Changes in the Composition of Unstimulated and Stimulated Saliva Due to Chewing Sour Cherry Gum and a Toothbrush Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
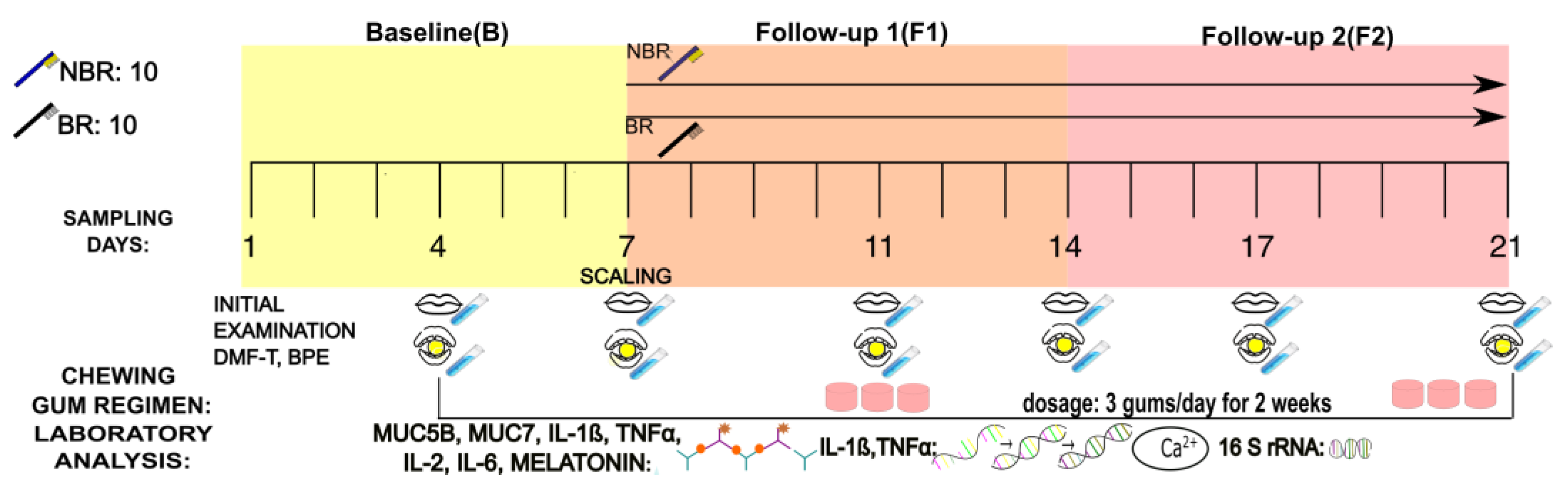
2.2. Study Design
2.3. Collection of Mixed Unstimulated and Stimulated Saliva
2.4. Preparation of the Chewing Gum with Sour Cherry Extract
2.5. ELISA Methods
2.5.1. Determination of MUC5B Concentration
2.5.2. Determination of MUC7 Concentration
2.5.3. Determination of IL-1β Concentration
2.5.4. Determination of TNFα Concentration
2.5.5. Determination of IL-2 Concentration
2.5.6. Determination of IL-6 Concentration
2.5.7. Determination of Melatonin Concentration
2.6. PCR Method
2.6.1. RNA Isolation with the MagMax Viral RNA Isolation Kit
2.6.2. RNA Transcription
2.7. Statistical Analysis of the PCR, ELISA Results and Clinical Data
2.8. Determination of Ca2+ Concentrations
3. 16S rRNA (Svedberg Ribonucleic Acid) Gene Sequencing Method
3.1. Isolation of Saliva Samples
3.2. Library Preparation
3.3. Bioinformatic Analysis
3.4. Core Microbiome
3.5. Correlation between the Microbiota, Cytokines, Mucins, and Melatonin
4. Results
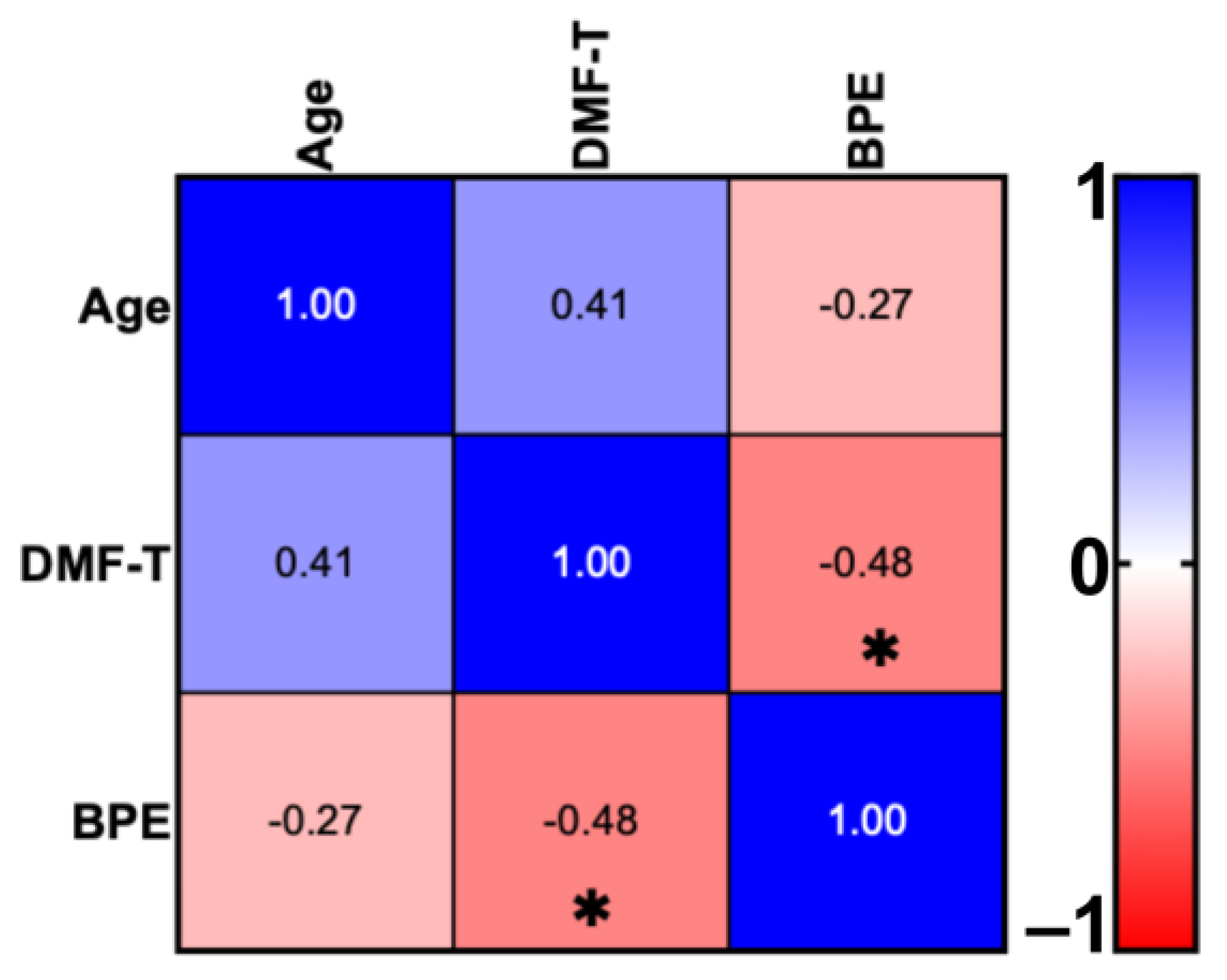
4.1. DMF-T Status of the Groups by Toothbrush Change and Age
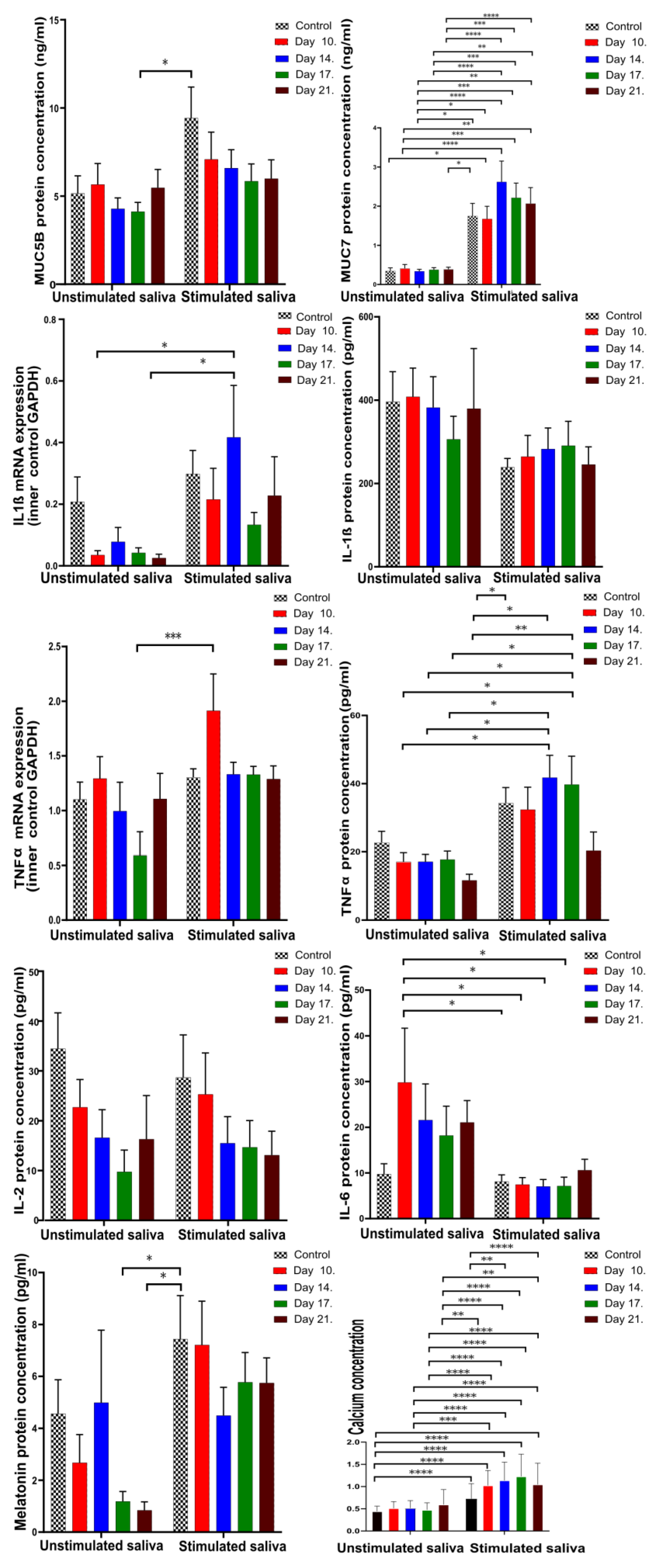
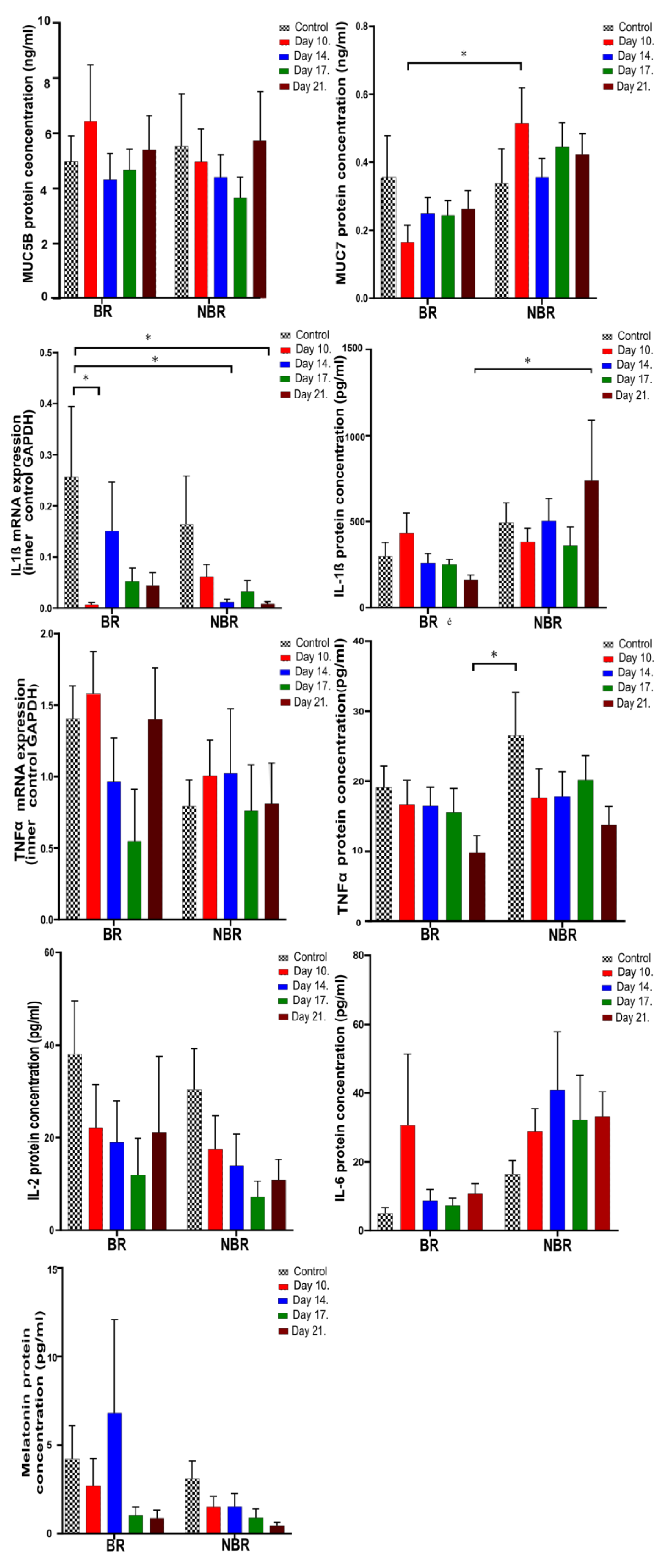
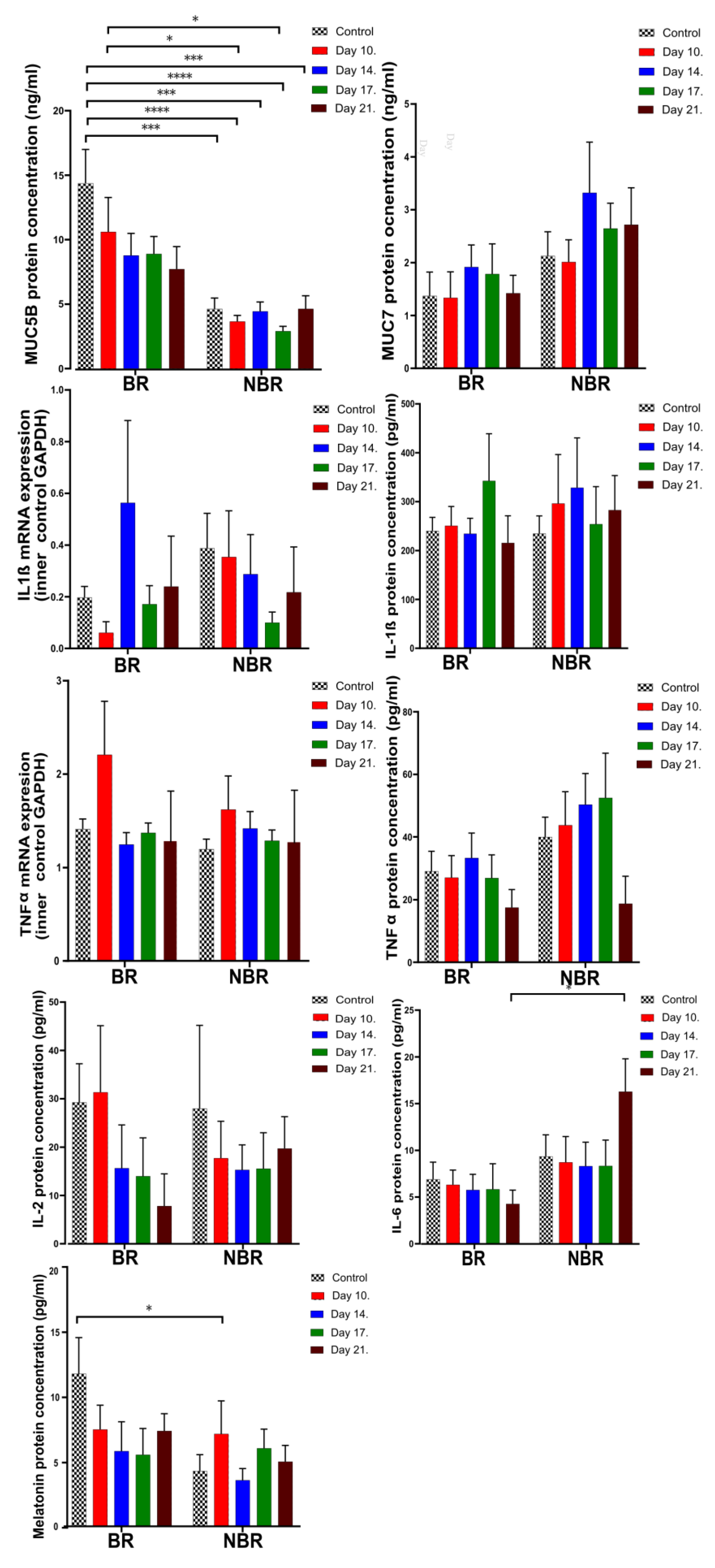
4.2. Pro-Inflammatory Cytokines, Mucins, and Melatonin Levels in Unstimulated and Stimulated Saliva
4.2.1. Comparison of Unstimulated and Stimulated Saliva
4.2.2. The Effects of Toothbrush Change on Unstimulated Saliva
4.2.3. The Effects of Toothbrush Change on Stimulated Saliva
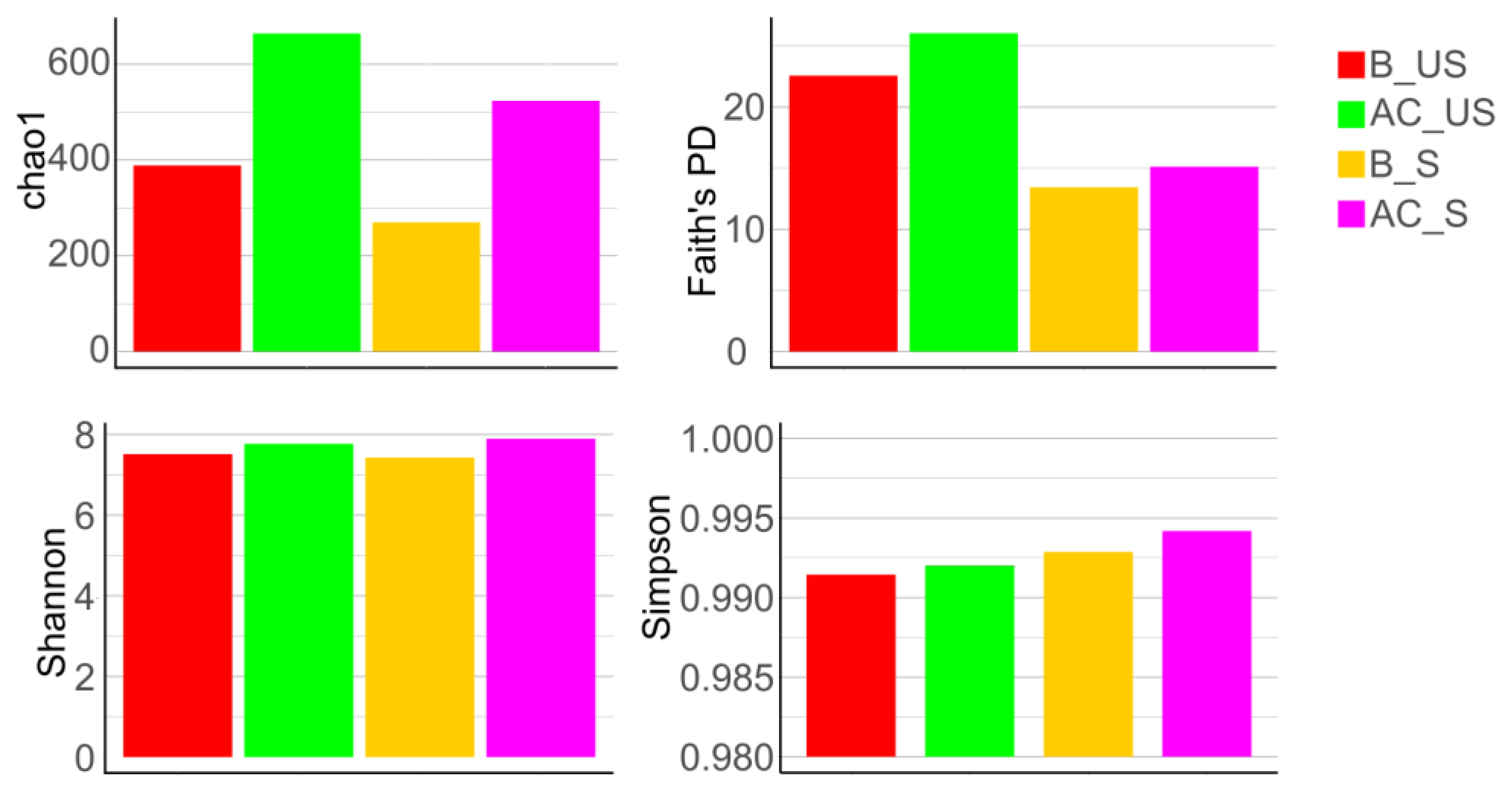
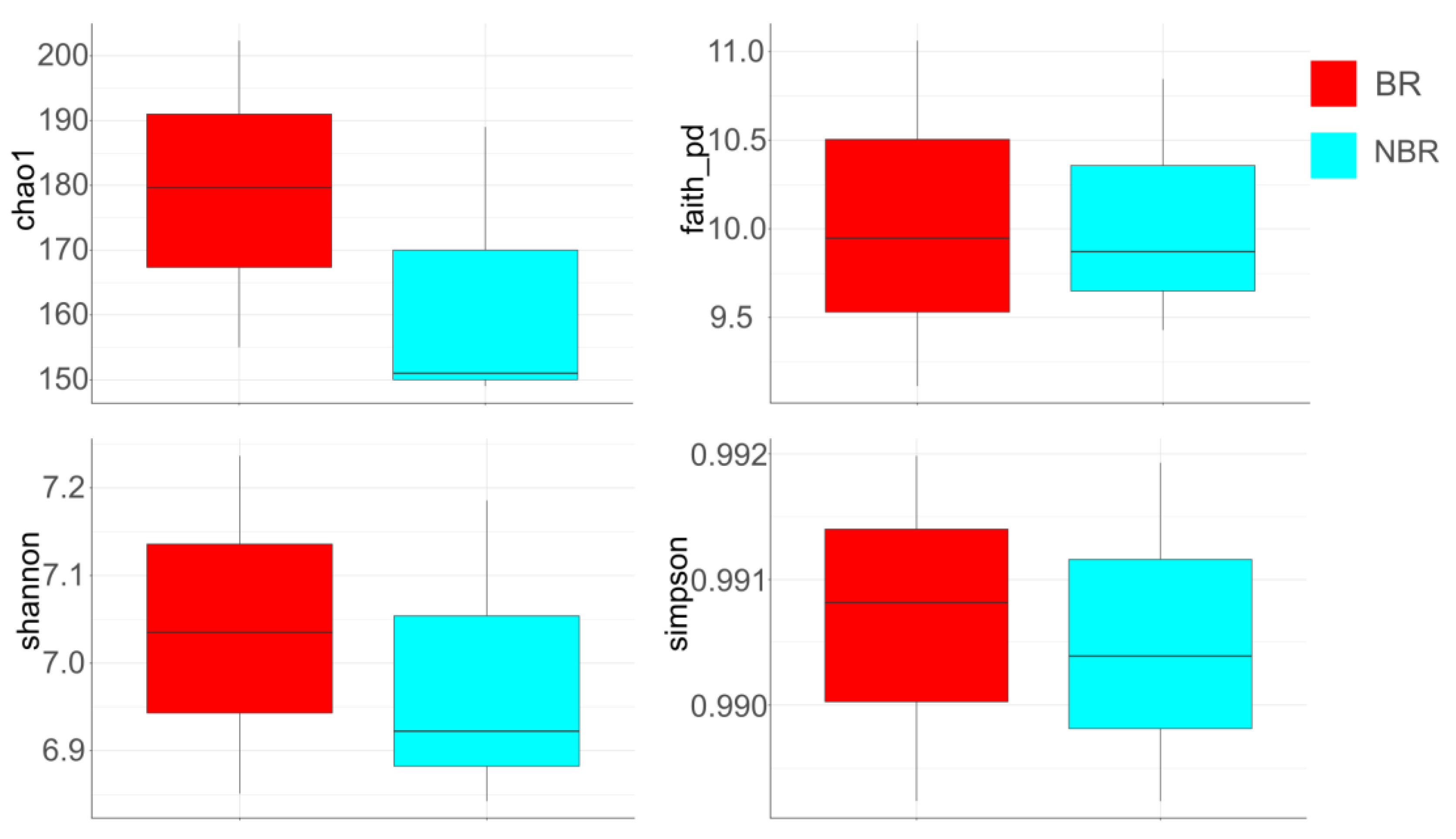
4.2.4. Alpha Diversity
Comparison of Unstimulated and Stimulated Saliva
Alpha Diversity of Saliva in Groups Based on Toothbrush Change
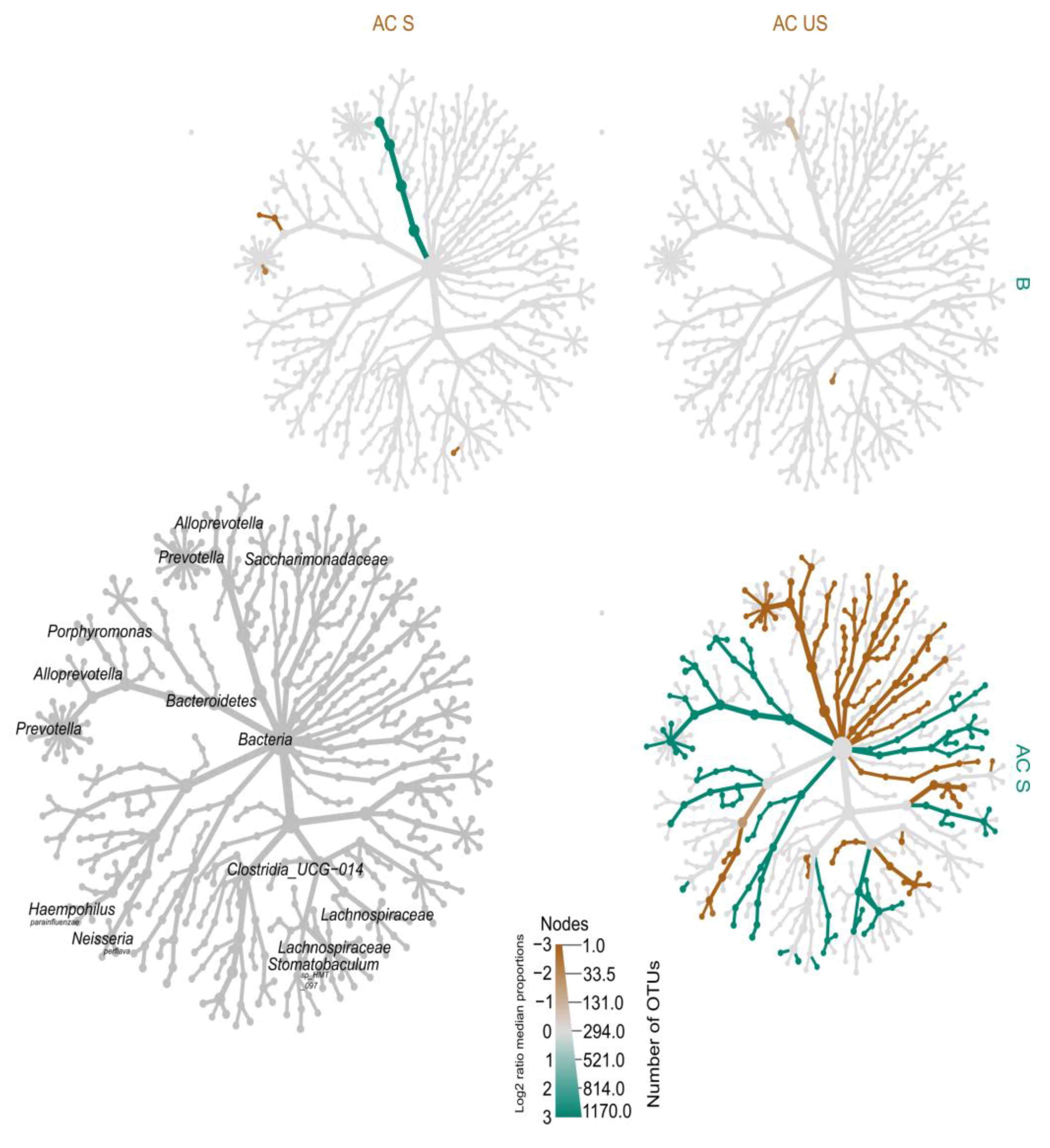
4.2.5. Heat-Tree of Microbiota Profiles in Saliva
Heat-Tree Demonstrating Unstimulated and Stimulated Saliva
Heat-Tree Demonstrating Stimulated Saliva
4.2.6. Core Microbiome
4.2.7. Correlation of the Microbiota with Cytokines, Mucins, and Melatonin
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, P.; Hussain, A.; Carrasco-Labra, A.; Siqueira, W.L. Salivary Proteins as Dental Caries Biomarkers: A Systematic Review. Caries Res. 2022, 56, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B.; Takahashi, N. Integrated hypothesis of dental caries and periodontal diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef] [PubMed]
- Kaidonis, J.; Townsend, G. The ‘sialo–microbial–dental complex’ in oral health and disease. Ann. Anat. 2016, 203, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins promote the coexistence of competing oral bacterial species. ISME J. 2017, 11, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins in host defense and disease prevention. J. Oral Microbiol. 2015, 7, 29759. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Passel, M.W.; van de Bovenkamp, J.H.; Schipper, R.G.; de Vos, W.M.; Dekker, J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes 2010, 1, 254–268. [Google Scholar] [CrossRef]
- Barrera, M.J.; Aguilera, S.; Veerman, E.; Quest, A.F.; Díaz-Jiménez, D.; Urzúa, U.; Cortés, J.; González, S.; Castro, I.; Molina, C.; et al. Salivary mucins induce a Toll-like receptor 4-mediated pro-inflammatory response in human submandibular salivary cells: Are mucins involved in Sjögren’s syndrome? Rheumatology 2015, 54, 1518–1527. [Google Scholar] [CrossRef]
- Simon-Soro, A.; Ren, Z.; Krom, B.P.; Hoogenkamp, M.A.; Cabello-Yeves, P.J.; Daniel, S.G.; Bittinger, K.; Tomas, I.; Koo, H.; Mira, A. Polymicrobial Aggregates in Human Saliva Build the Oral Biofilm. mBio 2022, 13, e0013122. [Google Scholar] [CrossRef]
- Li, S.; Bobek, L.A. Functional analysis of human MUC7 mucin gene 5’-flanking region in lung epithelial cells. Am. J. Respir. Cell Mol. Biol. 2006, 35, 593–601. [Google Scholar] [CrossRef]
- Kesimer, M.; Kiliç, N.; Mehrotra, R.; Thornton, D.J.; Sheehan, J.K. Identification of salivary mucin MUC7 binding proteins from Streptococcus gordonii. BMC Microbiol. 2009, 9, 163. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Bijle, M.N.; Ekambaram, M.; Lo, E.C.M.; Yiu, C.K.Y. Synbiotics in caries prevention: A scoping review. PLoS ONE 2020, 15, e0237547. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins protect surfaces from colonization by cariogenic bacteria. Appl. Environ. Microbiol. 2015, 81, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Van Seuningen, I.; Pigny, P.; Perrais, M.; Porchet, N.; Aubert, J.P. Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer? Front. Biosci. 2001, 6, D1216–D1234. [Google Scholar] [CrossRef] [PubMed]
- Slotwinska, S.M.; Zaleska, M. Salivary interleukin-1 beta and Streptococcus mutans level in oral cavity. Cent. Eur. J. Immunol. 2012, 37, 151–153. [Google Scholar]
- Boumba, D.; Skopouli, F.N.; Moutsopoulos, H.M. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren’s syndrome. Br. J. Rheumatol. 1995, 34, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Bielawska, A.; Bielawski, K.; Grabowska, S.Z.; Wójcicka, A.; Zalewska, M.; Maciorkowska, E. Pro-inflammatory cytokines in saliva of adolescents with dental caries disease. Ann. Agric. Environ. Med. AAEM 2012, 19, 711–716. [Google Scholar]
- Govula, K.; Anumula, L.; Swapna, S.; Kirubakaran, R. Interleukin-6: A potential salivary biomarker for dental caries progression—A Cross-sectional study. Int. J. Exp. Dent. Sci. 2021, 10, 8–13. [Google Scholar]
- Van Nieuw Amerongen, A.; Bolscher, J.G.; Veerman, E.C. Salivary proteins: Protective and diagnostic value in cariology? Caries Res. 2004, 38, 247–253. [Google Scholar] [CrossRef]
- Saeralaathan, S.; Rajkumar, A.; Balaji, T.M.; Raj, A.T.; Ganesh, A. Salivary melatonin is depleted in patients with dental caries due to the elevated oxidative stress. J. Oral Biol. Craniofacial Res. 2021, 11, 547–551. [Google Scholar] [CrossRef]
- Di Stefano, A.; Paulesu, L. Inhibitory effect of melatonin on production of IFN gamma or TNF alpha in peripheral blood mononuclear cells of some blood donors. J. Pineal Res. 1994, 17, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Bardow, A.V.A. Saliva and caries development. In Dental Caries The Disease and Its Clinical Management; Fejerskov, O., Nyvad, B., Kidd, E., Eds.; Wliey Blackwell: Oxford, UK, 2015. [Google Scholar]
- Morris, C.; Fichtel, S.L.; Taylor, A.J. Impact of Calcium on Salivary α-Amylase Activity, Starch Paste Apparent Viscosity, and Thickness Perception. Chemosens. Percept. 2011, 4, 116–122. [Google Scholar] [CrossRef]
- Dawes, C.; Dong, C. The flow rate and electrolyte composition of whole saliva elicited by the use of sucrose-containing and sugar-free chewing-gums. Arch. Oral Biol. 1995, 40, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Homoki, J.R.; Gyémánt, G.; Balogh, P.; Stündl, L.; Bíró-Molnár, P.; Paholcsek, M.; Váradi, J.; Fenyvesi, F.; Kelentey, B.; Nemes, J.; et al. Sour Cherry Extract Inhibits Human Salivary α-amylase and Growth of Streptococcus Mutans (A Pilot Clinical Study). Food Funct. 2018, 9, 4008–4016. [Google Scholar] [CrossRef] [PubMed]
- Bahador, A.; Lesan, S.; Kashi, N. Effect of Xylitol on Cariogenic and Beneficial Oral Streptococci: A Randomized, Double-Blind Crossover Trial. Iran. J. Microbiol. 2012, 4, 75–81. [Google Scholar] [PubMed]
- Skopkó, B.; Paholcsek, M.; Szilágyi-Rácz, A.; Fauszt, P.; Dávid, P.; Stündl, L.; Váradi, J.; Kovács, R.; Bágyi, K.; Remenyik, J. High-Throughput Sequencing Analysis of the Changes in the Salivary Microbiota of Hungarian Young and Adult Subpopulation by an Anthocyanin Chewing Gum and Toothbrush Change. Dent. J. 2023, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Lif Holgerson, P.; Sjöström, I.; Stecksén-Blicks, C.; Twetman, S. Dental plaque formation and salivary mutans streptococci in schoolchildren after use of xylitol-containing chewing gum. Int. J. Paediatr. Dent. 2007, 17, 79–85. [Google Scholar] [CrossRef]
- Söderling, E.; Hirvonen, A.; Karjalainen, S.; Fontana, M.; Catt, D.; Liisa, S. The effect of Xylitol on the Composition of the Oral Flora: A Pilot Study. Eur. J. Dent. 2011, 5, 24–31. [Google Scholar] [CrossRef]
- Pizzo, G.; Licata, M.E.; La Carar, M.; Pizzo, I. The effects of sugar-free chewing gums on dental plaque regrowth: A comparative study. J. Clin. Periodontol. 2007, 35, 503–508. [Google Scholar] [CrossRef]
- Yu-Hsiang, L.; Hui, Z.; Jeann, R.; Xinmin, Y.; Lei, Z.; David, C.; David, W. Direct Saliva Transcriptome Analysis. Clin. Chem. 2011, 57, 1295–1302. [Google Scholar] [CrossRef]
- Chirgwin, J.M.; Przybyla, A.E.; MacDonald, R.J.; Rutter, W.J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 18, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Chomoczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fidler, G.; Tolnai, E.; Stagel, A.; Remenyik, J.; Stundl, L.; Gal, F.; Biro, S.; Paholcsek, M. Tendentious effects of automated and manual metagenomic DNA purification protocols on broiler gut microbiome taxonomic profiling. Sci. Rep. 2020, 10, 3419. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Rayment, S.A.; Liu, B.; Soares, R.V.; Offner, G.D.; Oppenheim, F.G.; Troxler, R.F. The effects of duration and intensity of stimulation on total protein and mucin concentrations in resting and stimulated whole saliva. J. Dent. Res. 2001, 80, 1584–1587. [Google Scholar]
- Inoue, H.; Ono, K.; Masuda, W.; Inagaki, T.; Yokota, M.; Inenaga, K. Rheological Properties of Human Saliva and Salivary Mucins. J. Oral Biosci. 2008, 50, 134–141. [Google Scholar] [CrossRef]
- Chaudhury, N.M.; Shirlaw, P.; Pramanik, R.; Carpenter, G.H.; Proctor, G.B. Changes in Saliva Rheological Properties and Mucin Glycosylation in Dry Mouth. J. Dent. Res. 2015, 94, 1660–1667. [Google Scholar] [CrossRef]
- Wickström, C.; Christersson, C.; Davies, J.R.; Carlstedt, I. Macromolecular organization of saliva: Identification of ‘insoluble’ MUC5B assemblies and non-mucin proteins in the gel phase. Biochem. J. 2000, 351, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Vinke, J. Salivary Lubrication and Xerostomia. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2020. [Google Scholar]
- Wickström, C.; Herzberg, M.C.; Beighton, D.; Svensäter, G. Proteolytic degradation of human salivary MUC5B by dental biofilms. Microbiology 2009, 155, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Gabryel-Porowska, H.; Gornowicz, A.; Bielawska, A.; Wójcicka, A.; Maciorkowska, E.; Grabowska, S.Z.; Bielawski, K. Mucin levels in saliva of adolescents with dental caries. Med. Sci. Monit. 2014, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Roescher, N.; Tak, P.P.; Illei, G.G. Cytokines in Sjögren’s syndrome. Oral Dis. 2009, 15, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Horst, O.V.; Horst, J.A.; Samudrala, R.; Dale, B.A. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. 2011, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Veerayutthwilai, O.; Byers, M.R.; Pham, T.T.; Darveau, R.P.; Dale, B.A. Differential regulation of immune responses by odontoblasts. Oral Microbiol. Immunol. 2007, 22, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Nakashima, K.; Tamai, M.; Nakamura, H.; Iwanaga, N.; Fujikawa, K.; Aramaki, T.; Arima, K.; Iwamoto, N.; Ichinose, K.; et al. Toll-like receptor in salivary glands from patients with Sjögren’s syndrome: Functional analysis by human salivary gland cell line. J. Rheumatol. 2007, 34, 1019–1026. [Google Scholar] [PubMed]
- Biró, A.; Markovics, A.; Homoki, J.; Szőllősi, E.; Hegedűs, C.; Tarapcsák, S.; Lukács, J.; Stündl, L.; Gálné Remenyik, J. Anthocyanin-Rich Sour Cherry Extract Attenuates the Lipopolysaccharide-Induced Endothelial Inflammatory Response. Molecules 2019, 24, 3427. [Google Scholar] [CrossRef]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedűs, C.; Kovács, D.; Peitl, B.; Gál, F.; Stündl, L.; Szilvássy, Z.; Remenyik, J. Effect of Anthocyanin-Rich Tart Cherry Extract on Inflammatory Mediators and Adipokines Involved in Type 2 Diabetes in a High Fat Diet Induced Obesity Mouse Model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef]
- Syndergaard, B.; Al-Sabbagh, M.; Kryscio, R.J.; Xi, J.; Ding, X.; Ebersole, J.L.; Miller, C.S. Salivary biomarkers associated with gingivitis and response to therapy. J. Periodontol. 2014, 85, e295–e303. [Google Scholar] [CrossRef]
- Schoilew, K.; Ueffing, H.; Dalpke, A.; Wolff, B.; Frese, C.; Wolff, D.; Boutin, S. Bacterial biofilm composition in healthy subjects with and without caries experience. J. Oral Microbiol. 2019, 11, 1633194. [Google Scholar] [CrossRef] [PubMed]
- Caselli, B.; Fabbri, C.; D’Accolti, M.; Soffriti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Morton, J.T.; Dinis, M.; Alvarez, R.; Tran, N.C.; Knight, R.; Edlund, A. Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 2021, 31, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef] [PubMed]
- Love, R.; Tanner, A. Microbiology of Dental Caries and Dentinal Tubule Infection. In Endodontic Microbiology; Fouad, A.F., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; p. 30. [Google Scholar]
- Miller, C.S.; Danaher, R.J.; Kirakodu, S.; Carlson, C.R.; Mumper, R.J. Effect of chewing gum containing Xylitol and blackberry powder on oral bacteria: A randomized controlled crossover trial. Arch. Oral Biol. 2022, 143, 105523. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Maróti, G.; Lipták, L.; Mester, M.; Al Ayoubi, A.; Pap, B.; Madléna, M.; Minárovits, J.; Kovács, K.L. Microbiomes in supragingival biofilms and saliva of adolescents with gingivitis and gingival health. Oral Dis. 2021, 28, 2000–2014. [Google Scholar] [CrossRef]
- Campbell, K. Oral microbiome findings challenge dentistry dogma. Nature, 2021; online ahead of print. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Homer, K.A.; Marsh, P.D.; Beighton, D. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 1994, 140 Pt 12, 3407–3412. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Zhao, L.; Sun, X.; Feng, Q. Microbiome succession with increasing age in three oral sites. Aging 2020, 12, 7874–7907. [Google Scholar] [CrossRef]
- Hoskins, L.C.; Boulding, E.T. Degradation of blood group antigens in human colon ecosystems. I. In vitro production of ABH blood group-degrading enzymes by enteric bacteria. J. Clin. Investig. 1976, 57, 63–73. [Google Scholar] [CrossRef]
- Corfield, A.P.; Wagner, S.A.; Clamp, J.R.; Kriaris, M.R.; Hoskins, C. Mucin Degradation in the Human Colon: Production of Sialidase, Sialate O-Acetylesterase, N-Acetylneuraminate Lyase, Arylesterase, and Glycosulfatase Activities by Strains of Fecal Bacteria. Infect. Immun. 1992, 60, 3971–3978. [Google Scholar] [CrossRef] [PubMed]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Witkowska, E.; Kaveh, B.; Holgerson, P.; Tanner, A. The Microbiome in Populations with a Low and High Prevalence of Caries. J. Dent. Res. 2016, 95, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Rothballer, M.; Altenburger, M.J.; Woelber, J.P.; Karygianni, L.; Lagkouvardos, I.; Hellwig, E.; Al-Ahmad, A. In-vivo shift of the microbiota in oral biofilm in response to frequent sucrose consumption. Sci. Rep. 2018, 8, 14202. [Google Scholar] [CrossRef] [PubMed]
- He, X.; McLean, J.S.; Edlund, A.; Yooseph, S.; Hall, A.P.; Liu, S.Y.; Dorrestein, P.C.; Esquenazi, E.; Hunter, R.C.; Cheng, G.; et al. Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2015, 112, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G. Glycan-Dependent Helicobacter spp. and Streptococcus oralis Binding to Mucins in the Gastric and Oral Mucosal Niche. Ph.D. Dissertation, University of Gothenburg, Göteborg, Sweden, 2021. [Google Scholar]
- Sarkar, A.; Kuehl, M.N.; Alman, C.A.; Burkhardt, B.R. Linking the oral microbiome and salivary cytokine abundance to circadian oscillations. Sci. Rep. 2021, 11, 2658. [Google Scholar] [CrossRef]
- Yang, F.; Zeng, X.; Ning, K.; Liu, K.-L.; Lo, C.-C.; Wang, W.; Chen, J.; Wang, D.; Huang, R.; Chang, X.; et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012, 6, 1–10. [Google Scholar] [CrossRef]
- Hevesi, M.; Blázovics, A.; Kallay, E.; Végh, A.; Stéger-Máté, M.; Ficzek, G.; Tóth, M. Biological Activity of Sour Cherry Fruit on the Bacterial Flora of Human Saliva in vitro. Food Technol. Biotechnol. 2012, 50, 117–122. [Google Scholar]
- Ben Lagha, A.; LeBel, G.; Grenier, D. Tart cherry (Prunus cerasus L.) fractions inhibit biofilm formation and adherence properties of oral pathogens and enhance oral epithelial barrier function. Phytother. Res. 2020, 34, 886–895. [Google Scholar] [CrossRef]





| Cytokine | Probe (5′-3′) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| TNFα | CAATGGCGTGGAGCTGAGAG | AACCCCGAGTGACAAGC | TGGGAGTAGATGAGGTACAGG |
| IL-1β | TGATGGCCCTAAAACAGATGAAGTG | AATTCGGTACATCCTCGA | GATTTTCACCAGGCAAGTCTC |
| IL-2 | TGTGAGCATCCTGGTGAGTTTGGG | AAAGAAAACACAGCTACAACTGG | GAAGATGTTTCAGTTCTGTGGC |
| IL-6 | TGTTACATGTCTCCTTTCTCAGGGC | AATTCGGTACATCCTCGACG | GATTTTCACCAGGCAAGTCTC |
| GAPDH | CATTGCCCTCAACGACCACTTT | CCTCCACCTTTGACGCTG | CTCTTCCTCTTGTGCTCTTGC |
| Group | Participants Number | Sex | Age (M ± SD) | DMF-T (M ± SD) | D (T) (M ± SD) | M (T) (M ± SD) | F (T) (M ± SD) | BPE (M ± SD) | |
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | ||||||||
| AG I | 10 | 7 | 3 | 26.8 ± 2.04 | 4.9 ± 4.38 | 0.2 ± 0.63 | 0.8 ± 1.75 | 5 ± 4.76 | 0.42 ± 0.33 |
| AG II | 10 | 5 | 5 | 36.3 ± 3.83 | 8.9 ± 4.91 | 1.1 ± 1.1 | 1.1 ± 1.37 | 6.9 ± 3.66 | 0.33 ± 0.35 |
| ANOVA (p) | p = 0.0003 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | |||
| Total | 20 | 12 | 8 | 31.7 ± 5.71 | 6.9 ± 4.97 | 0.65 ± 0.99 | 0.95 ± 1.54 | 5.95 ± 4.24 | 0.42 ± 0.34 |
| Factor Type of Saliva | MUC5B | MUC7 | IL-1β mRNA | IL-1β | TNFα | TNFα mRNA | IL-2 | IL-6 | Melatonin | Ca2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Unstim. | 5.192 | 0.35 | 0.208 | 396.604 | 22.67 | 1.101 | 35.025 | 10.435 | 3.76 | * 0.431 |
| Stim. | 9.477 | 0.34 | 0.3 | 237.34 | 34.74 | 1.304 | 28.698 | 8.36 | 7.255 | * 1.26 |
| Genus/Mucin | MUC5B | MUC7 |
|---|---|---|
| Actinomyces | 0.258 | −0.462 |
| Lachnospiraceae_[G-3] | 0.775 | −0.644 |
| Eikenella | 0.658 | −0.301 |
| Capnocytophaga | 0.250 | −0.362 |
| Porphromonas | 0.415 | −0.288 |
| Peptrostreptococcae_[G-9] | 0.507 | −0.431 |
| Alloprevotellae | −0.537 | 0.308 |
| Bacteriodales_[G-2] | 0.424 | −0.19 |
| Veilonella | −0.428 | 0.476 |
| Bifidobacterium | −0.284 | 0.36 |
| Haemophilus | −0.641 | 0.615 |
| Bacteriodetes_[G-5] | −0.481 | 0.517 |
| Bifidobacterium | −0.284 | 0.36 |
| Mogibacterium | −0.374 | 0.242 |
| Ruminococcae_[G-1] | −0.1 | 0.32 |
| Peptococcus | −0.311 | 0.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopkó, B.E.; Homoki, J.R.; Fazekas, M.É.; Paholcsek, M.; Fauszt, P.; Dávid, P.; Stündl, L.; Molnár, P.B.; Forgács, I.N.; Váradi, J.; et al. Changes in the Composition of Unstimulated and Stimulated Saliva Due to Chewing Sour Cherry Gum and a Toothbrush Change. Cells 2024, 13, 251. https://doi.org/10.3390/cells13030251
Skopkó BE, Homoki JR, Fazekas MÉ, Paholcsek M, Fauszt P, Dávid P, Stündl L, Molnár PB, Forgács IN, Váradi J, et al. Changes in the Composition of Unstimulated and Stimulated Saliva Due to Chewing Sour Cherry Gum and a Toothbrush Change. Cells. 2024; 13(3):251. https://doi.org/10.3390/cells13030251
Chicago/Turabian StyleSkopkó, Boglárka Emese, Judit Rita Homoki, Mónika Éva Fazekas, Melinda Paholcsek, Péter Fauszt, Péter Dávid, László Stündl, Piroska Bíróné Molnár, Ildikó Noémi Forgács, Judit Váradi, and et al. 2024. "Changes in the Composition of Unstimulated and Stimulated Saliva Due to Chewing Sour Cherry Gum and a Toothbrush Change" Cells 13, no. 3: 251. https://doi.org/10.3390/cells13030251
APA StyleSkopkó, B. E., Homoki, J. R., Fazekas, M. É., Paholcsek, M., Fauszt, P., Dávid, P., Stündl, L., Molnár, P. B., Forgács, I. N., Váradi, J., Bágyi, K. Á., & Remenyik, J. (2024). Changes in the Composition of Unstimulated and Stimulated Saliva Due to Chewing Sour Cherry Gum and a Toothbrush Change. Cells, 13(3), 251. https://doi.org/10.3390/cells13030251








