Molecular Organization and Regulation of the Mammalian Synapse by the Post-Translational Modification SUMOylation
Abstract
1. Introduction
2. The SUMOylation/deSUMOylation Enzymatic Pathway at Synapses
2.1. SUMO Paralogs and the Associated Enzymatic SUMOylation/deSUMOylation Cascade
2.2. Synaptic Localization of the SUMOylation/deSUMOylation Machinery
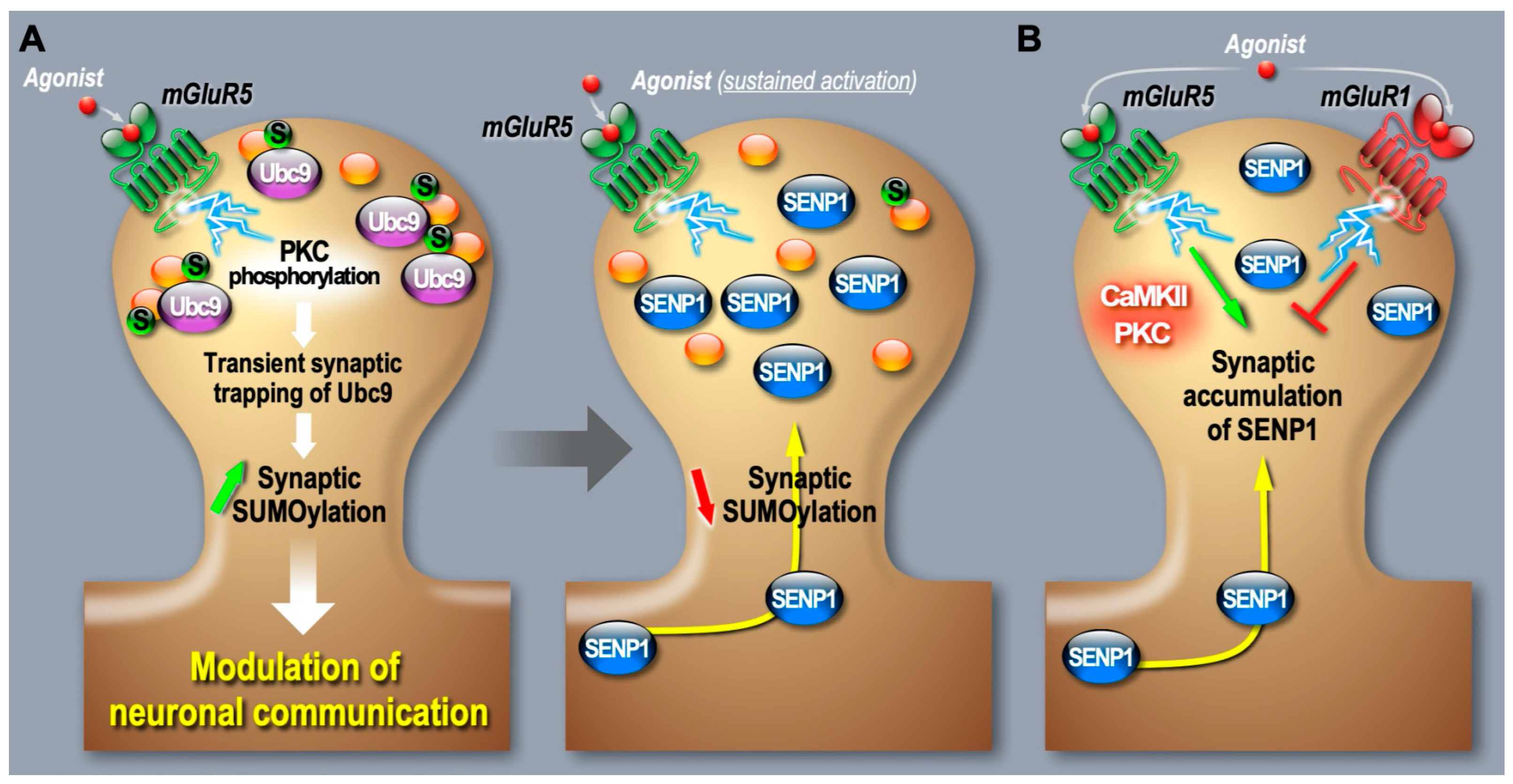
2.3. Synaptic Regulation of the SUMO Pathway
3. SUMOylation in Neurite Growth, Synapse Formation, Elimination and Maturation
3.1. SUMOylation of Transcription Factors in Neurite Growth and Branching
3.1.1. MEF2 SUMOylation
3.1.2. FOXP2 SUMOylation
3.1.3. MeCP2 SUMOylation
3.1.4. ZBTB20 SUMOylation
| Transcription Factor | SUMOylation Site | Effect of SUMOylation | References |
|---|---|---|---|
| MEF2A | K403 | Inhibits transcription activity, promotes dendritic claw differentiation | [31,32,33] |
| FOXP2 | K670, K673/674 | Transcriptional regulation and control of dendritic arborization | [36] |
| MeCP2 | K223, K412 | Repression of MeCP2 transcriptional activity, impact on spine density | [38,39] |
| ZBTB20 | K330, K371 | Affects the transcriptional activity of ZBTB20 and acts on neuritogenesis | [43] |
3.2. Extranuclear SUMOylation in Synapse Formation and Maturation
3.2.1. CASK SUMOylation
3.2.2. Local Protein Synthesis and Dendritic SUMOylation
- CPEB3 SUMOylation
- FMRP SUMOylation
3.3. SUMOylation and Microtubules (MTs)
4. SUMOylation, Biomolecular Condensates and Compartmentalization of the Synapse
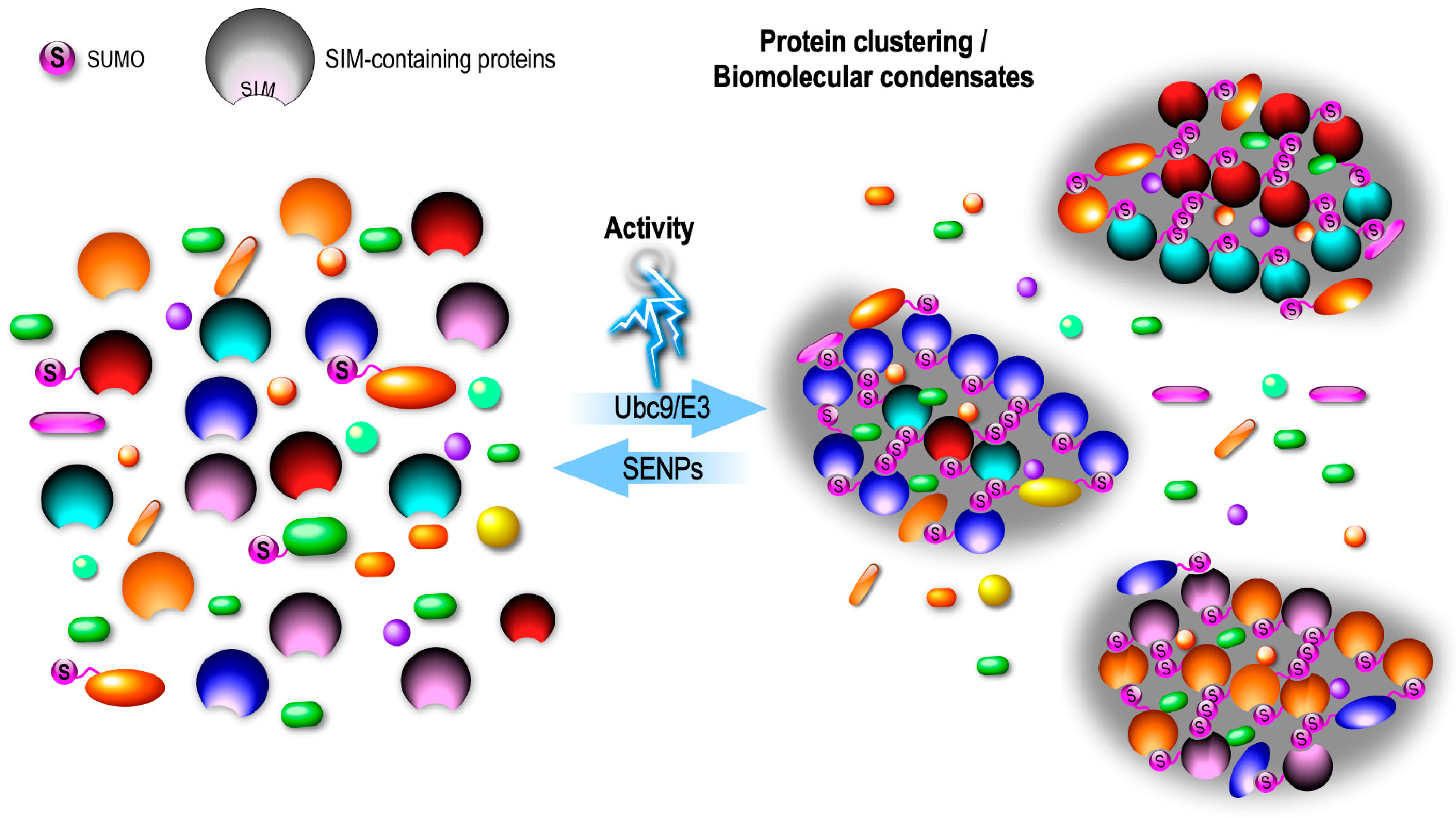
4.1. En Masse SUMOylation at Synapses?
4.2. SUMOylation and LLPS
4.3. SUMOylation and Compartmentalization of Pre- and Post-Synaptic Sites
| Target Protein | SUMOylation Site | Effect of SUMOylation | References |
|---|---|---|---|
| CASK | K679 | Prevents interaction with protein 4.1 and the association of CASK with the actin cytoskeleton; control of dendritic spine density | [45] |
| CPEB3 | K50, K294 | Regulates its oligomerization and acts as a local translational repressor | [47,48,49] |
| FMRP | K88, K130, K614 | Triggers the mGlu5R-dependent dissociation of FMRP-SUMO from mRNA granules, leading to local translation of mRNAs essential to spine maturation and elimination | [58] |
| KATNA1 | K330 | Enhances the activity that cleaves acetylated microtubules, leading to neurite outgrowth | [64] |
| Spastin | K427 | Abolishes the ability to cleave MTs, thus impacting their stability and consequently spine maturation | [68] |
| α-Tubulin | K96, K166, K304 | Reduces microtubule assembly affecting their length and subsequently neurite growth in neuronal cell lines | [71,72] |
| CRMP2 | K374 | Interaction with Voltage-Gated Calcium Channels and anchorage of Nav1.7 to the plasma membrane | [86] |
| Cav2.2 | K394 | Modulates neurotransmitter release by the activation of the presynaptic Ca2+ channels | [89] |
| RIM1α | K502 | Concentrates ion channels in the active zone; required for vesicle exocytosis | [93] |
| Synaptotagmin-1 | ? | Increases Synaptotagmin-1 SUMOylation in transgenic mice specifically overexpressing SUMO1 in neurons | [94] |
| Synapsin-1a | K687 | Maintains the synaptic vesicles in a reserved accessible pool ready to be activity-dependently delivered | [95] |
| Syntaxin-1A | K252, K253, K256 | Reduces the interaction with SNAP-25 and VAMP-2 SNARE proteins | [96] |
| mGluR7 | K889 | DeSUMOylation of mGlu7R leads to its internalization | [98] |
| GluK2 | K886 | Promotes the activity-dependent endocytosis of GluK2-containing Kainate receptors | [15,107,108] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matunis, M.J.; Coutavas, E.; Blobel, G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 1996, 135, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Delphin, C.; Guan, T.; Gerace, L.; Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 1997, 88, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Flotho, A.; Melchior, F. Sumoylation: A regulatory protein modification in health and disease. Annu. Rev. Biochem. 2013, 82, 357–385. [Google Scholar] [CrossRef] [PubMed]
- Celen, A.B.; Sahin, U. Sumoylation on its 25th anniversary: Mechanisms, pathology, and emerging concepts. FEBS J. 2020, 287, 3110–3140. [Google Scholar] [CrossRef] [PubMed]
- Gwizdek, C.; Casse, F.; Martin, S. Protein sumoylation in brain development, neuronal morphology and spinogenesis. Neuromol. Med. 2013, 15, 677–691. [Google Scholar] [CrossRef]
- Schorova, L.; Martin, S. Sumoylation in Synaptic Function and Dysfunction. Front. Synaptic Neurosci. 2016, 8, 9. [Google Scholar] [CrossRef]
- Henley, J.M.; Carmichael, R.E.; Wilkinson, K.A. Extranuclear SUMOylation in Neurons. Trends Neurosci. 2018, 41, 198–210. [Google Scholar] [CrossRef]
- Folci, A.; Mirabella, F.; Fossati, M. Ubiquitin and Ubiquitin-Like Proteins in the Critical Equilibrium between Synapse Physiology and Intellectual Disability. eNeuro 2020, 7, 1–25. [Google Scholar] [CrossRef]
- Henley, J.M.; Seager, R.; Nakamura, Y.; Talandyte, K.; Nair, J.; Wilkinson, K.A. SUMOylation of synaptic and synapse-associated proteins: An update. J. Neurochem. 2021, 156, 145–161. [Google Scholar] [CrossRef]
- Mandel, N.; Agarwal, N. Role of SUMOylation in Neurodegenerative Diseases. Cells 2022, 11, 3395. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, W.; Lin, W.; Mei, F. Paradoxes of Cellular SUMOylation Regulation: A Role of Biomolecular Condensates? Pharmacol. Rev. 2023, 75, 979–1006. [Google Scholar] [CrossRef] [PubMed]
- Colnaghi, L.; Russo, L.; Natale, C.; Restelli, E.; Cagnotto, A.; Salmona, M.; Chiesa, R.; Fioriti, L. Super Resolution Microscopy of SUMO Proteins in Neurons. Front. Cell. Neurosci. 2019, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Colnaghi, L.; Rondelli, D.; Muzi-Falconi, M.; Sertic, S. Tau and DNA Damage in Neurodegeneration. Brain Sci. 2020, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Conz, A.; Musi, C.A.; Russo, L.; Borsello, T.; Colnaghi, L. Super-resolution study of PIAS SUMO E3-ligases in hippocampal and cortical neurons. Eur. J. Histochem. 2021, 65, 3241. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Nishimune, A.; Mellor, J.R.; Henley, J.M. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 2007, 447, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Feligioni, M.; Nishimune, A.; Henley, J.M. Protein SUMOylation modulates calcium influx and glutamate release from presynaptic terminals. Eur. J. Neurosci. 2009, 29, 1348–1356. [Google Scholar] [CrossRef]
- Loriol, C.; Parisot, J.; Poupon, G.; Gwizdek, C.; Martin, S. Developmental regulation and spatiotemporal redistribution of the sumoylation machinery in the rat central nervous system. PLoS ONE 2012, 7, e33757. [Google Scholar] [CrossRef]
- Loriol, C.; Khayachi, A.; Poupon, G.; Gwizdek, C.; Martin, S. Activity-dependent regulation of the sumoylation machinery in rat hippocampal neurons. Biol. Cell 2013, 105, 30–45. [Google Scholar] [CrossRef]
- Loriol, C.; Casse, F.; Khayachi, A.; Poupon, G.; Chafai, M.; Deval, E.; Gwizdek, C.; Martin, S. mGlu5 receptors regulate synaptic sumoylation via a transient PKC-dependent diffusional trapping of Ubc9 into spines. Nat. Commun. 2014, 5, 5113. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Yoshida, D.; Nakamura, Y.; Sakakibara, S. Spatiotemporal distribution of SUMOylation components during mouse brain development. J. Comp. Neurol. 2014, 522, 3020–3036. [Google Scholar] [CrossRef] [PubMed]
- Josa-Prado, F.; Luo, J.; Rubin, P.; Henley, J.M.; Wilkinson, K.A. Developmental profiles of SUMOylation pathway proteins in rat cerebrum and cerebellum. PLoS ONE 2019, 14, e0212857. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Hildick, K.L.; Luo, J.; Dearden, L.; Wilkinson, K.A.; Henley, J.M. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J. 2013, 32, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.; Wilkinson, K.A.; Harding, A.L.; Carmichael, R.E.; Robinson, D.; Colley, H.E.; Guo, C. The SUMO protease SENP3 regulates mitochondrial autophagy mediated by Fis1. EMBO Rep. 2022, 23, e48754. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Jaafari, N.; Konopacki, F.A.; Owen, T.F.; Kantamneni, S.; Rubin, P.; Craig, T.J.; Wilkinson, K.A.; Henley, J.M. SUMOylation is required for glycine-induced increases in AMPA receptor surface expression (ChemLTP) in hippocampal neurons. PLoS ONE 2013, 8, e52345. [Google Scholar] [CrossRef]
- Schorova, L.; Pronot, M.; Poupon, G.; Prieto, M.; Folci, A.; Khayachi, A.; Brau, F.; Casse, F.; Gwizdek, C.; Martin, S. The synaptic balance between sumoylation and desumoylation is maintained by the activation of metabotropic mGlu5 receptors. Cell. Mol. Life Sci. 2019, 76, 3019–3031. [Google Scholar] [CrossRef]
- Pronot, M.; Poupon, G.; Pizzamiglio, L.; Prieto, M.; Chato-Astrain, I.; Lacagne, I.; Schorova, L.; Folci, A.; Brau, F.; Martin, S. Bidirectional regulation of synaptic SUMOylation by Group 1 metabotropic glutamate receptors. Cell. Mol. Life Sci. 2022, 79, 378. [Google Scholar] [CrossRef]
- Queiroz, L.Y.; Kageyama, R.; Cimarosti, H.I. SUMOylation effects on neural stem cells self-renewal, differentiation, and survival. Neurosci. Res. 2023, 199, 1–11. [Google Scholar] [CrossRef]
- Lyons, G.E.; Micales, B.K.; Schwarz, J.; Martin, J.F.; Olson, E.N. Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J. Neurosci. 1995, 15, 5727–5738. [Google Scholar] [CrossRef]
- Flavell, S.W.; Greenberg, M.E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 2008, 31, 563–590. [Google Scholar] [CrossRef]
- Shalizi, A.; Gaudilliere, B.; Yuan, Z.; Stegmuller, J.; Shirogane, T.; Ge, Q.; Tan, Y.; Schulman, B.; Harper, J.W.; Bonni, A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 2006, 311, 1012–1017. [Google Scholar] [CrossRef]
- Lu, H.; Liu, B.; You, S.; Chen, L.; Dongmei, Q.; Gu, M.; Lu, Y.; Chen, Y.; Zhang, F.; Yu, B. SENP2 regulates MEF2A de-SUMOylation in an activity dependent manner. Mol. Biol. Rep. 2013, 40, 2485–2490. [Google Scholar] [CrossRef]
- Yamada, T.; Yang, Y.; Huang, J.; Coppola, G.; Geschwind, D.H.; Bonni, A. Sumoylated MEF2A coordinately eliminates orphan presynaptic sites and promotes maturation of presynaptic boutons. J. Neurosci. 2013, 33, 4726–4740. [Google Scholar] [CrossRef]
- Meredith, L.J.; Wang, C.M.; Nascimento, L.; Liu, R.; Wang, L.; Yang, W.H. The Key Regulator for Language and Speech Development, FOXP2, is a Novel Substrate for SUMOylation. J. Cell. Biochem. 2016, 117, 426–438. [Google Scholar] [CrossRef]
- Estruch, S.B.; Graham, S.A.; Deriziotis, P.; Fisher, S.E. The language-related transcription factor FOXP2 is post-translationally modified with small ubiquitin-like modifiers. Sci. Rep. 2016, 6, 20911. [Google Scholar] [CrossRef]
- Usui, N.; Co, M.; Harper, M.; Rieger, M.A.; Dougherty, J.D.; Konopka, G. Sumoylation of FOXP2 Regulates Motor Function and Vocal Communication Through Purkinje Cell Development. Biol. Psychiatry 2017, 81, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Rocca, D.L.; Wilkinson, K.A.; Henley, J.M. SUMOylation of FOXP1 regulates transcriptional repression via CtBP1 to drive dendritic morphogenesis. Sci. Rep. 2017, 7, 877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, M.; Zhu, Y.; Xin, Y.J.; Zhao, Y.K.; Huang, J.; Yu, J.X.; Zhou, W.H.; Qiu, Z. SUMOylation of MeCP2 is essential for transcriptional repression and hippocampal synapse development. J. Neurochem. 2014, 128, 798–806. [Google Scholar] [CrossRef]
- Tai, D.J.; Liu, Y.C.; Hsu, W.L.; Ma, Y.L.; Cheng, S.J.; Liu, S.Y.; Lee, E.H. MeCP2 SUMOylation rescues Mecp2-mutant-induced behavioural deficits in a mouse model of Rett syndrome. Nat. Commun. 2016, 7, 10552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mi, J.; Li, N.; Sui, L.; Wan, T.; Zhang, J.; Chen, T.; Cao, X. Identification and characterization of DPZF, a novel human BTB/POZ zinc finger protein sharing homology to BCL-6. Biochem. Biophys. Res. Commun. 2001, 282, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.R.; Iossifov, I.; Levy, D.; Ronemus, M.; Wigler, M.; Vitkup, D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 2011, 70, 898–907. [Google Scholar] [CrossRef]
- Jones, K.A.; Luo, Y.; Dukes-Rimsky, L.; Srivastava, D.P.; Koul-Tewari, R.; Russell, T.A.; Shapiro, L.P.; Srivastava, A.K.; Penzes, P. Neurodevelopmental disorder-associated ZBTB20 gene variants affect dendritic and synaptic structure. PLoS ONE 2018, 13, e0203760. [Google Scholar] [CrossRef]
- Ripamonti, S.; Shomroni, O.; Rhee, J.S.; Chowdhury, K.; Jahn, O.; Hellmann, K.P.; Bonn, S.; Brose, N.; Tirard, M. SUMOylation controls the neurodevelopmental function of the transcription factor Zbtb20. J. Neurochem. 2020, 154, 647–661. [Google Scholar] [CrossRef]
- Biederer, T.; Sudhof, T.C. CASK and protein 4.1 support F-actin nucleation on neurexins. J. Biol. Chem. 2001, 276, 47869–47876. [Google Scholar] [CrossRef]
- Chao, H.W.; Hong, C.J.; Huang, T.N.; Lin, Y.L.; Hsueh, Y.P. SUMOylation of the MAGUK protein CASK regulates dendritic spinogenesis. J. Cell Biol. 2008, 182, 141–155. [Google Scholar] [CrossRef]
- Huang, Y.S.; Mendez, R.; Fernandez, M.; Richter, J.D. CPEB and translational control by cytoplasmic polyadenylation: Impact on synaptic plasticity, learning, and memory. Mol. Psychiatry 2023, 28, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Drisaldi, B.; Colnaghi, L.; Fioriti, L.; Rao, N.; Myers, C.; Snyder, A.M.; Metzger, D.J.; Tarasoff, J.; Konstantinov, E.; Fraser, P.E.; et al. SUMOylation Is an Inhibitory Constraint that Regulates the Prion-like Aggregation and Activity of CPEB3. Cell Rep. 2015, 11, 1694–1702. [Google Scholar] [CrossRef]
- Ford, L.; Ling, E.; Kandel, E.R.; Fioriti, L. CPEB3 inhibits translation of mRNA targets by localizing them to P bodies. Proc. Natl. Acad. Sci. USA 2019, 116, 18078–18087. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Schafer, N.P.; Wang, Q.; Song, S.S.; Chen, M.; Waxham, M.N.; Wolynes, P.G. Exploring the F-actin/CPEB3 interaction and its possible role in the molecular mechanism of long-term memory. Proc. Natl. Acad. Sci. USA 2020, 117, 22128–22134. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Schafer, N.P.; Bueno, C.; Lu, W.; Wolynes, P.G. A structural dynamics model for how CPEB3 binding to SUMO2 can regulate translational control in dendritic spines. PLoS Comput. Biol. 2022, 18, e1010657. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.M.; Roder, J.C.; Bear, M.F. Chemical induction of mGluR5- and protein synthesis--dependent long-term depression in hippocampal area CA1. J. Neurophysiol. 2001, 86, 321–325. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Bassell, G.J. Fragile balance: RNA editing tunes the synapse. Nat. Neurosci. 2011, 14, 1492–1494. [Google Scholar] [CrossRef]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef]
- The Dutch-Belgian Fragile X Consortium; Bakker, C.E.; Verheij, C.; Willemsen, R.; van der Helm, R.; Oerlemans, F.; Vermey, M.; Bygrave, A.; Hoogeveen, A.; Oostra, B.A.; et al. Fmr1 knockout mice: A model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 1994, 78, 23–33. [Google Scholar]
- Mientjes, E.J.; Nieuwenhuizen, I.; Kirkpatrick, L.; Zu, T.; Hoogeveen-Westerveld, M.; Severijnen, L.; Rife, M.; Willemsen, R.; Nelson, D.L.; Oostra, B.A. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol. Dis. 2006, 21, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; Folci, A.; Martin, S. Post-translational modifications of the Fragile X Mental Retardation Protein in neuronal function and dysfunction. Mol. Psychiatry 2020, 25, 1688–1703. [Google Scholar] [CrossRef]
- Khayachi, A.; Gwizdek, C.; Poupon, G.; Alcor, D.; Chafai, M.; Casse, F.; Maurin, T.; Prieto, M.; Folci, A.; De Graeve, F.; et al. Sumoylation regulates FMRP-mediated dendritic spine elimination and maturation. Nat. Commun. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Marmor-Kollet, H.; Siany, A.; Kedersha, N.; Knafo, N.; Rivkin, N.; Danino, Y.M.; Moens, T.G.; Olender, T.; Sheban, D.; Cohen, N.; et al. Spatiotemporal Proteomic Analysis of Stress Granule Disassembly Using APEX Reveals Regulation by SUMOylation and Links to ALS Pathogenesis. Mol. Cell 2020, 80, 876–891.e6. [Google Scholar] [CrossRef]
- Yang, K.; Shi, Y.; Du, X.; Wang, J.; Zhang, Y.; Shan, S.; Yuan, Y.; Wang, R.; Zhou, C.; Liu, Y.; et al. SENP1 in the retrosplenial agranular cortex regulates core autistic-like symptoms in mice. Cell Rep. 2021, 37, 109939. [Google Scholar] [CrossRef]
- Baas, P.W.; Rao, A.N.; Matamoros, A.J.; Leo, L. Stability properties of neuronal microtubules. Cytoskeleton 2016, 73, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Firestein, B.L.; Zheng, J.Q. Microtubules in dendritic spine development. J. Neurosci. 2008, 28, 12120–12124. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, Y.; Zou, J.; Cai, Z.; Yang, H.; Yang, J.; Zhang, Y.; Lin, H.; Zhang, G.; Tan, M. SUMOylation of microtubule-cleaving enzyme KATNA1 promotes microtubule severing and neurite outgrowth. J. Biol. Chem. 2022, 298, 102292. [Google Scholar] [CrossRef] [PubMed]
- McNally, F.J.; Vale, R.D. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 1993, 75, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ye, Y.; Ji, Z.; Tan, M.; Li, S.; Zhang, J.; Guo, G.; Lin, H. Katanin p60 promotes neurite growth and collateral formation in the hippocampus. Int. J. Clin. Exp. Med. 2014, 7, 2463–2470. [Google Scholar]
- Shin, S.C.; Im, S.K.; Jang, E.H.; Jin, K.S.; Hur, E.M.; Kim, E.E. Structural and Molecular Basis for Katanin-Mediated Severing of Glutamylated Microtubules. Cell Rep. 2019, 26, 1357–1367.e5. [Google Scholar] [CrossRef]
- Ji, Z.S.; Liu, Q.L.; Zhang, J.F.; Yang, Y.H.; Li, J.; Zhang, G.W.; Tan, M.H.; Lin, H.S.; Guo, G.Q. SUMOylation of spastin promotes the internalization of GluA1 and regulates dendritic spine morphology by targeting microtubule dynamics. Neurobiol. Dis. 2020, 146, 105133. [Google Scholar] [CrossRef]
- Yu, W.; Qiang, L.; Solowska, J.M.; Karabay, A.; Korulu, S.; Baas, P.W. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell 2008, 19, 1485–1498. [Google Scholar] [CrossRef]
- Rosas-Acosta, G.; Russell, W.K.; Deyrieux, A.; Russell, D.H.; Wilson, V.G. A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Mol. Cell. Proteom. 2005, 4, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, R.; Xie, X.; Diao, L.; Gao, N.; Cheng, J.; Zhang, X.; Li, Y.; Bao, L. SUMOylation of α-tubulin is a novel modification regulating microtubule dynamics. J. Mol. Cell Biol. 2021, 13, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pronot, M.; Kieffer, F.; Gay, A.S.; Debayle, D.; Forquet, R.; Poupon, G.; Schorova, L.; Martin, S.; Gwizdek, C. Proteomic Identification of an Endogenous Synaptic SUMOylome in the Developing Rat Brain. Front. Mol. Neurosci. 2021, 14, 780535. [Google Scholar] [CrossRef] [PubMed]
- Sferra, A.; Nicita, F.; Bertini, E. Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7354. [Google Scholar] [CrossRef]
- Vertegaal, A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat. Rev. Mol. Cell Biol. 2022, 23, 715–731. [Google Scholar] [CrossRef]
- Gasser, S.M.; Stutz, F. SUMO in the regulation of DNA repair and transcription at nuclear pores. FEBS Lett. 2023, 597, 2833–2850. [Google Scholar] [CrossRef] [PubMed]
- Esteras, M.; Liu, I.C.; Snijders, A.P.; Jarmuz, A.; Aragon, L. Identification of SUMO conjugation sites in the budding yeast proteome. Microb. Cell 2017, 4, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Psakhye, I.; Jentsch, S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 2012, 151, 807–820. [Google Scholar] [CrossRef]
- Keiten-Schmitz, J.; Roder, L.; Hornstein, E.; Muller-McNicoll, M.; Muller, S. SUMO: Glue or Solvent for Phase-Separated Ribonucleoprotein Complexes and Molecular Condensates? Front. Mol. Biosci. 2021, 8, 673038. [Google Scholar] [CrossRef]
- Bratek-Skicki, A.; Pancsa, R.; Meszaros, B.; Van Lindt, J.; Tompa, P. A guide to regulation of the formation of biomolecular condensates. FEBS J. 2020, 287, 1924–1935. [Google Scholar] [CrossRef]
- Yau, T.Y.; Sander, W.; Eidson, C.; Courey, A.J. SUMO Interacting Motifs: Structure and Function. Cells 2021, 10, 2825. [Google Scholar] [CrossRef] [PubMed]
- Lascorz, J.; Codina-Fabra, J.; Reverter, D.; Torres-Rosell, J. SUMO-SIM interactions: From structure to biological functions. Semin. Cell Dev. Biol. 2022, 132, 193–202. [Google Scholar] [CrossRef]
- Rodriguez, M.S.; Dargemont, C.; Hay, R.T. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J. Biol. Chem. 2001, 276, 12654–12659. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J. Mechanism of neurotransmitter release coming into focus. Protein Sci. 2018, 27, 1364–1391. [Google Scholar] [CrossRef]
- Benson, M.; Iniguez-Lluhi, J.A.; Martens, J. Sumo Modification of Ion Channels. Adv. Exp. Med. Biol. 2017, 963, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Kotler, O.; Khrapunsky, Y.; Shvartsman, A.; Dai, H.; Plant, L.D.; Goldstein, S.A.N.; Fleidervish, I. SUMOylation of Na(V)1.2 channels regulates the velocity of backpropagating action potentials in cortical pyramidal neurons. eLife 2023, 12, e81463. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Li, Q.; Wilson, S.M.; Brittain, J.M.; Meroueh, L.; Khanna, R. SUMOylation alters CRMP2 regulation of calcium influx in sensory neurons. Channels 2013, 7, 153–159. [Google Scholar] [CrossRef]
- Chew, L.A.; Khanna, R. CRMP2 and voltage-gated ion channels: Potential roles in neuropathic pain. Neuronal Signal. 2018, 2, NS20170220. [Google Scholar] [CrossRef]
- Ferron, L.; Nieto-Rostro, M.; Cassidy, J.S.; Dolphin, A.C. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat. Commun. 2014, 5, 3628. [Google Scholar] [CrossRef]
- Silveirinha, V.C.; Lin, H.; Tanifuji, S.; Mochida, S.; Cottrell, G.S.; Cimarosti, H.; Stephens, G.J. Ca(V)2.2 (N-type) voltage-gated calcium channels are activated by SUMOylation pathways. Cell Calcium 2021, 93, 102326. [Google Scholar] [CrossRef]
- Milovanovic, D.; Wu, Y.; Bian, X.; De Camilli, P. A liquid phase of synapsin and lipid vesicles. Science 2018, 361, 604–607. [Google Scholar] [CrossRef]
- McDonald, N.A.; Fetter, R.D.; Shen, K. Assembly of synaptic active zones requires phase separation of scaffold molecules. Nature 2020, 588, 454–458. [Google Scholar] [CrossRef]
- Imoto, Y.; Raychaudhuri, S.; Ma, Y.; Fenske, P.; Sandoval, E.; Itoh, K.; Blumrich, E.M.; Matsubayashi, H.T.; Mamer, L.; Zarebidaki, F.; et al. Dynamin is primed at endocytic sites for ultrafast endocytosis. Neuron 2022, 110, 2815–2835.e13. [Google Scholar] [CrossRef]
- Girach, F.; Craig, T.J.; Rocca, D.L.; Henley, J.M. RIM1α SUMOylation is required for fast synaptic vesicle exocytosis. Cell Rep. 2013, 5, 1294–1301. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Lee, L.; Knock, E.; Srikumar, T.; Sakurai, M.; Hazrati, L.N.; Katayama, T.; Staniszewski, A.; Raught, B.; Arancio, O.; et al. SUMO1 Affects Synaptic Function, Spine Density and Memory. Sci. Rep. 2015, 5, 10730. [Google Scholar] [CrossRef]
- Tang, L.T.; Craig, T.J.; Henley, J.M. SUMOylation of synapsin Ia maintains synaptic vesicle availability and is reduced in an autism mutation. Nat. Commun. 2015, 6, 7728. [Google Scholar] [CrossRef]
- Craig, T.J.; Anderson, D.; Evans, A.J.; Girach, F.; Henley, J.M. SUMOylation of Syntaxin1A regulates presynaptic endocytosis. Sci. Rep. 2015, 5, 17669. [Google Scholar] [CrossRef]
- Wilkinson, K.A.; Henley, J.M. Analysis of metabotropic glutamate receptor 7 as a potential substrate for SUMOylation. Neurosci. Lett. 2011, 491, 181–186. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, J.Y.; Park, S.P.; Lee, H.; Han, S.; Park, K.H.; Suh, Y.H. Regulation of mGluR7 trafficking by SUMOylation in neurons. Neuropharmacology 2016, 102, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Krumova, P.; Meulmeester, E.; Garrido, M.; Tirard, M.; Hsiao, H.H.; Bossis, G.; Urlaub, H.; Zweckstetter, M.; Kugler, S.; Melchior, F.; et al. Sumoylation inhibits α-synuclein aggregation and toxicity. J. Cell Biol. 2011, 194, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Rott, R.; Szargel, R.; Shani, V.; Hamza, H.; Savyon, M.; Abd Elghani, F.; Bandopadhyay, R.; Engelender, S. SUMOylation and ubiquitination reciprocally regulate α-synuclein degradation and pathological aggregation. Proc. Natl. Acad. Sci. USA 2017, 114, 13176–13181. [Google Scholar] [CrossRef] [PubMed]
- Sansevrino, R.; Hoffmann, C.; Milovanovic, D. Condensate biology of synaptic vesicle clusters. Trends Neurosci. 2023, 46, 293–306. [Google Scholar] [CrossRef]
- Rousseaux, M.W.C.; Vazquez-Velez, G.E.; Al-Ramahi, I.; Jeong, H.H.; Bajic, A.; Revelli, J.P.; Ye, H.; Phan, E.T.; Deger, J.M.; Perez, A.M.; et al. A Druggable Genome Screen Identifies Modifiers of α-Synuclein Levels via a Tiered Cross-Species Validation Approach. J. Neurosci. 2018, 38, 9286–9301. [Google Scholar] [CrossRef]
- Hoffmann, C.; Rentsch, J.; Tsunoyama, T.A.; Chhabra, A.; Aguilar Perez, G.; Chowdhury, R.; Trnka, F.; Korobeinikov, A.A.; Shaib, A.H.; Ganzella, M.; et al. Synapsin condensation controls synaptic vesicle sequestering and dynamics. Nat. Commun. 2023, 14, 6730. [Google Scholar] [CrossRef]
- Leenders, A.G.; Sheng, Z.H. Modulation of neurotransmitter release by the second messenger-activated protein kinases: Implications for presynaptic plasticity. Pharmacol. Ther. 2005, 105, 69–84. [Google Scholar] [CrossRef]
- Coppola, T.; Magnin-Luthi, S.; Perret-Menoud, V.; Gattesco, S.; Schiavo, G.; Regazzi, R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J. Biol. Chem. 2001, 276, 32756–32762. [Google Scholar] [CrossRef] [PubMed]
- Bolz, S.; Kaempf, N.; Puchkov, D.; Krauss, M.; Russo, G.; Soykan, T.; Schmied, C.; Lehmann, M.; Muller, R.; Schultz, C.; et al. Synaptotagmin 1-triggered lipid signaling facilitates coupling of exo- and endocytosis. Neuron 2023, 111, 3765–3774.e7. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.E.; Gonzalez-Gonzalez, I.M.; Wilkinson, K.A.; Konopacki, F.A.; Kantamneni, S.; Henley, J.M.; Mellor, J.R. SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat. Neurosci. 2012, 15, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Konopacki, F.A.; Jaafari, N.; Rocca, D.L.; Wilkinson, K.A.; Chamberlain, S.; Rubin, P.; Kantamneni, S.; Mellor, J.R.; Henley, J.M. Agonist-induced PKC phosphorylation regulates GluK2 SUMOylation and kainate receptor endocytosis. Proc. Natl. Acad. Sci. USA 2011, 108, 19772–19777. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Shang, Y.; Araki, Y.; Guo, T.; Huganir, R.L.; Zhang, M. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 2016, 166, 1163–1175.e12. [Google Scholar] [CrossRef]
- Zeng, M.; Diaz-Alonso, J.; Ye, F.; Chen, X.; Xu, J.; Ji, Z.; Nicoll, R.A.; Zhang, M. Phase Separation-Mediated TARP/MAGUK Complex Condensation and AMPA Receptor Synaptic Transmission. Neuron 2019, 104, 529–543.e6. [Google Scholar] [CrossRef]
- Wu, X.; Hu, S.; Kang, X.; Wang, C. Synaptotagmins: Beyond Presynaptic Neurotransmitter Release. Neuroscientist 2020, 26, 9–15. [Google Scholar] [CrossRef]
- Nicoll, R.A.; Tomita, S.; Bredt, D.S. Auxiliary subunits assist AMPA-type glutamate receptors. Science 2006, 311, 1253–1256. [Google Scholar] [CrossRef]
- Araki, Y.; Zeng, M.; Zhang, M.; Huganir, R.L. Rapid dispersion of SynGAP from synaptic spines triggers AMPA receptor insertion and spine enlargement during LTP. Neuron 2015, 85, 173–189. [Google Scholar] [CrossRef]
- Craig, T.J.; Henley, J.M. SUMOylation, Arc and the regulation homeostatic synaptic scaling: Implications in health and disease. Commun. Integr. Biol. 2012, 5, 634–636. [Google Scholar] [CrossRef]
- Nair, R.R.; Patil, S.; Tiron, A.; Kanhema, T.; Panja, D.; Schiro, L.; Parobczak, K.; Wilczynski, G.; Bramham, C.R. Dynamic Arc SUMOylation and Selective Interaction with F-Actin-Binding Protein Drebrin A in LTP Consolidation In Vivo. Front. Synaptic Neurosci. 2017, 9, 8. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chato-Astrain, I.; Pronot, M.; Coppola, T.; Martin, S. Molecular Organization and Regulation of the Mammalian Synapse by the Post-Translational Modification SUMOylation. Cells 2024, 13, 420. https://doi.org/10.3390/cells13050420
Chato-Astrain I, Pronot M, Coppola T, Martin S. Molecular Organization and Regulation of the Mammalian Synapse by the Post-Translational Modification SUMOylation. Cells. 2024; 13(5):420. https://doi.org/10.3390/cells13050420
Chicago/Turabian StyleChato-Astrain, Isabel, Marie Pronot, Thierry Coppola, and Stéphane Martin. 2024. "Molecular Organization and Regulation of the Mammalian Synapse by the Post-Translational Modification SUMOylation" Cells 13, no. 5: 420. https://doi.org/10.3390/cells13050420
APA StyleChato-Astrain, I., Pronot, M., Coppola, T., & Martin, S. (2024). Molecular Organization and Regulation of the Mammalian Synapse by the Post-Translational Modification SUMOylation. Cells, 13(5), 420. https://doi.org/10.3390/cells13050420








