The Role of Immune Cells Driving Electropathology and Atrial Fibrillation
Abstract
1. Introduction
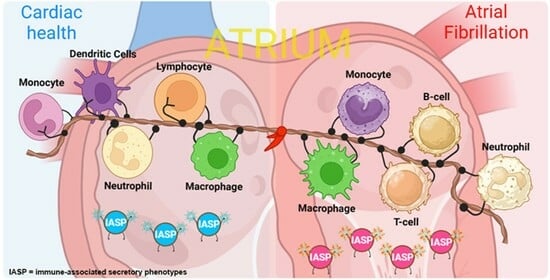
2. Composition and Distribution of Immune Cells in a Healthy Heart
3. Composition and Distribution of Immune Cells during AF and Cardiac Disease

4. What Is the Function of Various Immune Cells in Cardiac Function and AF?
4.1. Macrophages and Monocytes
4.2. Lymphocytes: T Cells, B Cells, and Natural Killer Cells
4.3. Neutrophils
4.4. Other Cells
5. Immune Markers as Potential Target for AF Diagnostics and Therapy
| Immune Cell | Study | IASPs | Sample Source | Comparison of IASP Concentration |
|---|---|---|---|---|
| Monocyte/macrophage | Yamashita et al. [41] | ICAM-1, VCAM-1, MCP-1, IL-6, TGF-β | LAA | SR with a history of PAF (n = 5) < PeAF (n = 11) |
| Hulsmanset al. [33] | SPP1, CCR2 | LAA | SR (n = 41) < PeAF with or without mitral regurgitation (n = 82) | |
| Zhang et al. [116] | CXCL-1, CXCR2 | Blood | SR (n = 31) < New-onset AF with resistant hypertension (n = 31) | |
| Wan et al. [117] | MIF | Blood | Control group (n = 103) < AF patients; PAF (n = 66) < PeAF (n = 68) < permanent AF (n = 52) | |
| Wang et al. [118] | Galectin-3 | Blood | PAF (n = 162) < PeAF (n = 51) | |
| Li et al. [119] | CXCL12 | Blood | Permanent AF (n = 68) > PAF (n = 74); PeAF (n = 128) | |
| B cell | Matsumori et al. [120] | Immunoglobulin-free light chains, kappa and lambda | Blood | SR (n = 28) < lone AF (n = 28); |
| Healthy volunteer (n = 28) > HF (n = 16) | ||||
| Kappa: controls (n = 75) < myocarditis (n = 111); lambda: controls (n = 75) > myocarditis (n = 111) | ||||
| T cell | Wu et al. [121] | Th17-related cytokines | Blood | Control group (n = 336) < AF (n = 336) |
| Neutrophil | Meulendijks et al. [95] | MPO, NET | LA | No AF (n = 20) < PeAF (n = 14) |
| Rudolph et al. [122] | MPO, elastase | RAA, Blood | No AF (n = 17) < PeAF (n = 10) | |
| He et al. [96] | NET | LAA, Blood | SR (n = 4) < AF (n = 4) | |
| Holzwirth et al. [123] | MPO | Blood | No AF (n = 37) < PeAF (n = 117) | |
| Uncategorized immune cells | Begieneman et al. [46] | CML, VCAM-1 | LAA | Control group (n = 9) < AF (n = 33) |
| Rahmutula et al. [124] | Total TGF-β1 and active TGF-β1 | RA | No AF (n = 11) < AF (n = 2); no AF (n = 11) < post-operative AF (n = 4) | |
| Zhao et al. [125] | β1-AR, pErk1/2, p38MAPK, p NF-κB | PV-MS | No AF (n = 12) < AF (n = 12) | |
| Kato et al. [126] | MMP2 | Blood | Chronic AF (n = 196) > control (n = 873) | |
| IL-10 | Chronic AF (n = 196) < control (n = 873) | |||
| Yalcin et al. [127] | Anti-M2-R, anti-β1-R | Blood | Healthy control (n = 75) < lone AF (n = 75) | |
| Serban et al. [128] | IL-1β, IL-6, VEGF | Blood | Significantly positively correlated with the duration of atrial depolarization | |
| Liu et al. [129] | IL-6 | Blood | In total population, >50% of AF patients (101/180) have blood IL-6 above median level |
6. Clinical and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Brundel, B.J.J.M.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef]
- Di Carlo, A.; Bellino, L.; Consoli, D.; Mori, F.; Zaninelli, A.; Baldereschi, M.; Cattarinussi, A.; D’Alfonso, M.G.; Gradia, C.; Sgherzi, B.; et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: The FAI Project. Europace 2019, 21, 1468–1475. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- De Groot, N.M.S.; Houben, R.P.M.; Smeets, J.L.; Boersma, E.; Schotten, U.; Schalij, M.J.; Crijns, H.; Allessie, M.A. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Epicardial breakthrough. Circulation 2010, 122, 1674–1682. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, M.; Liu, D.; Zhao, Q. Immune remodeling and atrial fibrillation. Front. Physiol. 2022, 13, 1497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Q.; Ma, Y.; Liu, Q. The role of immune cells in atrial fibrillation. J. Mol. Cell. Cardiol. 2018, 123, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-F.; Chen, Y.-J.; Lin, Y.-J.; Chen, S.-A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Francis Stuart, S.D.; De Jesus, N.M.; Lindsey, M.L.; Ripplinger, C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell. Cardiol. 2016, 91, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Wu, M.; Kang, L.; Xu, B. The effector cells and cellular mediators of immune system involved in cardiac inflammation and fibrosis after myocardial infarction. J. Cell. Physiol. 2020, 235, 8996–9004. [Google Scholar] [CrossRef]
- Zhong, W.; Ma, M.; Xie, J.; Huang, C.; Li, X.; Gao, M. Adipose-specific deletion of the cation channel TRPM7 inhibits TAK1 kinase-dependent inflammation and obesity in male mice. Nat. Commun. 2023, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, C.; Lu, X.; Chen, Z.; Zhang, N.; Wang, X.; Li, X.; Li, Y. Identification and Verification of Biomarkers and Immune Infiltration in Obesity-Related Atrial Fibrillation. Biology 2023, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Okumura, Y.; Watanabe, I.; Nakai, T.; Ohkubo, K.; Kofune, M.; Mano, H.; Sonoda, K.; Hiro, T.; Nikaido, M.; et al. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ. Arrhythm. Electrophysiol. 2012, 5, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Yamabe, H.; Enomoto, K.; Koyama, J.; Morihisa, K.; Hoshiyama, T.; Matsui, K.; Ogawa, H. Importance of pericardial fat in the formation of complex fractionated atrial electrogram region in atrial fibrillation. Int. J. Cardiol. 2014, 174, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Shipp, N.J.; Kelly, D.J.; Thanigaimani, S.; Neo, M.; Kuklik, P.; Lim, H.S.; Zhang, Y.; Drury, K.; Wong, C.X.; et al. Atrial Arrhythmia in Ageing Spontaneously Hypertensive Rats: Unraveling the Substrate in Hypertension and Ageing. PLoS ONE 2013, 8, e72416. [Google Scholar] [CrossRef] [PubMed]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Compagnucci, P.; MacDonald, B.; Mayedo, A.; Torlapati, P.G.; Bassiouny, M.; et al. Catheter ablation approach and outcome in HIV+ patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2023, 34, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Steffens, S.; Nahrendorf, M.; Madonna, R. Immune cells in cardiac homeostasis and disease: Emerging insights from novel technologies. Eur. Heart J. 2021, 43, 1533–1541. [Google Scholar] [CrossRef]
- Tucker, N.R.; Chaffin, M.; Fleming, S.J.; Hall, A.W.; Parsons, V.A.; Bedi, K.C.; Akkad, A.-D.; Herndon, C.N.; Arduini, A.; Papangeli, I.; et al. Transcriptional and Cellular Diversity of the Human Heart. Circulation 2020, 142, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Farbehi, N.; Patrick, R.; Dorison, A.; Xaymardan, M.; Janbandhu, V.; Wystub-Lis, K.; Ho, J.W.; Nordon, R.E.; Harvey, R.P. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. eLife 2019, 8, e43882. [Google Scholar] [CrossRef] [PubMed]
- Gladka, M.M.; Molenaar, B.; de Ruiter, H.; van der Elst, S.; Tsui, H.; Versteeg, D.; Lacraz, G.P.A.; Huibers, M.M.H.; van Oudenaarden, A.; van Rooij, E. Single-Cell Sequencing of the Healthy and Diseased Heart Reveals Cytoskeleton-Associated Protein 4 as a New Modulator of Fibroblasts Activation. Circulation 2018, 138, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Kunderfranco, P.; Peano, C.; Carullo, P.; Cremonesi, M.; Schorn, T.; Carriero, R.; Termanini, A.; Colombo, F.S.; Jachetti, E.; et al. Single-Cell Sequencing of Mouse Heart Immune Infiltrate in Pressure Overload-Driven Heart Failure Reveals Extent of Immune Activation. Circulation 2019, 140, 2089–2107. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.A.; Squiers, G.T.; McLellan, M.A.; Bolisetty, M.T.; Robson, P.; Rosenthal, N.A.; Pinto, A.R. Single-Cell Transcriptional Profiling Reveals Cellular Diversity and Intercommunication in the Mouse Heart. Cell Rep. 2018, 22, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Asp, M.; Giacomello, S.; Larsson, L.; Wu, C.; Fürth, D.; Qian, X.; Wärdell, E.; Custodio, J.; Reimegård, J.; Salmén, F.; et al. A Spatiotemporal Organ-Wide Gene Expression and Cell Atlas of the Developing Human Heart. Cell 2019, 179, 1647–1660.e19. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, Y.; Liu, X.; Yan, L.; Fan, X.; Yong, J.; Hu, Y.; Dong, J.; Li, Q.; Wu, X.; et al. Single-Cell Transcriptome Analysis Maps the Developmental Track of the Human Heart. Cell Rep. 2019, 26, 1934–1950.e5. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014, 40, 91–104. [Google Scholar] [CrossRef]
- Dick, S.A.; Wong, A.; Hamidzada, H.; Nejat, S.; Nechanitzky, R.; Vohra, S.; Mueller, B.; Zaman, R.; Kantores, C.; Aronoff, L.; et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 2022, 7, eabf7777. [Google Scholar] [CrossRef]
- Heidt, T.; Courties, G.; Dutta, P.; Sager, H.B.; Sebas, M.; Iwamoto, Y.; Sun, Y.; Da Silva, N.; Panizzi, P.; van der Lahn, A.M.; et al. Differential Contribution of Monocytes to Heart Macrophages in Steady-State and after Myocardial Infarction. Circ. Res. 2014, 115, 284–295. [Google Scholar] [CrossRef]
- Zaman, R.; Epelman, S. Resident cardiac macrophages: Heterogeneity and function in health and disease. Immunity 2022, 55, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Schloss, M.J.; Lee, I.-H.; Bapat, A.; Iwamoto, Y.; Vinegoni, C.; Paccalet, A.; Yamazoe, M.; Grune, J.; Pabel, S.; et al. Recruited macrophages elicit atrial fibrillation. Science 2023, 381, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Sakuma, J.; Takeuchi, I.; Yasukochi, Y.; Kato, K.; Oguri, M.; Fujimaki, T.; Horibe, H.; Muramatsu, M.; Sawabe, M.; et al. Identification of TNFSF13, SPATC1L, SLC22A25 and SALL4 as novel susceptibility loci for atrial fibrillation by an exome-wide association study. Mol. Med. Rep. 2017, 16, 5823–5832. [Google Scholar] [CrossRef] [PubMed]
- Fujiu, K.; Shibata, M.; Nakayama, Y.; Ogata, F.; Matsumoto, S.; Noshita, K.; Iwami, S.; Nakae, S.; Komuro, I.; Nagai, R.; et al. A heart–brain–kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat. Med. 2017, 23, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef]
- Chen, M.-C.; Chang, J.-P.; Liu, W.-H.; Yang, C.-H.; Chen, Y.-L.; Tsai, T.-H.; Wang, Y.-H.; Pan, K.-L. Increased inflammatory cell infiltration in the atrial myocardium of patients with atrial fibrillation. Am. J. Cardiol. 2008, 102, 861–865. [Google Scholar] [CrossRef]
- Yamashita, T.; Sekiguchi, A.; Iwasaki, Y.; Date, T.; Sagara, K.; Tanabe, H.; Suma, H.; Sawada, H.; Aizawa, T. Recruitment of immune cells across atrial endocardium in human atrial fibrillation. Circ. J. 2010, 74, 262–270. [Google Scholar] [CrossRef]
- Smorodinova, N.; Bláha, M.; Melenovský, V.; Rozsívalová, K.; Přidal, J.; Ďurišová, M.; Pirk, J.; Kautzner, J.; Kučera, T. Analysis of immune cell populations in atrial myocardium of patients with atrial fibrillation or sinus rhythm. PLoS ONE 2017, 12, e0172691. [Google Scholar] [CrossRef]
- Hohmann, C.; Pfister, R.; Mollenhauer, M.; Adler, C.; Kozlowski, J.; Wodarz, A.; Drebber, U.; Wippermann, J.; Michels, G. Inflammatory cell infiltration in left atrial appendageal tissues of patients with atrial fibrillation and sinus rhythm. Sci. Rep. 2020, 10, 1685. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhou, D.; Xie, X.; Wang, S.; Wang, Z.; Zhao, W.; Xu, H.; Zheng, L. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res. Cardiol. 2016, 111, 63. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Emmens, R.W.; van Wezenbeek, J.; Stooker, W.; Allaart, C.P.; Vonk, A.B.A.; van Rossum, A.C.; Niessen, H.W.M.; Krijnen, P.A.J. Atrial inflammation in different atrial fibrillation subtypes and its relation with clinical risk factors. Clin. Res. Cardiol. 2020, 109, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Begieneman, M.P.V.; Rijvers, L.; Kubat, B.; Paulus, W.J.; Vonk, A.B.A.; van Rossum, A.C.; Schalkwijk, C.G.; Stooker, W.; Niessen, H.W.M.; Krijnen, P.A.J. Atrial fibrillation coincides with the advanced glycation end product N(ε)-(carboxymethyl)lysine in the atrium. Am. J. Pathol. 2015, 185, 2096–2104. [Google Scholar] [CrossRef]
- Liu, A.; Jia, K.; Liang, H.; Jin, Q. Comprehensive analysis of autophagy-related genes and patterns of immune cell infiltration in valvular atrial fibrillation. BMC Cardiovasc. Disord. 2021, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Guo, W.; Yu, P.; Qiu, H.; Jiang, R.; Jiang, C. Characteristics of immune clusters and cell abundance in patients with different subtypes of nonparoxysmal atrial fibrillation. Sci. Rep. 2023, 13, 968. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, X.; Su, Y.; Liu, D.; Wu, L.; Li, S.; He, W.; Zhong, G.; Jiang, Z. PILRA is associated with immune cells infiltration in atrial fibrillation based on bioinformatics and experiment validation. Front. Cardiovasc. Med. 2023, 10, 1082015. [Google Scholar] [CrossRef]
- Shiba, M.; Sugano, Y.; Ikeda, Y.; Okada, H.; Nagai, T.; Ishibashi-Ueda, H.; Yasuda, S.; Ogawa, H.; Anzai, T. Presence of increased inflammatory infiltrates accompanied by activated dendritic cells in the left atrium in rheumatic heart disease. PLoS ONE 2018, 13, e0203756. [Google Scholar] [CrossRef]
- Zhang, K.M.; Hu, P.; Wang, S.W.; Wright, L.D.; Wechsler, A.S.; Spratt, J.A.; Briggs, F.N. Fast- and slow-twitch isoforms (SERCA1 and SERCA2a) of sarcoplasmic reticulum Ca-ATPase are expressed simultaneously in chronically stimulated muscle fibers. Pflug. Arch. 1997, 433, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Lüss, I.; Boknik, P.; Jones, L.R.; Kirchhefer, U.; Knapp, J.; Linck, B.; Lüss, H.; Meissner, A.; Müller, F.U.; Schmitz, W.; et al. Expression of cardiac calcium regulatory proteins in atrium v ventricle in different species. J. Mol. Cell. Cardiol. 1999, 31, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.; Massilamany, C.; Basavalingappa, R.H.; Gangaplara, A.; Rajasekaran, R.A.; Afzal, M.Z.; Khalilzad-Sharghi, V.; Zhou, Y.; Riethoven, J.-J.; Nandi, S.S.; et al. Epitope Mapping of SERCA2a Identifies an Antigenic Determinant that Induces Mainly Atrial Myocarditis in A/J mice. J. Immunol. 2018, 200, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Date, T.; Yamashita, T.; Sekiguchi, A.; Iwasaki, Y.; Aizawa, T.; Yamane, T.; Aramaki, Y.; Komukai, K.; Taniguchi, I.; Yoshimura, M. Infiltration of macrophages through the atrial endocardium of inflammation-induced rats: Contribution of fractalkine. Circ. J. 2009, 73, 932–937. [Google Scholar] [CrossRef]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Sun, X.; Ravaud, C.; Villa del Campo, C.; Klaourakis, K.; Lupu, I.-E.; Lord, A.M.; Browne, C.; Jacobsen, S.E.W.; Greaves, D.R.; et al. Tissue-resident macrophages regulate lymphatic vessel growth and patterning in the developing heart. Development 2021, 148, dev194563. [Google Scholar] [CrossRef]
- Leid, J.; Carrelha, J.; Boukarabila, H.; Epelman, S.; Jacobsen, S.E.W.; Lavine, K.J. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ. Res. 2016, 118, 1498–1511. [Google Scholar] [CrossRef]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–1392. [Google Scholar] [CrossRef]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef]
- Wan, E.; Yeap, X.Y.; Dehn, S.; Terry, R.; Novak, M.; Zhang, S.; Iwata, S.; Han, X.; Homma, S.; Drosatos, K.; et al. Enhanced Efferocytosis of Apoptotic Cardiomyocytes Through MER Tyrosine Kinase Links Acute Inflammation Resolution to Cardiac Repair After Infarction. Circ. Res. 2013, 113, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Chakarov, S.; Lim, H.Y.; Tan, L.; Lim, S.Y.; See, P.; Lum, J.; Zhang, X.-M.; Foo, S.; Nakamizo, S.; Duan, K.; et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019, 363, eaau0964. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, M.J.; Harmsen, M.C.; van Rooijen, N.; Petersen, A.H.; van Luyn, M.J.A. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 2007, 170, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.-L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, P.; Swirski, F.K.; Figueiredo, J.-L.; Waterman, P.; Sosnovik, D.E.; Aikawa, E.; Libby, P.; Pittet, M.; Weissleder, R.; Nahrendorf, M. Impaired infarct healing in atherosclerotic mice with Ly-6Chi monocytosis. J. Am. Coll. Cardiol. 2010, 55, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, Z.; Yuan, J.; Li, C.; Zhao, R.; Liu, W.; Deng, W.; Gu, N.; Zhang, W.; Hu, S.; et al. Hypoxia-reoxygenation induces macrophage polarization and causes the release of exosomal miR-29a to mediate cardiomyocyte pyroptosis. In Vitro Cell Dev. Biol. Anim. 2021, 57, 30–41. [Google Scholar] [CrossRef]
- Monnerat, G.; Alarcón, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepúlveda, M.; et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Xie, A.; Kim, T.-Y.; Terentyeva, R.; Liu, M.; Shi, G.; Feng, F.; Choi, B.-R.; Terentyev, D.; et al. Interleukin-1β, Oxidative Stress, and Abnormal Calcium Handling Mediate Diabetic Arrhythmic Risk. JACC Basic Transl. Sci. 2021, 6, 42–52. [Google Scholar] [CrossRef]
- Kume, O.; Teshima, Y.; Abe, I.; Ikebe, Y.; Oniki, T.; Kondo, H.; Saito, S.; Fukui, A.; Yufu, K.; Miura, M.; et al. Role of atrial endothelial cells in the development of atrial fibrosis and fibrillation in response to pressure overload. Cardiovasc. Pathol. 2017, 27, 18–25. [Google Scholar] [CrossRef]
- Yan, X.; Anzai, A.; Katsumata, Y.; Matsuhashi, T.; Ito, K.; Endo, J.; Yamamoto, T.; Takeshima, A.; Shinmura, K.; Shen, W.; et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 2013, 62, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Beyersdorf, N.; Weirather, J.; Podolskaya, A.; Bauersachs, J.; Ertl, G.; Kerkau, T.; Frantz, S. Activation of CD4+ T Lymphocytes Improves Wound Healing and Survival After Experimental Myocardial Infarction in Mice. Circulation 2012, 125, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Laroumanie, F.; Douin-Echinard, V.; Pozzo, J.; Lairez, O.; Tortosa, F.; Vinel, C.; Delage, C.; Calise, D.; Dutaur, M.; Parini, A.; et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 2014, 129, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Nevers, T.; Salvador, A.M.; Grodecki-Pena, A.; Knapp, A.; Velazquez, F.; Aronovitz, M.; Kapur, N.K.; Karas, R.H.; Blanton, R.M.; Alcaide, P. Left Ventricular T Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ. Heart Fail. 2015, 8, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Curato, C.; Slavic, S.; Dong, J.; Skorska, A.; Altarche-Xifró, W.; Miteva, K.; Kaschina, E.; Thiel, A.; Imboden, H.; Wang, J.; et al. Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. J. Immunol. 2010, 185, 6286–6293. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ogawa, M.; Suzuki, J.; Hirata, Y.; Nagai, R.; Isobe, M. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int. Heart J. 2011, 52, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Xia, Y.; Bujak, M.; Gonzalez-Quesada, C.; Frangogiannis, N.G. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am. J. Pathol. 2010, 176, 2177–2187. [Google Scholar] [CrossRef]
- Sulzgruber, P.; Koller, L.; Winter, M.-P.; Richter, B.; Blum, S.; Korpak, M.; Hülsmann, M.; Goliasch, G.; Wojta, J.; Niessner, A. The impact of CD4+CD28null T-lymphocytes on atrial fibrillation and mortality in patients with chronic heart failure. Thromb. Haemost. 2017, 117, 349–356. [Google Scholar] [CrossRef]
- Frisancho-Kiss, S.; Davis, S.E.; Nyland, J.F.; Frisancho, J.A.; Cihakova, D.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Cutting Edge: Cross-Regulation by TLR4 and T cell Ig Mucin-3 Determines Sex Differences in Inflammatory Heart Disease1. J. Immunol. 2007, 178, 6710–6714. [Google Scholar] [CrossRef]
- Bansal, S.S.; Ismahil, M.A.; Goel, M.; Zhou, G.; Rokosh, G.; Hamid, T.; Prabhu, S.D. Dysfunctional and Proinflammatory Regulatory T-Lymphocytes Are Essential for Adverse Cardiac Remodeling in Ischemic Cardiomyopathy. Circulation 2019, 139, 206–221. [Google Scholar] [CrossRef]
- Hammer, A.; Niessner, A.; Sulzgruber, P. The impact of CD4+CD28null T lymphocytes on atrial fibrillation: A potential pathophysiological pathway. Inflamm. Res. 2021, 70, 1011–1014. [Google Scholar] [CrossRef]
- Kazem, N.; Sulzgruber, P.; Thaler, B.; Baumgartner, J.; Koller, L.; Laufer, G.; Steinlechner, B.; Hohensinner, P.; Wojta, J.; Niessner, A. CD8+CD28null T Lymphocytes are Associated with the Development of Atrial Fibrillation after Elective Cardiac Surgery. Thromb. Haemost. 2020, 120, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; LePrince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guérin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, T.; Loveland, P.; Kanellakis, P.; Cao, A.; Kallies, A.; Huang, A.L.; Peter, K.; Toh, B.-H.; Bobik, A. Alarmin-activated B cells accelerate murine atherosclerosis after myocardial infarction via plasma cell-immunoglobulin-dependent mechanisms. Eur. Heart J. 2021, 42, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Bouchentouf, M.; Forner, K.-A.; Cuerquis, J.; Michaud, V.; Zheng, J.; Paradis, P.; Schiffrin, E.L.; Galipeau, J. Induction of cardiac angiogenesis requires killer cell lectin-like receptor 1 and α4β7 integrin expression by NK cells. J. Immunol. 2010, 185, 7014–7025. [Google Scholar] [CrossRef]
- Jeong, Y.-M.; Cheng, X.W.; Lee, K.H.; Lee, S.; Cho, H.; Kim, W. Substance P enhances the local activation of NK1R-expressing c-kit+ cardiac progenitor cells in right atrium of ischemia/reperfusion-injured heart. BMC Mol. Cell. Biol. 2020, 21, 41. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef]
- Liu, P.; Aitken, K.; Kong, Y.-Y.; Opavsky, M.A.; Martino, T.; Dawood, F.; Wen, W.-H.; Kozieradzki, I.; Bachmaier, K.; Straus, D.; et al. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 2000, 6, 429–434. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Lindsey, M.L.; Michael, L.H.; Youker, K.A.; Bressler, R.B.; Mendoza, L.H.; Spengler, R.N.; Smith, C.W.; Entman, M.L. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 1998, 98, 699–710. [Google Scholar] [CrossRef]
- Gwechenberger, M.; Mendoza, L.H.; Youker, K.A.; Frangogiannis, N.G.; Smith, C.W.; Michael, L.H.; Entman, M.L. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 1999, 99, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Entman, M.L.; Youker, K.; Shoji, T.; Kukielka, G.; Shappell, S.B.; Taylor, A.A.; Smith, C.W. Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J. Clin. Investig. 1992, 90, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Saito, Y.; Kishimoto, I.; Harada, M.; Kuwahara, K.; Takahashi, N.; Nakagawa, Y.; Nakanishi, M.; Tanimoto, K.; Usami, S.; et al. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation 2004, 110, 3306–3312. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Meulendijks, E.R.; Al-Shama, R.F.M.; Kawasaki, M.; Fabrizi, B.; Neefs, J.; Wesselink, R.; Ernault, A.C.; Piersma, S.; Pham, T.V.; Jimenez, C.R.; et al. Atrial epicardial adipose tissue abundantly secretes myeloperoxidase and activates atrial fibroblasts in patients with atrial fibrillation. J. Transl. Med. 2023, 21, 366. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, R.; Yue, H.; Zhang, X.; Pan, X.; Sun, Y.; Shi, J.; Zhu, G.; Qin, C.; Guo, Y. Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct. Target. Ther. 2023, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Vafadarnejad, E.; Rizzo, G.; Krampert, L.; Arampatzi, P.; Arias-Loza, A.-P.; Nazzal, Y.; Rizakou, A.; Knochenhauer, T.; Bandi, S.R.; Nugroho, V.A.; et al. Dynamics of Cardiac Neutrophil Diversity in Murine Myocardial Infarction. Circ. Res. 2020, 127, e232–e249. [Google Scholar] [CrossRef] [PubMed]
- Pfirschke, C.; Engblom, C.; Gungabeesoon, J.; Lin, Y.; Rickelt, S.; Zilionis, R.; Messemaker, M.; Siwicki, M.; Gerhard, G.M.; Kohl, A.; et al. Tumor-Promoting Ly-6G+ SiglecFhigh Cells Are Mature and Long-Lived Neutrophils. Cell Rep. 2020, 32, 108164. [Google Scholar] [CrossRef]
- Engblom, C.; Pfirschke, C.; Zilionis, R.; Da Silva Martins, J.; Bos, S.A.; Courties, G.; Rickelt, S.; Severe, N.; Baryawno, N.; Faget, J.; et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 2017, 358, eaal5081. [Google Scholar] [CrossRef]
- Horckmans, M.; Ring, L.; Duchene, J.; Santovito, D.; Schloss, M.J.; Drechsler, M.; Weber, C.; Soehnlein, O.; Steffens, S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 2017, 38, 187–197. [Google Scholar] [CrossRef]
- Wei, X.; Zou, S.; Xie, Z.; Wang, Z.; Huang, N.; Cen, Z.; Hao, Y.; Zhang, C.; Chen, Z.; Zhao, F.; et al. EDIL3 deficiency ameliorates adverse cardiac remodelling by neutrophil extracellular traps (NET)-mediated macrophage polarization. Cardiovasc. Res. 2022, 118, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Adamsson Eryd, S.; Smith, J.G.; Melander, O.; Hedblad, B.; Engström, G. Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophil counts. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Chen, K.; Rha, S.-W.; Lim, H.-E.; Li, G.; Liu, T. Usefulness of Neutrophil/Lymphocyte Ratio as a Predictor of Atrial Fibrillation: A Meta-analysis. Arch. Med. Res. 2015, 46, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-N.; Tong, M.-S.; Sung, P.-H.; Chen, Y.-L.; Chen, C.-H.; Tsai, N.-W.; Huang, C.-J.; Chang, Y.-T.; Chen, S.-F.; Chang, W.-N.; et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomed. J. 2017, 40, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Halova, I.; Rönnberg, E.; Draberova, L.; Vliagoftis, H.; Nilsson, G.P.; Draber, P. Changing the threshold-Signals and mechanisms of mast cell priming. Immunol. Rev. 2018, 282, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, J.; Kalkkinen, N.; Welgus, H.G.; Kovanen, P.T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J. Biol. Chem. 1994, 269, 18134–18140. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, K.A.; Wang, Y.; Shiota, N.; Saarinen, J.; Hyytiäinen, M.; Kokkonen, J.O.; Keski-Oja, J.; Kovanen, P.T. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: A novel function for chymase. FASEB J. 2001, 15, 1377–1388. [Google Scholar] [CrossRef]
- Tatler, A.L.; Porte, J.; Knox, A.; Jenkins, G.; Pang, L. Tryptase activates TGFbeta in human airway smooth muscle cells via direct proteolysis. Biochem. Biophys. Res. Commun. 2008, 370, 239–242. [Google Scholar] [CrossRef]
- Uemura, K.; Kondo, H.; Ishii, Y.; Kobukata, M.; Haraguchi, M.; Imamura, T.; Otsubo, T.; Ikebe-Ebata, Y.; Abe, I.; Ayabe, R.; et al. Mast Cells Play an Important Role in the Pathogenesis of Hyperglycemia-Induced Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2016, 27, 981–989. [Google Scholar] [CrossRef]
- Legere, S.A.; Haidl, I.D.; Castonguay, M.C.; Brunt, K.R.; Légaré, J.-F.; Marshall, J.S. Increased mast cell density is associated with decreased fibrosis in human atrial tissue. J. Mol. Cell. Cardiol. 2020, 149, 15–26. [Google Scholar] [CrossRef]
- Lee, S.A.; Fitzgerald, S.M.; Huang, S.K.; Li, C.; Chi, D.S.; Milhorn, D.M.; Krishnaswamy, G. Molecular regulation of interleukin-13 and monocyte chemoattractant protein-1 expression in human mast cells by interleukin-1beta. Am. J. Respir. Cell. Mol. Biol. 2004, 31, 283–291. [Google Scholar] [CrossRef]
- Lin, T.J.; Befus, A.D. Differential regulation of mast cell function by IL-10 and stem cell factor. J. Immunol. 1997, 159, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.C.; Peronet, R.; David, B.; Namane, A.; Mécheri, S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001, 166, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Van Wagoner, D.R. Oxidant and inflammatory mechanisms and targeted therapy in AF: An update. J. Cardiovasc. Pharmacol. 2015, 66, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.-S.; Zacharowski, K.; Angelini, G.D. Inflammatory response and cardioprotection during open-heart surgery: The importance of anaesthetics. Br. J. Pharmacol. 2008, 153, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Cao, H.-J.; Han, X.; Teng, F.; Chen, C.; Yang, J.; Yan, X.; Li, P.-B.; Liu, Y.; Xia, Y.-L.; et al. Chemokine Receptor CXCR-2 Initiates Atrial Fibrillation by Triggering Monocyte Mobilization in Mice. Hypertension 2020, 76, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, Z. Serum macrophage migration inhibitory factor is correlated with atrial fibrillation. J. Clin. Lab. Anal. 2018, 32, e22225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, L.; Dong, Y.; Fu, Y.; Pan, Y.; Luan, Q.; Liu, Y.; Liu, Z.; Yang, X.; Chen, M.; et al. Plasma Galectin-3 is associated with progression from paroxysmal to persistent atrial fibrillation. BMC Cardiovasc. Disord. 2021, 21, 226. [Google Scholar] [CrossRef]
- Li, D.; Bjørnager, L.; Langkilde, A.; Andersen, O.; Jøns, C.; Agner, B.F.R.; Dixen, U.; Landex, N.L. Stromal cell-derived factor 1α (SDF-1α): A marker of disease burden in patients with atrial fibrillation. Scand. Cardiovasc. J. 2016, 50, 36–41. [Google Scholar] [CrossRef]
- Matsumori, A.; Shimada, T.; Nakatani, E.; Shimada, M.; Tracy, S.; Chapman, N.M.; Drayson, M.T.; Hartz, V.L.; Mason, J.W. Immunoglobulin free light chains as an inflammatory biomarker of heart failure with myocarditis. Clin. Immunol. 2020, 217, 108455. [Google Scholar] [CrossRef]
- Wu, N.; Xu, B.; Liu, Y.; Chen, X.; Tang, H.; Wu, L.; Xiang, Y.; Zhang, M.; Shu, M.; Song, Z.; et al. Elevated plasma levels of Th17-related cytokines are associated with increased risk of atrial fibrillation. Sci. Rep. 2016, 6, 26543. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, V.; Andrié, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K.; et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Holzwirth, E.; Kornej, J.; Erbs, S.; Obradovic, D.; Bollmann, A.; Hindricks, G.; Thiele, H.; Büttner, P. Myeloperoxidase in atrial fibrillation: Association with progression, origin and influence of renin-angiotensin system antagonists. Clin. Res. Cardiol. 2020, 109, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Rahmutula, D.; Marcus, G.M.; Wilson, E.E.; Ding, C.-H.; Xiao, Y.; Paquet, A.C.; Barbeau, R.; Barczak, A.J.; Erle, D.J.; Olgin, J.E. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-β1. Cardiovasc. Res. 2013, 99, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Yin, Z.; Zhu, Y.; Xin, F.; Zhang, H.; Po, S.S.; Wang, H. Impact of proinflammatory epicardial adipose tissue and differentially enhanced autonomic remodeling on human atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2023, 165, e158–e174. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Oguri, M.; Hibino, T.; Yajima, K.; Matsuo, H.; Segawa, T.; Watanabe, S.; Yoshida, H.; Satoh, K.; Nozawa, Y.; et al. Genetic factors for lone atrial fibrillation. Int. J. Mol. Med. 2007, 19, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Kesikli, S.A.; Ates, A.H.; Evranos, B.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Oto, M.A.; et al. Elevated M2-muscarinic and β1-adrenergic receptor autoantibody levels are associated with paroxysmal atrial fibrillation. Clin. Res. Cardiol. 2015, 104, 226–233. [Google Scholar] [CrossRef]
- Serban, R.C.; Balan, A.I.; Perian, M.; Pintilie, I.; Somkereki, C.; Huţanu, A.; Scridon, A. Atrial electrical remodeling induced by chronic ischemia and inflammation in patients with stable coronary artery disease. Chin. J. Physiol. 2019, 62, 11. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Q.; Liu, J.; Zhang, Q.; Zhang, J. To Assess the Role of Immune Infiltrating Immune Cells of Patients With Chronic Heart Failure With Atrial Fibrillation and Nursing Care in These Patients. Cell. Mol. Biol. 2023, 69, 49–56. [Google Scholar] [CrossRef]
- Pretorius, M.; Murray, K.T.; Yu, C.; Byrne, J.G.; Billings, F.T.I.; Petracek, M.R.; Greelish, J.P.; Hoff, S.J.; Ball, S.K.; Mishra, V.; et al. Angiotensin-converting enzyme inhibition or mineralocorticoid receptor blockade do not affect prevalence of atrial fibrillation in patients undergoing cardiac surgery. Crit. Care Med. 2012, 40, 2805. [Google Scholar] [CrossRef]
- Roşianu, Ş.H.; Roşianu, A.-N.; Aldica, M.; Căpâlneanu, R.; Buzoianu, A.D. Inflammatory markers in paroxysmal atrial fibrillation and the protective role of renin-angiotensin-aldosterone system inhibitors. Clujul Med. 2013, 86, 217–221. [Google Scholar]
- Miceli, A.; Capoun, R.; Fino, C.; Narayan, P.; Bryan, A.J.; Angelini, G.D.; Caputo, M. Effects of angiotensin-converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol. 2009, 54, 1778–1784. [Google Scholar] [CrossRef]
- Liu, T.; Korantzopoulos, P.; Shao, Q.; Zhang, Z.; Letsas, K.P.; Li, G. Mineralocorticoid receptor antagonists and atrial fibrillation: A meta-analysis. EP Eur. 2016, 18, 672–678. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Ferrazzi, P.; Rovere, M.E.; Gandino, A.; Cemin, R.; Ferrua, S.; Belli, R.; Maestroni, S.; Simon, C.; et al. Colchicine Reduces Postoperative Atrial Fibrillation. Circulation 2011, 124, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.; Giannopoulos, G.; Kossyvakis, C.; Efremidis, M.; Panagopoulou, V.; Kaoukis, A.; Raisakis, K.; Bouras, G.; Angelidis, C.; Theodorakis, A.; et al. Colchicine for Prevention of Early Atrial Fibrillation Recurrence After Pulmonary Vein Isolation: A Randomized Controlled Study. J. Am. Coll. Cardiol. 2012, 60, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Eikelboom, J.W.; Nidorf, S.M.; Al-Omran, M.; Gupta, N.; Teoh, H.; Friedrich, J.O. Colchicine in cardiac disease: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Dernellis, J.; Panaretou, M. Relationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur. Heart J. 2004, 25, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Halonen, J.; Halonen, P.; Järvinen, O.; Taskinen, P.; Auvinen, T.; Tarkka, M.; Hippeläinen, M.; Juvonen, T.; Hartikainen, J.; Hakala, T. Corticosteroids for the Prevention of Atrial Fibrillation after Cardiac Surgery—A Randomized Controlled Trial. JAMA 2007, 297, 1562–1567. [Google Scholar] [CrossRef]
- Won, H.; Kim, J.-Y.; Shim, J.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; Joung, B. Effect of a Single Bolus Injection of Low-Dose Hydrocortisone for Prevention of Atrial Fibrillation Recurrence after Radiofrequency Catheter Ablation. Circ. J. 2013, 77, 53–59. [Google Scholar] [CrossRef]
- Iskandar, S.; Reddy, M.; Afzal, M.R.; Rajasingh, J.; Atoui, M.; Lavu, M.; Atkins, D.; Bommana, S.; Umbarger, L.; Jaeger, M.; et al. Use of Oral Steroid and its Effects on Atrial Fibrillation Recurrence and Inflammatory Cytokines Post Ablation—The Steroid AF Study. J. Atr. Fibrill. 2017, 9, 1604. [Google Scholar] [CrossRef]
- Suleiman, M.; Koestler, C.; Lerman, A.; Lopez-Jimenez, F.; Herges, R.; Hodge, D.; Bradley, D.; Cha, Y.-M.; Brady, P.A.; Munger, T.M.; et al. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: A double-blind, placebo-controlled, randomized trial. Heart Rhythm 2012, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Stoner, J.A.; Humphrey, M.B.; Morris, L.; Filiberti, A.; Reynolds, J.C.; Elkholey, K.; Javed, I.; Twidale, N.; Riha, P.; et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin. Electrophysiol. 2020, 6, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Gwechenberger, M.; Socas, A.; Zorn, G.; Albinni, S.; Marx, M.; Bergler-Klein, J.; Binder, T.; Wojta, J.; Gössinger, H.D. Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin. Res. Cardiol. 2012, 101, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K. Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur. J. Cardio-Thorac. Surg. 2006, 29, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Navarro-Garcia, J.A.; Wu, J.; Saljic, A.; Abu-Taha, I.; Li, L.; Lahiri, S.K.; Keefe, J.A.; Aguilar-Sanchez, Y.; Moore, O.M.; et al. Chronic kidney disease promotes atrial fibrillation via inflammasome pathway activation. J. Clin. Investig. 2023, 133, e167517. [Google Scholar] [CrossRef] [PubMed]
- Aschar-Sobbi, R.; Izaddoustdar, F.; Korogyi, A.S.; Wang, Q.; Farman, G.P.; Yang, F.; Yang, W.; Dorian, D.; Simpson, J.A.; Tuomi, J.M.; et al. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat. Commun. 2015, 6, 6018. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; LaCanna, R.; Powers, J.C.; Vrakas, C.; Liu, F.; Berretta, R.; Chacko, G.; Holten, J.; Jadiya, P.; Wang, T.; et al. Class I Histone Deacetylase Inhibition for the Treatment of Sustained Atrial Fibrillation. J. Pharmacol. Exp. Ther. 2016, 358, 441–449. [Google Scholar] [CrossRef]
- Sakabe, M.; Shiroshita-Takeshita, A.; Maguy, A.; Dumesnil, C.; Nigam, A.; Leung, T.-K.; Nattel, S. Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation 2007, 116, 2101–2109. [Google Scholar] [CrossRef]
- Baczko, I.; Liknes, D.; Yang, W.; Hamming, K.C.; Searle, G.; Jaeger, K.; Husti, Z.; Juhasz, V.; Klausz, G.; Pap, R.; et al. Characterization of a novel multifunctional resveratrol derivative for the treatment of atrial fibrillation. Br. J. Pharmacol. 2014, 171, 92–106. [Google Scholar] [CrossRef]
- Frommeyer, G.; Wolfes, J.; Ellermann, C.; Kochhäuser, S.; Dechering, D.G.; Eckardt, L. Acute electrophysiologic effects of the polyphenols resveratrol and piceatannol in rabbit atria. Clin. Exp. Pharmacol. Physiol. 2019, 46, 94–98. [Google Scholar] [CrossRef]
- Carnes, C.A.; Chung, M.K.; Nakayama, T.; Nakayama, H.; Baliga, R.S.; Piao, S.; Kanderian, A.; Pavia, S.; Hamlin, R.L.; McCarthy, P.M.; et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ. Res. 2001, 89, E32–E38. [Google Scholar] [CrossRef]
- Shiroshita-Takeshita, A.; Schram, G.; Lavoie, J.; Nattel, S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 2004, 110, 2313–2319. [Google Scholar] [CrossRef]
- Obokata, M.; Negishi, K.; Kurosawa, K.; Tateno, R.; Tange, S.; Arai, M.; Amano, M.; Kurabayashi, M. Left Atrial Strain Provides Incremental Value for Embolism Risk Stratification over CHA2DS2-VASc Score and Indicates Prognostic Impact in Patients with Atrial Fibrillation. J. Am. Soc. Echocardiogr. 2014, 27, 709–716.e4. [Google Scholar] [CrossRef]
- Donal, E.; Lip, G.Y.H.; Galderisi, M.; Goette, A.; Shah, D.; Marwan, M.; Lederlin, M.; Mondillo, S.; Edvardsen, T.; Sitges, M.; et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 355–383. [Google Scholar] [CrossRef]
- Koh, A.S.; Siau, A.; Gao, F.; Chioh, F.W.J.; Leng, S.; Zhao, X.; Zhong, L.; Tan, R.S.; Koh, P.L.; Kovalik, J.-P.; et al. Left Atrial Phasic Function in Older Adults Is Associated with Fibrotic and Low-Grade Inflammatory Pathways. Gerontology 2023, 69, 47–56. [Google Scholar] [CrossRef]
- Gonzalez, F.A.; Ângelo-Dias, M.; Martins, C.; Gomes, R.; Bacariza, J.; Fernandes, A.; Borrego, L.M.; EchoCrit Group. Left atrial strain is associated with distinct inflammatory and immune profile in patients with COVID-19 pneumonia. Ultrasound J. 2023, 15, 2. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Study Type | Inflammation Markers | AF Outcome | Patient Cohort | Study | |
|---|---|---|---|---|---|---|
| ACE inhibitors or MRAs | pre- and post-operative ramipril or spironolactone | RDBPC | CRP ↓ | POAF | n = 432, SR: valve/CABG | Pretorius et al. [130] |
| ACEI or ARB therapy | clinical study | CRP ↓, IL-6 ↓ | AF burden ↓ | n = 64, PAF | Roşianu et al. [131] | |
| pre-operative ACEI | retrospective | n.a. | POAF ↑ | n = 6104, CABG | Miceli et al. [132] | |
| post-procedural eplerenone or spirolactone | meta-analysis | n.a. | POAF ↓, AF recurrence ↓, AF burden ↓ * | n = 3640, HF: cardiac surgery or lsPeAF: ablation | Liu et al. [133] | |
| Colchicine | post-operative | RDBPC substudy | n.a. | POAF ↓ | n = 336, SR: cardiac surgery | Imazio et al. [134] |
| post-procedural | RDBPC | CRP ↓, IL-6 ↓ | AF recurrence ↓ | n = 161, AF: RF ablation | Deftereos et al. [135] | |
| post-operative | systematic review | n.a. | POAF ↓, AF recurrence ↓ | n = 1118, SR: pericardiotomy or PAF: ablation | Verma et al. [136] | |
| Corticosteroids | post-operative low-dose prednisone | RBPC ** | CRP ↓ | AF recurrence ↓ | n = 104, PeAF: cardioversion | Dernelis & Panaretou et al. [137] |
| post-operative hydrocortisone | RDBPC | CRP ↓ | POAF ↓ | n = 241, SR: valve/CABG | Halonen et al. [138] | |
| post-operative single low-dose hydrocortisone injection | clinical study | n.a. | AF recurrence | n = 209, PAF: RF ablation | Won et al. [139] | |
| pre- and post-operative oral prednisone | RDBPC | IL-1 , IL-6 ↓, IL-8 ↓, TNF-α | AF recurrence | n = 60, PAF: ablation | Iskandar et al. [140] | |
| Statins | post-operative atorvastatin | RDBPC | CRP ↓ | AF recurrence | n = 108, PAF and PeAF: ablation | Suleiman et al. [141] |
| Vagus nerve stimulation | tragus stimulation, parasym device | RDBPC | IL-6 ↓, TNF-α | time spent in AF ↓ | n = 47, PAF | Stavrakis et al. [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Huiskes, F.G.; de Groot, N.M.S.; Brundel, B.J.J.M. The Role of Immune Cells Driving Electropathology and Atrial Fibrillation. Cells 2024, 13, 311. https://doi.org/10.3390/cells13040311
Huang M, Huiskes FG, de Groot NMS, Brundel BJJM. The Role of Immune Cells Driving Electropathology and Atrial Fibrillation. Cells. 2024; 13(4):311. https://doi.org/10.3390/cells13040311
Chicago/Turabian StyleHuang, Mingxin, Fabries G. Huiskes, Natasja M. S. de Groot, and Bianca J. J. M. Brundel. 2024. "The Role of Immune Cells Driving Electropathology and Atrial Fibrillation" Cells 13, no. 4: 311. https://doi.org/10.3390/cells13040311
APA StyleHuang, M., Huiskes, F. G., de Groot, N. M. S., & Brundel, B. J. J. M. (2024). The Role of Immune Cells Driving Electropathology and Atrial Fibrillation. Cells, 13(4), 311. https://doi.org/10.3390/cells13040311