Recapitulation of Structure–Function–Regulation of Blood–Brain Barrier under (Patho)Physiological Conditions
Abstract
1. Introduction
2. Structure and Functions of BBB under Healthy Condition
2.1. The Discovery of the Barrier between Circulating Blood and the Brain
2.2. The Proposal of the Term BBB
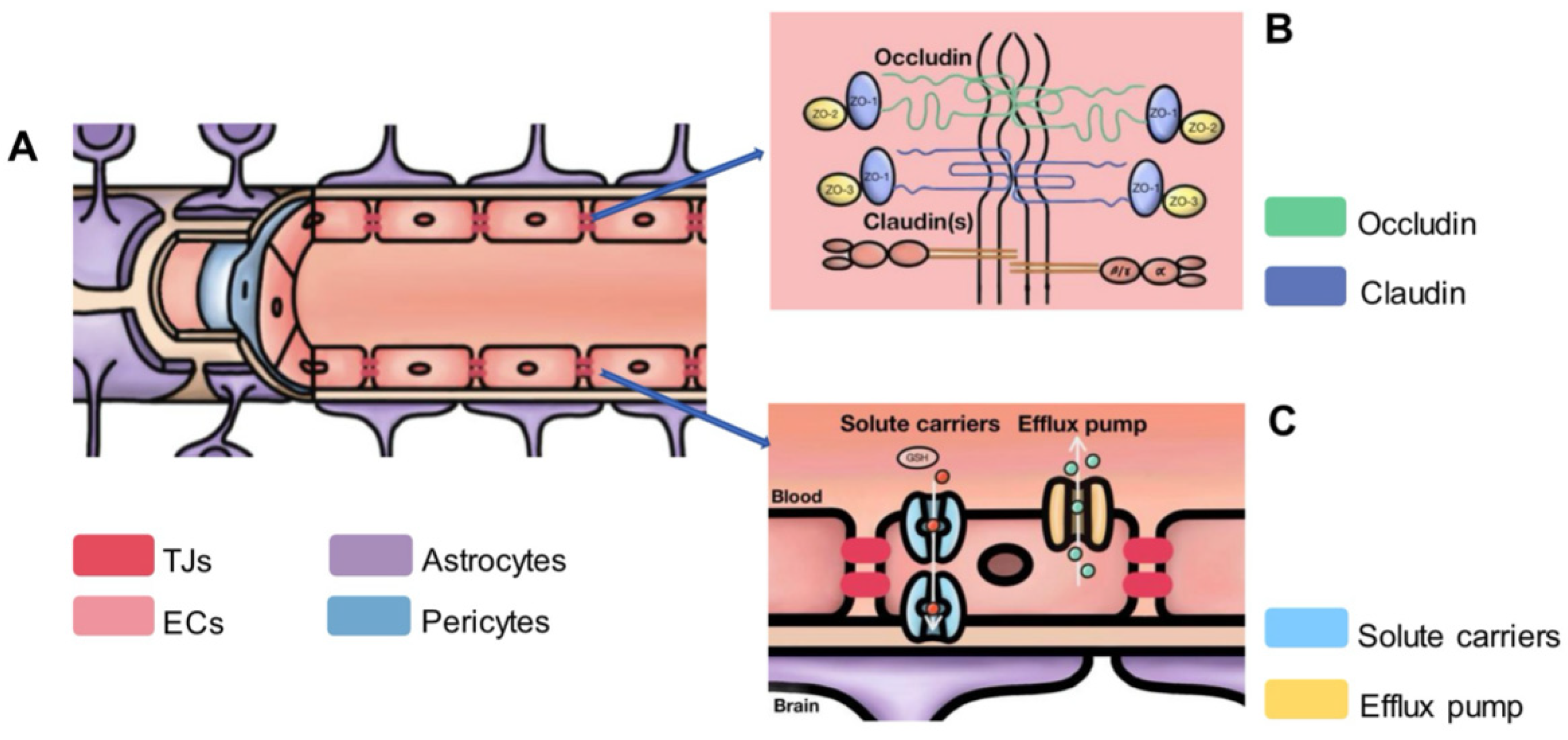
2.3. The Microstructure of the BBB
2.4. Study on Neighboring Cells Involved in BBB
2.4.1. Pericytes
2.4.2. Astrocytes
2.4.3. Microglia
2.4.4. Interactions among BBB and Neighboring Cells
3. Regulation of BBB’s Integrity under Normal Condition
3.1. Neural Regulation of BBB
3.2. Humoral Regulation of BBB
3.3. Auto-Regulation of BBB
3.4. Factors That Affect the BBB under Normal Conditions
3.4.1. Circadian Rhythm
3.4.2. Psychological Stress Factors
3.4.3. Diet and Nutrients
3.4.4. Intestinal Microbiota
3.4.5. Exercise and Environmental Exploration
4. Blood–Brain Barrier under Disease Conditions
4.1. Types of BBB Damage and Underlying Mechanisms
4.1.1. Direct Disruption of Tight Junctions
4.1.2. Oxidative Stress
4.1.3. Inflammation
4.1.4. Metalloproteinase Activation
4.1.5. Disruption of Transporters
| Toxic Factors | Mechanisms of Increased BBB Permeability | BBB Model | Dosage | Representative Reference |
|---|---|---|---|---|
| Ethanol | By increasing oxidative stresses | In vitro (human stem cells) | 50 mM | Stoffel, R. D., Bell, K. T. and Canfield, S. G. (2020) [121]. |
| Lead | By damaging tight junctions | In vivo (mice) | 7-day exposure to Pb at 54 mg/kg and 4 weeks | Gu, H., Territo, P. R., Persohn, S. A., et al. (2020) [122]. |
| Mercury | By increasing oxidative stresses | In vitro (porcine) | 3 µM of organic mercury compounds and 100 µM of inorganic HgCl2 | Lohren, H., Bornhorst, J., Fitkau, R., et al. (2016) [123]. |
| Carbon monoxide | By damaging endothelial cell function and tight junctions | In vivo (rats) | Absorption of 2.5–3.0 mL/L CO for 1 h | Wang, X., Tie, X., Zhang, J., Wan, J. and Liu, Y. (2004) [124]. |
| Cocaine | By downregulating TJPs, altering the expression of adhesion molecules, and promoting neuroinflammation | In vitro (rat) | 5–20 mg/kg, ip | Barr JK, Brailoiu GC, Abood ME, et al. (2020) [125]. |
| Nicotine | By altering tight-junction protein distribution and promoting inflammation | In vivo (rat) | 4.5 mg free base per kilogram of body weight per day | Hawkins, B. T., Abbruscato, T. J., et al. (2004) [126]. |
| PM 2.5 1 | By downregulating tight junctions and disrupting tight junctions in the BBB | In vitro | 10 ug/mL | Kang, Y. J., Tan, H. Y., Lee, C. Y. and Cho, H. (2021) [127]. |
| Pesticides 2 | By inducing oxidative stress and inflammation and disrupting tight junctions | In vivo (rat) | 1/50th of the LD50 for the pesticides quinalphos, cypermethrin, and lindane. | Gupta, A., Agarwal, R. and Shukla, G. S. (1999) [128]. |
4.2. Restoration of BBB under Disease Conditions
4.2.1. Endothelial Cells
4.2.2. Pericytes
4.2.3. Astrocytes
4.2.4. Microglia
4.3. Therapeutic Strategies Targeted to BBB Integrity under Disease Condition
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Y.; Liu, E.; Pei, Y.; Yao, Q.; Ma, H.; Mu, Y.; Wang, Y.; Zhang, Y.; Yang, X.; Wang, X. The impairment of intramural periarterial drainage in brain after subarachnoid hemorrhage. Acta Neuropathol. Commun. 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The glymphatic system and waste clearance with brain aging: A review. Gerontology 2019, 65, 106–119. [Google Scholar] [CrossRef]
- Ehrlich, P. Ueber die Beziehungen von Chemischer Constitution, Vertheilung, und Pharmakologischen Wirkung; Collected Studies on Immunity; John Wiley: New York, NY, USA, 1906; pp. 567–595. [Google Scholar]
- Goldmann, E.E. Die äussere und innere Sekretion des gesunden und kranken Organismus im Lichte der ‘vitalen Färbung. Beitr. Klin. Chir. 1909, 64, 192–265. [Google Scholar]
- Goldmann, E.E. Vitalfärbung am Zentralnervensystem: Beitrag zur Physio-Pathologie des Plexus Chorioideus und der Hirnhäute; Königl. Akademie der Wissenschaften: Berlin, Germany, 1913. [Google Scholar]
- Stewart, P.; Wiley, M. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail-chick transplantation chimeras. Dev. Biol. 1981, 84, 183–192. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. JNrn: Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Stern, L.; Gautier, R. II.–Les Rapports Entre Le Liquide Céphalo-Rachidien et Les éléments Nerveux De L’axe Cerebrospinal. Arch. Int. Physiol. Biochim. 1922, 17, 391–448. [Google Scholar] [CrossRef]
- Bernard, C. Etude sur la physiologie du coeur. Rev. Deux Mondes (1829–1971) 1865, 56, 236–252. [Google Scholar]
- Segal, M.B. The choroid plexuses and the barriers between the blood and the cerebrospinal fluid. Cell. Mol. Neurobiol. 2000, 20, 183–196. [Google Scholar] [CrossRef]
- Reese, T.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- Brightman, M.; Reese, T. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef]
- Gerhardt, G.A.; Oke, A.F.; Nagy, G.; Moghaddam, B.; Adams, R.N. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984, 290, 390–395. [Google Scholar] [CrossRef]
- Farrell, C.; Shivers, R. Capillary junctions of the rat are not affected by osmotic opening of the blood-brain barrier. Acta Neuropathol. 1984, 63, 179–189. [Google Scholar] [CrossRef]
- Butt, A.M.; Jones, H.C.; Abbott, N. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and-2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Morita, K.; Furuse, M.; Fujimoto, K.; Tsukita, S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 1999, 96, 511–516. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef]
- Wolburg, H.; Wolburg-Buchholz, K.; Kraus, J.; Rascher-Eggstein, G.; Liebner, S.; Hamm, S.; Duffner, F.; Grote, E.-H.; Risau, W.; Engelhardt, B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003, 105, 586–592. [Google Scholar] [CrossRef]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M. Wnt/β-catenin signaling controls development of the blood–brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Simpson, I.A.; Dwyer, D.; Malide, D.; Moley, K.H.; Travis, A.; Vannucci, S. Metabolism: The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E242–E253. [Google Scholar] [CrossRef]
- Dempsey, E.W.; Wislocki, G.B. An electron microscopic study of the blood-brain barrier in the rat, employing silver nitrate as a vital stain. J. Cell Biol. 1955, 1, 245–256. [Google Scholar] [CrossRef]
- Van Breemen, V.L.; Clemente, C.D. Silver deposition in the central nervous system and the hematoencephalic barrier studied with the electron microscope. J. Biophys. Biochem. Cytol. 1955, 1, 161–166. [Google Scholar] [CrossRef]
- Luse, S.A. Electron microscopic observations of the central nervous system. J. Cell Biol. 1956, 2, 531–542. [Google Scholar] [CrossRef]
- Gerschenfeld, H.; Wald, F.; Zadunaisky, J.; De Robertis, E.P. Function of astroglia in the water-ion metabolism of the central nervous system: An electron microscope study. Neurology 1959, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- McConnell, H.L.; Mishra, A. Cells of the blood–brain barrier: An overview of the neurovascular unit in health and disease. Methods Protoc. 2022, 2492, 3–24. [Google Scholar]
- Naranjo, O.; Osborne, O.; Torices, S.; Toborek, M. In vivo targeting of the neurovascular unit: Challenges and advancements. Cell. Mol. Neurobiol. 2022, 42, 2131–2146. [Google Scholar] [CrossRef]
- Eberth, C. Handbuch der Lehre von der Gewegen des Menschen und der Tiere; Engelmann: Leipzig, Germany, 1871. [Google Scholar]
- Rouget, C. Memoire sur les Development, la Structure et la Proprietes Physiologiques des Capillaires Sanguines et Lymphatiques; Archives of Physiology and Biochemistry: Frankfurt, Germany, 1873; pp. 603–663. [Google Scholar]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Underly, R.G.; Grant, R.I.; Watson, A.N.; Lindner, V.; Shih, A.Y. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015, 2, 041402. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Guo, Z.-N.; Zhang, D.-H.; Qu, Y.; Jin, H. The role of pericytes in ischemic stroke: Fom cellular functions to therapeutic targets. Front. Mol. Neurosci. 2022, 15, 866700. [Google Scholar] [CrossRef]
- Lees, K.R.; Bluhmki, E.; Von Kummer, R.; Brott, T.G.; Toni, D.; Grotta, J.C.; Albers, G.W.; Kaste, M.; Marler, J.R.; Hamilton, S.A. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010, 375, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Prinz, M. Microglia heterogeneity in the single-cell era. Cell Rep. 2020, 30, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Paza, E.; Perkins, E.M.; James, O.G.; Kenkhuis, B.; Lloyd, A.F.; Burr, K.; Story, D.; Yusuf, D.; He, X.; et al. Generation of pure monocultures of human microglia-like cells from induced pluripotent stem cells. Stem Cell Res. 2020, 49, 102046. [Google Scholar] [CrossRef]
- Bathini, P.; Dupanloup, I.; Zenaro, E.; Terrabuio, E.; Fischer, A.; Ballabani, E.; Doucey, M.A.; Alberi, L. Systemic Inflammation Causes Microglial Dysfunction with a Vascular AD phenotype. Brain Behav. Immun. Health 2023, 28, 100568. [Google Scholar] [CrossRef] [PubMed]
- Benmamar-Badel, A.; Owens, T.; Wlodarczyk, A. Protective microglial subset in development, aging, and disease: Lessons from transcriptomic studies. Front. Immunol. 2020, 11, 430. [Google Scholar] [CrossRef]
- Bouchè, V.; Aldegheri, G.; Donofrio, C.A.; Fioravanti, A.; Roberts-Thomson, S.; Fox, S.B.; Schettini, F.; Generali, D. BRAF signaling inhibition in glioblastoma: Which clinical perspectives? Front. Oncol. 2021, 11, 772052. [Google Scholar] [CrossRef]
- Bíró, T.; Oláh, A.; Tóth, B.I.; Szöllősi, A.G. Endogenous Factors That Can Influence Skin pH. Curr. Probl. Dermatol. 2018, 54, 54–63. [Google Scholar]
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.W.; Wijmenga, C.; van Schooten, F.J. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef]
- Engelhardt, B.; Liebner, S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014, 355, 687–699. [Google Scholar] [CrossRef]
- Raab, S.; Beck, H.; Gaumann, A.; Yüce, A.; Gerber, H.P.; Plate, K.; Hammes, H.P.; Ferrara, N.; Breier, G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. J. Thromb. Haemost. 2004, 91, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.; Mikhailova, N. The Role of Dll4/Notch Signaling in Normal and Pathological Ocular Angiogenesis: Dll4 Controls Blood Vessel Sprouting and Vessel Remodeling in Normal and Pathological Conditions. J. Ophthalmol. 2018, 2018, 3565292. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat. Rev. Neurosci. 2020, 21, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Whiteus, C.; Freitas, C.; Grutzendler, J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature 2014, 505, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hogan-Cann, A.D.; Globa, A.K.; Lu, P.; Nagy, J.I.; Bamji, S.X.; Anderson, C.M. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J. Cereb. Blood Flow Metab. 2019, 39, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Vazana, U.; Veksler, R.; Pell, G.S.; Prager, O.; Fassler, M.; Chassidim, Y.; Roth, Y.; Shahar, H.; Zangen, A.; Raccah, R.; et al. Glutamate-Mediated Blood-Brain Barrier Opening: Implications for Neuroprotection and Drug Delivery. J. Neurosci. 2016, 36, 7727–7739. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.S.; Sherrington, C.S. On the Regulation of the Blood-supply of the Brain. J. Physiol. 1890, 11, 85–158.117. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, J.; Zafar, R.; Kantorovich, S.; Jiang, R.; Carney, P.R.; Jiang, H. Cortical neurovascular coupling driven by stimulation of channelrhodopsin-2. PLoS ONE 2012, 7, e46607. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Toussay, X.; Anenberg, E.; Lecrux, C.; Ferreirós, N.; Karagiannis, A.; Plaisier, F.; Chausson, P.; Jarlier, F.; Burgess, S.A.; et al. COX-2-Derived Prostaglandin E2 Produced by Pyramidal Neurons Contributes to Neurovascular Coupling in the Rodent Cerebral Cortex. J. Neurosci. 2015, 35, 11791–11810. [Google Scholar] [CrossRef]
- Banks, W.A. Brain meets body: The blood-brain barrier as an endocrine interface. Endocrinology 2012, 153, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Maggioli, E.; McArthur, S.; Mauro, C.; Kieswich, J.; Kusters, D.; Reutelingsperger, C.; Yaqoob, M.; Solito, E. Estrogen protects the blood–brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav. Immun. 2016, 51, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Lee, J.Y.; Kim, W.-S.; Yune, T.Y.; Ju, B.-G. 17β-estradiol ameliorates tight junction disruption via repression of MMP transcription. J. Mol. Endocrinol. 2015, 29, 1347–1361. [Google Scholar] [CrossRef]
- Abi-Ghanem, C.; Robison, L.S.; Zuloaga, K.L. Androgens’ effects on cerebrovascular function in health and disease. Biol. Sex Differ. 2020, 11, 35. [Google Scholar] [CrossRef]
- Atallah, A.; Mhaouty-Kodja, S.; Grange-Messent, V. Metabolism: Chronic depletion of gonadal testosterone leads to blood–brain barrier dysfunction and inflammation in male mice. J. Cereb. Blood Flow Metab. 2017, 37, 3161–3175. [Google Scholar] [CrossRef]
- Liu, W.Y.; Wang, Z.B.; Zhang, L.C.; Wei, X.; Li, L. Tight junction in blood-brain barrier: An overview of structure, regulation, and regulator substances. CNS Neurosci. Ther. 2012, 18, 609–615. [Google Scholar] [CrossRef]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565 Pt 2, 475–486. [Google Scholar] [CrossRef]
- Jeftha, T.; Makhathini, K.B.; Fisher, D. The Effect of Dexamethasone on Lipopolysaccharide-induced Inflammation of Endothelial Cells of the Blood-brain Barrier/Brain Capillaries. Curr. Neurovasc. Res. 2023, 20, 334–345. [Google Scholar] [CrossRef]
- Langston, J.W.; Li, W.; Harrison, L.; Aw, T.Y. Activation of promoter activity of the catalytic subunit of γ-glutamylcysteine ligase (GCL) in brain endothelial cells by insulin requires antioxidant response element 4 and altered glycemic status: Implication for GCL expression and GSH synthesis. Free Radic. Biol. Med. 2011, 51, 1749–1757. [Google Scholar] [CrossRef]
- Helms, H.C.; Waagepetersen, H.S.; Nielsen, C.U.; Brodin, B. Paracellular tightness and claudin-5 expression is increased in the BCEC/astrocyte blood-brain barrier model by increasing media buffer capacity during growth. AAPS J. 2010, 12, 759–770. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef]
- Ding, G.R.; Qiu, L.B.; Wang, X.W.; Li, K.C.; Zhou, Y.C.; Zhou, Y.; Zhang, J.; Zhou, J.X.; Li, Y.R.; Guo, G.Z. EMP-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Toxicol. Lett. 2010, 196, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Stanness, K.A.; Westrum, L.E.; Fornaciari, E.; Mascagni, P.; Nelson, J.A.; Stenglein, S.G.; Myers, T.; Janigro, D. Morphological and functional characterization of an in vitro blood-brain barrier model. Brain Res. 1997, 771, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Krizanac-Bengez, L.; Kapural, M.; Parkinson, F.; Cucullo, L.; Hossain, M.; Mayberg, M.R.; Janigro, D. Effects of transient loss of shear stress on blood-brain barrier endothelium: Role of nitric oxide and IL-6. Brain Res. 2003, 977, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Jeliazkova-Mecheva, V.V.; Hymer, W.C.; Nicholas, N.C.; Bobilya, D.J. Brief heat shock affects the permeability and thermotolerance of an in vitro blood-brain barrier model of porcine brain microvascular endothelial cells. Microvasc. Res. 2006, 71, 108–114. [Google Scholar] [CrossRef]
- Carver, K.A.; Lourim, D.; Tryba, A.K.; Harder, D.R. Rhythmic expression of cytochrome P450 epoxygenases CYP4x1 and CYP2c11 in the rat brain and vasculature. Am. J. Physiol. Cell Physiol. 2014, 307, C989–C998. [Google Scholar] [CrossRef]
- Schurhoff, N.; Toborek, M. Circadian rhythms in the blood-brain barrier: Impact on neurological disorders and stress responses. Mol. Brain 2023, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.L.; Pulaski, L.; Gottesman, M.M.; Pastan, I. Analysis of the properties of the N-terminal nucleotide-binding domain of human P-glycoprotein. Biochemistry 2000, 39, 5518–5526. [Google Scholar] [CrossRef]
- Kervezee, L.; Hartman, R.; van den Berg, D.J.; Shimizu, S.; Emoto-Yamamoto, Y.; Meijer, J.H.; de Lange, E.C. Diurnal variation in P-glycoprotein-mediated transport and cerebrospinal fluid turnover in the brain. AAPS J. 2014, 16, 1029–1037. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yue, Z.; Arnold, D.M.; Artiushin, G.; Sehgal, A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 2018, 173, 130–139.e110. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Chen, Y.; Hao, C.; Shen, J.; Chen, W.; Cheng, W.; Yan, S.; Li, J.; Li, Y.; Gulizhaerkezi, T.; et al. The effects of acupuncture on depression by regulating BDNF-related balance via lateral habenular nucleus BDNF/TrkB/CREB signaling pathway in rats. Behav. Brain Res. 2023, 451, 114509. [Google Scholar] [CrossRef]
- Perlegos, A.E.; Shields, E.J.; Shen, H.; Liu, K.F.; Bonini, N.M. Mettl3-dependent m(6)A modification attenuates the brain stress response in Drosophila. Nat. Commun. 2022, 13, 5387. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Miller, G.E.; Cohen, S.; Ritchey, A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002, 21, 531–541. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Powell, N.D.; Godbout, J.P.; Sheridan, J.F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 2013, 33, 13820–13833. [Google Scholar] [CrossRef] [PubMed]
- McKim, D.B.; Weber, M.D.; Niraula, A.; Sawicki, C.M.; Liu, X.; Jarrett, B.L.; Ramirez-Chan, K.; Wang, Y.; Roeth, R.M.; Sucaldito, A.D.; et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 2018, 23, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Wang, Y.; Godbout, J.P.; Sheridan, J.F. Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression. J. Neurosci. 2018, 38, 2328–2340. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, L.; Yang, H.; Zhang, Z.; Yang, X.; Zhang, L.; He, S. Induction of IL-13 production and upregulation of gene expression of protease activated receptors in P815 cells by IL-6. Cytokine 2010, 50, 138–145. [Google Scholar] [CrossRef]
- Esposito, P.; Gheorghe, D.; Kandere, K.; Pang, X.; Connolly, R.; Jacobson, S.; Theoharides, T.C. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001, 888, 117–127. [Google Scholar] [CrossRef]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood-Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Zhang, Y.; Zheng, W.; Davidson, T.L. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J. Alzheimers Dis. 2010, 21, 207–219. [Google Scholar] [CrossRef]
- Chen, X.; Ghribi, O.; Geiger, J.D. Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer’s and Parkinson’s diseases. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S127–S141. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M. For lack of gut microbes, the blood-brain barrier ‘leaks’. Lab. Anim. 2015, 44, 6. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A. The tantalizing links between gut microbes and the brain. Nature 2015, 526, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Wang, F.; Ding, G.; Chen, W.; Fang, R.; Yao, Y.; Pang, M.; Lu, Z.Q.; Liu, J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016, 1642, 70–78. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; Carro, E.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef]
- Nishijima, T.; Piriz, J.; Duflot, S.; Fernandez, A.M.; Gaitan, G.; Gomez-Pinedo, U.; Verdugo, J.M.; Leroy, F.; Soya, H.; Nuñez, A.; et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 2010, 67, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Monnier, A.; Garnier, P.; Quirie, A.; Pernet, N.; Demougeot, C.; Marie, C.; Prigent-Tessier, A. Effect of short-term exercise training on brain-derived neurotrophic factor signaling in spontaneously hypertensive rats. J. Hypertens. 2017, 35, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hrvatin, S.; Hochbaum, D.R.; Nagy, M.A.; Cicconet, M.; Robertson, K.; Cheadle, L.; Zilionis, R.; Ratner, A.; Borges-Monroy, R.; Klein, A.M.; et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci. 2018, 21, 120–129. [Google Scholar] [CrossRef]
- Cash, A.; Theus, M.H. Mechanisms of blood–brain barrier dysfunction in traumatic brain injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Price, L.; Wilson, C.; Grant, G. Blood–brain barrier pathophysiology following traumatic brain injury. In Translational Research in Traumatic Brain Injury; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Harvey, L.A.; Close, J.C. Traumatic brain injury in older adults: Characteristics, causes and consequences. Injury 2012, 43, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Impact of inflammation on the blood–neural barrier and blood–nerve interface: From review to therapeutic preview. Int. Rev. Neurobiol. 2017, 137, 29–45. [Google Scholar]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Spindler, K.R.; Hsu, T.-H. Viral disruption of the blood–brain barrier. Trends Microbiol. 2012, 20, 282–290. [Google Scholar] [CrossRef]
- Nottet, H.S. Interactions between macrophages and brain microvascular endothelial cells: Role in pathogenesis of HIV-1 infection and blood-brain barrier function. J. Neurovirol. 1999, 5, 659–669. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nian, K.; Harding, I.C.; Herman, I.M.; Ebong, E.E. Blood-brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front. Physiol. 2020, 11, 605398. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; De Silva, T.M.; Chen, J.; Faraci, F.M. Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ. Res. 2017, 120, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Gundert-Remy, U.; Stahlmann, R. The blood-brain barrier in toxicology. Front. Pharmacol. 2010, 1. [Google Scholar] [CrossRef]
- Liu, J.T.; Dong, M.H.; Zhang, J.Q.; Bai, Y.; Kuang, F.; Chen, L.W. Microglia and astroglia: The role of neuroinflammation in lead toxicity and neuronal injury in the brain. Neuroimmunol. Neuroinflamm. 2015, 2, 131–137. [Google Scholar]
- Wu, Y.-C.; Sonninen, T.-M.; Peltonen, S.; Koistinaho, J.; Lehtonen, Š. Blood–brain barrier and neurodegenerative diseases—Modeling with iPSC-derived brain cells. Int. J. Mol. Sci. 2021, 22, 7710. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O.; Mastorakis, N.E. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol. Res. 2020, 157, 104769. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, Z.; Fan, T.; Wang, K.; Li, S.; Zhao, J.; Zhu, W. Childhood social isolation causes anxiety-like behaviors via the damage of blood-brain barrier in amygdala in female mice. Front. Cell Dev. Biol. 2022, 10, 943067. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Byun, H.-M.; Chung, E.-C.; Chung, H.-Y.; Bae, O.-N. Loss of integrity: Impairment of the blood-brain barrier in heavy metal-associated ischemic stroke. Toxicol. Res. 2013, 29, 157–164. [Google Scholar] [CrossRef][Green Version]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part, I. mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Tobwala, S.; Wang, H.-J.; Carey, J.W.; Banks, W.A.; Ercal, N. Effects of lead and cadmium on brain endothelial cell survival, monolayer permeability, and crucial oxidative stress markers in an in vitro model of the blood-brain barrier. Toxics 2014, 2, 258–275. [Google Scholar] [CrossRef]
- Sun, X.; He, Y.; Guo, Y.; Li, S.; Zhao, H.; Wang, Y.; Zhang, J.; Xing, M. Arsenic affects inflammatory cytokine expression in Gallus gallus brain tissues. BMC Vet. Res. 2017, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Aschner, J.L. Mercury neurotoxicity: Mechanisms of blood-brain barrier transport. Neurosci. Biobehav. Rev. 1990, 14, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, R.D.; Bell, K.; Canfield, S. The Effects of Multiple Exposure Ethanol on Barrier Tightness and Passive Permeability in a Human Stem Cell Derived Blood-Brain Barrier Model. In Proceedings of the IMPRS, Indianapolis, IN, USA, 15 December 2020; Volume 3. [Google Scholar]
- Gu, H.; Territo, P.R.; Persohn, S.A.; Bedwell, A.A.; Eldridge, K.; Speedy, R.; Chen, Z.; Zheng, W.; Du, Y. Evaluation of chronic lead effects in the blood brain barrier system by DCE-CT. J. Trace Elem. Med. Biol. 2020, 62, 126648. [Google Scholar] [CrossRef] [PubMed]
- Lohren, H.; Bornhorst, J.; Fitkau, R.; Pohl, G.; Galla, H.-J.; Schwerdtle, T. Effects on and transfer across the blood-brain barrier in vitro—Comparison of organic and inorganic mercury species. BMC Pharmacol. Toxicol. 2016, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Xin-Xin, T.; Jing-Ping, Z.; Jian-Hua, W.; Yu, L. Effects of L-NAME on blood-brain barrier permeability after acute carbon monoxide poisoning in rats. Acad. J. Second Mil. Med. Univ. 2004, 25, 896–897. [Google Scholar]
- Barr, J.L.; Brailoiu, G.C.; Abood, M.E.; Rawls, S.M.; Unterwald, E.M.; Brailoiu, E. Acute cocaine administration alters permeability of blood-brain barrier in freely-moving rats—Evidence using miniaturized fluorescence microscopy. Drug Alcohol. Depend. 2020, 206, 107637. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Abbruscato, T.J.; Egleton, R.D.; Brown, R.C.; Huber, J.D.; Campos, C.R.; Davis, T.P. Nicotine increases in vivo blood–brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004, 1027, 48–58. [Google Scholar] [CrossRef]
- Kang, Y.J.; Tan, H.Y.; Lee, C.Y.; Cho, H. An air particulate pollutant induces neuroinflammation and neurodegeneration in human brain models. Adv. Sci. 2021, 8, 2101251. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Agarwal, R.; Shukla, G.S. Functional impairment of blood-brain barrier following pesticide exposure during early development in rats. Hum. Exp. Toxicol. 1999, 18, 174–179. [Google Scholar] [PubMed]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163, 144–171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F. Autophagy in vascular endothelial cells. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1021–1028. [Google Scholar] [CrossRef]
- Liu, D.Z.; Ander, B.P.; Xu, H.; Shen, Y.; Kaur, P.; Deng, W.; Sharp, F.R. Blood–brain barrier breakdown and repair by Src after thrombin-induced injury. Ann. Neurol. 2010, 67, 526–533. [Google Scholar] [CrossRef]
- Guo, R.; Wang, X.; Fang, Y.; Chen, X.; Chen, K.; Huang, W.; Chen, J.; Hu, J.; Liang, F.; Du, J.; et al. rhFGF20 promotes angiogenesis and vascular repair following traumatic brain injury by regulating Wnt/β-catenin pathway. Biomed. Pharmacother. 2021, 143, 112200. [Google Scholar] [CrossRef]
- Geranmayeh, M.H.; Rahbarghazi, R.; Farhoudi, M. Targeting pericytes for neurovascular regeneration. Cell Commun. Signal. 2019, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Pisani, F.; Castagnola, V.; Simone, L.; Loiacono, F.; Svelto, M.; Benfenati, F. Role of pericytes in blood–brain barrier preservation during ischemia through tunneling nanotubes. Cell Death Dis. 2022, 13, 582. [Google Scholar] [CrossRef]
- Lamagna, C.; Bergers, G. The bone marrow constitutes a reservoir of pericyte progenitors. J. Leukoc. Biol. 2006, 80, 677–681. [Google Scholar] [CrossRef]
- Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 2015, 33, 1962–1974. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Zhang, S.L.; Sehgal, A. Regulation of the blood–brain barrier by circadian rhythms and sleep. Trends Neurosci. 2019, 42, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Oh-oka, K.; Kono, H.; Ishimaru, K.; Miyake, K.; Kubota, T.; Ogawa, H.; Okumura, K.; Shibata, S.; Nakao, A. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: Implications in intestinal permeability and susceptibility to colitis. PLoS ONE 2014, 9, e98016. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, Y.; Ying, H.; Xue, Y.; Zhang, Z.; Wang, P.; Liu, L.; Zhang, H. Increasing of blood-tumor barrier permeability through paracellular pathway by low-frequency ultrasound irradiation in vitro. J. Mol. Neurosci. 2011, 43, 541–548. [Google Scholar] [CrossRef] [PubMed]


| Types of Damage | Potential Causes | Main Mechanisms | Related Diseases | Representative References |
|---|---|---|---|---|
| Mechanical damage | Falls, transportation accidents | Disruption of tight junctions; activation of immune cells; promotion of mast cell degranulation. | Traumatic brain injury Stroke Brain tumors Epilepsy | Cash, A. and Theus, M. H. (2020) [99]. Price, L.; Wilson, C.; Grant, G. (2016) [100]. Harvey, L. A. and Close, J. C. T. (2012) [101]. |
| Inflammatory and immune-mediated damage | Injuries, infections, neurodegenerative processes | Disruption of tight junctions; activation of immune cells; promotion of mast cell degranulation. | Multiple sclerosis Meningitis Encephalitis HIV-associated cerebral vasculitis | Skaper, S. D. (2017) [102]. Huang, X., Hussain, B., and Chang, J. (2020) [103]. |
| Infection-related damage | Neutrophil microbes such as Neisseria meningitides viruses or bacteria-mediated damage | Release of cytokines and chemokines by immune cells; disruption of tight junctions; physical disruption; formation of Neutrophil Extracellular Traps. | Meningitis Encephalitis Neurosyphilis Toxoplasmosis AIDS | Goverdhan, P. and Pachter, J. S. (2012) [104]. H. S. L. M. (1999) [105]. Varatharaj, A. and Galea, I. (2016) [106]. |
| Ischemic and vascular BBB damage | BBB disruption via ischemia or elevated blood pressure | BBB breakdown due to ischemia and high blood pressure, affecting ECs and basal lamina. | Ischemic stroke Hypertension | Nian, K., Harding, I. C., Herman, I. M. and Ebong, E. E. (2020) [107]. Xiaoming Hu, T., Michael De Silva, Jun Chen (2017) [108]. |
| Toxic-factor-induced damage | Heavy metals, pesticides, chemicals, radiation | Induction of cellular damage, oxidative stress, or inflammation within BBB cells. | Diabetes mellitus Brain tumors Amyotrophic lateral sclerosis | Gundert-Remy, U. and Stahlmann, R. (2010) [109]. Jin-Tao Liu, Mo-Han Dong, Jie-Qiong Zhang (2015) [110]. |
| Protein aggregate-related damage | Aging, genetic-related protein aggregates | Accumulation of protein aggregates in certain neurodegenerative diseases, which can directly interact with the BBB and disrupt its functions. | Alzheimer’s disease Multiple sclerosis Parkinson’s disease Huntington’s disease Prion diseases | Wu, Y.-C., Sonninen, T.-M., Peltonen, S., Koistinaho, J., and Lehtonen, Š. (2021) [111]. |
| Mental-health induced-damage | Social isolation stress | Decreased expression of Claudin-5; microglial activation in the amygdala in female mice. | Multiple sclerosis PTSD Epilepsy | Welcome, M.O. and Mastorakis, N.E. (2020) [112]. Wu, X., Ding, Z., Fan, T. (2022) [113]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fong, H.; Zhou, B.; Feng, H.; Luo, C.; Bai, B.; Zhang, J.; Wang, Y. Recapitulation of Structure–Function–Regulation of Blood–Brain Barrier under (Patho)Physiological Conditions. Cells 2024, 13, 260. https://doi.org/10.3390/cells13030260
Fong H, Zhou B, Feng H, Luo C, Bai B, Zhang J, Wang Y. Recapitulation of Structure–Function–Regulation of Blood–Brain Barrier under (Patho)Physiological Conditions. Cells. 2024; 13(3):260. https://doi.org/10.3390/cells13030260
Chicago/Turabian StyleFong, Hin, Botao Zhou, Haixiao Feng, Chuoying Luo, Boren Bai, John Zhang, and Yuechun Wang. 2024. "Recapitulation of Structure–Function–Regulation of Blood–Brain Barrier under (Patho)Physiological Conditions" Cells 13, no. 3: 260. https://doi.org/10.3390/cells13030260
APA StyleFong, H., Zhou, B., Feng, H., Luo, C., Bai, B., Zhang, J., & Wang, Y. (2024). Recapitulation of Structure–Function–Regulation of Blood–Brain Barrier under (Patho)Physiological Conditions. Cells, 13(3), 260. https://doi.org/10.3390/cells13030260






