Molecular Markers in Embryo Non-Development: Analysis of Gene Expressions (Ki-67, hTERT, HIF-1α) in Spent Embryo Culture Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigation of Characteristics of SpentEmbryo Culture Medium Samples
2.2. Collection of Culture Medium Samples Containing Human Embryos
2.3. Total RNA Isolation from Human Spent Embryo Culture Medium and Determination of Gene Expression by Real Time Polymerase Chain Reaction
2.4. Statistical Evaluations
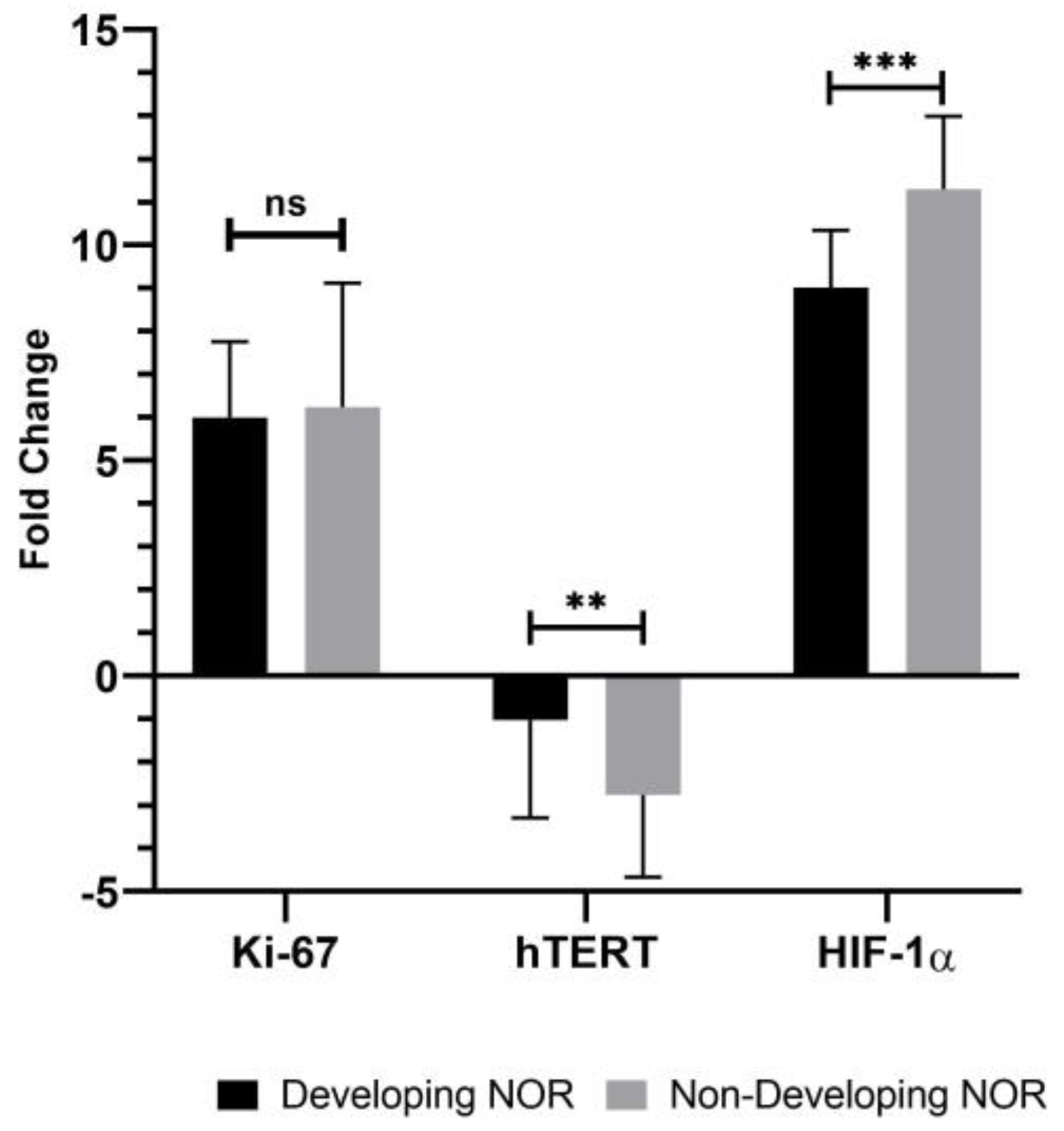
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Chiu, W.T.; Hsu, P.L.; Lin, S.C.; Peng, I.C.; Wang, C.Y.; Tsai, S.J. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 2022, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- Peciuliene, I.; Jakubauskiene, E.; Vilys, L.; Zinkeviciute, R.; Kvedaraviciute, K.; Kanopka, A. Short-term hypoxia in cells induces expression of genes which are enhanced in stressed cells. Genes 2022, 13, 1596. [Google Scholar] [CrossRef] [PubMed]
- Stantic, M.; Wolfsberger, J.; Sakil, H.A.; Wilhelm, M.T. ΔNp73 enhances HIF-1α protein stability through repression of the ECV complex. Oncogene 2018, 37, 3729–3739. [Google Scholar] [CrossRef]
- Paredes, F.; Williams, H.C.; Martin, A.S. Metabolic adaptation in hypoxia and cancer. Cancer Lett. 2021, 502, 133–142. [Google Scholar] [CrossRef]
- MacCallum, D.E.; Hall, P.A. The biochemical characterization of the DNA binding activity of pKi67. J. Pathol. 2000, 191, 286–298. [Google Scholar] [CrossRef]
- Booth, D.G.; Takagi, M.; Sanchez-Pulido, L.; Petfalski, E.; Vargiu, G.; Samejima, K.; Imamoto, N.; Ponting, C.P.; Tollervey, D.; Earnshaw, W.C.; et al. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. Elife 2014, 3, e01641. [Google Scholar] [CrossRef]
- Scholzen, T.; Endl, E.; Wohlenberg, C.; van der Sar, S.; Cowell, I.G.; Gerdes, J.; Singh, P.B. The Ki-67 protein interacts with members of the heterochromatin protein 1 (HP1) family: A potential role in the regulation of higher-order chromatin structure. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2002, 196, 135–144. [Google Scholar] [CrossRef]
- Csonka, T.; Murnyák, B.; Szepesi, R.; Bencze, J.; Bognár, L.; Klekner, Á.; Hortobágyi, T. Assessment of candidate immunohistochemical prognostic markers of meningioma recurrence. Folia Neuropathol. 2016, 54, 114–126. [Google Scholar] [CrossRef]
- Küçükosmanoğlu, İ.; Karanis, M.İ.E.; Ünlü, Y.; Çöven, İ. Evaluation of P57, P53 and Ki67 Expression in Meningiomas. J. Korean Neurosurg. Soc. 2022, 65, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Duronio, R.J.; Xiong, Y. Signaling pathways that control cell proliferation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008904. [Google Scholar] [CrossRef] [PubMed]
- Lander, A.D.; Gokoffski, K.K.; Wan FY, M.; Nie, Q.; Calof, A.L. Cell lineages and the logic of proliferative control. PLoS Biol. 2009, 7, e1000015. [Google Scholar] [CrossRef]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Shay, J.W.; Zou, Y.; Hiyama, E.; Wright, W.E. Telomerase and cancer. Hum. Mol. Genet. 2001, 10, 677–685. [Google Scholar] [CrossRef]
- Vasilopoulos, E.; Fragkiadaki, P.; Kalliora, C.; Fragou, D.; Docea, A.O.; Vakonaki, E.; Tsoukalas, D.; Calina, D.; Buga, A.M.; Georgiadis, G.; et al. The association of female and male infertility with telomere length. Int. J. Mol. Med. 2019, 44, 375–389. [Google Scholar] [CrossRef]
- Ito, M.; Muraki, M.; Takahashi, Y.; Imai, M.; Tsukui, T.; Yamakawa, N.; Nakagawa, K.; Ohgi, S.; Horikawa, T.; Iwasaki, W.; et al. Glutathione S-transferase theta 1 expressed in granulosa cells as a biomarker for oocyte quality in age-related infertility. Fertil. Steril. 2008, 90, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Butts, S.; Riethman, H.; Ratcliffe, S.; Shaunik, A.; Coutifaris, C.; Barnhart, K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J. Clin. Endocrinol. Metab. 2009, 94, 4835–4843. [Google Scholar] [CrossRef]
- Martínez, P.; Blasco, M.A. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat. Rev. Cancer 2011, 11, 161–176. [Google Scholar] [CrossRef]
- Hidema, S.; Fukuda, T.; Date, S.; Tokitake, Y.; Matsui, Y.; Sasaki, H.; Nishimori, K. Transgenic expression of Telomerase reverse transcriptase (Tert) improves cell proliferation of primary cells and enhances reprogramming efficiency into the induced pluripotent stem cell. Biosci. Biotechnol. Biochem. 2016, 80, 1925–1933. [Google Scholar] [CrossRef]
- Jaijyan, D.K.; Selariu, A.; Cruz-Cosme, R.; Tong, M.; Yang, S.; Stefa, A.; Kekich, D.; Sadoshima, J.; Herbig, U.; Tang, Q.; et al. New intranasal and injectable gene therapy for healthy life extension. Proc. Natl. Acad. Sci. USA 2022, 119, e2121499119. [Google Scholar] [CrossRef]
- Relitti, N.; Saraswati, A.P.; Federico, S.; Khan, T.; Brindisi, M.; Zisterer, D.; Brogi, S.; Gemma, S.; Butini, S.; Campiani, G. Telomerase-based Cancer Therapeutics: A Review on their Clinical Trials. Curr. Top. Med. Chem. 2020, 20, 433–457. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.; Kumar, V.; Khan, A.M.; Joo, M.D.; Lee, K.W.; Sohn, S.H.; Kong, I.K. Cycloastragenol activation of telomerase improves β-Klotho protein level and attenuates age-related malfunctioning in ovarian tissues. Mech. Ageing Dev. 2023, 209, 111756. [Google Scholar] [CrossRef] [PubMed]
- DK, G. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil Steril 2000, 73, 1155–1158. [Google Scholar]
- Olcay, I.O.; Akcay, B.; Bahceci, M.; Arici, A.; Boynukalin, K.; Yakicier, C.; Ozpinar, A.; Basar, M. Noninvasive amino acid turnover predicts human embryo aneuploidy. Gynecol. Endocrinol. 2022, 38, 461–466. [Google Scholar] [CrossRef]
- Ates, B.; Öner, Ç.; Akbulut, Z.; Çolak, E. Capsaicin Alters the Expression of Genetic and Epigenetic Molecules in Hepatocellular Carcinoma Cell. Int. J. Mol. Cell. Med. 2022, 11, 236. [Google Scholar]
- Khurana, P.; Smyth, N.R.; Sheth, B.; Velazquez, M.A.; Eckert, J.J.; Fleming, T.P. Advanced maternal age perturbs mouse embryo development and alters the phenotype of derived embryonic stem cells. J. Dev. Orig. Health Dis. 2022, 13, 395–405. [Google Scholar] [CrossRef]
- Xing, J.; Qiao, G.; Luo, X.; Liu, S.; Chen, S.; Ye, G.; Zhang, C.; Yi, J. Ferredoxin 1 regulates granulosa cell apoptosis and autophagy in polycystic ovary syndrome. Clin. Sci. 2023, 137, 453–468. [Google Scholar] [CrossRef]
- Toupance, S.; Fattet, A.J.; Thornton, S.N.; Benetos, A.; Guéant, J.L.; Koscinski, I. Ovarian telomerase and female fertility. Biomedicines 2021, 9, 842. [Google Scholar] [CrossRef]
- Goto, H.; Iwata, H.; Takeo, S.; Nisinosono, K.; Murakami, S.; Monji, Y.; Kuwayama, T. Effect of bovine age on the proliferative activity, global DNA methylation, relative telomere length and telomerase activity of granulosa cells. Zygote 2013, 21, 256–264. [Google Scholar] [CrossRef]
- Kosebent, E.G.; Uysal, F.; Ozturk, S. The altered expression of telomerase components and telomere-linked proteins may associate with ovarian aging in mouse. Exp. Gerontol. 2020, 138, 110975. [Google Scholar] [CrossRef] [PubMed]
- Uysal, F.; Kosebent, E.G.; Toru, H.S.; Ozturk, S. Decreased expression of TERT and telomeric proteins as human ovaries age may cause telomere shortening. J. Assist. Reprod. Genet. 2021, 38, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.C.C.; Santana, V.P.; Donaires, F.S.; Picinato, M.C.; Giorgenon, R.C.; Santana, B.A.; Pimentel, R.N.; Keefe, D.L.; Calado, R.T.; Ferriani, R.A.; et al. Telomere Length and Telomerase Activity in Immature Oocytes and C umulus Cells of Women with Polycystic Ovary Syndrome. Reprod. Sci. 2020, 27, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, C.; Murakami, T.; Okamura, K.; Yajima, A. Telomerase activity in normal ovaries and premature ovarian failure. Tohoku J. Exp. Med. 2000, 190, 231–238. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Zhang, X.; Liu, Y.; Wang, Z.; Wang, P.; Du, Y.; Qin, Y.; Chen, Z.-J. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum. Reprod. 2017, 32, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, S.; Gong, C.; Hu, Y.; Liu, J.; Wang, W.; Chen, Y.; Liao, Q.; He, B.; Huang, Y.; et al. Effects of environmental and pathological hypoxia on male fertility. Front. Cell Dev. Biol. 2021, 9, 725933. [Google Scholar] [CrossRef]
- Becker, C.M.; Rohwer, N.; Funakoshi, T.; Cramer, T.; Bernhardt, W.; Birsner, A.; Folkman, J.; D’Amato, R.J. 2-Methoxyestradiol inhibits hypoxia-inducible factor-1α and suppresses growth of lesions in a mouse model of endometriosis. Am. J. Pathol. 2008, 172, 534–544. [Google Scholar] [CrossRef]
- Li, W.-N.; Wu, M.-H.; Tsai, S.-J. Hypoxia and reproductive health: The role of hypoxia in the development and progression of endometriosis. Reproduction 2021, 161, F19–F31. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Wu, G.; Zang, Z.; Zhang, J.Q.; Li, X.; Tao, J.; Shen, M.; Liu, H. FOXO1 mediates hypoxia-induced G0/G1 arrest in ovarian somatic granulosa cells by activating the TP53INP1-p53-CDKN1A pathway. Development 2021, 148, dev199453. [Google Scholar] [CrossRef]
- Xin, Y.; Zhao, L.; Peng, R. HIF-1 signaling: An emerging mechanism for mitochondrial dynamics. J. Physiol. Biochem. 2023, 79, 489–500. [Google Scholar] [CrossRef]
- Yao, F.; Chu, M.; Xi, G.; Dai, J.; Wang, Z.; Hao, J.; Yang, Q.; Wang, W.; Tang, Y.; Zhang, J.; et al. Single-embryo transcriptomic atlas of oxygen response reveals the critical role of HIF-1α in prompting embryonic zygotic genome activation. Redox Biol. 2024, 72, 103147. [Google Scholar] [CrossRef] [PubMed]
- Albogami, S.M.; Al-Kuraishy, H.M.; Al-Maiahy, T.J.; Al-Buhadily, A.K.; Al-Gareeb, A.I.; Alorabi, M.; Alotaibi, S.S.; De Waard, M.; Sabatier, J.M.; Saad, H.M.; et al. Hypoxia-Inducible Factor 1 and Preeclampsia: A New Perspective. Curr. Hypertens. Rep. 2022, 24, 687–692. [Google Scholar] [CrossRef]
- Zhu, F.; Gao, J.; Zeng, F.; Lai, Y.; Ruan, X.; Deng, G. Hyperoside protects against cyclophosphamide induced ovarian damage and reduced fertility by suppressing HIF-1α/BNIP3-mediated autophagy. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 156, 113743. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, H.; Pan, Y.; Zhang, T.; Yang, S.; Liu, M.; Robert, N.; Wang, J.; Zhao, T.; Zhao, L.; et al. Low oxygen concentration improves yak oocyte maturation and inhibits apoptosis through HIF-1 and VEGF. Reprod. Domest. Anim. Zuchthyg. 2022, 57, 381–392. [Google Scholar] [CrossRef]
- Zhu, S.M.; Rao, T.; Yang, X.; Ning, J.Z.; Yu, W.M.; Ruan, Y.; Yuan, R.; Li, C.L.; Jiang, K.; Hu, W.; et al. Autophagy may play an important role in varicocele. Mol. Med. Rep. 2017, 16, 5471–5479. [Google Scholar] [CrossRef]
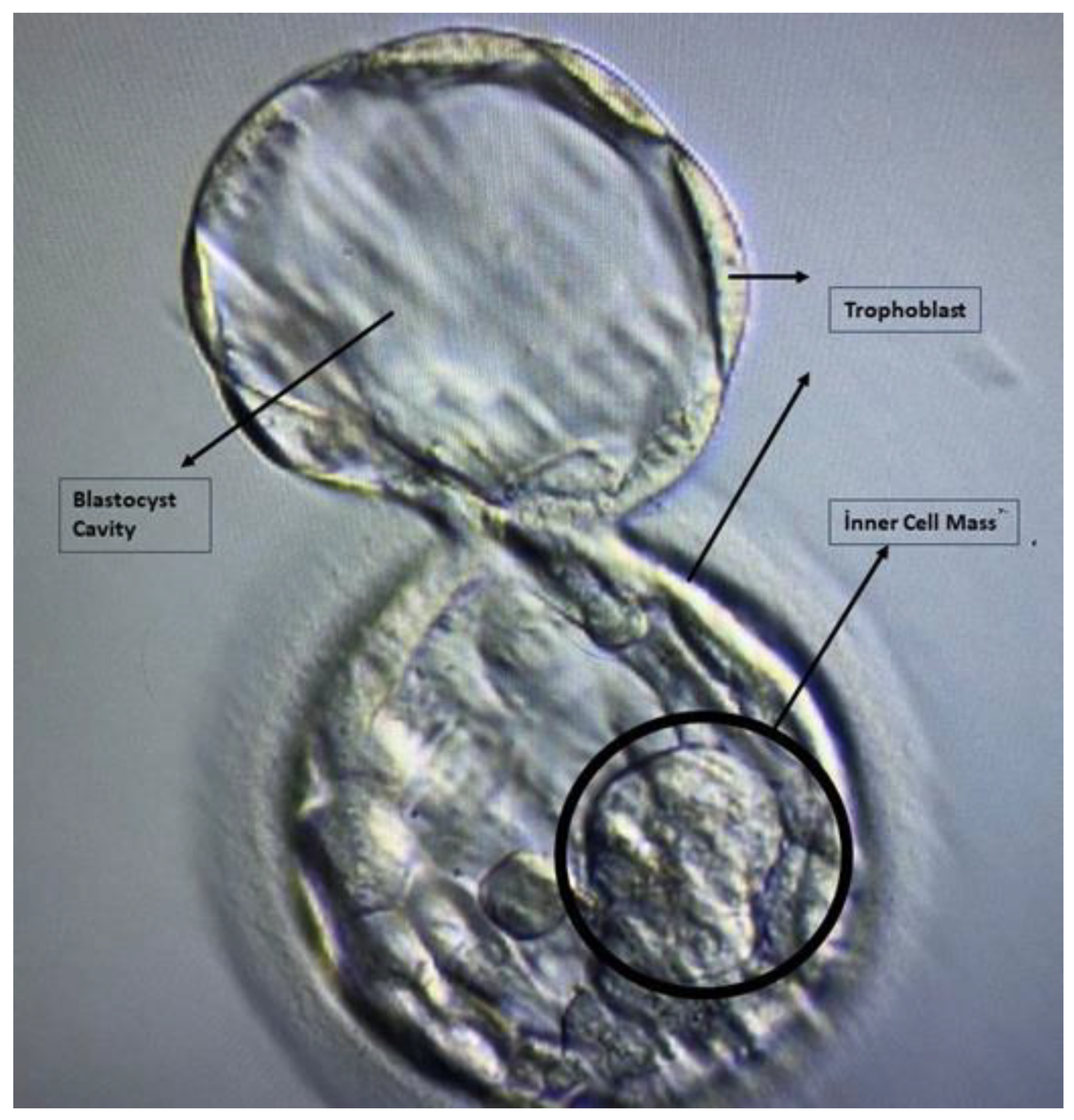
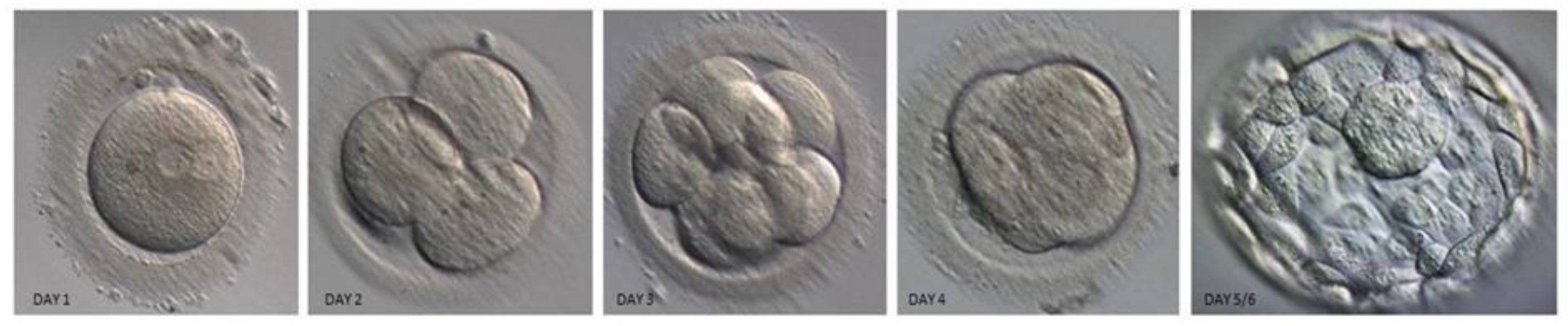
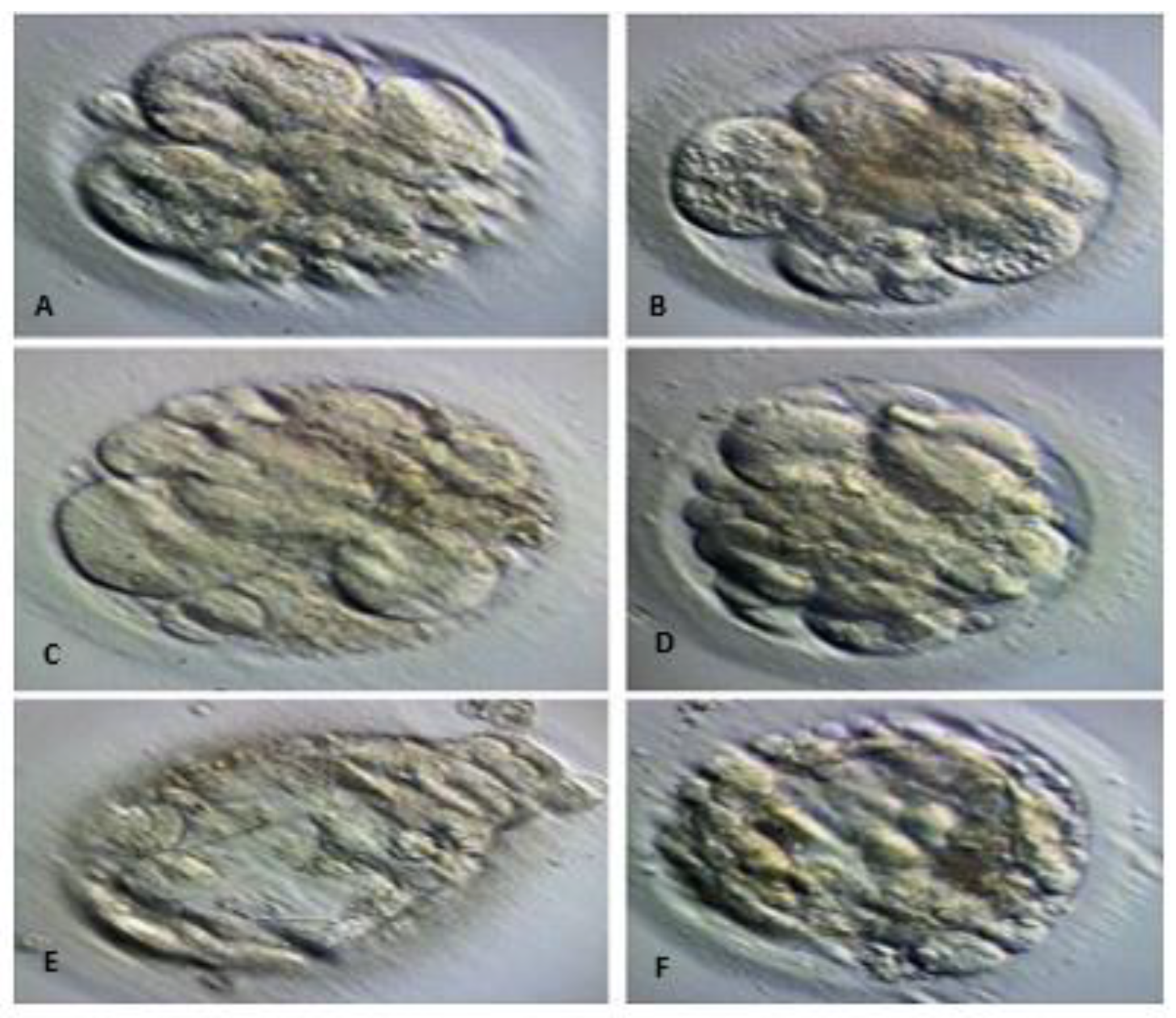
| Quality Grade | Blastocyst Stage | Description |
|---|---|---|
| 1 | Early blastocyst | The blastocyst cavity’s volume is less than half of the embryo’s. |
| 2 | Blastocyst | The blastocyst cavity is greater than or equal to half of the volume of the embryo |
| 3 | Full blastocyst | The blastocyst cavity completely fills the embryo |
| 4 | Expanded blastocyst | The volume of the blastocyst cavity is larger than that of the early embryo, and the membrane around it is becoming thinner |
| 5 | Hatching blastocyst | Herniation of the outer layer of cells through the surrounding membrane has begun. |
| 6 | Hatched blastocyst | Complete separation of the blastocyst from the surrounding membrane occurred. |
| Blastocyst Structure | Grade | Description |
| Inner Cell Mass | A | Tightly packed, many cells |
| Inner Cell Mass | B | Loosely grouped, several cells |
| Inner Cell Mass | C | Very few cells |
| Blastocyst Structure | Grade | Description |
| Trophectoderm | A | Numerous cells creating a closely linked epithelium |
| Trophectoderm | B | Few cells |
| Trophectoderm | C | The loose epithelium is made up of very few cells |
| Accession Number | Gene | Forward | Reverse | Tm (Basic) |
|---|---|---|---|---|
| NM-0024117.1 | Ki-67 | 5′-TCCTTTGGTGGGCACCTAAGACCTG-3′ | 5′-TGATGGTTGAGGTCGTTCCTTGATG-3′ | 55 °C |
| AB085628.1 | hTERT | 5′-TGCCAGCCCCAGCGTCAAAG-3′ | 5′-TGCCAGCCCCAGCGTCAAAG-3′ | 55.88 °C |
| OR762216.1 | HIF-1 α | 5′-GGCGCGAACGACAAGAAAAAG-3′ | 5′-CCTTATCAAGATGCGAACTCACA-3′ | 54.36 °C |
| NG-007073.2 | GAPDH | 5′-CGAGGGGGGAGCCAAAAGGG-3′ | 5′-TGCCAGCCCCAGCGTCAAAG-3′ | 56 °C |
| Spent Embryo Culture Medium Containing Developing Normoresponder Embryos | ||||
|---|---|---|---|---|
| Ki-67 | hTERT | HIF-1α | ||
| Ki-67 | Pearson correlation | 1 | −0.364 | 0.313 |
| Sig. (2-tailed) | 0.115 | 0.179 | ||
| N | 20 | 20 | 20 | |
| hTERT | Pearson correlation | −0.364 | 1 | −0.021 |
| Sig. (2-tailed) | 0.115 | 0.931 | ||
| N | 20 | 20 | 20 | |
| HIF-1α | Pearson correlation | 0.313 | −0.021 | 1 |
| Sig. (2-tailed) | 0.179 | 0.931 | ||
| N | 20 | 20 | 20 | |
| Spent Embryo Culture Medium Containing Non-Developing Normoresponder Embryos | ||||
| Ki-67 | hTERT | HIF-1α | ||
| Ki-67 | Pearson correlation | 1 | −0.181 | 0.762 ** |
| Sig. (2-tailed) | 0.444 | 0.000 | ||
| N | 20 | 20 | 20 | |
| hTERT | Pearson correlation | −0.181 | 1 | 0.123 |
| Sig. (2-tailed) | 0.444 | 0.605 | ||
| N | 20 | 20 | 20 | |
| HIF-1α | Pearson correlation | 0.762 ** | 0.123 | 1 |
| Sig. (2-tailed) | 0.000 | 0.605 | ||
| N | 20 | 20 | 20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kılıç, N.Ö.; Kütük, D.; Öner, Ç.; Öztürk, S.A.; Selam, B.; Çolak, E. Molecular Markers in Embryo Non-Development: Analysis of Gene Expressions (Ki-67, hTERT, HIF-1α) in Spent Embryo Culture Medium. Cells 2024, 13, 2093. https://doi.org/10.3390/cells13242093
Kılıç NÖ, Kütük D, Öner Ç, Öztürk SA, Selam B, Çolak E. Molecular Markers in Embryo Non-Development: Analysis of Gene Expressions (Ki-67, hTERT, HIF-1α) in Spent Embryo Culture Medium. Cells. 2024; 13(24):2093. https://doi.org/10.3390/cells13242093
Chicago/Turabian StyleKılıç, Nergis Özlem, Duygu Kütük, Çağrı Öner, Senem Aslan Öztürk, Belgin Selam, and Ertuğrul Çolak. 2024. "Molecular Markers in Embryo Non-Development: Analysis of Gene Expressions (Ki-67, hTERT, HIF-1α) in Spent Embryo Culture Medium" Cells 13, no. 24: 2093. https://doi.org/10.3390/cells13242093
APA StyleKılıç, N. Ö., Kütük, D., Öner, Ç., Öztürk, S. A., Selam, B., & Çolak, E. (2024). Molecular Markers in Embryo Non-Development: Analysis of Gene Expressions (Ki-67, hTERT, HIF-1α) in Spent Embryo Culture Medium. Cells, 13(24), 2093. https://doi.org/10.3390/cells13242093







