CycloZ Suppresses TLR4-Driven Inflammation to Reduce Asthma-Like Responses in HDM-Exposed Mouse Models
Abstract
1. Introduction
2. Materials and Method
2.1. Animals
2.2. Allergen Instillation and Drug Administration
2.3. BALF Collection, and Total Cell Count in BALF
2.4. Lung Collection
2.5. RNA Analysis
2.6. ELISA
2.7. Histology
2.8. Western Blot
2.9. Statistics
3. Results
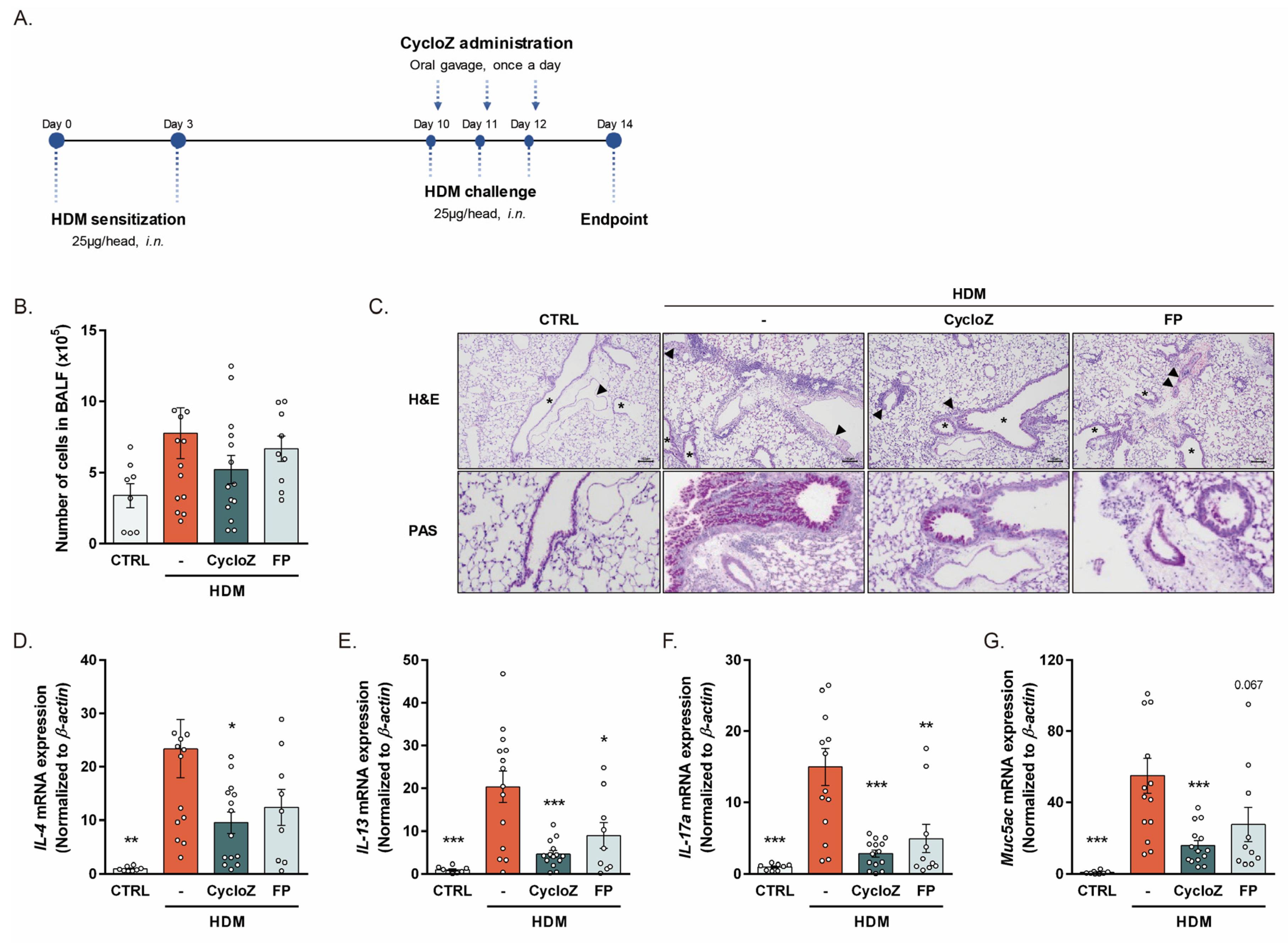
3.1. CycloZ Alleviates Allergic Asthma Responses in Acute Asthma Models
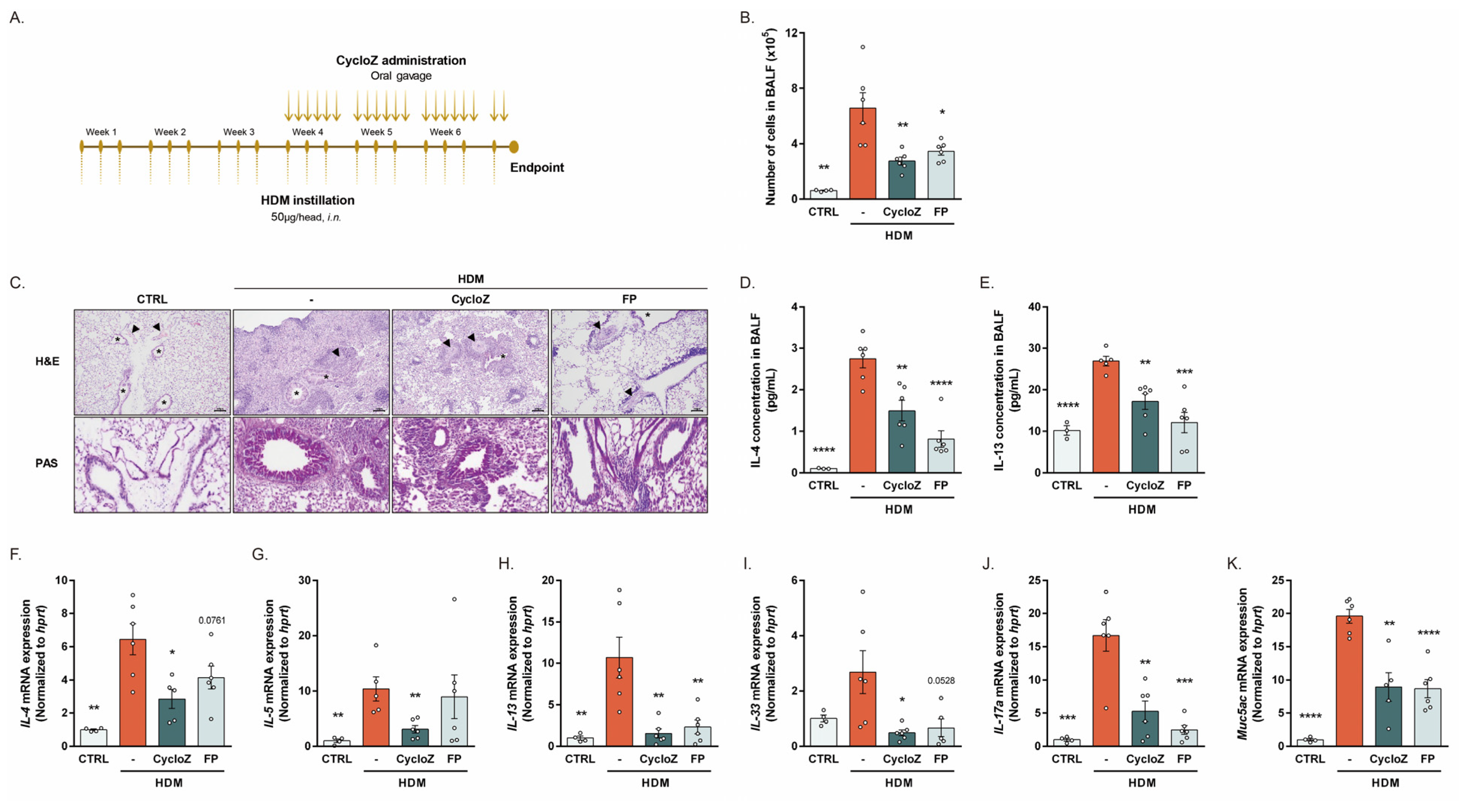
3.2. CycloZ Dramatically Ameliorates Asthma Phenotypes in Chronic Asthma Model
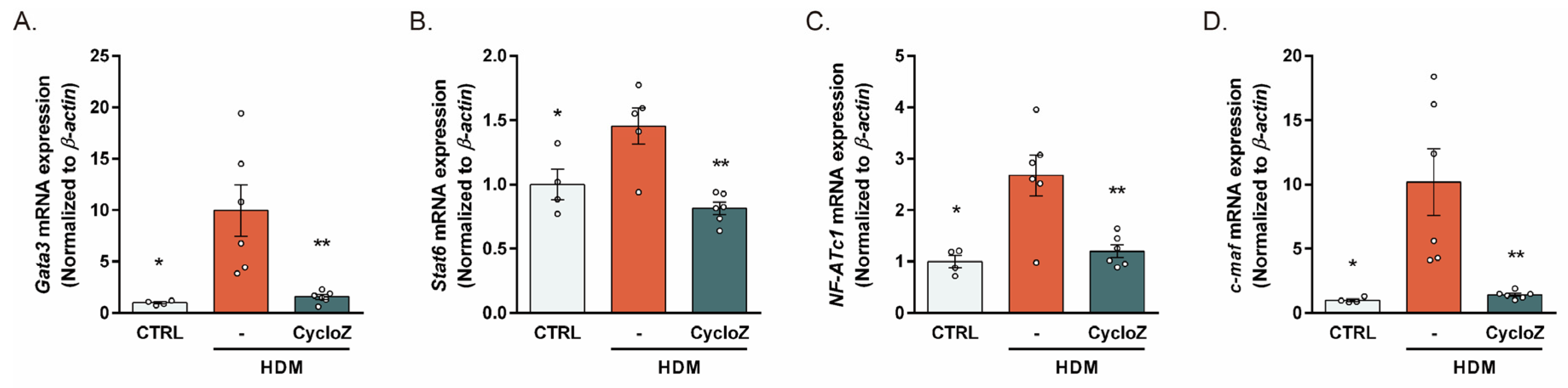
3.3. CycloZ Reduced the HDM-Induced Upregulation of Th2 Response Modulators
3.4. CycloZ Inhibited HDM-Driven TLR4 Pathway Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Heart, Lung, and Blood Institute. What Is Asthma? Available online: https://www.nhlbi.nih.gov/health/asthma (accessed on 14 October 2024).
- Mayo Clinic. Asthma. Available online: https://www.mayoclinic.org/diseases-conditions/asthma/symptoms-causes/syc-20369653 (accessed on 14 October 2024).
- Venkatesan, P. GINA report for asthma. Lancet Respir. Med. 2023, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Toskala, E.; Kennedy, D.W. Asthma risk factors. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S11–S16. [Google Scholar] [CrossRef]
- De Alba, J.; Raemdonck, K.; Dekkak, A.; Collins, M.; Wong, S.; Nials, A.T.; Knowles, R.G.; Belvisi, M.G.; Birrell, M.A. House dust mite induces direct airway inflammation in vivo: Implications for future disease therapy? Eur. Respir. J. 2010, 35, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Gregory, L.G.; Lloyd, C.M. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011, 32, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef]
- Schaub, B.; Bellou, A.; Gibbons, F.K.; Velasco, G.; Campo, M.; He, H.; Liang, Y.; Gillman, M.W.; Gold, D.; Weiss, S.T.; et al. TLR2 and TLR4 stimulation differentially induce cytokine secretion in human neonatal, adult, and murine mononuclear cells. J. Interf. Cytokine Res. 2004, 24, 543–552. [Google Scholar] [CrossRef]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma—Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [CrossRef]
- AbuJabal, R.; Ramakrishnan, R.K.; Bajbouj, K.; Hamid, Q. Role of IL-5 in asthma and airway remodelling. Clin. Exp. Allergy 2024, 54, 538–549. [Google Scholar] [CrossRef]
- Corren, J. Role of interleukin-13 in asthma. Curr. Allergy Asthma Rep. 2013, 13, 415–420. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Mayo Clinic. Asthma Medications: Know Your Options. Available online: https://www.mayoclinic.org/diseases-conditions/asthma/in-depth/asthma-medications/art-20045557 (accessed on 14 October 2024).
- O’Byrne, P.M.; Jenkins, C.; Bateman, E.D. The paradoxes of asthma management: Time for a new approach? Eur. Respir. J. 2017, 50, 1701103. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Conte, C.; Grottelli, S.; Bellezza, I.; Cacciatore, I.; Bolanos, J.P. Cyclo(His-Pro) promotes cytoprotection by activating Nrf2-mediated up-regulation of antioxidant defence. J. Cell. Mol. Med. 2009, 13, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- De Masi, A.; Li, X.; Lee, D.; Jeon, J.; Wang, Q.; Baek, S.; Park, O.; Mottis, A.; Strotjohann, K.; Rapin, A.; et al. Cyclo(His-Pro): A further step in the management of steatohepatitis. JHEP Rep. 2023, 5, 100815. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Han, D.; Kim, K.H.; Seo, A.; Moon, J.J.; Jeong, J.S.; Kim, J.H.; Kang, E.; Bae, E.; Kim, Y.C.; et al. Protective effect of Cyclo(His-Pro) on peritoneal fibrosis through regulation of HDAC3 expression. FASEB J. 2024, 38, e23819. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Jung, H.Y.; Lee, D.; Jeon, J.; Kim, J.; Baek, S.; Lee, J.Y.; Kim, J.Y.; Kwon, H.J. Inhibition of chloride intracellular channel protein 1 (CLIC1) ameliorates liver fibrosis phenotype by activating the Ca2+-dependent Nrf2 pathway. Biomed. Pharmacother. 2023, 168, 115776. [Google Scholar] [CrossRef]
- Song, M.K.; Hwang, I.K.; Rosenthal, M.J.; Harris, D.M.; Yamaguchi, D.T.; Yip, I.; Go, V.L. Antidiabetic actions of arachidonic acid and zinc in genetically diabetic Goto-Kakizaki rats. Metabolism 2003, 52, 7–12. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Zinc Supplementation in Children with Asthma Exacerbation. Pediatr. Rep. 2016, 8, 6685. [Google Scholar] [CrossRef]
- Khanbabaee, G.; Omidian, A.; Imanzadeh, F.; Adibeshgh, F.; Ashayeripanah, M.; Rezaei, N. Serum level of zinc in asthmatic patients: A case-control study. Allergol. Immunopathol. 2014, 42, 19–21. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, D.; Kim, B.; Park, B.-Y.; Oh, C.J.; Kim, M.-J.; Jeon, J.-H.; Lee, I.-K.; Park, O.; Baek, S.; et al. CycloZ Improves Hyperglycemia and Lipid Metabolism by Modulating Lysine Acetylation in KK-Ay Mice. Diabetes Metab. J. 2023, 47, 653–667. [Google Scholar] [CrossRef]
- Sagar, S.; Akbarshahi, H.; Uller, L. Translational value of animal models of asthma: Challenges and promises. Eur. J. Pharmacol. 2015, 759, 272–277. [Google Scholar] [CrossRef]
- Zheng, W.; Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef]
- Saito, T.; Yamamoto, T.; Kazawa, T.; Gejyo, H.; Naito, M. Expression of toll-like receptor 2 and 4 in lipopolysaccharide-induced lung injury in mouse. Cell Tissue Res. 2005, 321, 75–88. [Google Scholar] [CrossRef]
- Berndt, A.; Derksen, F.J.; Venta, P.J.; Ewart, S.; Yuzbasiyan-Gurkan, V.; Robinson, N.E. Elevated amount of Toll-like receptor 4 mRNA in bronchial epithelial cells is associated with airway inflammation in horses with recurrent airway obstruction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L936–L943. [Google Scholar] [CrossRef]
- Hammad, H.; Chieppa, M.; Perros, F.; Willart, M.A.; Germain, R.N.; Lambrecht, B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009, 15, 410–416. [Google Scholar] [CrossRef]
- Lapi, F.; Kezouh, A.; Suissa, S.; Ernst, P. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur. Respir. J. 2013, 42, 79–86. [Google Scholar] [CrossRef]
- Verma, A. Top 10 Asthma Drugs in the World. Available online: https://www.marketresearchreports.com/blog/2019/09/07/top-10-asthma-drugs-world?srsltid=AfmBOooztMX3EboJRb2KOFCHnQ33TL8Wx0YDmN0ZuxYN1DSoBrq0AoXx (accessed on 14 October 2024).
- NCT00878605; Development of A Novel Anti-Hyperglycemic Agent. VA Office of Research and Development: Washington, DC, USA, 2018.
- NCT02784275; A Study to Evaluate the Efficacy and Safety of Cyclo-Z in Patients with Obese Type 2 Diabetes. NovMetaPharma Co., Ltd.: Seoul, Republic of Korea, 2018.
- NCT03560271; A Phase 2 Study of Cyclo-Z in Subjects with Type 2 Diabetes. NovMetaPharma Co., Ltd.: Seoul, Republic of Korea, 2019.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Jeon, J.; Baek, S.; Park, O.; Kim, A.-R.; Do, M.-S.; Jung, H.-Y. CycloZ Suppresses TLR4-Driven Inflammation to Reduce Asthma-Like Responses in HDM-Exposed Mouse Models. Cells 2024, 13, 2034. https://doi.org/10.3390/cells13232034
Lee D, Jeon J, Baek S, Park O, Kim A-R, Do M-S, Jung H-Y. CycloZ Suppresses TLR4-Driven Inflammation to Reduce Asthma-Like Responses in HDM-Exposed Mouse Models. Cells. 2024; 13(23):2034. https://doi.org/10.3390/cells13232034
Chicago/Turabian StyleLee, Dohyun, Jongsu Jeon, Seoyeong Baek, Onyu Park, Ah-Ram Kim, Myoung-Sool Do, and Hoe-Yune Jung. 2024. "CycloZ Suppresses TLR4-Driven Inflammation to Reduce Asthma-Like Responses in HDM-Exposed Mouse Models" Cells 13, no. 23: 2034. https://doi.org/10.3390/cells13232034
APA StyleLee, D., Jeon, J., Baek, S., Park, O., Kim, A.-R., Do, M.-S., & Jung, H.-Y. (2024). CycloZ Suppresses TLR4-Driven Inflammation to Reduce Asthma-Like Responses in HDM-Exposed Mouse Models. Cells, 13(23), 2034. https://doi.org/10.3390/cells13232034






