Excess Weight Impairs Oocyte Quality, as Reflected by mtDNA and BMP-15
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Treatment Protocol
2.3. Blood and Follicular Fluid Collection and Cumulus Cell Isolation
2.4. Measuring Hormone and Lipid Levels in Plasma and Follicular Fluid
2.5. DNA Extraction
2.6. Quantification of mtDNA
2.7. ELISA (Enzyme-Linked Immunosorbent Assay)
2.7.1. BMP-15
2.7.2. HSPG2
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Relation of Age to BMP-15 and mtDNA
4.2. Lipid Profile
4.3. Treatment Outcomes
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Volume 894. [Google Scholar]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef]
- Purcell, S.H.; Moley, K.H. The impact of obesity on egg quality. J. Assist. Reprod. Genet. 2011, 28, 517–524. [Google Scholar] [CrossRef]
- Metwally, M.; Cutting, R.; Tipton, A. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reprod. Biomed. Online 2007, 15, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Brown, M.B.; Stern, J.E.; Missmer, S.A.; Fujimoto, V.Y.; Leach, R.; A Sart Writing Group. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum. Reprod. 2011, 26, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Zhu, P.; Wang, J.; Liu, J.; Li, N.; Wang, W.; Zhang, W.; Zhang, C.; Wang, Y.; et al. Human follicular fluid proteome reveals association between overweight status and oocyte maturation abnormality. Clin. Proteom. 2020, 17, 22. [Google Scholar] [CrossRef]
- Valckx, S.D.M.; De Pauw, I.; De Neubourg, D.; Inion, I.; Berth, M.; Fransen, E.; Bols, P.E.J.; Leroy, J.L.M.R. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum. Reprod. 2012, 27, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- Marteil, G.; Richard-Parpaillon, L.; Kubiak, J.Z. Role of oocyte quality in meiotic maturation and embryonic development. Reprod. Biol. 2009, 9, 203–224. [Google Scholar] [CrossRef]
- Wilding, M.; Dale, B.; Marino, M.; di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef]
- Desquiret-Dumas, V.; Clément, A.; Seegers, V.; Boucret, L.; Ferré-L’Hotellier, V.; Bouet, P.E.; Descamps, P.; Procaccio, V.; Reynier, P.; May-Panloup, P. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum. Reprod. 2017, 32, 607–614. [Google Scholar] [CrossRef]
- Taugourdeau, A.; Desquiret-Dumas, V.; Hamel, J.F.; Chupin, S.; Boucret, L.; Ferré-L’Hotellier, V.; Bouet, P.E.; Descamps, P.; Procaccio, V.; Reynier, P.; et al. The mitochondrial DNA content of cumulus cells may help predict embryo implantation. J. Assist. Reprod. Genet. 2019, 36, 223–228. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Smitz, J. Molecular control of oogenesis. Biochim. Biophys. Acta 2012, 1822, 1896–1912. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, R.; Di Pasquale, E.; Marozzi, A.; Bione, S.; Toniolo, D.; Grammatico, P.; Nelson, L.M.; Beck-Peccoz, P.; Persani, L. BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum. Mutat. 2009, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Tang, L.; Cai, J.; Lu, X.-E.; Xu, J.; Zhu, X.-M.; Luo, Q.; Huang, H.-F. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum. Reprod. 2007, 22, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Bayasula Iwase, A.; Kobayashi, H.; Goto, M.; Nakahara, T.; Nakamura, T.; Kondo, M.; Nagatomo, Y.; Kotani, T.; Kikkawa, F. A proteomic analysis of human follicular fluid: Comparison between fertilized oocytes and non-fertilized oocytes in the same patient. J. Assist. Reprod. Genet. 2013, 30, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Bentov, Y.; Beharier, O.; Moav-Zafrir, A.; Kabessa, M.; Godin, M.; Greenfield, C.S.; Ketzinel-Gilad, M.; Ash Broder, E.; Holzer, H.E.; Wolf, D.; et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum. Reprod. 2021, 36, 2506–2513. [Google Scholar] [CrossRef]
- Gowri, V.; Rizvi, S.G.; Squib, S.; Al Futaisi, A. High-sensitivity C-reactive protein is a marker of obesity and not of polycystic ovary syndrome per se. Fertil. Steril. 2010, 94, 2832–2834. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Xu, F.; Duffy, D.M. Molecular control of ovulation and luteinization in the primate follicle. Front. Biosci. 2007, 12, 297–307. [Google Scholar] [CrossRef]
- Cherian-Shaw, M.; Puttabyatappa, M.; Greason, E.; Rodriguez, A.; VandeVoort, C.A.; Chaffin, C.L. Expression of scavenger receptor-BI and low-density lipoprotein receptor and differential use of lipoproteins to support early steroidogenesis in luteinizing macaque granulosa cells. Endocrinology 2009, 150, 957–965. [Google Scholar] [CrossRef]
- Azhar, S.; Nomoto, A.; Leers-Sucheta, S.; Reaven, E. Simultaneous induction of an HDL receptor protein (SR-BI) and the selective uptake of HDL-cholesteryl esters in a physiologically relevant steroidogenic cell model. J. Lipid Res. 1998, 39, 1616–1628. [Google Scholar] [CrossRef]
- Li, X.; Peegel, H.; Menon, K.M. Regulation of high density lipoprotein receptor messenger ribonucleic acid expression and cholesterol transport in theca-interstitial cells by insulin and human chorionic gonadotropin. Endocrinology 2001, 142, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Racowsky, C.; Stern, J.E.; Gibbons, W.E.; Behr, B.; Pomeroy, K.O.; Biggers, J.D. National collection of embryo morphology data into Society for Assisted Reproductive Technology Clinic Outcomes Reporting System: Associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertil Steril. 2011, 95, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Alikani, M.; Calderon, G.; Tomkin, G.; Garrisi, J.; Kokot, M.; Cohen, J. Cleavage anomalies in early human embryos and survival after prolonged culture in-vitro. Hum. Reprod. 2000, 15, 2634–2643. [Google Scholar] [CrossRef]
- Leuthner, T.C.; Hartman, J.H.; Ryde, I.T.; Meyer, J.N. PCR-Based Determination of Mitochondrial DNA Copy Number in Multiple Species. Methods Mol. Biol. 2021, 2310, 91–111. [Google Scholar] [PubMed]
- Ogino, M.; Tsubamoto, H.; Sakata, K.; Oohama, N.; Hayakawa, H.; Kojima, T.; Shigeta, M.; Shibahara, H. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J. Assist. Reprod. Genet. 2016, 33, 367–371. [Google Scholar] [CrossRef]
- Bellver, J.; Busso, C.; Pellicer, A.; Remohí, J.; Simón, C. Obesity and assisted reproductive technology outcomes. Reprod. Biomed. Online 2006, 12, 562–568. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Hashim, Z.H.; Amer, L.; Al-Wasiti, E.A. Relation of serum and follicular level of BMP15 with Oocyte quality, embryo grading and pregnancy rate. J. Contemp. Med. Sci. 2022, 8, 337–342. [Google Scholar] [CrossRef]
- Bentov, Y.; Jurisicova, A.; Kenigsberg, S.; Casper, R.F. What maintains the high intra-follicular estradiol concentration in pre-ovulatory follicles? J. Assist. Reprod. Genet. 2016, 33, 85–94. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Irving-Rodgers, H.F.; Russell, D.L. Extracellular matrix of the developing ovarian follicle. Reproduction 2003, 126, 415–424. [Google Scholar] [CrossRef]
- Ma, Y.; Jin, J.; Tong, X.; Yang, W.; Ren, P.; Dai, Y.; Pan, Y.; Zhang, Y.; Zhang, S. ADAMTS1 and HSPG2 mRNA levels in cumulus cells are related to human oocyte quality and controlled ovarian hyperstimulation outcomes. J. Assist. Reprod. Genet. 2020, 37, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Boucret, L.; Chao de la Barca, J.M.; Morinière, C.; Desquiret, V.; Ferré-L’Hôtellier, V.; Descamps, P.; Marcaillou, C.; Reynier, P.; Procaccio, V.; May-Panloup, P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015, 30, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- El Shourbagy, S.H.; Spikings, E.C.; Freitas, M.; St John, J.C. Mitochondria directly influence fertilisation outcome in the pig. Reproduction 2006, 131, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Lamas-Toranzo, I.; Pericuesta, E.; Bermejo-Álvarez, P. Mitochondrial and metabolic adjustments during the final phase of follicular development prior to IVM of bovine oocytes. Theriogenology 2018, 119, 156–162. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Chrétien, M.F.; Jacques, C.; Vasseur, C.; Malthièry, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Guedikian, A.A.; Madrigal, V.K.; Phan, J.D.; Hill, D.L.; Alvarez, J.P.; Chazenbalk, G.D. Cumulus cell mitochondrial resistance to stress in vitro predicts oocyte development during assisted reproduction. J. Clin. Endocrinol. Metab. 2016, 101, 2235–2245. [Google Scholar] [CrossRef]
- Mobarak, H.; Heidarpour, M.; Tsai, P.-S.J.; Rezabakhsh, A.; Rahbarghazi, R.; Nouri, M.; Mahdipour, M. Autologous mitochondrial microinjection; a strategy to improve the oocyte quality and subsequent reproductive outcome during aging. Cell Biosci. 2019, 9, 95. [Google Scholar] [CrossRef]
- Leridon, H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum. Reprod. 2004, 19, 1548–1553. [Google Scholar] [CrossRef]
- Steiner, A.Z.; Pritchard, D.; Stanczyk, F.Z.; Kesner, J.S.; Meadows, J.W.; Herring, A.H.; Baird, D.D. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017, 318, 1367–1376. [Google Scholar] [CrossRef]
- Hashim, Z.H.; Al-Wasiti, E.A.; Amery, L. Effect of Age on Serum and Follicular Fluid BMP15 and GDF9, The Oocyte Secreted Factors. J. Pharm. Negat. Results 2022, 13, 432–436. [Google Scholar] [CrossRef]
- Martínez-Moro, Á.; Lamas-Toranzo, I.; González-Brusi, L.; Pérez-Gómez, A.; Padilla-Ruiz, E.; García-Blanco, J.; Bermejo-Álvarez, P. mtDNA content in cumulus cells does not predict development to blastocyst or implantation. Hum. Reprod. Open 2022, 2022, hoac029. [Google Scholar] [CrossRef] [PubMed]
- Simsek-Duran, F.; Li, F.; Ford, W.; Swanson, R.J.; Jones, H.W.; Castora, F.J. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE 2013, 8, e64955. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Goto, H.; Tanaka, H.; Sakaguchi, Y.; Kimura, K.; Kuwayama, T.; Monji, Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod. Fertil. Dev. 2011, 23, 424–432. [Google Scholar] [CrossRef]
- Zeng, H.; Ren, Z.; Yeung, W.S.B.; Shu, Y.; Xu, Y.; Zhuang, G.; Liang, X.Y. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 2007, 22, 1681–1686. [Google Scholar] [CrossRef]
- Das, U.N. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Combelles, C.M.H.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef] [PubMed]
- Schon, S.B.; Yang, K.; Schindler, R.; Jiang, L.; Neff, L.M.; Seeley, R.J.; Marsh, E.E. Obesity-related alterations in protein expression in human follicular fluid from women undergoing in vitro fertilization. FS Sci. 2022, 3, 331–339. [Google Scholar] [CrossRef]
- Raviv, S.; Hantisteanu, S.; Sharon, S.M.; Atzmon, Y.; Michaeli, M.; Shalom-Paz, E. Lipid droplets in granulosa cells are correlated with reduced pregnancy rates. J. Ovarian. Res. 2020, 13, 4. [Google Scholar] [CrossRef]
- Haikin Herzberger, E.; Miller, N.; Ghetler, Y.; Tamir Yaniv, R.; Neumark, E.; Shulman, A.; Wiser, A. A prospective study of C-reactive protein in patients with obesity during IVF. Hum. Fertil. 2021, 24, 182–187. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhang, F.; Li, F.; Jin, H.; Su, Y.; Li, G. Serum C-reactive protein levels are associated with clinical pregnancy rate after in vitro fertilization among normal-weight women. Front. Endocrinol. 2023, 14, 934766. [Google Scholar] [CrossRef]
- Svensson, H.; Einarsson, S.; Olausson, D.; Kluge, L.; Bergh, C.; Edén, S.; Lönn, M.; Thurin-Kjellberg, A. Inflammatory and metabolic markers in relation to outcome of in vitro fertilization in a cohort of predominantly overweight and obese women. Sci. Rep. 2022, 12, 13331. [Google Scholar] [CrossRef] [PubMed]
- Macklon, N.S.; Brosens, J.J. The human endometrium as a sensor of embryo quality. Biol. Reprod. 2014, 91, 98. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Jiang, X.; Hameed, U.; Shi, Q. Role of lipid metabolism and signaling in mammalian oocyte maturation, quality, and acquisition of competence. Front. Cell Dev. Biol. 2021, 9, 639704. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qu, J.; Tian, M.; Yang, R.; Song, X.; Li, R.; Yan, J.; Qiao, J. Lipid metabolic process involved in oocyte maturation during folliculogenesis. Front. Cell Dev. Biol. 2022, 10, 806890. [Google Scholar] [CrossRef]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef]
- Sermondade, N.; Huberlant, S.; Bourhis-Lefebvre, V.; Arbo, E.; Gallot, V.; Colombani, M.; Fréour, T. Female obesity is negatively associated with live birth rate following IVF: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 439–451. [Google Scholar] [CrossRef]
| Characteristic * | BMI < 24.9 n = 28 | BMI > 25 n = 22 | p-Value | 95% CI |
|---|---|---|---|---|
| Age (y) mean ± SD | 31.86 ± 5.42 | 29.14 ± 5.88 | 0.09 | |
| BMI (kg/m2) | 21.44 ± 2.19 | 31.13 ± 4.69 | <0.001 | [−11.7, −7.7] |
| Chronic disease | 12 (41.3%) | 10 (47.6%) | 0.775 | |
| Medications | 9 (31%) | 8 (38.1%) | 0.76 | |
| Smoking | 10 (37%) | 4 (21%) | 0.33 | |
| Gravidity | 0.97 ± 1.43 | 0.62 ± 1.12 | 0.34 | |
| Parity | 0.48 ± 0.63 | 0.29 ± 0.46 | 0.21 | |
| Cesarean section | 3 (10.3%) | 3 (14.3%) | 0.69 | |
| Miscarriage | ||||
| 1 | 6 (20.7%) | 2 (9.5%) | 0.3 | |
| 2 or more | 2 (6.9%) | 2 (9.5%) | 0.8 | |
| Cause of infertility | ||||
| Male factor | 17 (58.3%) | 10 (47.6%) | 0.56 | |
| Mechanical factor | 3 (10.3%) | 2 (9.5%) | 1.0 | |
| Unexplained | 9 (31%) | 5 (23.8%) | 0.75 | |
| Anovulation | 0 | 4 (19%) | 0.026 |
| Characteristic * | BMI < 24.9 | BMI > 25 | p-Value | 95% CI |
|---|---|---|---|---|
| Hormonal profile | ||||
| FSH baseline (IU/L) | 7.88 ± 2.2 | 6.42 ± 1.91 | 0.019 | [0.25, 2.7] |
| LH baseline (IU/L) | 6.2 ± 2.9 | 4.6 ± 2.4 | 0.46 | |
| E2 baseline pg/mL) | 30 ± 75.8 | 40 ± 77.5 | 0.11 | [−14, 1216] |
| E2 on trigger day (pg/mL) | 2469 ± 1186 | 1868 ± 878 | 0.055 | |
| E2 on ovum pick-up day (pg/mL) | 1399 ± 1123 | 1157 ± 525 | 0.38 | |
| Antral follicle count | 16 ± 9.1 | 18 ± 9.3 | 0.475 | |
| Total days of treatment | 10.6 ± 2.5 | 9.7 ± 1.8 | 0.17 | |
| Total gonadotropin dose (units) | 2833 ± 1515 | 2415 ± 1195 | 0.29 | |
| Treatment protocol | ||||
| Antagonist | 24 (82.1%) | 19 (90.5%) | 0.35 | |
| Other | 5 (17.9%) | 2 (9.5%) | ||
| Gonadotropin treatment | 0.581 | |||
| Menopur | 17 (58.6%) | 13 (61.9%) | ||
| Pergoveris | 11 (37.9%) | 6 (28.6%) | ||
| Gonal F | 1 (3.4%) | 2 (9.5%) | ||
| Ovulation induction | 0.33 | |||
| Ovitrelle | 5 (17.2%) | 1 (4.8%) | ||
| GnRH agonist | 4 (13.8%) | 2 (9.5%) | ||
| Ovitrelle + GnRH agonist | 20 (69%) | 18 (85.7%) | ||
| Endometrial thickness (mm) | 9.7 ±1.9 | 10.1 ±2.5 | 0.55 | |
| Number of follicles (>14 mm) | 8.4 ± 4.6 | 7.9 ± 3.4 | 0.69 | |
| Number of oocytes collected | 13 ± 7.5 | 14 ± 7.5 | 0.62 | |
| Number of M2 oocytes | 11.14 ±7.4 | 11.29 ±6.8 | 0.94 | |
| Number of fertilized oocytes (2PN) | 8.07 ±6.6 | 8.05 ±5.6 | 0.99 | |
| Usable embryos | 3.7 ± 2.6 | 3.7 ± 2.7 | 0.92 | |
| Number of top-quality embryos D3 + D5 | 3 ± 3.6 | 3 ± 2.8 | 0.94 | |
| KID score | 4.25 ± 1.26 | 4.68 ± 0.749 | 0.192 | |
| Frozen embryos transferred D3 + D5 | 0.65 ± 1 | 0.50 ± 0.7 | 0.62 | |
| Chemical pregnancy | ||||
| Fresh embryo transfer | 15 (65.2%) | 7 (50%) | 0.49 | |
| Frozen embryo transfer | 9 (69.2%) | 8 (61.5%) | 1.0 | |
| Clinical pregnancy | ||||
| Fresh embryo transfer | 11 (47.8%) | 4 (28.6%) | 0.31 | |
| Frozen embryo transfer | 9 (69.2%) | 7 (53.8%) | 0.69 |
| Characteristic | BMI < 24.9 | BMI > 25 | p-Value | 95% CI |
|---|---|---|---|---|
| Blood chemistry on OPU day | ||||
| Chemistry on OPU day in follicular fluid | ||||
| C-reactive protein (mg/L) | 1.5 ± 1.6 | 5.2 ± 3.8 | 0.002 | [−5.82, −1.61] |
| Cholesterol (mg/dL ± SD) | 31 ± 35 | 23 ± 8.6 | 0.4 | [−0.07, 25.40] |
| Triglycerides (mg/dL ± SD) | 23 ± 8.6 | 14.7 ± 7.1 | 0.63 | |
| HDL (mg/dL ± SD) | 34 ± 26 | 20.9 ± 7.2 | 0.05 | |
| LDL (mg/dL ± SD) | 10 ± 29 | 11 ± 28.6 | 0.99 | |
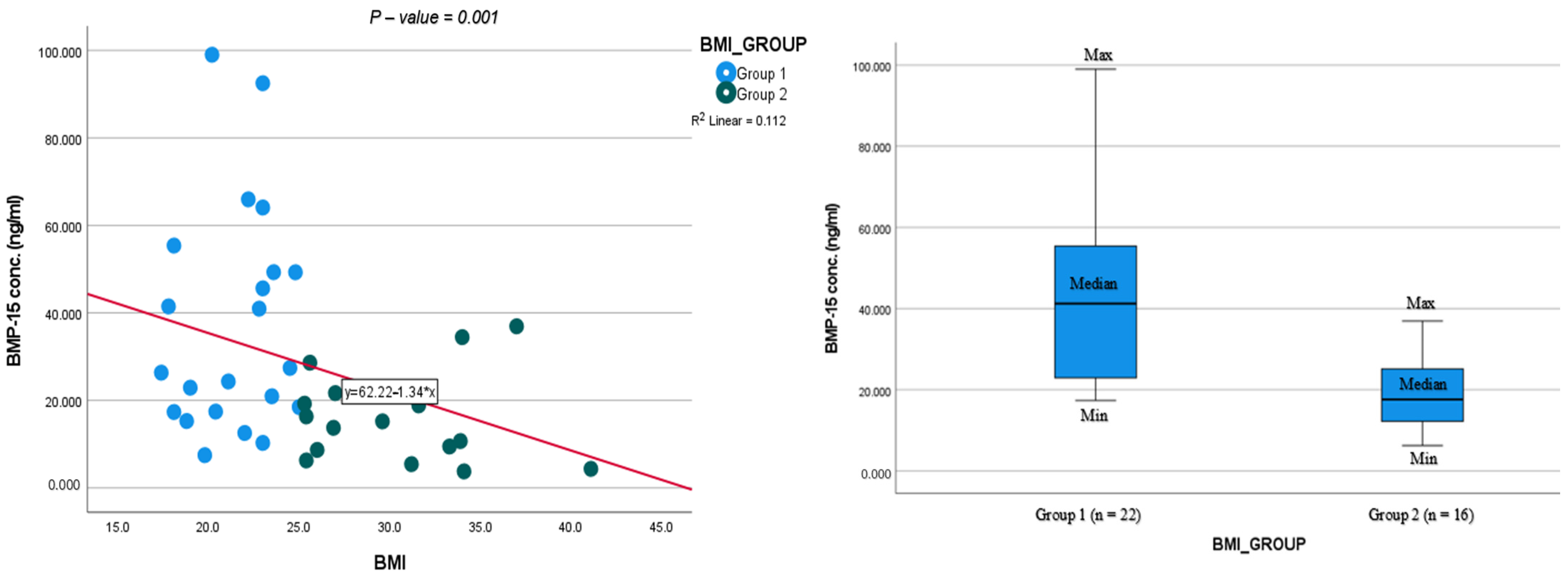
| BMP-15 concentration in FF (ng/mL) | 34.8 ± 26.2 | 15.1 ± 10.5 | 0.006 | [10.24, 38.70] |
| HSPG2 concentration in FF (ng/mL) | 360.8 ± 119.2 | 440.6 ± 271 | 0.264 | |
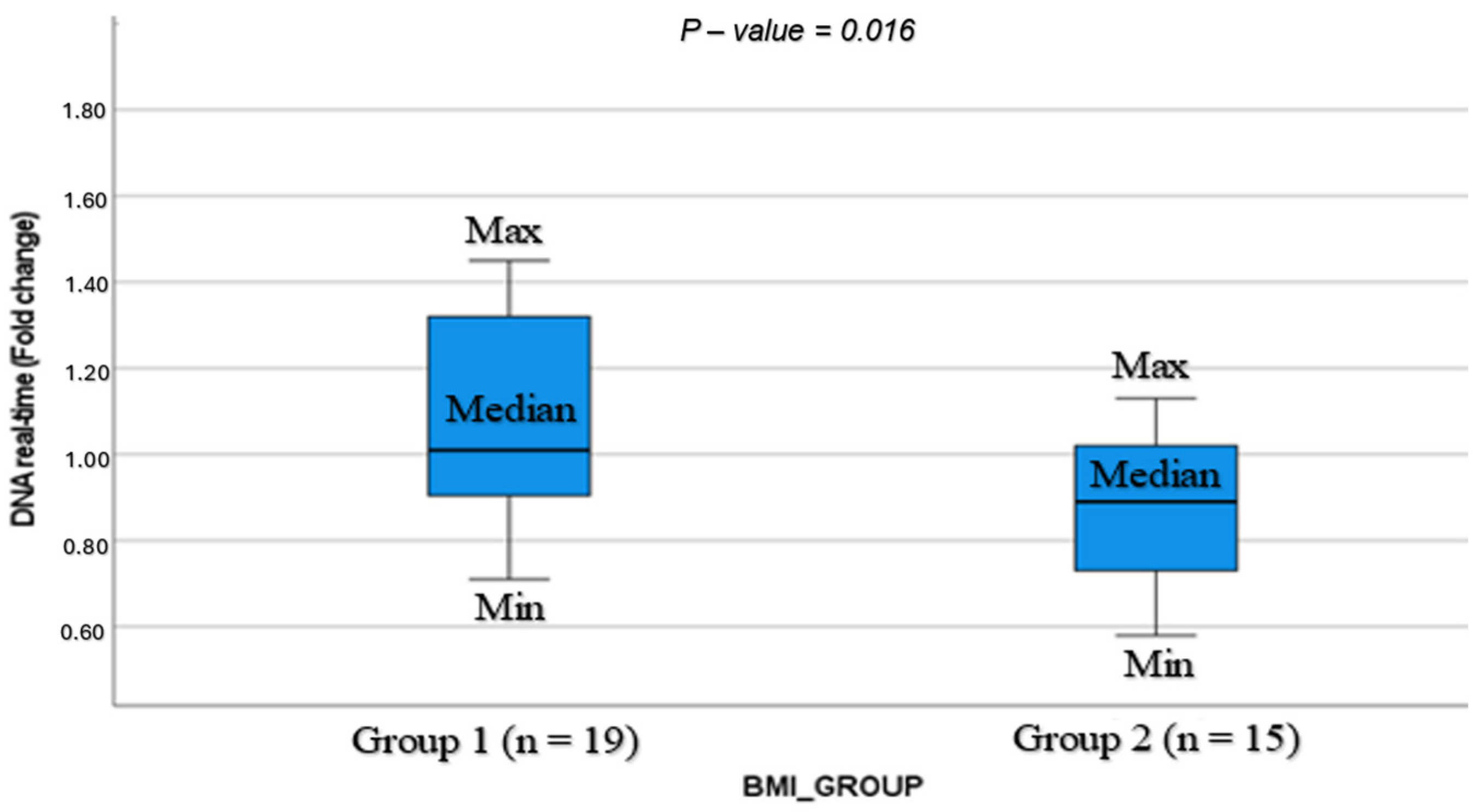
| mtDNA expression in CC (fold change) | 1.10 ± 0.3 | 0.87 ± 0.18 | 0.016 | [0.04, 0.41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigal, E.; Shavit, M.; Atzmon, Y.; Aslih, N.; Bilgory, A.; Estrada, D.; Michaeli, M.; Rotfarb, N.; Shibli Abu-Raya, Y.; Meisel-Sharon, S.; et al. Excess Weight Impairs Oocyte Quality, as Reflected by mtDNA and BMP-15. Cells 2024, 13, 1872. https://doi.org/10.3390/cells13221872
Sigal E, Shavit M, Atzmon Y, Aslih N, Bilgory A, Estrada D, Michaeli M, Rotfarb N, Shibli Abu-Raya Y, Meisel-Sharon S, et al. Excess Weight Impairs Oocyte Quality, as Reflected by mtDNA and BMP-15. Cells. 2024; 13(22):1872. https://doi.org/10.3390/cells13221872
Chicago/Turabian StyleSigal, Emiliya, Maya Shavit, Yuval Atzmon, Nardin Aslih, Asaf Bilgory, Daniella Estrada, Mediea Michaeli, Nechama Rotfarb, Yasmin Shibli Abu-Raya, Shilhav Meisel-Sharon, and et al. 2024. "Excess Weight Impairs Oocyte Quality, as Reflected by mtDNA and BMP-15" Cells 13, no. 22: 1872. https://doi.org/10.3390/cells13221872
APA StyleSigal, E., Shavit, M., Atzmon, Y., Aslih, N., Bilgory, A., Estrada, D., Michaeli, M., Rotfarb, N., Shibli Abu-Raya, Y., Meisel-Sharon, S., & Shalom-Paz, E. (2024). Excess Weight Impairs Oocyte Quality, as Reflected by mtDNA and BMP-15. Cells, 13(22), 1872. https://doi.org/10.3390/cells13221872






