Abstract
Glioblastoma (GB) is a rare but extremely aggressive brain tumor that significantly impacts patient outcomes, affecting both duration and quality of life. The protocol established by Stupp and colleagues in 2005, based on radiotherapy and chemotherapy with Temozolomide, following maximum safe surgical resection remains the gold standard for GB treatment; however, it is evident nowadays that the extreme intratumoral and intertumoral heterogeneity, as well as the invasiveness and tendency to recur, of GB are not compatible with a routine and unfortunately ineffective treatment. This review article summarizes the main challenges in the search for new valuable therapies for GB and focuses on the impact that extracellular vesicle (EV) research and exploitation may have in the field. EVs are natural particles delimited by a lipidic bilayer and filled with functional cellular content that are released and uptaken by cells as key means of cell communication. Furthermore, EVs are stable in body fluids and well tolerated by the immune system, and are able to cross physiological, interspecies, and interkingdom barriers and to target specific cells, releasing inherent or externally loaded functionally active molecules. Therefore, EVs have the potential to be ideal allies in the fight against GB and to improve the prognosis for GB patients. The present work describes the main preclinical results obtained so far on the use of EVs for GB treatment, focusing on both the EV sources and molecular cargo used in the various functional studies, primarily in vivo. Finally, a SWOT analysis is performed, highlighting the main advantages and pitfalls of developing EV-based GB therapeutic strategies. The analysis also suggests the main directions to explore to realize the possibility of exploiting EVs for the treatment of GB.
1. Introduction
Gliomas are the most frequent intracranial tumors in adults. The first reports of gliomas date back to the early 19th century thanks to the British scientists Berns and Abernethy; however, the first comprehensive histomorphological description was made by the German pathologist Rudolf Virchow in 1865. Studying gliomas, Virchow first introduced the concept of “neuroglia”, from which gliomas derive, as a connective tissue of the brain and the spinal cord that is formed by star-shaped units, interconnected by fine fibers, in which the nervous elements are located [1,2]. The term glioblastoma (GB) was first used in 1927 by the neuropathologist Percival Bailey and the neurosurgeon Harvey Cushing, who developed the first systematic classification and histological description of gliomas, providing the basis for the modern classification of gliomas [3]. Since then, glioma classification has been updated several times by the World Health Organization (WHO), introducing different nomenclature and diagnostic criteria. In the fourth edition of the WHO Classification of CNS Tumors (WHO CNS4), the term “multiforme” was abolished [4]. Furthermore, in the WHO CNS4, and even more so in the fifth and latest 2021 edition (WHO CNS5), besides the traditionally used histological and immunohistochemical features, molecular (genetic and expression data) parameters were also incorporated, establishing a different approach to both CNS tumor nomenclature and grading, and highlighting the significance of integrated diagnoses [5,6,7].
According to the WHO Classification of CNS Tumors, GB is the highest and most severe prognostic-grade, namely “grade IV”, glioma and the most aggressive and lethal among all primary brain tumors, with a median overall survival without treatment of at most 4 months, otherwise it is 14–17 months after diagnosis, and it has a 5-year survival rate of 5–6% [4,5,8,9]. Although considered a rare tumor, with an incidence rate of approximately 3 per 100,000 people, GB accounted for 14.5% of all CNS tumors and for 48.6% of the malignant ones based on the data collected in the Central Brain Tumor Registry of the United States (CBTRUS) from 2014 to 2018 [8]. The tumor has a slightly higher incidence in men than in women and a higher incidence in Caucasian than in African or Asian populations. The annual age-adjusted incidence of GB increases with age from 0.15 per 100,000 in children to 15.03 per 100,000 in patients over 75, with a median age at diagnosis of 65 years. In the pediatric age range, however, although rare, GB constitutes one of the groups of neoplasms with the worst prognosis [8,10]. Unfortunately, recent studies indicate an increase in the incidence of both GB and brain tumors in general, as highlighted by the Global Cancer Statistics of 2020 [11]. The etiology of GB is unknown, with the only identifiable risk factor being exposure to ionizing radiation. Other possible contributing factors include aging, non-ionizing radiation, and air pollution [12,13].
GB primarily develops in the cerebral hemispheres. Depending on the affected brain area and increased intracranial pressure, patients may exhibit a variety of rapidly developing and life-altering symptoms. Neurological symptoms, such as severe headache, loss of vision, language alteration, and persistent weakness, as well as psychological and psychiatric symptoms, including unpredictable personality changes, can severely compromise the quality of life and even the autonomy of patients, with a strong impact on the patient‘s family and friends [14,15,16,17]. Diagnosis typically occurs only at the onset of symptoms and is primarily based on magnetic resonance imaging (MRI), which has technical limitations in terms of specificity (difficulties in excluding other diagnosis) and sensitivity (spatial resolution of 2–3 mm) [18]. The most widely adopted therapy for GB since 2005 involves the surgical removal of the tumor mass, followed by radiotherapy and chemotherapy, according to the protocol identified by Stupp and coworkers [19] (Stupp et al., 2005). The surgical removal of GB is a complex procedure due to the large number of cells that make up the tumor and their high infiltration power in surrounding healthy tissues. This is a major cause of the high recurrence rate, and the success of the procedure is strongly dependent on the location and accessibility of the tumor mass. Extensive surgical therapy, where possible, is crucial to improve patient prognosis and reduce the risk of recurrence, although this may increase the risk of postoperative neurological deficits [20]. After surgical resection, and if this is not possible, patients receive radiotherapy in combination with chemotherapy, of which Temozolomide (TMZ), a DNA alkylating agent, is the chemotherapy of choice [19].
Recently, clinical trials have tested alternative therapies, such as extended adjuvant TMZ treatment, the monoclonal anti-VEGFA antibody Bevacizumab, the receptor tyrosine kinases inhibitor Regorafenib, magnetic tumor-treating fields, and immunotherapies, alone or in combination with established treatments [21,22,23,24]. Moreover, in order to identify the most effective interventions, several meta-analyses have compared the efficacy of different treatments across different randomized clinical trials. These studies have highlighted some promising results, such as the ability of Bevacizumab in combination with TMZ to slightly increase progression-free survival (but not overall patient survival), making it a potential salvage regimen for recurrent GB [25,26,27,28].
Novel approaches based on photodynamic and sonodynamic therapies, also in combination, have shown promising results in preclinical studies of GB, both in vitro and in vivo [29,30,31,32,33]. Photodynamic therapy (PDT) induces cell death in the target tissue through the production and accumulation of reactive oxygen species by a combination of light energy, oxygen, and photosensitizing cytotoxic agents [29,34,35]. Recent studies have shown that phytocompounds, such as berberine and curcumin, can be used as efficient photosensitizers in PDT against highly malignant gliomas [29,36,37]. Sonodynamic therapy (SDT) is a noninvasive procedure that uses focused ultrasounds to activate sonosensitizers (i.e., 5-Aminolevulinic Acid or 5-ALA) to block tumor growth, although the mechanism is still unclear [32]. These approaches provide the valuable opportunity for spatially and temporally specific GB treatments with limited systemic toxicity. Several clinical trials are ongoing or have recently ended to evaluate the feasibility and effectiveness of PDT (NCT01966809 terminated; NCT05363826, NCT04391062, NCT04469699, and NCT03897491 recruiting; and NCT05736406 not yet recruiting) and SDT (NCT04845919, NCT06039709, NCT05370508, and NCT05362409, all recruiting) for GB treatments. The results of these studies will be fundamental for the integration of these therapies into clinical practice.
So far, no breakthrough therapies leading to the extensive and durable survival of patients have been discovered, and survival rates for GB patients have not shown a notable improvement in population-based studies [10]. Therefore, there is a significant need for effective and minimally invasive diagnostic and therapeutic tools to detect early GB onset, and, even more, to block GB progression and avoid its recurrence after surgery.
Improving our understanding of GB onset and progression at the molecular, cellular, and systemic levels is critical in identifying new key therapeutic targets and implementing effective treatment strategies. In this scenario, the discovery of natural and functional nanoparticles surrounded by a lipid membrane, called extracellular vesicles (EVs), which are released and uptaken by cells in both physiological and pathological conditions, including tumorigenesis, results in the strong potential for inspiring new, more effective, and less invasive diagnostic and therapeutic approaches for GB patient treatment. In this review, we will focus on the significance of EVs in the development of new promising therapeutic strategies against GB.
2. Main Issues in GB Therapy
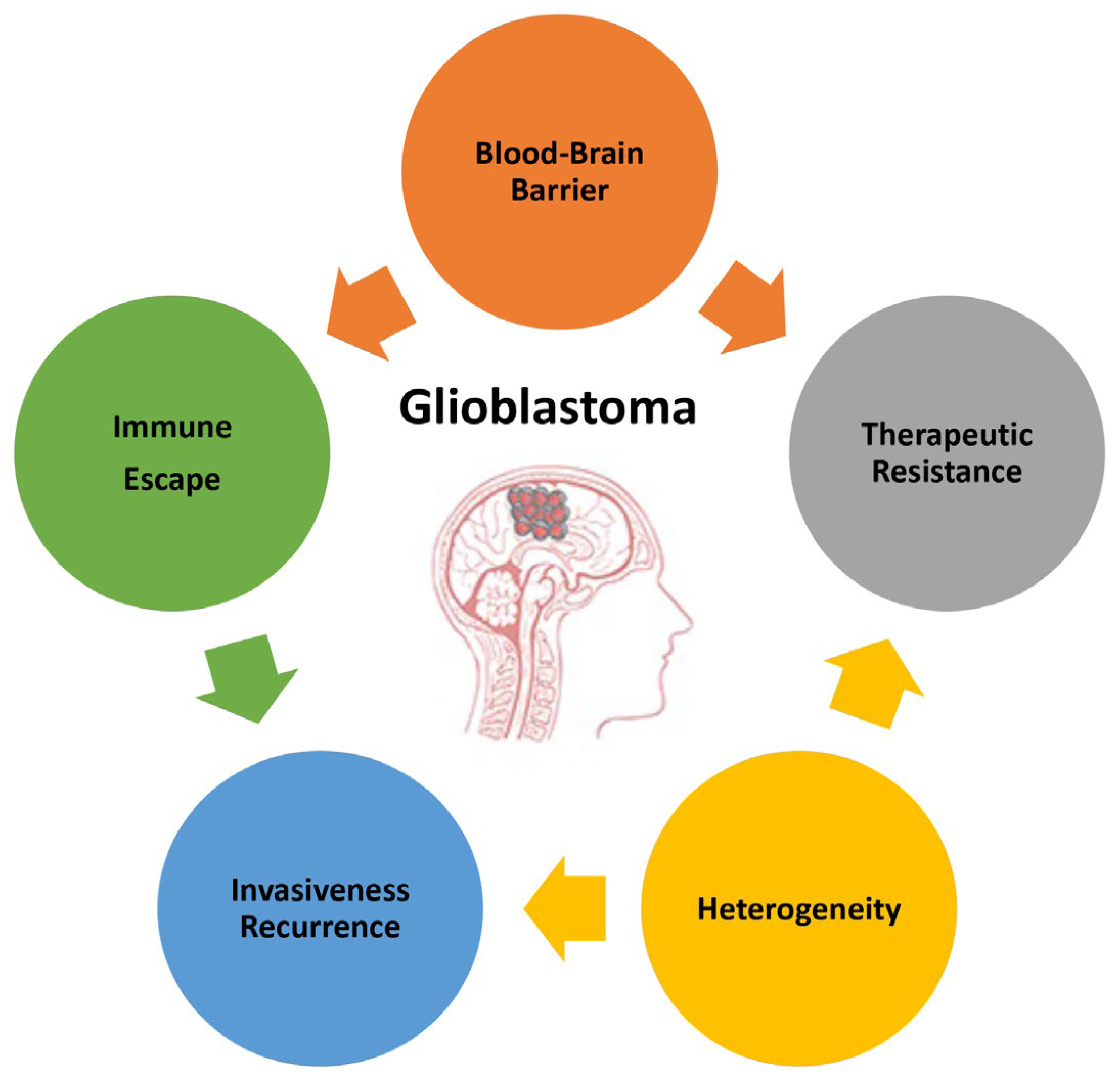
The poor prognosis of GB and the objective difficulties in developing successful therapies are due to the complex and unique features characterizing this type of tumor. This paragraph summarizes the main attributes of GB (Figure 1) to highlight the direction that basic investigations, research and development, and technology transfers should take.
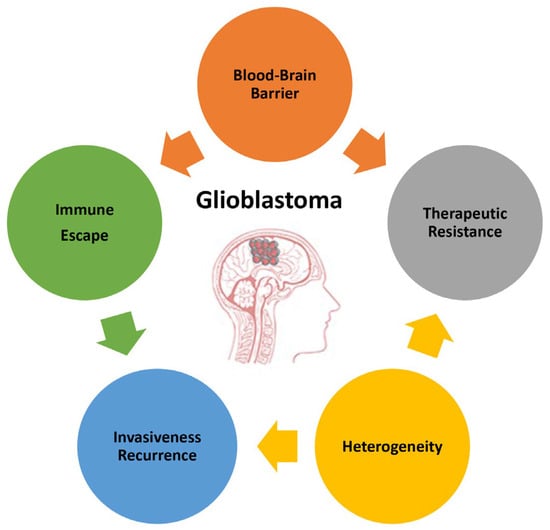
Figure 1.
Main features of glioblastoma responsible for its high malignancy and poor prognosis.
2.1. Blood–Brain Barrier
The blood–brain barrier (BBB) is a highly selective and specialized barrier that maintains the homeostasis of the CNS, protecting and isolating the brain from potentially harmful agents transported in the blood, such as viruses and other pathogens. The BBB has a complex structure formed by multiple layers, including brain microvascular non-fenestrated endothelial cells sealed by junctional complexes, and is surrounded by the end feet of glial cells on the outer face. The physiological barrier is the result of a series of physical, transport, and metabolic processes within endothelial cells that are highly coordinated by interactions with different vascular, immune, and neural cell types [38,39,40]. Therefore, the BBB is not only a physical barrier, but it also actively uses endothelial efflux transporters to pump small lipophilic molecules that can diffuse through the plasma membrane back in the bloodstream. Even though the BBB plays an evolutionarily important role in preserving brain health and function, in the case of brain pathologies, such as GB, it can hinder the delivery of therapeutic molecules to the brain, limiting their clinical success [39,40,41]. Moreover, as the barrier works in both directions, the flow of molecules from the brain to the bloodstream is also restricted, reducing the diagnostic potential of liquid biopsies based on blood samples for brain diseases [18].
Interestingly, the integrity of the barrier is compromised in many brain pathologies, including GB; however, this alone is not enough to enhance drug accumulation and efficacy. This is in part due to the highly heterogeneous barrier disruption and the persistence of an intact barrier in areas surrounding the tumor [40,42,43,44]. Additionally, in the case of GB, the impaired BBB, also known as the blood–tumor barrier (BTB), can function synergistically with the intact BBB to create a tumor-supportive adaptive environment, paradoxically allowing for the transport of pro-tumoral molecules while limiting the entry of antitumoral ones [45,46].
2.2. Heterogeneity
GB is a highly dynamic and rapidly evolving tumor characterized by strong molecular and cellular heterogeneity within an individual tumor at a given time, as well as at different times during tumor development and even more so in different patients [47,48]. The high inter- and intratumoral heterogeneity of GB can affect tumor diagnosis and characterization, as it is challenging to achieve a comprehensive description of the whole molecular signature in such a heterogeneous tumor through surgical biopsies. To have a better chance of accurate diagnosis, multiple biopsies should be performed and repeated during the progression of GB, a practice that is neither feasible nor sustainable for the patient [18]. Moreover, GBs’ heterogeneity makes it extremely difficult to target all tumor cells inside the same patient and to cure different patients with the same drug [49]. With regard to GB, we can distinguish between molecular and a cellular heterogeneity.
2.2.1. Molecular
In 2016, the WHO CNS4 classified gliomas into low-grade gliomas (LGGs) and GB, with GB further subdivided into primary and secondary tumors [4]. Primary and secondary GBs are histologically indistinguishable, but develop from different genetic precursors and therefore show different genetic alterations. Primary, or de novo, GB is characterized by the absence of a mutation in the isocitrate dehydrogenase 1 (IDH1) gene, accounts for 90% of all GBs, affects older patients, and has a worse prognosis. The most frequent genetic alterations include the loss of heterozygosity (LOH) on chromosome 10q, mutations or deletions in the Phosphatase and Tensin Homolog (PTEN) gene, the mutation or amplification of the Epidermal Growth Factor Receptor (EGFR) gene, and the deletion of the Cyclin-Dependent Kinase Inhibitor 2A/B (CDKN2A/B) gene. Secondary GB, arising from LGGs or astrocytomas, are rarer, usually diagnosed at a younger age, and have a more favorable outcome. The most common genetic alterations include IDH1 gene mutations, an LOH at 22q and 19q chromosomes, and the methylation of the O6 Methylguanine-DNA Methyltransferase (MGMT) promoter [50].
GBs’ molecular signature was identified and listed in The Cancer Genome Atlas (TCGA) project, which reported a large-scale multi-dimensional analysis at the genomic, epigenomic, and transcriptomic level [51,52]. These studies highlighted frequent alterations in core oncogenic pathways involving the tumor protein p53, the receptor tyrosine kinase/Ras/phosphoinositide 3-kinase, and retinoblastoma. Furthermore, a bulk gene expression profile analysis identified three main GB molecular subtypes, namely proneural (TCGA-PN), classical (TCGA-CL), and mesenchymal (TCGA-MES), harboring mutations in the Platelet-derived growth factor receptor A (PDGFRA) and IDH1 genes (PN), EGFR gene (MES), and Neurofibromatosis 1 (NF1) gene (CL), respectively, with PN having a better prognosis than the MES subtype [48,53]. However, over time, the switching of a GB tumor from one subtype to another has been described, e.g., from PN to MES, and is one of the main causes of resistance to treatments [54,55]. Recently, single-cell RNA sequencing pointed out the high level of intratumoral complexity by showing the presence of transcriptionally distinct subclones, each with specific features, in the same tumor sample [56].
2.2.2. Cellular
A second layer of heterogeneity is due to the developmental state of GB cells in the tumor. GBs contain subsets of GB stem cells (GSCs) that resemble and could be derived from neural stem cells (NSCs) [57,58,59,60]. GSCs are considered to represent the driving force of GBs, as they are able to maintain stemness and proliferation, as well as to remain quiescent and escape both chemotherapy and radiotherapy. These abilities can be transferred to other tumor cells, conditioning the tumor microenvironment to support GB growth, progression, and recurrence [61]. GSCs could be isolated thanks to the presence of CD133, CD44, and L1 cell adhesion molecules (L1CAMs) on the cell surface, but it is unknown whether different GSC markers isolate different cellular states or subtypes and whether tumors generated with different subpopulations of GSCs give rise to GBs with a comparable or distinct cellular composition. Several studies have shown that GSCs in recurrent GB differ from those that initiated and maintained in the primary tumor, displaying different surface markers (CD15, BMI1, and SOX2, instead of CD133) and being more aggressive. Finally, GB single-cell sequencing analyses suggested that tumor cell hierarchy arising from GSCs might account for the intratumoral cellular and molecular heterogeneity [46,62,63].
2.3. Invasiveness and Recurrence
GB cells have been shown to invade normal brain tissues, penetrating the brain parenchyma and the perivascular space by degrading the extracellular matrix through the release of proteolytic factors and by forming membrane-derived extensions known as invadopodia, which are involved in cell migration [64,65]. The patterns, directionality, and mechanisms responsible for this invasive behavior involve the extracellular matrix (ECM) and cytoskeletal remodeling, cell-to-cell and cell-to-ECM adhesion mechanisms, and the overall features of epithelial–mesenchymal transition (EMT). GB cells show a preference toward specific brain regions (the subventricular zone) compared to others, such as the hippocampus and cerebellum. Due to their aggressive migratory behavior, GB cells can escape complete surgical resection, resulting in recurrence within a few centimeters of the original location. Almost all GB tumors return after surgery, radiotherapy, and chemotherapy, and show a lower response rate to conventional therapies, leaving very few options of treatment. Currently, management protocols for recurrent GB patients are still lacking, and most patients are not eligible for surgical re-resection [66]. Multiple studies have identified the tumor-initiating and clonogenic potential of GSCs, as well as their role in therapeutic resistance, making them the main contributors to GB recurrence. After debulking, the unremoved GSCs migrate within the resection cavity and initiate and recapitulate the entire tumor [67,68,69].
2.4. Therapeutic Resistance
Therapeutic resistance is one of the main causes of poor prognosis for patients with GB. This feature increases over time, weakening current therapeutic protocols for recurrent GB. In most cases, surgery is no longer possible, and chemo- and radiotherapy fail to counteract tumor progression [46]. Many of the features of GB described here have also been linked to radio- and/or chemoresistance. First of all, the presence of the BBB limits the access of chemotherapeutics to the brain [40]. Moreover, therapeutic resistance tends to increase over time due to the molecular changes that occur during GB progression, including the switching from the PN to the MES subtype, and the accumulation of O6-methylguanine-DNA methyltransferase (MGMT). MGMT is in charge of DNA repair, and increased levels of MGMT are associated with increased resistance to TMZ chemotherapy [70]. GSCs play a key role in therapeutic resistance, showing low sensitivity to both chemo- and radiotherapies, which further decreases following repeated chemoradiation treatments [71,72]. Therapeutic resistance is mediated by the increase in MGMT, as well as anti-apoptotic proteins drug-efflux transporters, which reduce drug sensitivity, and the activation of both DNA damage checkpoints and DNA repair mechanism induced by radiation [73,74].
2.5. Immune Escape
The immunosurveillance of the CNS is naturally designed to preserve neuronal function and minimize non-specific immune responses. Additionally, GBs have been described as an immunologically “cold” tumor that employs multiple immunosuppressive mechanisms [75]. GB cells present few new tumoral immunogenic antigens on the surface, likely due to a low mutational burden, the post-transcriptional downregulation of antigen expression, and, last but not least, the post-translational mechanisms that interfere with antigen exposure on the cell membrane. This is the case for the family of ligands of the Natural Killer group 2D (NKG2D) receptor, which act as stress-induced ligands expressed by damaged or transformed cells to be recognized and cleared by immune cells. The ligands bind and activate the NKG2D receptor on NK cells, triggering cytotoxicity, and T cell differentiation and expansion [76]. However, GB cells have various mechanisms to limit the expression and/or the exposure on the membrane of NGK2 ligands and to escape immune surveillance. These mechanisms include translational regulation by miRNAs, intracellular retention, proteolytic degradation, as well as shedding from the membrane through proteolytic cleavage or EV encapsulation and release [76]. Moreover, GB cells contribute to creating an immunosuppressive microenvironment by actively secreting soluble factors, interleukins, prostaglandins, and EVs that target microglia, monocytes, and macrophages, thereby inducing a tumor-supportive M2 phenotype; as well as T lymphocytes, sequestering them in the bone marrow and impairing their antitumoral activity. The complex GB microenvironment, conditioned by the GB itself, facilitates the ability of the tumor to evade the immune response, to progress, and also to recur [46,77,78,79,80]. Immunotherapy against GB is still challenging due to its characteristics. To date, clinical trials involving immune checkpoint inhibitors, vaccine-based therapy, and viral therapy have not yielded the desirable positive outcomes observed in other tumors. Ongoing studies are investigating different combinatorial approaches to overcome GB features [80].
3. Investigating EVs for GB Treatment: Strategies, Advantages, and Future Perspectives
GB cells communicate with each other and with the tumor microenvironment. EVs play key roles in this process, as they transfer functional molecules (DNA, mRNAs, microRNAs or miRNAs, long non-coding RNAs or lncRNAs, proteins, lipids, and metabolites) and their related properties between cells [81]. EVs are released by cells, mainly through two mechanisms that involve budding from the cell membrane (microvesicles and ectosomes), or the exocytosis of intraluminal vesicles formed inside the multivesicular bodies present in the cytoplasm (exosomes) [82,83,84]. The first mechanism produces both small and large EVs, while the second one specifically gives rise to smaller vesicles due to physical cellular constraints [85]. EVs can travel through the bloodstream and other biological fluids, transporting the messengers contained within over a considerable distance from the site of origin. They can target specific cells through cell membrane interactions and can be internalized in the target cells, where they release their functional content [85,86,87].
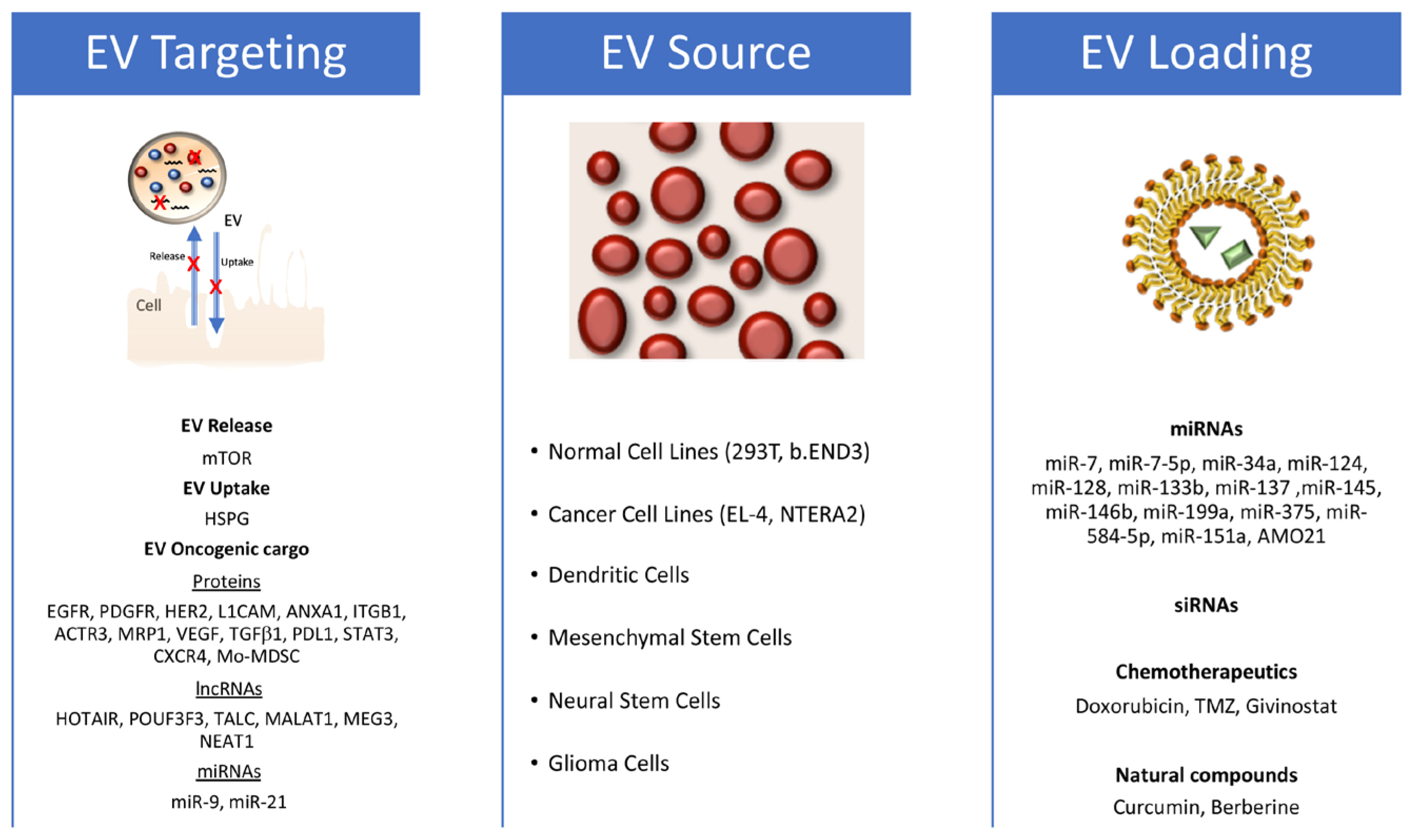
The release of EVs by GB cells, particularly GSCs, can promote the oncogenic transformation of normal brain cells and increase the malignancy of other GB cells by inducing proliferation, motility, and chemoresistance. Additionally, GB-derived EVs (GDEVs) can target and condition non-tumoral cells in the GB microenvironment, such as macrophages, immune cells, astrocytes, and endothelial cells, inducing angiogenesis and immune escape, and creating a supportive microenvironment for GB growth and progression. In turn, cells in the conditioned tumor microenvironment release EVs that are uptaken by GB cells, increasing their proliferation, motility, invasiveness, and resistance to therapy. Several review articles have explored the role played by EVs in the cellular cross-talk within GB microenvironments [65,81,88,89]. This review, instead, aims to focus on how EV biology can be exploited to overcome current limitations in GB therapy and develop innovative and breakthrough therapeutic approaches (Figure 2).
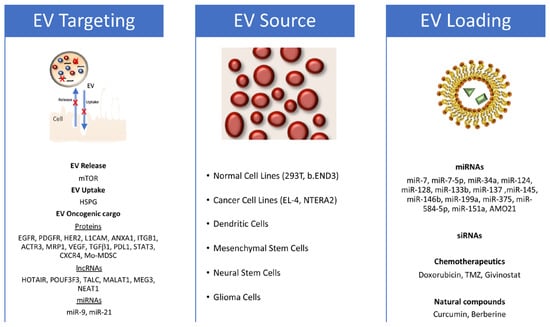
Figure 2.
EV exploitation strategy for the treatment of glioblastoma. Figure schematizes the identified targets that block EV release and uptake or impair specific oncogenic glioblastoma (GB) features on the left; the EV sources used in different approaches against GB in the middle; and the therapeutic molecules used in functional assays in glioblastoma models on the right (both in vitro and in vivo).
3.1. Targeting EV Biology and Cargo
EVs are very much implicated in the establishment of a supportive microenvironment for GB, as well as tumors in general, including their progression and the induction of key malignant features, such as proliferation, invasiveness, chemoresistance, and immune escape. Therefore, therapeutic strategies aimed at blocking EV release, uptake, and circulation may counteract GB progression and improve patient outcomes, regardless of the high tumor heterogeneity.
Several strategies have been identified that are based on targeting EVs on route (i.e., hemodialysis) or inhibiting EV release and uptake, including the use of natural substances (i.e., the antifungal agent ketoconazole or the alkaloid berberine extracted from herbal plants), as well as based on repurposed drugs such as heparin and reserpine, which are already used as anticoagulant and anti-hypertensive drugs, respectively [64,90,91,92]. Alone, berberine, an efficient sensitizer used in PDT, can also suppress cancer cell proliferation by reducing the synthesis of fatty acids and inhibiting the formation and secretion of extracellular vesicles in cancer cells [91].
In glioma cells heparan sulfate proteoglycans (HSPGs) have been identified as key modulators of EV uptake [93]. A decrease in HSPGs on the cell membrane of GBs and the use of free heparin, which competes with cell-membrane HSPGs for the binding to EVs, strongly reduces EV uptake [94]; however, the heparin-mediated blocking of EV binding to target cells and the subsequent internalization is not specific to cancer cells [95,96]. Identifying selective strategies to address tumor-derived EV biology, without affecting normal EVs, might be fundamental for developing clinically suitable, effective, and safe interventions, avoiding undesired off-target effects. In GB, increased levels of the mammalian Target of Rapamycin (mTOR) and consequent pathway activation are required for promoting EV release through the downregulation of the autophagy pathway, as well as for maintaining GSC tumor features (self-renewal, proliferation, migration, and invasiveness) [67]. This mechanism is potentially relevant for the development of novel approaches that selectively target GDEV production.
In parallel, defining specific functionally relevant GB-associated EV cargo that regulates the complex crosstalk within GB environment may allow for specific and targeted therapeutic approaches. Several GDEV-associated proteins have been identified as key players in the induction of the proliferation (EGFRvIII, PDGFR, and Human Epidermal Growth Factor Receptor 2 (HER2)), invasiveness (L1CAM, Annexin A1 or ANXA1, Integrin Beta 1 (ITB1), and Actin-related protein 3 (ACTR3)), and chemoresistance (multidrug resistance protein 1 (MRP1)) of GB cells. GDEVs are also enriched in pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), transforming growth factor beta 1 (TGFβ1), C-X-C chemokine receptor type 4 (CXCR4), and plasminogen activators and proteases, which induce endothelial cells proliferation and tube formation as well as in immune modulators, such as Programmed Cell Death Ligand 1 (PDL1) and monocytic myeloid-derived suppressor cells (Mo-MDSC), which inhibit inflammatory and immune responses. In the last decade, gene/RNA therapy has gained significant attention in the GB therapeutic field. Several lncRNAs with oncogenic activity have been found inside GDEVs [97]. Among these, the lncRNAs POUF3F3 and TALC can remodel the GB microenvironment. POUF3F3 acts on endothelial cells, inducing angiogenesis and GB progression, while TALC acts on microglia, inducing M2 polarization with the consequent secretion of the complement components C5/C5a, leading to the induction of TMZ chemoresistance in GB cells [98,99]. Other GDEV-associated lncRNAs, such as MALAT1, MEG3, NEAT1, and HOTAIR, play a key role in promoting EMT and possibly chemoresistance when uptaken by GB cells both in vitro and in vivo [97]. There is accumulating evidence that miRNAs also play a significant role in GB pathogenesis and have a high potential for use in targeted therapeutic approaches [100]. Among the others, miR-9, upregulated in GSCs, has been found in EVs isolated from both GB cell cultures and patients, and is strongly implied in the phenotypic traits of malignant GBs including cell proliferation and migration [101]. miR-9 is also involved in the expression of the drug efflux protein P-glycoprotein, which contributes to an increase in TMZ drug resistance in GB cells. Anti-miR-9 molecules encapsulated in EVs derived from Mesenchymal Stem Cells (MSCs) were shown to be successfully delivered to GB cells in vitro, causing a reduction in the levels of P-glycoprotein and enhancing TMZ sensitivity [102]. Therefore, a promising direction is represented by therapeutic approaches based on EVs loaded with small interfering RNAs (siRNAs) targeting GB cells and downregulating the expression of the inherent oncogenic lncRNAs or miRNAs, thus inhibiting their transfer via EVs to other tumor and non-tumor cells within the GB microenvironment.
3.2. EVs as Drug Delivery Systems in GB Therapy
Recently, nanomedicine has dominated the field of tumor therapy with respect to different approaches, thanks to the abilities of nanoparticles to be loaded with specific therapeutic agents as well as surfaces decorated with specific targeting molecules. First, synthetic nanocarriers such as liposomes, have been used, due to their high loading and surface functionalization efficiency; however, they have important limitations, such as low tolerability, relatively short circulation times in biological environments, fast body clearance, and, last but not least, a low capability for BBB. Even though different strategies have been developed to increase BBB permeability and enhance nanoparticle access to the brain tissue, low biocompatibility and a high risk of off-target effects are still key issues to overcome [39,103].
EVs offer key advantages in this regard. Several studies have shown their low immunogenicity and cytotoxicity, low clearance from the phagocytic system, prolonged circulation time, high biocompatibility, and efficient cell uptake [104,105]. Interestingly, EVs are able to cross interspecies and even interkingdom boundaries; EVs isolated from different sources, including microalgae, bacteria, and plants, are easily uptaken by mammalian cells [106,107,108,109,110]. Through several physical/chemical/genetic strategies, EVs can be loaded with various functional cargoes, such as nucleic acids, proteins, natural substances, chemotherapeutics, photosensitizers, and sonosensitizers, that can be delivered to the cells. Moreover, the EV surface can be also functionalized with cell-type-specific targeting ligands to enhance the interaction with specific cellular types [39,111]. The encapsulation of therapeutic molecules within EVs can improve the molecules’ solubility, stability, bioavailability, and therapeutic effect. Furthermore, EVs are capable of crossing the BBB, making them the ideal candidates for delivering therapeutic molecules to the brain for the treatment of brain pathologies [103].
The first study on the ability of engineered EVs to cross the BBB and efficiently delivery functional molecules to the brain was performed by Alvarez-Erviti and coauthors by using EVs isolated from mouse bone-marrow-derived dendritic cells [112]. To confer brain-targeting capabilities, dendritic cells were previously engineered to express recombinant fusion proteins. These proteins contained the central nervous system-specific rabies viral glycoprotein (RVG) peptide, which specifically binds to the acetylcholine receptor, fused to the N terminus of a murine dendritic cell (DC)-derived lysosome-associated membrane protein (Lamp2b), which is a highly EV-associated protein. Isolated EVs exposing the RVG-Lamp2b fusion protein on the membrane were electroporated with specific siRNAs and then injected intravenously into mice. The EVs were capable of specific targeting and siRNA delivery to brain neurons, microglia, and oligodendrocytes [112]. A similar strategy was used in mouse models for Parkinson’s disease [113]. This approach has shown great potential in penetrating the BBB and could be exploited for the treatment of brain pathologies. Recently, Kim and coworkers [114] used the same Lamp2b-based approach to decorate EVs released from 293T cells with the Transferrin Receptor-binding peptide T7, which is able to target transferrin receptors naturally located on GB cells’ surface. The engineered T7-EVs were further modified to encapsulate antisense miRNA oligonucleotides against miR-21 (AMO-21). When injected intravenously into an intracranial GB xenotransplanted rat model, T7-EVs were much more efficient than both unmodified and RVG-decorated EVs in reaching the brain, targeting GB cells, and delivering AMO21, resulting in a reduction in GB size [114].
EVs from other cell types have also been successfully tested for GB treatment. EVs derived from a mouse lymphoma cell line (EL-4) were loaded with the anti-inflammatory drug curcumin or with an inhibitor of the signal transducer and activator of transcription 3 (STAT3). These EVs were efficiently and noninvasively delivered, via the intranasal route, to microglia cells in mice brains, causing a significantly delayed brain tumor growth in a glioma mouse model [115]. In addition, EVs from a brain endothelial cell line (b.END3), which are naturally enriched with the CD3 tetraspanin, were loaded with doxorubicin. These EVs were capable of crossing the BBB into a xenotransplanted zebrafish brain tumor model (obtained by injecting GB cells into the zebrafish brain ventricle), significantly decreasing the fluorescent intensity of xenotransplanted cancer cells and tumor growth markers [116].
MSCs are a well-studied source of EVs that have been utilized in several clinical trials, both in their native form or after engineering, to target a wide range of pathologies, including tumors [64,117]. MSCs can communicate with different cell types, including GB cells, through direct contact via gap junctions and/or contact-independent pathways [102,118]. Stromal cells resembling MSCs are a key component of the GB microenvironment, where they can play both tumor-supportive and -suppressive roles [119,120]. MSC-derived EVs carrying specific miRNAs (miR-7, miR-34a, miR-124, miR-133b, miR-145, miR-146b miR-199a, miR-375, or miR-584-5p) were found in vitro to counteract GB tumor features, including cell proliferation, migration, and invasion. Specific molecules or pathways were targeted by miRNAs, including Forkhead box (FOX)A2 (miR-124a), Wnt pathway (miR-133b), EGFR (miR-146b), SLC31A (miR-375), and MMP2 (miR-584-5p). Many of these MSC-EVs engineered with miRNAs were also tested in vivo in xenotransplanted mouse models, where they showed the ability to cross the BBB and promote tumor regression [100]. A very promising study is the one involving MSC-EVs loaded with miR-124a and systemically delivered in mouse models xenotransplanted intracranially with GB cells; the loaded MSC-EVs were able to suppress tumor growth and prolong overall animal survival [121].
Tumor-derived EVs (TDEVs) can be also used as natural carriers for the delivery of antioncogenic molecules [64], thanks to their high tumor targeting and permeability, making them a technological Trojan horse, as evoked by Simionescu and coauthors [100]. EVs isolated from engineered patient-derived GSCs and carrying the miR-302-367 cluster are rapidly internalized by neighboring GB cells, and are able to inhibit GB cell proliferation, stemness, and invasion through the repression of the Cyclin A, Cyclin D1, E2F1, and CXCR4 pathways [122]. In addition, GDEVs that were engineered with miR-124, miR-128, or miR-137 improved the survival rate of a GB mouse model when combined with chemotherapy [123], whereas GDEVs carrying miR-151a reduced chemoresistance in GB xenograft mouse models [124]. Cell treatment with miRNA-specific inductors can also be used as a valid alternative to external loading to obtain EVs carrying specific miRNA molecules. This is the approach used by Wang and coauthors, which employed traditional medicine substances to induce GDEVs’ miR-7-5p enrichment [125]. As a result, GDEVs carrying miR-7-5p reduced GB formation and metastasis in GB nude mouse models [125]. The exploitation of GDEVs as drug carriers for GB therapy requires an extremely careful evaluation of the antioncogenic properties of the loaded therapeutic molecule vs. the oncogenic potential of the GDEVs’ intrinsic cargo as well as a relative cost–benefit assessment. Guo and coworkers recently identified a saponin-mediated cargo elimination strategy to improve the biosafety of GDEVs in GB therapy applications [126]. A systematic analysis of the original proteins and RNAs together with functional in vitro and in vivo assays confirmed the high efficiency of the method in eliminating GB-EV cargo, and its inherited abilities in promoting GB progression, without affecting uptake by GB cells. Furthermore, saponin-treated GDEVs loaded with doxorubycin displayed an effective tumor-suppressive role in both subcutaneous and orthotopic GB mouse models [126]. These data are extremely promising and could be exploited to develop novel visionary therapeutic pipelines. These could involve isolating TDEVs directly from the patients, disarming their oncogenic properties through cargo elimination protocols, loading and/or functionalizing them with anticancer molecules, and administrating them to the patient. Finally, although TDEVs preferentially target (and are uptaken by) the cells of the same tumor type, TDEVs’ surface can be functionalized with specific molecules involved in EV–cell interactions to improve or alter natural tumor tropisms. Geng and coauthors have shown that GDEVs are more easily uptaken by GB cells than other type of tumor cells such as pancreatic cancer (PC) cells, and vice versa [127]. However, functionalizing EVs with cyclic arginine-glycine-aspartic acid-tyrosine-cysteine (cRGDyC), a ligand for the integrin αvβ3 enriched in GB cells, enhances the ability of PC cells (and also the GB cells themselves) to target and be internalized into GB cells [127].
Other cells that naturally show GB tropism are neural stem cells (NSCs), from which the GB might be derived [57,58], and teratocarcinoma cells. Both NSCs and NTERA2 human teratocarcinoma cells, which resemble NSCs as they can differentiate into both glia and neurons [128,129], demonstrate GB tropism in vivo and have been proposed for cell-based delivery systems in anti-GB therapies [130,131,132]. EVs released by NSCs and engineered with antisense oligonucleotides (ASOs) targeting oncogenic and tolerogenic signal transducers and activators of transcription 3 (STAT3) were effective, after intracranial injection at a distant site, in reaching the glioma microenvironment, targeting and activating microglia to inhibit tumor growth [133]. Even more interestingly, native NTERA2-derived EVs, naturally carrying the oncodevelopmental factor Cripto [134,135,136], have been recently shown to impair GB cell migration in vitro without inducing undesirable effects such as increased GB cell proliferation or enhanced TMZ drug resistance [137]. Cripto is a membrane-bound glycosylphosphatidyl inositol-anchored protein that can be also cleaved and released as a soluble factor [138], and it only recently has been found to be associated with EVs [137,139]. Mouse Cripto gene targeting was associated with neural differentiation in embryonic stem cell cultures, the specification of anterior neural identities in vivo, and Cripto null embryos, which mostly comprise anterior neuroectoderm, including NSCs [140,141,142]. Cripto has been mostly identified as being associated with oncogenic features, particularly in GB, but it is also able to act as an antioncogene [143,144,145,146]. Although these data have not yet been confirmed in vivo, they suggest new unobvious and even paradoxical paradigms in GB, as well as in tumor in general and their progression and therapies. First, EV sorting and delivery could be an alternative route for regulating the spread and activity of soluble and/or membrane-bound signaling molecules, modulating their final impact on target cells and then on cancer development and progression [64,137]. Secondly, specific subsets of TDEVs might possess antitumoral potential and could be used per se or eventually enhanced through ad hoc modifications [64]. Therefore, molecularly identifying and isolating these specific subsets of TDEVs, and then inducing or bio-mimicking them is certainly a novel, ambitious, and promising therapeutic direction to explore.
4. Mitigating the Pitfalls and Realizing the Potential of EV Exploitation in GB Treatment
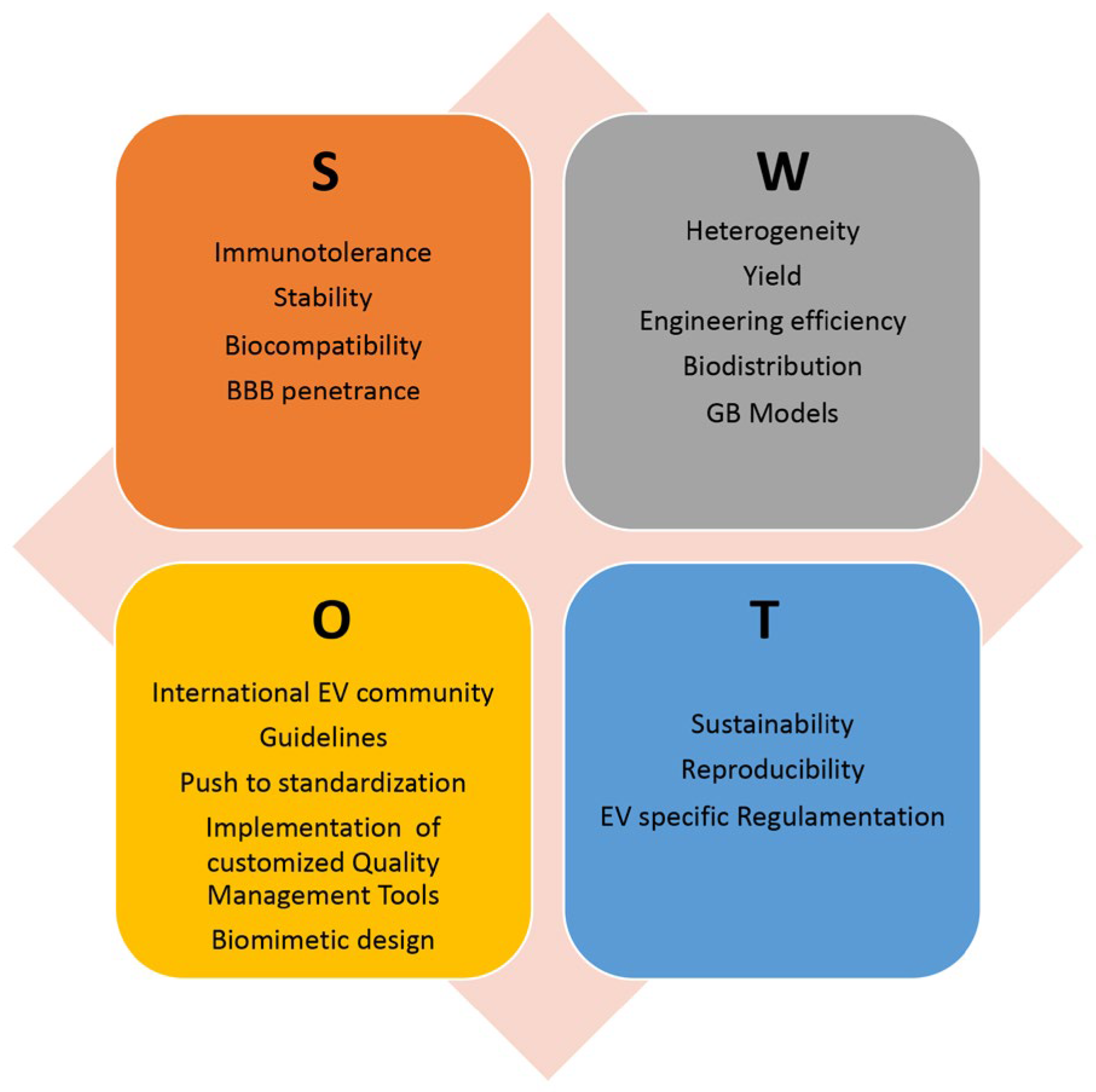
Despite these promising results, there are currently no ongoing clinical trials for EV-based GB treatment; however, EV-based therapies are being evaluated in clinical trials for the treatment of other types of tumors. This paragraph outlines the main issues and directions to explore in order to find the optimal solutions and fully realize the potential of EVs as an advanced therapeutic platform for GB treatment. Figure 3 shows an analysis of the Strengths, Weaknesses, Opportunities, and Threats (SWOT) of using EVs in novel GB therapeutic strategies.
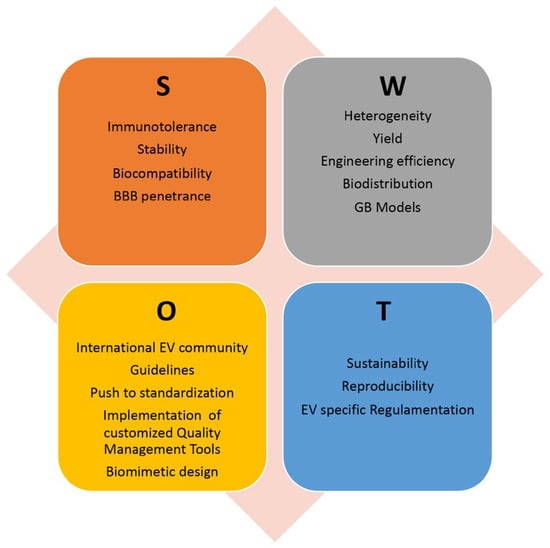
Figure 3.
SWOT analysis of EV exploitation in GB treatment, highlighting the relative Strengths (S), Weaknesses (W), Opportunities (O), and Threats (T). BBB: blood-brain barrier; GB: glioblastoma.
4.1. Extracellular Vesicle Source
The previous subsection has listed the main EV sources that have been used for GB therapeutic assays both in vitro and in vivo. They are all derived from mammalian cells, including cell lines of brain endothelial cells, tumor cells (both GB and teratocarcinoma), and stem cells (both neural and mesenchymal). To be exploited in patient therapy, EVs must be produced in high quantities and with extremely high quality, thereby complying with good manufacturing practices (GMP), pharmaceutical biologics regulation, and ongoing specific regulations for EV therapeutics [106]. The process needs careful scaling up and out, standardization, and quality assurance of the experimental pipeline, as well as quality control of the final products, requiring a high commitment in terms of cost, resources, and time. Alternative sources of EVs, such as plants, microalgae, and bacteria, have also been identified, and their biocompatibility and biodistribution in animal models have been studied [106]. These alternative EV sources have a lower cost of production, but unfortunately, no brain tropism has been proved yet. Therefore, the production pipeline must be extended to include an EV surface functionalization phase, followed by the isolation and characterization of the engineered EVs. A careful evaluation of the advantage and disadvantages of each strategy must be performed, in terms of therapeutic efficacy and efficiency as well as of cost and sustainability.
4.2. Extracellular Vesicle Handling Procedures
Various techniques have been used until now for EV isolation, including differential ultracentrifugation (dUC), which is still considered the gold standard, as well as different commercial kits, exclusion chromatography, and tangential flow filtration [147]. EV loading is usually subdivided into endogenous or indirect (the manipulation of the parental cell) and exogenous or direct (EV engineering), the latter of which can be further subdivided into passive (co-incubation of molecules and EVs) and active (i.e., electroporation and sonication) [148]. Standardized procedures should be identified to ensure low inter-batch variability and the high reproducibility of the results. The International Society for EVs (ISEV) has been committed to this direction for years, providing the EV scientific community with minimal information for studying EVs and several position papers dedicated to specific aspects of EV research and exploitation [149,150,151,152,153]. Recently, it is becoming increasingly evident how quality management tools, which have already been used in other scientific contexts [154,155,156], can assist in resolving the main issues in EV research and development [157,158]. Applying the Failure Mode and Effect Analysis, for the risk assessment and management of scientific procedures [159] to the individual steps of the EV production pipeline might help in avoiding and preventing pitfalls and failures in EV bioprocessing and manipulation [158,160]. Similarly, the Design of Experiments mathematical model [161,162] might be used to identify and optimize key factors in multivariable processes, such as production, engineering or functional assays of EVs [158,160,163]. Recently, a new tool, called EV decision-making grid (EV-DMG), inspired to multicriteria decision making, has been proposed as a novel, customizable, efficient and easy-to-use model to support responsible EV research and innovation [164]. All together, these instruments and guidelines can greatly improve the selection, optimization, standardization, quality assurance and control, and ultimately the reliability of the EV landscape.
4.3. EV Biodistribution
The biodistribution of EVs was evaluated and found to be concentrated in the spleen, liver, lungs, kidneys, and gastrointestinal tract [165]. TDEVs, instead, have a high tropism towards tumor cells, especially those of the same type. Additionally, the final biodistribution of EVs can be influenced by the source and handling of EVs as well as the “ad hoc” cell surface functionalization [166]. EV size is also an important variable to consider: smaller EVs are easily eliminated via blood circulation, whereas larger EVs may have difficulties in passing through the BBB but are more efficient in delivering their cargo content [39,102]. EVs have been shown to penetrate the BBB and deliver therapeutic agents into the brain, making them suitable drug delivery vectors for intravenous administration. Determining the appropriate quantity of EVs to administer and the frequency of administration requires an evaluation of the amount of EVs that can pass through the barrier and enter to the brain, as well as the amount of EV cargo that can be effectively delivered to the GB cells. However, as GB therapy typically involves surgical tumor resection as a first step, it may also be a suitable strategy to concomitantly inject therapeutic EVs at the tumor site to prevent recurrence.
4.4. Biomimetic Nanoparticles
Recently, an important research direction is exploring the possibility of obtaining biomimetic nanocarriers that combine the advantages of both artificial (e.g., high yield and loading efficiency) and natural (e.g., low immunogenicity, biosafety, and biocompatibility) delivery vectors through the development of technologically advanced ad hoc nanoplatforms. Several methods can be used to create EV-like biomimetic nanoparticles (EBPs), including parental cell loading and consequent extrusion, which is the most common. This also includes the possibility of wrapping organic or inorganic synthesized nanoparticles in a cell membrane, by which the biocompatibility and targeting properties of the parental cells are transferred to the obtained EBPs [167,168,169]. Biomimetic nanovesicles were obtained by subjecting brain endothelial cells to serial extrusion through filters with diminishing pore sizes, after the cells had been loaded with chemotherapeutic agents. The resulting EBPs had a similar drug-loading capacity and pharmacokinetics profiles to EVs naturally produced by the same cells and were able to cross the BBB in orthotopic xenograft mouse models, but they achieved a higher yield than natural EVs [170,171,172]. Recently, a nanoplatform called EV-DNs was developed, in which doxorubicin-loaded heparin nanoparticles (DNs) were attached to the surface of native grapefruit EVs. EV-DNs were capable of penetrating the BBB in vivo, reduce GB cell proliferation and increase animal model survival [108]. Another innovative biomimetic design developed for targeting GB included the co-loading of the antitumoral Cu synthetic chelator di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT) with Regadenoson (Reg), which transiently opens the BBB, and lastly, Angiopeptide-2 functionalized red blood cell membrane (Ang-M) camouflaging. When injected into orthotopic xenografted mouse models, the obtained Ang-M@(Dp44mT/Reg) nanoparticles actively traversed the BBB and delivered Dp44mT specifically to GBM cells with negligible systemic drug toxicity [173].
Finally, EBPs are a promising delivery platform for cancer combination therapy, which has emerged as a relevant trend in cancer and GB treatment. Biomimetic nanovesicles can be derived from GB cells and used for the encapsulation of both photosensitizers (i.e., berberine) and chemotherapeutics (i.e., Temozolomide and Givinostat) to combine different drugs and approaches, thus achieving higher therapeutic effects [174]. Biomimetic theranostic nanovectors composed of iron-modified polydopamine nanoparticles loaded with the anticancer drug doxorubicin (MPDAFe@DOX) and coated with cancer cell membranes (MPDAFe@DOX@Mem) were found to be effective in killing cancer cells through a combination of chemotherapy and PDT while also enabling MRI imaging [175]. A biomimetic system, named RGE-Exo-SPION/Cur, was implemented by loading curcumin (Cur) and superparamagnetic iron oxide nanoparticles (SPIONs) into EVs further functionalized by click chemistry to be carried on the surface of a neuropilin-1-targeted peptide (RGERPPR, RGE). The RGE-Exo-SPION/Cur nanoparticles proved effective in targeting GB cells and reducing GB tumor size in orthotopic GB-bearing nude mouse models, and they were also relevant for simultaneous GB MRI-based diagnosis and therapy [176]. Combination cancer therapy utilizing biomimetic nanoparticles and sonodynamic-enhanced chemotherapy has demonstrated synergistic antitumor efficacy and significantly prolonged the survival rate of mouse models. The biomimetic nanosonosensitizer systems were fabricated with biodegradable and pH-sensitive polyglutamic acid (PGA) and the chemotherapeutic agent and sonosensitizer doxorubicin (DOX), camouflaged with human GB U87 cell membranes. The resulting EBPs in combination with noninvasive ultrasounds were capable of passing through the BBB, induce cancer cell apoptosis, and increase chemosensitivity in orthotopic GBM xenograft mouse models [177].
4.5. Glioblastoma Models
One of the main reasons for the limited success of novel therapeutic molecules entering clinical trials is the poor correlation between their efficacy in in vitro, ex vivo, and in vivo tumor models, and the patient treatment scenarios. Several in vitro and ex vivo models have been developed to study GB, each one with its own strengths and weaknesses, ranging from conventional GB cell lines to emerging technologies, such as bioprinting and microfluidics [7,178]. In 2006, primary early passage cells from fresh GB biopsies were isolated. The maintenance of cells in serum-free supplemented medium preserved the genetic heterogeneity and gene expression profiles of the tumor of origin [179]. GB-patient-derived cell lines (GPDCLs) were cultured in both bi-dimensional (2D) and three-dimensional (3D) cell cultures (spheroids or gliomaspheres) as well as used for mouse xenografts [7,178]. In 2015, a biobank of 48 GPDCLs representing all four transcriptional GB subtypes was created and made freely available [180]. Recently, a reference collection of 12 GPDCLs, the different molecular subtypes, and relative xenograft mouse models was developed for the identification and evaluation of novel GB therapeutics [181]; however, establishing and maintaining these cells in vitro as well as growing them in vivo in mouse models is challenging and limits their utility [182]. Furthermore, 3D structures that include not only GB cells but also non-tumor cells can mimic the GB tumor microenvironment and are definitely better models for therapeutic studies. In this context, organotypic glioma slice cultures derived directly from tumor biopsies have the great advantage of comprising several cell types and structures of the GB tumoral microenvironment. They were used to study GB cell proliferation, migration, invasion, and susceptibility to treatment [7,178,183]. In 2016, the first GB organoids were obtained by adapting the methodology used to develop cerebral organoids or mini-brains from induced pluripotent stem cells to GB cells [184,185]. These organoids were successfully implanted into mouse brains, resulting in models that were more sophisticated and representative of the parental tumors than simple GPDCLs [185]. A biobank of patient-derived glioblastoma organoids, with each one showing parental tumor heterogeneity, was establish and is of high value for identifying personalized target and treatment strategies [186]. However, GB organoids self-assemble, resulting in high variability in cellular composition and structure. Recently more and more sophisticated and high-cost models have been developed based on emerging technologies such as bioprinting and microfluidics. 3D bio-printed GB models exploit novel biomaterials and tissue engineering methodologies to reconstruct 3D models of the tumors on the basis of clinical images. This allows for a high control over both cellular and the extracellular matrix disposition and the high level of homogeneity between the bio-printed models [187,188]. Microfluidic devices take advantages of a central chamber where tumor cells are cultured in a 3D collagen hydrogel perfused with microliters of circulating media whose composition is controlled in terms of nutrients, pH, and growth factors to mimic vasculature and create a more realistic and dynamic GB microenvironment [189,190,191] Microfluidics has also been applied to enrich and characterize specific GB cells and also GDEVs [192,193]. This technology has many advantages, such as simplified on-chip processing, guaranteeing minimal cell samples, high sensitivity, and rapid analysis [178]. Furthermore, microfluidics, tissue engineering, and biomaterials research have been converging to develop more sophisticated 3D cancer-on-a-chip tissue models with the potential to significantly advance the understanding of cancer biology and allowing for accelerated and cost-effective drug discovery [194].
Finally, to assess the ability of therapeutic EVs to efficiently deliver therapeutic molecules across the BBB, without inducing immune reactions or other undesired systemic effects, in vivo studies are absolutely needed. Orthotopic xenotransplanted immunocompetent mouse models have been shown to recapitulate patient GB–microenvironment interactions and are therefore suitable for this purpose [195,196]. Recently, zebrafish xenografts have been proposed as an alternative in vivo model due to several advantages. The transparency in the early stages, which allows easy the visualization of the tumor; the high number and rapid development of offspring; and the small size make them extremely suitable for high-throughput screening [197,198,199].
In conclusion, there is no single optimal model that is suitable for all needs, but researchers have to carefully select the best model(s) for their specific study purpose. Ad hoc grants, collaborations, and access to platforms for testing specific GB models will help in the collection of more and more robust data and strengthen preclinical studies in a short time.
5. Conclusions
GB is a rare but extremely aggressive brain tumor that has a significant impact on patient outcomes, affecting both the duration and quality of life, and no breakthrough therapies have yet been discovered that can prolong patient survival. Functional and preclinical studies based on the isolation and engineering of EVs from different cellular sources, as well as the design and production of more complex and sophisticated EBPs, are definitely a promising direction to explore. Furthermore, EVs and EBPs could efficiently deliver more than one anticancer molecule with different mechanisms of action to GB cells, and other cells within the GB microenvironment, to synergistically enhance their therapeutic effects. The use of EVs and EBPs, in combination with chemotherapy, photodynamic, as well as sonodynamic therapies, might have a desirable higher impact on GB patients’ survival, relapse-free time, and quality of life. In addition, the combination of different ex vivo and in vivo models of GB may contribute to the identification and study of the most promising anti-GB drugs and the production of more robust and reliable preclinical data. This could help to increase the success rate of subsequent clinical trials and advance EV-based anti-GB combination approaches into the GB therapeutic pipeline.
Funding
This work was funded by the European Union—NextGenerationEU—under the CEVITA project within the framework of the AMICO 2 program of CNR—UVR supported by the PoC—PNRR measure of the Ministry of Enterprise and Made in Italy.
Acknowledgments
The author acknowledges Anna Maria Aliperti for English proofreading and Mariarosaria Aletta for bibliographic support.
Conflicts of Interest
The author has filed the patent PCT/IB2023/053735 related to the use of lipidic vesicles for the treatment of aggressive tumors.
References
- Stoyanov, G.S.; Dzhenkov, D.L. On the Concepts and History of Glioblastoma Multiforme—Morphology, Genetics and Epigenetics. Folia Med. 2018, 60, 48–66. [Google Scholar] [CrossRef]
- Chvátal, A.; Verkhratsky, A. An Early History of Neuroglial Research: Personalities. Neuroglia 2018, 1, 245–281. [Google Scholar] [CrossRef]
- Bailey, P.; Cushing, H. A Classification of the Tumors of the Glioma Group on a Histogenetic Basis with a Correlated Study of Prognosis. J. Am. Med. Assoc. 1926, 87, 268. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Sahm, F.; Brandner, S.; Bertero, L.; Capper, D.; French, P.J.; Figarella-branger, D.; Giangaspero, F.; Haberler, C.; Hegi, M.E.; Kristensen, B.W.; et al. Molecular Diagnostic Tools for the World Health Organization (WHO) 2021 Classification of Gliomas, Glioneuronal and Neuronal Tumors; an EANO Guideline. Neuro-Oncology 2023, 25, 1731–1749. [Google Scholar] [CrossRef] [PubMed]
- Boccellato, C.; Rehm, M. Glioblastoma, from Disease Understanding towards Optimal Cell-Based in Vitro Models. Cell. Oncol. 2022, 45, 527–541. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, III1–III105. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.; Barnholtz-Sloan, J.S. Epidemiology of Intracranial Gliomas. Intracranial Gliomas Part I Surg. 2017, 30, 1–11. [Google Scholar]
- Tamimi, A.F.; Juweid, M. Epidemiology and Outcome of Glioblastoma. Glioblastoma 2017, 143–153. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer Risk in 680,000 People Exposed to Computed Tomography Scans in Childhood or Adolescence: Data Linkage Study of 11 Million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef]
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.; Bender, S.; Jones, D.T.W.; Lichter, P.; Grill, J.; Becher, O.; Hawkins, C.; Majewski, J.; Jones, C.; Costello, J.F.; et al. Paediatric and Adult Glioblastoma: Multiform (Epi)Genomic Culprits Emerge. Nat. Rev. Cancer 2014, 14, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Golla, H.; Nettekoven, C.; Bausewein, C.; Tonn, J.C.; Thon, N.; Feddersen, B.; Schnell, O.; Böhlke, C.; Becker, G.; Rolke, R.; et al. Effect of Early Palliative Care for Patients with Glioblastoma (EPCOG): A Randomised Phase III Clinical Trial Protocol. BMJ Open 2020, 10, e034378. [Google Scholar] [CrossRef] [PubMed]
- Golla, H.; Ale Ahmad, M.; Galushko, M.; Hampl, J.; Maarouf, M.; Schroeter, M.; Herrlinger, U.; Hellmich, M.; Voltz, R. Glioblastoma Multiforme from Diagnosis to Death: A Prospective, Hospital-Based, Cohort, Pilot Feasibility Study of Patient Reported Symptoms and Needs. Support. Care Cancer 2014, 22, 3341–3352. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, M.; Osti, D.; Faletti, S.; Beznoussenko, G.V.; Dimeco, F.; Surgery, N.; Hopkins, J.; Medicine, T.; Orientale, P. Extracellular Vesicles: The Key for Precision Medicine in Glioblastoma. Neuro-Oncology 2022, 24, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant te-mozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.A.; Khan, A.; Azmy, S.; Gilbert, O.; Khan, S.; Goliber, R.; Szczecinski, E.J.; Durrani, H.; Burke, S.; Salem, A.A.; et al. Meta-Analysis of Overall Survival and Postoperative Neurologic Deficits after Resection or Biopsy of Butterfly Glioblastoma. Neurosurg. Rev. 2022, 45, 3511–3521. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib Compared with Lomustine in Patients with Relapsed Glioblastoma (REGOMA): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef]
- Badruddoja, M.A.; Pazzi, M.; Sanan, A.; Schroeder, K.; Kuzma, K.; Norton, T.; Scully, T.; Mahadevan, D.; Ahmadi, M.M. Phase II Study of Bi-Weekly Temozolomide plus Bevacizumab for Adult Patients with Recurrent Glioblastoma. Cancer Chemother. Pharmacol. 2017, 80, 715–721. [Google Scholar] [CrossRef]
- Chen, E.; Ling, A.L.; Reardon, D.A.; Chiocca, E.A. Lessons Learned from Phase 3 Trials of Immunotherapy for Glioblastoma: Time for Longitudinal Sampling? Neuro-Oncology 2023, 20, 1–15. [Google Scholar] [CrossRef]
- Kaka, N.; Hafazalla, K.; Samawi, H.; Simpkin, A.; Perry, J.; Sahgal, A.; Das, S. Progression-Free but No Overall Survival Benefit for Adult Patients with Bevacizumab Therapy for the Treatment of Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 1723. [Google Scholar] [CrossRef]
- Schritz, A.; Aouali, N.; Fischer, A.; Dessenne, C.; Adams, R.; Berchem, G.; Huiart, L.; Schmitz, S. Systematic Review and Network Meta-Analysis of the Efficacy of Existing Treatments for Patients with Recurrent Glioblastoma. Neuro-Oncol. Adv. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Gupta, T.; Mona, J.; Selvarajan, P.; Kannan, S.; Menon, N.; Tmh, A.; Memorial, T. Updated Systematic Review and Meta-Analysis of Extended Aduvant Temozolomide in Newly Diagnosed Glioblastoma. Neuro-Oncol. Adv. 2023, 5, vdad086. [Google Scholar] [CrossRef]
- Begagić, E.; Pugonja, R.; Bečulić, H.; Čeliković, A.; Tandir Lihić, L.; Kadić Vukas, S.; Čejvan, L.; Skomorac, R.; Selimović, E.; Jaganjac, B.; et al. Molecular Targeted Therapies in Glioblastoma Multiforme: A Systematic Overview of Global Trends and Findings. Brain Sci. 2023, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, D.; Wilding, H.; Baroz, A.; Trifoi, M.; Shenoy, G.; Slagle-Webb, B.; Hayes, D.; Soudagar, Y.; Connor, J.; Mansouri, A. Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability. Cancers 2023, 15, 3427. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.L.; Dai, X.L.; Yin, M.Y.; Cheng, H.W.; Qian, H.S.; Wang, H.; Zhu, D.M.; Wang, X.W. Nanosensitizers for Sonodynamic Therapy for Glioblastoma Multiforme: Current Progress and Future Perspectives. Mil. Med. Res. 2022, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.M.; Cacaccio, J.; Durrani, F.A.; Bshara, W.; Turowski, S.G.; Spernyak, J.A.; Pandey, R.K. Sonodynamic Therapy in Combination with Photodynamic Therapy Shows Enhanced Long-Term Cure of Brain Tumor. Sci. Rep. 2020, 10, 21791. [Google Scholar] [CrossRef] [PubMed]
- Keenlyside, A.; Marples, T.; Gao, Z.; Hu, H.; Nicely, L.G.; Nogales, J.; Li, H.; Landgraf, L.; Solth, A.; Melzer, A.; et al. Development and Optimisation of in Vitro Sonodynamic Therapy for Glioblastoma. Sci. Rep. 2023, 13, 20215. [Google Scholar] [CrossRef]
- Miretti, M.; González Graglia, M.A.; Suárez, A.I.; Prucca, C.G. Photodynamic Therapy for Glioblastoma: A Light at the End of the Tunnel. J. Photochem. Photobiol. 2023, 13, 100161. [Google Scholar] [CrossRef]
- Juarranz, Á.; Gilaberte, Y.; González, S. Photodynamic Therapy (PDT) in Oncology. Cancers 2020, 12, 3341. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Oncologic Photodynamic Therapy Photosensitizers: A Clinical Review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Carriero, F.; Martinelli, C.; Gabriele, F.; Barbieri, G.; Zanoletti, L.; Milanesi, G.; Casali, C.; Azzalin, A.; Manai, F.; Paolillo, M.; et al. Berberine Photo-activation Potentiates Cytotoxicity in Human Astrocytoma Cells through Apoptosis Induction. J. Pers. Med. 2021, 11, 942. [Google Scholar] [CrossRef]
- Kielbik, A.; Wawryka, P.; Przystupski, D.; Rossowska, J.; Szewczyk, A.; Saczko, J.; Kulbacka, J.; Chwiłkowska, A. Effects of Photosensitization of Curcumin in Human Glioblastoma Multiforme Cells. In Vivo 2019, 33, 1857–1864. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Liu, F.; Gan, S.; Roy, S.; Hasan, I.; Zhang, B.; Guo, B. Emerging Extracellular Vesicle-Based Carriers for Glioblastoma Diagnosis and Therapy. Nanoscale 2023, 15, 10904–10938. [Google Scholar] [CrossRef]
- Luo, H.; Shusta, E.V. Blood–Brain Barrier Modulation to Improve Glioma Drug Delivery. Pharmaceutics 2020, 12, 1085. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of Molecularly Targeted Therapy to Malignant Glioma, a Disease of the Whole Brain. Expert Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef] [PubMed]
- Santarosa, C.; Castellano, A.; Conte, G.M.; Cadioli, M.; Iadanza, A.; Terreni, M.R.; Franzin, A.; Bello, L.; Caulo, M.; Falini, A.; et al. Dynamic Contrast-Enhanced and Dynamic Susceptibility Contrast Perfusion MR Imaging for Glioma Grading: Preliminary Comparison of Vessel Compartment and Permeability Parameters Using Hotspot and Histogram Analysis. Eur. J. Radiol. 2016, 85, 1147–1156. [Google Scholar] [CrossRef]
- Law, M.; Yang, S.; Babb, J.S.; Knopp, E.A.; Golfinos, J.G.; Zagzag, D.; Johnson, G. Comparison of Cerebral Blood Volume and Vascular Permeability from Dynamic Susceptibility Contrast-Enhanced Perfusion MR Imaging with Glioma Grade. Am. J. Neuroradiol. 2004, 25, 746–755. [Google Scholar] [PubMed]
- Onda, K.; Tanaka, R.; Takahashi, H.; Takeda, N.; Ikuta, F. Cerebral Glioblastoma with Cerebrospinal Fluid Dissemination: A Clinicopathological Study of 14 Cases Examined by Complete Autopsy. Neurosurgery 1989, 25, 533–540. [Google Scholar] [CrossRef]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef]
- Yekula, A.; Taylor, A.; Beecroft, A.; Kang, K.M.; Small, J.L.; Muralidharan, K.; Rosh, Z.; Carter, B.S.; Balaj, L. The Role of Extracellular Vesicles in Acquisition of Resistance to Therapy in Glioblastomas. Cancer Drug Resist. 2021, 4, 1–16. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-Cell RNA-Seq Highlights Intratumoral Heterogeneity in Primary Glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Reardon, D.A.; Wen, P.Y. Glioma in 2014: Unravelling Tumour Heterogeneity-Implications for Therapy. Nat. Rev. Clin. Oncol. 2015, 12, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N. Glioblastoma Multiforme and Genetic Mutations: The Issue Is Not over YetAn Overview of the Current Literature. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2020, 81, 64–70. [Google Scholar] [CrossRef] [PubMed]
- McLendon, R.; Friedman, A.; Bigner, D.; Van Meir, E.G.; Brat, D.J.; Mastrogianakis, G.M.; Olson, J.J.; Mikkelsen, T.; Lehman, N.; Aldape, K.; et al. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Lee, E.; Yong, R.L.; Paddison, P.; Zhu, J. Comparison of Glioblastoma (GBM) Molecular Classification Methods. Semin. Cancer Biol. 2018, 53, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.L.; Balasubramaniyan, V.; Vaillant, B.; Ezhilarasan, R.; Hummelink, K.; Hollingsworth, F.; Wani, K.; Heathcock, L.; James, J.D.; Goodman, L.D.; et al. Mesenchymal Differentiation Mediated by NF-ΚB Promotes Radiation Resistance in Glioblastoma. Cancer Cell 2013, 24, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Riester, M.; Cheng, Y.K.; Huse, J.T.; Squatrito, M.; Helmy, K.; Charles, N.; Michor, F.; Holland, E.C. Most Human Non-GCIMP Glioblastoma Subtypes Evolve from a Common Proneural-like Precursor Glioma. Cancer Cell 2014, 26, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.Y.; Kim, W.K.; Lee, J.K.; Park, J.; et al. Human Glioblastoma Arises from Subventricular Zone Cells with Low-Level Driver Mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef]
- Almengló, C.; Caamaño, P.; Fraga, M.; Devesa, J.; Costoya, J.A.; Arce, V.M.; José Costoya, C.A. From Neural Stem Cells to Glioblastoma: A Natural History of GBM Recapitulated in Vitro. J. Cell. Physiol. 2021, 236, 7390–7404. [Google Scholar] [CrossRef]
- Yan, K.; Yang, K.; Rich, J.N. The Evolving Landscape of Glioblastoma Stem Cells. Curr. Opin. Neurol. 2013, 26, 701–707. [Google Scholar] [CrossRef]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef]
- Parada, L.F.; Dirks, P.B.; Wechsler-Reya, R.J. Brain Tumor Stem Cells Remain in Play. J. Clin. Oncol. 2017, 35, 2428–2431. [Google Scholar] [CrossRef]
- Meyer, M.; Reimand, J.; Lan, X.; Head, R.; Zhu, X.; Kushida, M.; Bayani, J.; Pressey, J.C.; Lionel, A.C.; Clarke, I.D.; et al. Single Cell-Derived Clonal Analysis of Human Glioblastoma Links Functional and Genomic Heterogeneity. Proc. Natl. Acad. Sci. USA 2015, 112, 851–856. [Google Scholar] [CrossRef]
- Huang, Q.; Bin Zhang, Q.; Dong, J.; Wu, Y.Y.; Shen, Y.T.; Zhao, Y.D.; Zhu, Y.D.; Diao, Y.; Wang, A.D.; Lan, Q. Glioma Stem Cells Are More Aggressive in Recurrent Tumors with Malignant Progression than in the Primary Tumor, and Both Can Be Maintained Long-Term in Vitro. BMC Cancer 2008, 8, 304. [Google Scholar] [CrossRef]
- Liguori, G.L.; Kralj-Iglič, V. Pathological and Therapeutic Significance of Tumor-Derived Extracellular Vesicles in Cancer Cell Migration and Metastasis. Cancers 2023, 15, 4425. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Cifola, I.; Caratelli, S.; Sconocchia, G.; D’Agnano, I.; Cenciarelli, C. Glioma Extracellular Vesicles for Precision Medicine: Prognostic and Theragnostic Application. Discov. Oncol. 2022, 13, 49. [Google Scholar] [CrossRef]
- Mallick, S.; Benson, R.; Hakim, A.; Rath, G.K. Management of Glioblastoma after Recurrence: A Changing Paradigm. J. Egypt. Natl. Canc. Inst. 2016, 28, 199–210. [Google Scholar] [CrossRef]
- Ryskalin, L.; Biagioni, F.; Lenzi, P.; Frati, A.; Fornai, F. MTOR Modulates Intercellular Signals for Enlargement and Infiltration in Glioblastoma Multiforme. Cancers 2020, 12, 2486. [Google Scholar] [CrossRef]
- Jackson, M.; Hassiotou, F.; Nowak, A. Glioblastoma Stem-like Cells: At the Root of Tumor Recurrence and a Therapeutic Target. Carcinogenesis 2014, 36, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A Restricted Cell Population Propagates Glioblastoma Growth after Chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Fedele, M.; Cerchia, L.; Pegoraro, S.; Sgarra, R.; Manfioletti, G. Molecular Sciences Proneural-Mesenchymal Transition: Phenotypic Plasticity to Acquire Multitherapy Resistance in Glioblastoma. Int. J. Mol. Sci. 2019, 20, 2746. [Google Scholar] [CrossRef] [PubMed]
- Osuka, S.; Sampetrean, O.; Shimizu, T.; Saga, I.; Onishi, N.; Sugihara, E.; Okubo, J.; Fujita, S.; Takano, S.; Matsumura, A.; et al. IGF1 Receptor Signaling Regulates Adaptive Radioprotection in Glioma Stem Cells. Stem Cells 2013, 31, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.A.; Vora, P.; Venugopal, C.; McFarlane, N.; Subapanditha, M.K.; Murty, N.K.; Hassell, J.A.; Hallett, R.M.; Singh, S.K. A Novel Stem Cell Culture Model of Recurrent Glioblastoma. J. Neurooncol. 2016, 126, 57–67. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of Gene Expression and Chemoresistance of CD133+ Cancer Stem Cells in Glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of Immunotherapy Resistance: Lessons from Glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Schmiedel, D.; Mandelboim, O. NKG2D Ligands-Critical Targets for Cancer Immune Escape and Therapy. Front. Immunol. 2018, 9, 2040. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Vashist, S.; Panjiyar, B.K. Navigating the Immune Challenge in Glioblastoma: Exploring Immunotherapeutic Avenues for Overcoming Immune Suppression. Cureus 2023, 15, e46089. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, T.; Therkelsen, K.E.; Ahmad, S.; Nagpal, S. Current State of Immunotherapy for Treatment of Glioblastoma. Curr. Treat. Options Oncol. 2019, 20, 24. [Google Scholar] [CrossRef]
- Pearson, J.R.D.; Cuzzubbo, S.; McArthur, S.; Durrant, L.G.; Adhikaree, J.; Tinsley, C.J.; Pockley, A.G.; McArdle, S.E.B. Immune Escape in Glioblastoma Multiforme and the Adaptation of Immunotherapies for Treatment. Front. Immunol. 2020, 11, 582106. [Google Scholar] [CrossRef]
- Sener, U.; Ruff, M.W.; Campian, J.L. Immunotherapy in Glioblastoma: Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 7046. [Google Scholar] [CrossRef]
- Tankov, S.; Walker, P.R. Glioma-Derived Extracellular Vesicles—Far More Than Local Mediators. Front. Immunol. 2021, 12, 679954. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Kalluri, R. The Biology and Function of Exosomes in Cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Mantile, F.; Franco, P.; Stoppelli, M.P.; Liguori, G.L. Biological Role and Clinical Relevance of Extracellular Vesicles as Key Mediators of Cell Communication in Cancer. In Biological Membrane Vesicles: Scientific, Biotechnological and Clinical Considerations. Advances in Biomembranes and Lipid Self-Assembly; Elsiever: Amsterdam, The Netherlands, 2020; Volume 33. [Google Scholar]
- Kahlert, C.; Kalluri, R. Exosomes in Tumor Microenvironment Influence Cancer Progression and Metastasis. J. Mol. Med. 2013, 91, 431–437. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms Associated with Biogenesis of Exosomes in Cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef]
- Catalano, M.; Serpe, C.; Limatola, C. Microglial Extracellular Vesicles as Modulators of Brain Microenvironment in Glioma. Int. J. Mol. Sci. 2022, 23, 13165. [Google Scholar] [CrossRef] [PubMed]
- Kopper, T.J.; Yu, X.; Graner, M.W. Immunopathology of Extracellular Vesicles in Macrophage and Glioma Cross-Talk. J. Clin. Med. 2023, 12, 3430. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Kim, H.; McGee, L.; Johnson, A.E.; Talwar, S.; Marugan, J.; Southall, N.; Hu, X.; Lal, M.; Mondal, D.; et al. High-Throughput Screening Identified Selective Inhibitors of Exosome Biogenesis and Secretion: A Drug Repurposing Strategy for Advanced Cancer. Sci. Rep. 2018, 8, 8161. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Song, X.; Xie, R.; Ouyang, C.; Xie, L.; Li, Q.; Su, T.; Xu, M.; Xu, T.; Huang, D.; et al. Berberine Inhibits Cancer Cells Growth by Suppressing Fatty Acid Synthesis and Biogenesis of Extracellular Vesicles. Life Sci. 2020, 257, 118122. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E. Extracellular Vesicles in Cancer Therapy. Semin. Cancer Biol. 2022, 86, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Svensson, K.J.; Van Kuppevelt, T.H.; Li, J.P.; Belting, M. Cancer Cell Exosomes Depend on Cell-Surface Heparan Sulfate Proteoglycans for Their Internalization and Functional Activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef] [PubMed]
- Atai, N.A.; Balaj, L.; Van Veen, H.; Breakefield, X.O.; Jarzyna, P.A.; Van Noorden, C.J.F.; Skog, J.; Maguire, C.A. Heparin Blocks Transfer of Extracellular Vesicles between Donor and Recipient Cells. J. Neurooncol. 2013, 115, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.W.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-Associated AAV Vector as a Novel Gene Delivery System. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Multhaupt, H.; Sanderson, R.D. Recent Insights into Cell Surface Heparan Sulphate Proteoglycans and Cancer. F1000Research 2016, 5, 1541. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Pillai, P.P. Current Insights on Extracellular Vesicle-Mediated Glioblastoma Progression: Implications in Drug Resistance and Epithelial-Mesenchymal Transition. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130065. [Google Scholar] [CrossRef]
- Lang, H.L.; Hu, G.W.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.M.; Wu, L.; Xu, G.H. Glioma Cells Promote Angiogenesis through the Release of Exosomes Containing Long Non-Coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 959–972. [Google Scholar]
- Li, Z.; Meng, X.; Wu, P.; Zha, C.; Han, B.; Li, L.; Sun, N.; Qi, T.; Qin, J.; Zhang, Y.; et al. Glioblastoma Cell-Derived LncRNA-Containing Exosomes Induce Microglia to Produce Complement C5, Promoting Chemotherapy Resistance. Cancer Immunol. Res. 2021, 9, 1383–1399. [Google Scholar] [CrossRef]
- Simionescu, N.; Zonda, R.; Petrovici, A.R.; Georgescu, A. The Multifaceted Role of Extracellular Vesicles in Glioblastoma: Microrna Nanocarriers for Disease Progression and Gene Therapy. Pharmaceutics 2021, 13, 988. [Google Scholar] [CrossRef]
- Geng, L.; Xu, J.; Zhu, Y.; Hu, X.; Liu, Y.; Yang, K.; Xiao, H.; Zou, Y.; Liu, H.; Ji, J.; et al. Targeting MiR-9 in Glioma Stem Cell-Derived Extracellular Vesicles: A Novel Diagnostic and Therapeutic Biomarker. Transl. Oncol. 2022, 22, 101451. [Google Scholar] [CrossRef]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-MiR-9 by Mesenchymal Stem Cell-Derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef]
- Shahjin, F.; Chand, S.; Yelamanchili, S.V. Extracellular Vesicles as Drug Delivery Vehicles to the Central Nervous System. J. Neuroimmune Pharmacol. 2020, 15, 443–458. [Google Scholar] [CrossRef]
- Macedo-Pereira, A.; Martins, C.; Lima, J.; Sarmento, B. Digging the Intercellular Crosstalk via Extracellular Vesicles: May Exosomes Be the Drug Delivery Solution for Target Glioblastoma? J. Control. Release 2023, 358, 98–115. [Google Scholar] [CrossRef]
- Benecke, L.; Coray, M.; Umbricht, S.; Chiang, D.; Figueiró, F.; Muller, L. Exosomes: Small Evs with Large Immunomodulatory Effect in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 3600. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, E.Y.; Chiou, T.W.; Harn, H.J. Exosomes in Clinical Trial and Their Production in Compliance with Good Manufacturing Practice. Tzu Chi Med. J. 2020, 32, 113–120. [Google Scholar]
- Picciotto, S.; Santonicola, P.; Paterna, A.; Rao, E.; Raccosta, S.; Romancino, D.P.; Noto, R.; Touzet, N.; Manno, M.; Di Schiavi, E.; et al. Extracellular Vesicles From Microalgae: Uptake Studies in Human Cells and Caenorhabditis Elegans. Front. Bioeng. Biotechnol. 2022, 10, 830189. [Google Scholar] [CrossRef]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Giancaterino, S.; Boi, C. Alternative Biological Sources for Extracellular Vesicles Production and Purification Strategies for Process Scale-Up. Biotechnol. Adv. 2023, 63, 108092. [Google Scholar] [CrossRef] [PubMed]
- Adamo, G.; Fierli, D.; Romancino, D.P.; Picciotto, S.; Barone, M.E.; Aranyos, A.; Božič, D.; Morsbach, S.; Raccosta, S.; Stanly, C.; et al. Nanoalgosomes: Introducing Extracellular Vesicles Produced by Microalgae. J. Extracell. Vesicles 2021, 10, e12081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered Extracellular Vesicles for Cancer Therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic Exosomal SiRNA Delivery Reduced Alpha-Synuclein Aggregates in Brains of Transgenic Mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic Delivery of MicroRNA-21 Antisense Oligonucleotides to the Brain Using T7-Peptide Decorated Exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-Inflammatory Drugs from the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Xunian, Z.; Kalluri, R. Biology and Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes. Cancer Sci. 2020, 111, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Biancone, L.; Bruno, S.; Deregibus, M.C.; Tetta, C.; Camussi, G. Therapeutic Potential of Mesenchymal Stem Cell-Derived Microvesicles. Nephrol. Dial. Transplant. 2012, 27, 3037–3042. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; von der Ohe, J.; Hass, R. Msc-Derived Extracellular Vesicles in Tumors and Therapy. Cancers 2021, 13, 5212. [Google Scholar] [CrossRef] [PubMed]
- Pavon, L.F.; Sibov, T.T.; De Souza, A.V.; Da Cruz, E.F.; Malheiros, S.M.F.; Cabral, F.R.; De Souza, J.G.; Boufleur, P.; De Oliveira, D.M.; De Toledo, S.R.C.; et al. Tropism of Mesenchymal Stem Cell toward CD133+ Stem Cell of Glioblastoma in Vitro and Promote Tumor Proliferation in Vivo. Stem Cell Res. Ther. 2018, 9, 310. [Google Scholar] [CrossRef]
- Lang, F.M.; Hossain, A.; Gumin, J.; Momin, E.N.; Shimizu, Y.; Ledbetter, D.; Shahar, T.; Yamashita, S.; Parker Kerrigan, B.; Fueyo, J.; et al. Mesenchymal Stem Cells as Natural Biofactories for Exosomes Carrying MiR-124a in the Treatment of Gliomas. Neuro. Oncol. 2018, 20, 380–390. [Google Scholar] [CrossRef]
- Fareh, M.; Almairac, F.; Turchi, L.; Burel-Vandenbos, F.; Paquis, P.; Fontaine, D.; Lacas-Gervais, S.; Junier, M.P.; Chneiweiss, H.; Virolle, T. Cell-Based Therapy Using MiR-302-367 Expressing Cells Represses Glioblastoma Growth. Cell Death Dis. 2017, 8, e2713. [Google Scholar] [CrossRef]
- Bhaskaran, V.; Nowicki, M.O.; Idriss, M.; Jimenez, M.A.; Lugli, G.; Hayes, J.L.; Mahmoud, A.B.; Zane, R.E.; Passaro, C.; Ligon, K.L.; et al. The Functional Synergism of MicroRNA Clustering Provides Therapeutically Relevant Epigenetic Interference in Glioblastoma. Nat. Commun. 2019, 10, 442. [Google Scholar] [CrossRef]
- Zeng, A.; Wei, Z.; Yan, W.; Yin, J.; Huang, X.; Zhou, X.; Li, R.; Shen, F.; Wu, W.; Wang, X.; et al. Exosomal Transfer of MiR-151a Enhances Chemosensitivity to Temozolomide in Drug-Resistant Glioblastoma. Cancer Lett. 2018, 436, 10–21. [Google Scholar] [CrossRef]
- Wang, H.; Feng, J.; Ao, F.; Tang, Y.; Xu, P.; Wang, M.; Huang, M. Tumor-Derived Exosomal MicroRNA-7-5p Enhanced by Verbascoside Inhibits Biological Behaviors of Glioblastoma in Vitro and in Vivo. Mol. Ther. Oncolytics 2021, 20, 569–582. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, G.; Xia, Y.; Li, H.Y.; Yuan, J.; Zhang, J.; Chen, Y.; Guo, H.; Yang, Y.; Wang, Y.; et al. Eliminating the Original Cargos of Glioblastoma Cell-Derived Small Extracellular Vesicles for Efficient Drug Delivery to Glioblastoma with Improved Biosafety. Bioact. Mater. 2022, 16, 204–217. [Google Scholar] [CrossRef]
- Geng, T.; Leung, E.; Chamley, L.W.; Wu, Z. Functionalisation of Extracellular Vesicles with Cyclic-RGDyC Potentially for Glioblastoma Targeted Intracellular Drug Delivery. Biomater. Adv. 2023, 149, 213388. [Google Scholar] [CrossRef]
- Andrews, P.W. Retinoic Acid Induces Neuronal Differentiation of a Cloned Human Embryonal Carcinoma Cell Line in Vitro. Dev. Biol. 1984, 103, 285–293. [Google Scholar] [CrossRef]
- Langlois, A.; Duval, D. Differentiation of the Human NT2 Cells into Neurons and Glia. Methods Cell Sci. 1997, 219, 213–219. [Google Scholar] [CrossRef]
- Tyler, M.A.; Ulasov, I.V.; Sonabend, A.M.; Nandi, S.; Han, Y.; Marler, S.; Roth, J.; Lesniak, M.S. Neural Stem Cells Target Intracranial Glioma to Deliver an Oncolytic Adenovirus in Vivo. Gene Ther. 2009, 16, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Mashal, M.; Grijalvo, S.; Eritja, R.; Puras, G.; Pedraz, J.L. Cationic Niosome-Based HBMP7 Gene Transfection of Neuronal Precursor NT2 Cells to Reduce the Migration of Glioma Cells in Vitro. J. Drug Deliv. Sci. Technol. 2019, 53, 101219. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S. Human NT2 Neural Precursor-Derived Tumor-Infiltrating Cells as Delivery Vehicles for Treatment of Glioblastoma. Hum. Gene Ther. 2010, 21, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Adamus, T.; Hung, C.-Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K.; et al. Glioma-Targeted Delivery of Exosome-Encapsulated Antisense Oligonucleotides Using Neural Stem Cells. Mol. Ther. Nucleic Acids 2022, 27, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Persico, M.G.; Liguori, G.L.; Parisi, S.; D’Andrea, D.; Salomon, D.S.; Minchiotti, G. Cripto in Tumors and Embryo Development. Biochim. Biophys. Acta Rev. Cancer 2001, 1552, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Minchiotti, G.; Parisi, S.; Liguori, G.L.; D’Andrea, D.; Persico, M.G. Role of the EGF-CFC Gene Cripto in Cell Differentiation and Embryo Development. Gene 2002, 287, 33–37. [Google Scholar] [CrossRef]
- de Castro, N.P.; Rangel, M.C.; Nagaoka, T.; Salomon, D.S.; Bianco, C. Cripto-1: An Embryonic Gene That Promoted Tumorigeneis. Future Oncol. 2010, 6, 1127–1142. [Google Scholar] [CrossRef]
- Mantile, F.; Kisovec, M.; Adamo, G.; Romancino, D.P.; Hočevar, M.; Božič, D.; Bedina Zavec, A.; Podobnik, M.; Stoppelli, M.P.; Kisslinger, A.; et al. A Novel Localization in Human Large Extracellular Vesicles for the EGF-CFC Founder Member CRIPTO and Its Biological and Therapeutic Implications. Cancers 2022, 14, 3700. [Google Scholar] [CrossRef]
- Minchiotti, G.; Parisi, S.; Liguori, G.; Signore, M.; Lania, G.; Adamson, E.D.; Lago, C.T.; Persico, M.G. Membrane-Anchorage of Cripto Protein by Glycosylphosphatidylinositol and Its Distribution during Early Mouse Development. Mech. Dev. 2000, 90, 133–142. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Zhang, M.; Li, T.; Zheng, X.; Guo, Q.; Zhang, X. Exosomal Cripto-1 Serves as a Potential Biomarker for Perihilar Cholangiocarcinoma. Front. Oncol. 2021, 11, 730615. [Google Scholar] [CrossRef]
- Parisi, S.; D’Andrea, D.; Lago, C.T.; Adamson, E.D.; Persico, M.G.; Minchiotti, G. Nodal-Dependent Cripto Signaling Promotes Cardiomyogenesis and Redirects the Neural Fate of Embryonic Stem Cells. J. Cell Biol. 2003, 163, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.L.; Echevarría, D.; Improta, R.; Signore, M.; Adamson, E.; Martínez, S.; Persico, M.G. Anterior Neural Plate Regionalization in Cripto Null Mutant Mouse Embryos in the Absence of Node and Primitive Streak. Dev. Biol. 2003, 264, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.L.; Echevarria, D.; Bonilla, S.; D’Andrea, D.; Liguoro, A.; Persico, M.G.; Martinez, S. Characterization of the Functional Properties of the Neuroectoderm in Mo Use Cripto−/− Embryos Showing Severe Gastrulation Defects. Int. J. Dev. Biol. 2009, 53, 549–557. [Google Scholar] [CrossRef]
- Pilgaard, L.; Mortensen, J.H.; Henriksen, M.; Olesen, P.; Sørensen, P.; Laursen, R.; Vyberg, M.; Agger, R.; Zachar, V.; Moos, T.; et al. Cripto-1 Expression in Glioblastoma Multiforme. Brain Pathol. 2014, 24, 360–370. [Google Scholar] [CrossRef]
- Tysnes, B.B.; Sætran, H.A.; Mørk, S.J.; Margaryan, N.V.; Eide, G.E.; Petersen, K.; Strizzi, L.; Hendrix, M.J. Age-Dependent Association between Protein Expression of the Embryonic Stem Cell Marker Cripto-1 and Survival of Glioblastoma Patients 1,2. Transl. Oncol. 2013, 6, 732. [Google Scholar] [CrossRef]
- Bianco, C.; Rangel, M.C.; Castro, N.P.; Nagaoka, T.; Rollman, K.; Gonzales, M.; Salomon, D.S. Role of Cripto-1 in Stem Cell Maintenance and Malignant Progression. Am. J. Pathol. 2010, 177, 532–540. [Google Scholar] [CrossRef]
- Giorgio, E.; Liguoro, A.; D’Orsi, L.; Mancinelli, S.; Barbieri, A.; Palma, G.; Arra, C.; Liguori, G.L. Cripto Haploinsufficiency Affects in Vivo Colon Tumor Development. Int. J. Oncol. 2014, 45, 31–40. [Google Scholar] [CrossRef]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stemcells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular Vesicles in Cancer Nanomedicine. Semin. Cancer Biol. 2019, 69, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A Framework for Standardized Reporting of Extracellular Vesicle Flow Cytometry Experiments; MIFlowCyt-EV: A Framework for Standardized Reporting of Extracellular Vesicle Flow Cytometry Experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzás, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V.; et al. Updating the MISEV Minimal Requirements for Extracellular Vesicle Studies: Building Bridges to Reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Boilard, E.; Buzas, E.I.; Cheng, L.; Falcón-Perez, J.M.; Gardiner, C.; Gustafson, D.; Gualerzi, A.; Hendrix, A.; Hoffman, A.; et al. Considerations towards a Roadmap for Collection, Handling and Storage of Blood Extracellular Vesicles. J. Extracell. Vesicles 2019, 8, 1647027. [Google Scholar] [CrossRef]
- Nieuwland, R.; Siljander, P.R.M.; Falcón-Pérez, J.M.; Witwer, K.W. Reproducibility of Extracellular Vesicle Research. Eur. J. Cell Biol. 2022, 101, 151226. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.; Colotti, G.; Liguori, G.L.; Di Carlo, M.; Digilio, F.A.; Lacerra, G.; Mascia, A.; Cirafici, A.M.; Barra, A.; Lanati, A.; et al. Applying Quality and Project Management Methodologies in Biomedical Research Laboratories: A Public Research Network’s Case Study. Accredit. Qual. Assur. 2015, 20, 203–213. [Google Scholar] [CrossRef]
- Hollmann, S.; Regierera, B.; D’Elia, D.; Kisslinger, A.; Liguori, G.L. Toward the Definition of Common Strategies for Improving Reproducibility, Standardization, Management, and Overall Impact of Academic Research. Adv. Biomembr. Lipid Self-Assem. 2022, 35, 2–24. [Google Scholar]
- Digilio, F.A.; Lanati, A.; Bongiovanni, A.; Mascia, A.; Di Carlo, M.; Barra, A.; Cirafici, A.M.; Colotti, G.; Kisslinger, A.; Lacerra, G.; et al. Quality-Based Model for Life Sciences Research Guidelines. Accredit. Qual. Assur. 2016, 21, 221–230. [Google Scholar] [CrossRef]
- Liguori, G.L.; Kisslinger, A. Standardization and Reproducibility in EV Research: The Support of a Quality Management System. In Biological Membrane Vesicles: Scientific, Biotechnological and Clinical Considerations. Advances in Biomembranes and Lipid Self-Assembly; Elsiever: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Liguori, G.L.; Kisslinger, A. Quality Management Tools on the Stage: Old but New Allies for Rigor and Standardization of Extracellular Vesicle Studies. Front. Bioeng. Biotechnol. 2022, 10, 826252. [Google Scholar] [CrossRef] [PubMed]
- Mascia, A.; Cirafici, A.M.; Bongiovanni, A.; Colotti, G.; Lacerra, G.; Di Carlo, M.; Digilio, F.A.; Liguori, G.L.; Lanati, A.; Kisslinger, A. A Failure Mode and Effect Analysis (FMEA)-Based Approach for Risk Assessment of Scientific Processes in Non-Regulated Research Laboratories. Accredit. Qual. Assur. 2020, 25, 311–322. [Google Scholar] [CrossRef]
- Herwig, C.; Pörtner, R.; Möller, J. Digital Twins Tools and Concepts for Smart Biomanufacturing; Springer International Publishing: Cham, Switzerland, 2022; ISBN 9783030716592. [Google Scholar]
- Mancinelli, S.; Zazzu, V.; Turcato, A.; Lacerra, G.; Digilio, F.A.; Mascia, A.; Di Carlo, M.; Cirafici, A.M.; Bongiovanni, A.; Colotti, G.; et al. Applying Design of Experiments Methodology to PEI Toxicity Assay on Neural Progenitor Cells. In Mathematical Models in Biology: Bringing Mathematics to Life; Zazzu, V., Ferraro, M.B., Guarracino, M.R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–63. ISBN 978-3-319-23497-7. [Google Scholar]
- Mancinelli, S.; Turcato, A.; Kisslinger, A.; Bongiovanni, A.; Zazzu, V.; Lanati, A.; Liguori, G.L. Design of Transfections: Implementation of Design of Experiments for Cell Transfection Fine Tuning. Biotechnol. Bioeng. 2021, 118, 4488–4502. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Faruqu, F.N.; Liam-or, R.; Abu Abed, O.; Li, D.; Venner, K.; Errington, R.J.; Summers, H.; Wang, J.T.-W.; Al-Jamal, K.T. Design of Experiment (DoE)-Driven in Vitro and in Vivo Uptake Studies of Exosomes for Pancreatic Cancer Delivery Enabled by Copper-Free Click Chemistry-Based Labelling. J. Extracell. Vesicles 2020, 9, 1779458. [Google Scholar] [CrossRef]
- Loria, F.; Picciotto, S.; Adamo, G.; Zendrini, A.; Raccosta, S.; Manno, M. A Decision-Making Tool to Navigate through Extracellular Vesicle Research and Product Development. bioRxiv 2023, 2023, 11. [Google Scholar]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.A.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Busatto, S.; Huang, J.; Morad, G.; Moses, M.A. A Facile Magnetic Extrusion Method for Preparing Endosome-Derived Vesicles for Cancer Drug Delivery. Adv. Funct. Mater. 2021, 31, 2008326. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.K.; Winnik, F.M. Strategies in Biomimetic Surface Engineering of Nanoparticles for Biomedical Applications. Nanoscale 2012, 4, 360–368. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, C.; Xiao, K. Engineered Extracellular Vesicles-like Biomimetic Nanoparticles as an Emerging Platform for Targeted Cancer Therapy. J. Nanobiotechnol. 2023, 21, 287. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Goh, W.J.; Zou, S.; Ong, W.Y.; Torta, F.; Alexandra, A.F.; Schiffelers, R.M.; Storm, G.; Wang, J.W.; Czarny, B.; Pastorin, G. Bioinspired Cell-Derived Nanovesicles versus Exosomes as Drug Delivery Systems: A Cost-Effective Alternative. Sci. Rep. 2017, 7, 14322. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Bin Hu, X.; Huang, S.; Luo, S.; Tang, T.; Xiang, D.X. Exosomes and Biomimetic Nanovesicles-Mediated Anti-Glioblastoma Therapy: A Head-to-Head Comparison. J. Control. Release 2021, 336, 510–521. [Google Scholar] [CrossRef]
- Ismail, M.; Yang, W.; Li, Y.; Wang, Y.; He, W.; Wang, J.; Muhammad, P.; Chaston, T.B.; Rehman, F.U.; Zheng, M.; et al. Biomimetic Dp44mT-Nanoparticles Selectively Induce Apoptosis in Cu-Loaded Glioblastoma Resulting in Potent Growth Inhibition. Biomaterials 2022, 289, 121760. [Google Scholar] [CrossRef]
- Martinelli, C.; Gabriele, F.; Dini, E.; Carriero, F.; Bresciani, G.; Slivinschi, B.; Dei Giudici, M.; Zanoletti, L.; Manai, F.; Paolillo, M.; et al. Development of Artificial Plasma Membranes Derived Nanovesicles Suitable for Drugs Encapsulation. Cells 2020, 9, 1626. [Google Scholar] [CrossRef]
- Bigaj-Józefowska, M.J.; Coy, E.; Załęski, K.; Zalewski, T.; Grabowska, M.; Jaskot, K.; Perrigue, P.; Mrówczyński, R.; Grześkowiak, B.F. Biomimetic Theranostic Nanoparticles for Effective Anticancer Therapy and MRI Imaging. J. Photochem. Photobiol. B Biol. 2023, 249, 112813. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 Targeted and Cargo-Loaded Exosomes Facilitate Simultaneous Imaging and Therapy of Glioma in Vitro and in Vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, S.; Fang, Q.; He, H.; Ren, J.; Sun, D.; Lai, J.; Ma, A.; Chen, Z.; Liu, L.; et al. Biomimetic Nanosonosensitizers Combined with Noninvasive Ultrasound Actuation to Reverse Drug Resistance and Sonodynamic-Enhanced Chemotherapy against Orthotopic Glioblastoma. ACS Nano 2023, 17, 421–436. [Google Scholar] [CrossRef]
- Liu, P.; Griffiths, S.; Veljanoski, D.; Vaughn-Beaucaire, P.; Speirs, V.; Brüning-Richardson, A. Preclinical Models of Glioblastoma: Limitations of Current Models and the Promise of New Developments. Expert Rev. Mol. Med. 2021, 23, e20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor Stem Cells Derived from Glioblastomas Cultured in BFGF and EGF More Closely Mirror the Phenotype and Genotype of Primary Tumors than Do Serum-Cultured Cell Lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bergström, T.; Jiang, Y.; Johansson, P.; Marinescu, V.D.; Lindberg, N.; Segerman, A.; Wicher, G.; Niklasson, M.; Baskaran, S.; et al. The Human Glioblastoma Cell Culture Resource: Validated Cell Models Representing All Molecular Subtypes. EBioMedicine 2015, 2, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Stringer, B.W.; Day, B.W.; D’Souza, R.C.J.; Jamieson, P.R.; Ensbey, K.S.; Bruce, Z.C.; Lim, Y.C.; Goasdoué, K.; Offenhäuser, C.; Akgül, S.; et al. A Reference Collection of Patient-Derived Cell Line and Xenograft Models of Proneural, Classical and Mesenchymal Glioblastoma. Sci. Rep. 2019, 9, 4902. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Mayrhofer, M.; Kultima, H.G.; Bergström, T.; Elfineh, L.; Cavelier, L.; Isaksson, A.; Nelander, S. Primary Glioblastoma Cells for Precision Medicine: A Quantitative Portrait of Genomic (in)Stability during the First 30 Passages. Neuro-Oncology 2018, 20, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Merz, F.; Gaunitz, F.; Dehghani, F.; Renner, C.; Meixensberger, J.; Gutenberg, A.; Giese, A.; Schopow, K.; Hellwig, C.; Schäfer, M.; et al. Organotypic Slice Cultures of Human Glioblastoma Reveal Different Susceptibilities to Treatments. Neuro-Oncology 2013, 15, 670–681. [Google Scholar] [CrossRef]
- Lancaster, A.M.; Juergen, A. Knoblich1 Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found in Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-Tumoral Heterogeneity. Cell 2020, 180, 188–204.e22. [Google Scholar] [CrossRef]
- Hermida, M.A.; Kumar, J.D.; Schwarz, D.; Laverty, K.G.; Di Bartolo, A.; Ardron, M.; Bogomolnijs, M.; Clavreul, A.; Brennan, P.M.; Wiegand, U.K.; et al. Three Dimensional in Vitro Models of Cancer: Bioprinting Multilineage Glioblastoma Models. Adv. Biol. Regul. 2020, 75, 100658. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.A.; Bansal, R.; Lammers, T.; Zhang, Y.S.; Michel Schiffelers, R.; Prakash, J. 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv. Mater. 2019, 31, e1806590. [Google Scholar] [CrossRef]
- Li, Q.; Lin, H.; Rauch, J.; Deleyrolle, L.P.; Reynolds, B.A.; Viljoen, H.J.; Zhang, C.; Gu, L.; Van Wyk, E.; Lei, Y. Scalable Culturing of Primary Human Glioblastoma Tumor-Initiating Cells with a Cell-Friendly Culture System. Sci. Rep. 2018, 8, 3531. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Monge, R.; Martínez-González, A.; Virumbrales-Muñoz, M.; Llamazares, G.A.; Berganzo, J.; Hernández-Laín, A.; Santolaria, J.; Doblaré, M.; Hubert, C.; et al. Glioblastoma on a Microfluidic Chip: Generating Pseudopalisades and Enhancing Aggressiveness through Blood Vessel Obstruction Events. Neuro-Oncology 2017, 19, 503–513. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Virumbrales-munoz, M.; Mcminn, P.H.; Rehman, S.; Gomez, I.; Karim, M.R.; Trusttchel, R.; Wisinski, K.B.; Beebe, J.; Skala, M.C. Tumor-on-a-Chip: A Microfluidic Model to Study Cell Response to Environmental Gradients. Lab Chip 2020, 19, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.P.; Nahed, B.V.; Madden, M.W.; Oliveira, S.M.; Springer, S.; Bhere, D.; Chi, A.S.; Wakimoto, H.; Michael Rothenberg, S.; Sequist, L.V.; et al. Brain Tumor Cells in Circulation Are Enriched for Mesenchymal Gene Expression. Cancer Discov. 2014, 4, 1299–1309. [Google Scholar] [CrossRef]
- Reátegui, E.; Van Der Vos, K.E.; Lai, C.P.; Zeinali, M.; Atai, N.A.; Aldikacti, B.; Floyd, F.P.; Khankhel, A.; Thapar, V.; Hochberg, F.H.; et al. Engineered Nanointerfaces for Microfluidic Isolation and Molecular Profiling of Tumor-Specific Extracellular Vesicles. Nat. Commun. 2018, 9, 175. [Google Scholar] [CrossRef]
- Seaman, K.; Sun, Y.; You, L. Recent Advances in Cancer-on-a-Chip Tissue Models to Dissect the Tumour Microenvironment. Med-X 2023, 1, 11. [Google Scholar] [CrossRef]
- Garcia, C.; Dubois, G.G.; Xavier, L.L.; Geraldo, H.H.; da Fonseca, C.C.C.; Correia, H.H.; Meirelles, F.; Ventura, G.; Romão, L.; Canedo, S.H.S.; et al. The Orthotopic Xenotransplant of Human Glioblastoma Successfully Recapitulates Glioblastoma-Microenvironment Interactions in a Non-Immunosuppressed Mouse Model. BMC Cancer 2014, 14, 923. [Google Scholar] [CrossRef]
- Valdor, R.; García-Bernal, D.; Bueno, C.; Ródenas, M.; Moraleda, J.M.; Macian, F.; Martínez, S. Glioblastoma Progression Is Assisted by Induction of Immunosuppressive Function of Pericytes through Interaction with Tumor Cells. Oncotarget 2017, 8, 68614–68626. [Google Scholar] [CrossRef]
- Vargas-Patron, L.A.; Agudelo-Dueñãs, N.; Madrid-Wolff, J.; Venegas, J.A.; González, J.M.; Forero-Shelton, M.; Akle, V. Xenotransplantation of Human Glioblastoma in Zebrafish Larvae: In Vivo Imaging and Proliferation Assessment. Biol. Open 2019, 8, bio043257. [Google Scholar] [CrossRef]
- Zeng, A.; Ye, T.; Cao, D.; Huang, X.; Yang, Y.; Chen, X.; Xie, Y.; Yao, S.; Zhao, C. Identify a Blood-Brain Barrier Penetrating Drug-TNB Using Zebrafish Orthotopic Glioblastoma Xenograft Model. Sci. Rep. 2017, 7, 14372. [Google Scholar] [CrossRef]
- Pudelko, L.; Edwards, S.; Balan, M.; Nyqvist, D.; Al-Saadi, J.; Dittmer, J.; Almlöf, I.; Helleday, T.; Bräutigam, L. An Orthotopic Glioblastoma Animal Model Suitable for High-Throughput Screenings. Neuro-Oncology 2018, 20, 1475–1484. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).