complex I Activity Independently of p53. Mol. Cell 2018, 69, 594–609. [Google Scholar] [CrossRef]
- Fasano, C.; Disciglio, V.; Bertora, S.; Lepore Signorile, M.; Simone, C. FOXO3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ni, Z.; Li, B.S.; Yong, X.; Yang, X.; Zhang, J.W.; Zhang, D.; Qin, Y.; Jie, M.M.; Dong, H.; et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut 2017, 66, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Tackmann, N.R.; Zhang, Y. Mouse modelling of the MDM2/MDMX-p53 signalling axis. J. Mol. Cell Biol. 2017, 9, 34–44. [Google Scholar] [CrossRef]
- Huang, W.; Xie, W.; Zhong, H.; Cai, S.; Huang, Q.; Liu, Y.; Zeng, Z.; Liu, Y. Cytosolic p53 Inhibits Parkin-Mediated Mitophagy and Promotes Acute Liver Injury Induced by Heat Stroke. Front. Immunol. 2022, 13, 859231. [Google Scholar] [CrossRef] [PubMed]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2003193118. [Google Scholar] [CrossRef]
- Chen, H.; Lin, X.; Yi, X.; Liu, X.; Yu, R.; Fan, W.; Ling, Y.; Liu, Y.; Xie, W. SIRT1-mediated p53 deacetylation inhibits ferroptosis and alleviates heat stress-induced lung epithelial cells injury. Int. J. Hyperth. 2022, 39, 977–986. [Google Scholar] [CrossRef]
- Jin, Y.; Gu, W.; Chen, W. Sirt3 is critical for p53-mediated ferroptosis upon ROS-induced stress. J. Mol. Cell Biol. 2021, 13, 151–154. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, D.; Yang, Y.T.; Li, X.Y.; Li, H.N.; Zhang, X.P.; Long, J.Y.; Lu, Y.Q.; Liu, L.; Yang, G.; et al. Suppression of microRNA-222-3p ameliorates ulcerative colitis and colitis-associated colorectal cancer to protect against oxidative stress via targeting BRG1 to activate Nrf2/HO-1 signaling pathway. Front. Immunol. 2023, 14, 1089809. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Xin, T.; Xu, S.; Liu, X.; Gao, Y.; Huang, H. Eleutheroside E functions as anti-cervical cancer drug by inhibiting the phosphatidylinositol 3-kinase pathway and reprogramming the metabolic responses. J. Pharm. Pharmacol. 2022, 74, 1251–1260. [Google Scholar] [CrossRef]
- Chen, B.; Song, Y.; Zhan, Y.; Zhou, S.; Ke, J.; Ao, W.; Zhang, Y.; Liang, Q.; He, M.; Li, S.; et al. Fangchinoline inhibits non-small cell lung cancer metastasis by reversing epithelial-mesenchymal transition and suppressing the cytosolic ROS-related Akt-mTOR signaling pathway. Cancer Lett. 2022, 543, 215783. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Feng, H.; Wu, H.; Jin, Z.; Shen, X.; Kuang, J.; Huo, Z.; Chen, X.; Gao, H.; Ye, F.; et al. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 2018, 431, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Tan, M.J.; Sng, M.K.; Teo, Z.; Phua, T.; Choo, C.C.; Li, L.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. 2017, 8, e2562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shen, X.; Zhi, F.; Wen, Z.; Gao, Y.; Xu, J.; Yang, B.; Bai, Y. An overview of arsenic trioxide-involved combined treatment algorithms for leukemia: Basic concepts and clinical implications. Cell Death Discov. 2023, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Pandit, B.; Royzen, M. Recent Development of Prodrugs of Gemcitabine. Genes 2022, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Skwarski, M.; McGowan, D.R.; Belcher, E.; Di Chiara, F.; Stavroulias, D.; McCole, M.; Derham, J.L.; Chu, K.-Y.; Teoh, E.; Chauhan, J.; et al. Mitochondrial Inhibitor Atovaquone Increases Tumor Oxygenation and Inhibits Hypoxic Gene Expression in Patients with Non–Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.T.; Huang, H.C.; Lin, J.K. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010, 49, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dey, D.; Biswas, P.K.; Rahaman, T.I.; Saha, S.; Parvez, A.; Khan, D.A.; Lily, N.J.; Saha, K.; Sohel, M.; et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int. J. Mol. Sci. 2022, 23, 11746. [Google Scholar] [CrossRef]
- Pricci, M.; Girardi, B.; Giorgio, F.; Losurdo, G.; Ierardi, E.; Di Leo, A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020, 21, 2364. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Edupuganti, V.; Tyndall, J.; Gamble, A.B. Self-immolative Linkers in Prodrugs and Antibody Drug Conjugates in Cancer Treatment. Recent Pat. Anticancer Drug Discov. 2021, 16, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.; Newell, H. Do molecularly targeted agents in oncology have reduced attrition rates? Nat. Rev. Drug Discov. 2009, 8, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef] [PubMed]
- Camarillo, J.M.; Rose, K.L.; Galligan, J.J.; Xu, S.; Marnett, L.J. Covalent Modification of CDK2 by 4-Hydroxynonenal as a Mechanism of Inhibition of Cell Cycle Progression. Chem. Res. Toxicol. 2016, 29, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Schexnayder, C.; Broussard, K.; Onuaguluchi, D.; Poché, A.; Ismail, M.; McAtee, L.; Llopis, S.; Keizerweerd, A.; McFerrin, H.; Williams, C. Metformin Inhibits Migration and Invasion by Suppressing ROS Production and COX2 Expression in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3692. [Google Scholar] [CrossRef]
- Weng, M.S.; Chang, J.H.; Hung, W.Y.; Yang, Y.C.; Chien, M.H. The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 61. [Google Scholar] [CrossRef]
- Briere, D.M.; Li, S.; Calinisan, A.; Sudhakar, N.; Aranda, R.; Hargis, L.; Peng, D.H.; Deng, J.; Engstrom, L.D.; Hallin, J.; et al. The KRAS(G12C) Inhibitor MRTX849 Reconditions the Tumor Immune Microenvironment and Sensitizes Tumors to Checkpoint Inhibitor Therapy. Mol. Cancer Ther. 2021, 20, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.B.; Cheng, N.; Markosyan, N.; Sor, R.; Kim, I.K.; Hallin, J.; Shoush, J.; Quinones, L.; Brown, N.V.; Bassett, J.B.; et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov. 2023, 13, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Alqahtani, A.M.; Youssif, B.G.M.; Gouda, A.M. Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism. Molecules 2021, 26, 6677. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Zhong, P.; Wang, J.; Weng, Q.; Qian, Y.; Han, J.; Zou, C.; Liang, G. EGFR inhibition attenuates diabetic nephropathy through decreasing ROS and endoplasmic reticulum stress. Oncotarget 2017, 8, 32655–32667. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, K.; Wang, X.; Jia, Z.; Yang, Y.; Duan, Y.; Huang, L.; Wu, Z.X.; Zhang, J.Y.; Ding, X. Cholesterol promotes EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1 signaling-mediated ERRα re-expression. Mol. Cancer 2022, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Cho, P.; Selfors, L.M.; Kuiken, H.J.; Kaul, R.; Fujiwara, T.; Harris, I.S.; Zhang, T.; Gygi, S.P.; Brugge, J.S. 3D Culture Models with CRISPR Screens Reveal Hyperactive NRF2 as a Prerequisite for Spheroid Formation via Regulation of Proliferation and Ferroptosis. Mol. Cell 2020, 80, 828–844. [Google Scholar] [CrossRef]
- Hiebert, P.; Wietecha, M.S.; Cangkrama, M.; Haertel, E.; Mavrogonatou, E.; Stumpe, M.; Steenbock, H.; Grossi, S.; Beer, H.D.; Angel, P.; et al. Nrf2-Mediated Fibroblast Reprogramming Drives Cellular Senescence by Targeting the Matrisome. Dev. Cell 2018, 46, 145–161. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). J. Am. Med. Assoc. 2009, 301, 39–51. [Google Scholar] [CrossRef]
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). J. Am. Med. Assoc. 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Dentro, S.C.; Leshchiner, I.; Haase, K.; Tarabichi, M.; Wintersinger, J.; Deshwar, A.G.; Yu, K.; Rubanova, Y.; Macintyre, G.; Demeulemeester, J.; et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 2021, 184, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Dando, I.; Cordani, M.; Dalla Pozza, E.; Biondani, G.; Donadelli, M.; Palmieri, M. Antioxidant Mechanisms and ROS-Related MicroRNAs in Cancer Stem Cells. Oxid. Med. Cell Longev. 2015, 2015, 425708. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates 2018, 41, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Tuveson, D.A. The promise and perils of antioxidants for cancer patients. N. Engl. J. Med. 2014, 371, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, Z.; Zhang, S.; Zhang, X.; Li, P.; Yang, H.; Ma, Y. Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis. Cell. Mol. Immunol. 2023, 20, 51–64. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Coleman, J.A.; Yip, W.; Wong, N.C.; Sjoberg, D.D.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Herr, H.W.; Cha, E.K.; Donahue, T.F.; et al. Multicenter Phase II Clinical Trial of Gemcitabine and Cisplatin as Neoadjuvant Chemotherapy for Patients with High-Grade Upper Tract Urothelial Carcinoma. J. Clin. Oncol. 2023, 41, 1618–1625. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Q.; Zhao, H.; Liu, T.; Wu, H.; Shen, Q.; Wang, C.; Yin, T. Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-κB/STAT3 signaling cascade. Cancer Lett. 2016, 382, 53–63. [Google Scholar] [CrossRef]
- Guo, B.; Yang, F.; Zhang, L.; Zhao, Q.; Wang, W.; Yin, L.; Chen, D.; Wang, M.; Han, S.; Xiao, H.; et al. Cuproptosis Induced by ROS Responsive Nanoparticles with Elesclomol and Copper Combined with αPD-L1 for Enhanced Cancer Immunotherapy. Adv. Mater. 2023, 35, e2212267. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, Y.; Dai, M.; Li, Y.; Peng, R.; Yu, S.; Liu, H. Rotenone restrains colon cancer cell viability, motility and epithelial-mesenchymal transition and tumorigenesis in nude mice via the PI3K/AKT pathway. Int. J. Mol. Med. 2020, 46, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Pawar, J.S.; Ghosh, I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int. J. Biol. Macromol. 2021, 181, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Peta, K.T.; Durandt, C.; van Heerden, M.B.; Joubert, A.M.; Pepper, M.S.; Ambele, M.A. Effect of 2-methoxyestradiol treatment on early- and late-stage breast cancer progression in a mouse model. Cell Biochem. Funct. 2023, 41, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lai, J.; Su, J.; Li, J.; Li, C.; Zhu, B.; Li, Y. Naringenin-induced Oral Cancer Cell Apoptosis Via ROS-mediated Bid and Bcl-xl Signaling Pathway. Curr. Cancer Drug Targets 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Y.; Chen, Y.Y.; Wang, M.S.; Tong, J.J.; Xu, M.; Zhao, C.; Lin, H.Y.; Mei, L.C.; Dong, J.; Zhang, W.L.; et al. A catalase inhibitor: Targeting the NADPH-binding site for castration-resistant prostate cancer therapy. Redox Biol. 2023, 63, 102751. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, Y. Atovaquone exerts its anticancer effect by inhibiting Na(+)/K(+)-ATPase ion transport in canine cancer cells. Vet. World 2023, 16, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.G.; Shende, M.; Inci, G.; Park, S.H.; Jung, J.S.; Kim, S.B.; Kim, J.H.; Mo, Y.W.; Seo, J.H.; Feng, J.H.; et al. Combination of metformin/efavirenz/fluoxetine exhibits profound anticancer activity via a cancer cell-specific ROS amplification. Cancer Biol. Ther. 2023, 24, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cancer prevention with rapamycin. Oncotarget 2023, 14, 342–350. [Google Scholar] [CrossRef]
- Antar, S.A.; Saleh, M.A.; Al-Karmalawy, A.A. Investigating the possible mechanisms of pirfenidone to be targeted as a promising anti-inflammatory, anti-fibrotic, anti-oxidant, anti-apoptotic, anti-tumor, and/or anti-SARS-CoV-2. Life Sci. 2022, 309, 121048. [Google Scholar] [CrossRef]
- Pant, S.; Burris, H.A., 3rd; Moore, K.; Bendell, J.C.; Kurkjian, C.; Jones, S.F.; Moreno, O.; Kuhn, J.G.; McMeekin, S.; Infante, J.R. A first-in-human dose-escalation study of ME-143, a second generation NADH oxidase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.H.; Zhu, J.J.; Lister, N.L.; Harrison, S.G.; Keerthikumar, S.; Goode, D.L.; Urban, R.Q.; Byrne, D.J.; Azad, A.; Vela, I.; et al. Low-dose carboplatin modifies the tumor microenvironment to augment CAR T cell efficacy in human prostate cancer models. Nat. Commun. 2023, 14, 5346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ren, S.; Jiang, T.; Zhu, B.; Li, X.; Zhao, C.; Jia, Y.; Shi, J.; Zhang, L.; Liu, X.; et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol. Res. 2019, 7, 630–643. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Liu, P.; Sun, C.; Pei, L.J.; Huang, Y.G. Propofol Augments Paclitaxel-Induced Cervical Cancer Cell Ferroptosis In Vitro. Front. Pharmacol. 2022, 13, 816432. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Dass, C.R. Doxorubicin Action on Mitochondria: Relevance to Osteosarcoma Therapy? Curr. Drug Targets 2018, 19, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, Y.; Okazaki, T.; Ebihara, S.; Komatsu, R.; Nihei, M.; Kobayashi, M.; Hirano, T.; Sugiura, H.; Tamada, T.; Tanaka, N.; et al. Beneficial effects of sunitinib on tumor microenvironment and immunotherapy targeting death receptor5. Oncoimmunology 2019, 8, e1543526. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Tang, T.; Sheng, L.; Ma, Y.; Liu, Y.; Yan, L.; Qi, S.; Ling, L.; Zhang, Y. Salidroside inhibits the proliferation and migration of gastric cancer cells via suppression of Src-associated signaling pathway activation and heat shock protein 70 expression. Mol. Med. Rep. 2018, 18, 147–156. [Google Scholar] [CrossRef]
- Zong, L.; Li, J.; Chen, X.; Chen, K.; Li, W.; Li, X.; Zhang, L.; Duan, W.; Lei, J.; Xu, Q.; et al. Lipoxin A4 Attenuates Cell Invasion by Inhibiting ROS/ERK/MMP Pathway in Pancreatic Cancer. Oxid. Med. Cell Longev. 2016, 2016, 6815727. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Yang, F.; Yu, Y.; Zeng, A.; Ye, T.; Yin, W.; Xie, Y.; Fu, Z.; Zhao, C. Lobaplatin induces BGC-823 human gastric carcinoma cell apoptosis via ROS- mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed. Pharmacother. 2016, 83, 1239–1246. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, S.; Ni, X.; Shen, S.; Wang, J.; Sun, W.; Suo, T.; Liu, H.; Ni, X.; Liu, H. KRAS G12D mutation eliminates reactive oxygen species through the Nrf2/CSE/H (2)S axis and contributes to pancreatic cancer growth. Acta Biochim. Biophys. Sin. 2022, 54, 1731–1739. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Min, S.; Kim, J.Y.; Cho, H.M.; Park, S.; Hwang, J.M.; You, H.; Chan Chae, Y.; Lee, W.J.; Sun, W.; Kang, D.; et al. Heat shock protein 60 couples an oxidative stress-responsive p38/MK2 signaling and NF-κB survival machinery in cancer cells. Redox Biol. 2022, 51, 102293. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Rong, J.; Lai, Y.; Tao, L.; Yuan, X.; Shu, X. The Degree of Helicobacter pylori Infection Affects the State of Macrophage Polarization through Crosstalk between ROS and HIF-1α. Oxid. Med. Cell Longev. 2020, 2020, 5281795. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Xie, X. AMPK and Cancer. Exp. Suppl. 2016, 107, 203–226. [Google Scholar] [PubMed]
- Khanna-Chopra, R.; Jajoo, A.; Semwal, V.K. Chloroplasts and mitochondria have multiple heat tolerant isozymes of SOD and APX in leaf and inflorescence in Chenopodium album. Biochem. Biophys. Res. Commun. 2011, 412, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Yu, J.; Wang, H.; Yu, A.; Xiao, L.; Li, Z.; Zhang, H.; Zhang, Y.; Wu, Y. Rational design of a reversible fluorescent probe for sensing GSH in mitochondria. Anal. Chim. Acta 2022, 1220, 340081. [Google Scholar] [CrossRef]
- Mäntymaa, P.; Siitonen, T.; Guttorm, T.; Säily, M.; Kinnula, V.; Savolainen, E.R.; Koistinen, P. Induction of mitochondrial manganese superoxide dismutase confers resistance to apoptosis in acute myeloblastic leukaemia cells exposed to etoposide. Br. J. Haematol. 2000, 108, 574–581. [Google Scholar] [CrossRef]
- Ale-Agha, N.; Jakobs, P.; Goy, C.; Zurek, M.; Rosen, J.; Dyballa-Rukes, N.; Metzger, S.; Greulich, J.; von Ameln, F.; Eckermann, O.; et al. Mitochondrial Telomerase Reverse Transcriptase Protects from Myocardial Ischemia/Reperfusion Injury by Improving Complex I Composition and Function. Circulation 2021, 144, 1876–1890. [Google Scholar] [CrossRef]
- Missiroli, S.; Perrone, M.; Gafà, R.; Nicoli, F.; Bonora, M.; Morciano, G.; Boncompagni, C.; Marchi, S.; Lebiedzinska-Arciszewska, M.; Vezzani, B.; et al. PML at mitochondria-associated membranes governs a trimeric complex with NLRP3 and P2X7R that modulates the tumor immune microenvironment. Cell Death Differ. 2023, 30, 429–441. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Moll, U.M. Identification of p53 in mitochondria. Methods Mol. Biol. 2013, 962, 75–84. [Google Scholar] [PubMed]
- Savu, D.I.; Moisoi, N. Mitochondria–Nucleus communication in neurodegenerative disease. Who talks first, who talks louder? Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148588. [Google Scholar] [CrossRef] [PubMed]
- Correia-Melo, C.; Marques, F.D.; Anderson, R.; Hewitt, G.; Hewitt, R.; Cole, J.; Carroll, B.M.; Miwa, S.; Birch, J.; Merz, A.; et al. Mitochondria are required for pro-ageing features of the senescent phenotype. Embo J. 2016, 35, 724–742. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Breda, C.N.S.; Davanzo, G.G.; Basso, P.J.; Saraiva Câmara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as central hub of the immune system. Redox Biol. 2019, 26, 101255. [Google Scholar] [CrossRef]
- Corpet, A.; Kleijwegt, C.; Roubille, S.; Juillard, F.; Jacquet, K.; Texier, P.; Lomonte, P. PML nuclear bodies and chromatin dynamics: Catch me if you can! Nucleic Acids Res. 2020, 48, 11890–11912. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).