Environmental Enrichment Attenuates Repetitive Behavior and Alters the Functional Connectivity of Pain and Sensory Pathways in C58 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing
2.2. Behavioral Assessment and Analyses
2.3. Magnetic Resonance Imaging
2.3.1. Image Acquisition
2.3.2. Image Preprocessing
2.4. Functional Connectivity Analysis
3. Results
3.1. Repetitive Motor Behavior
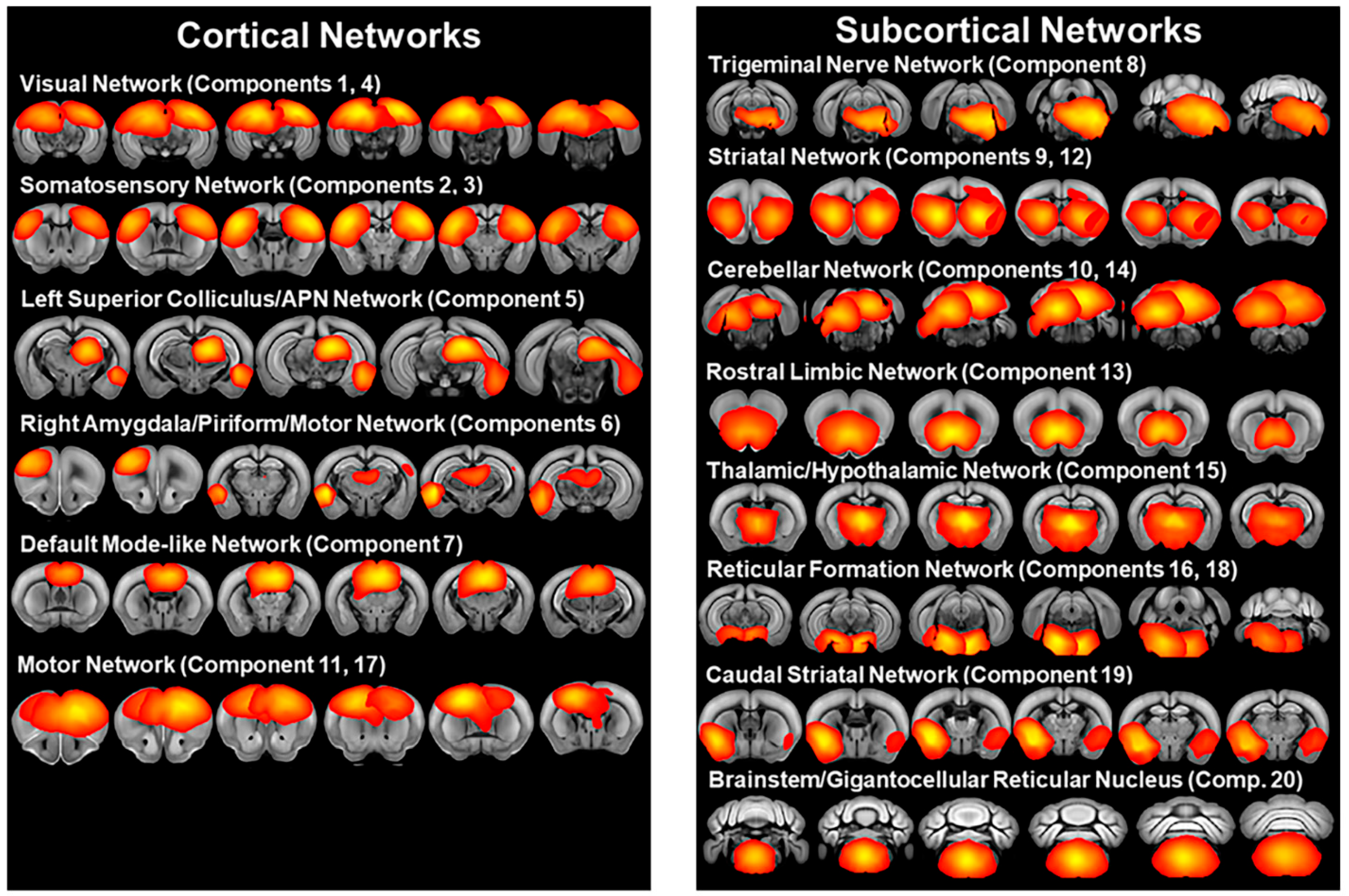
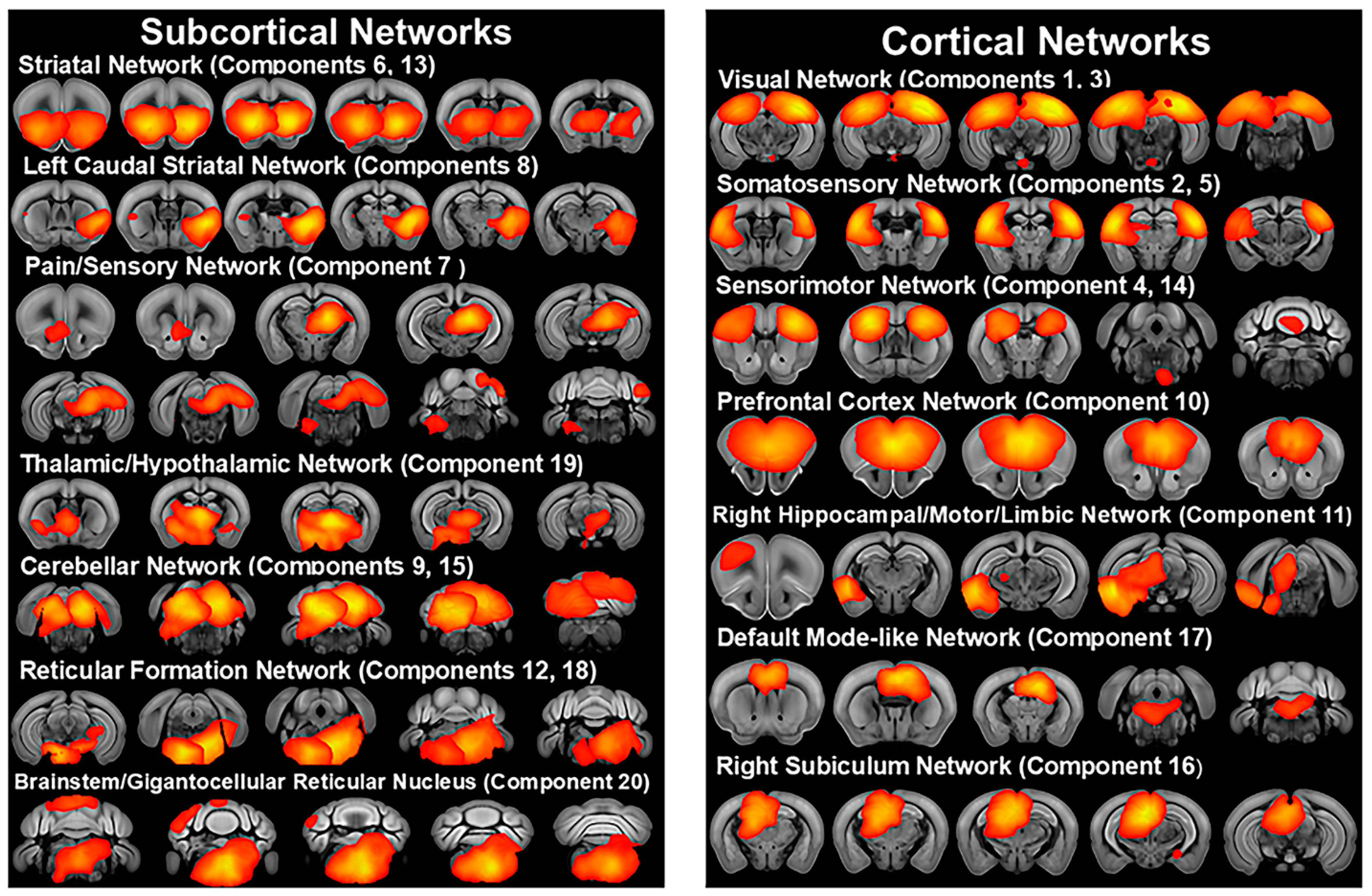
3.2. Independent Component Analysis
3.3. Mouse Strain Differences in Functional Connectivity
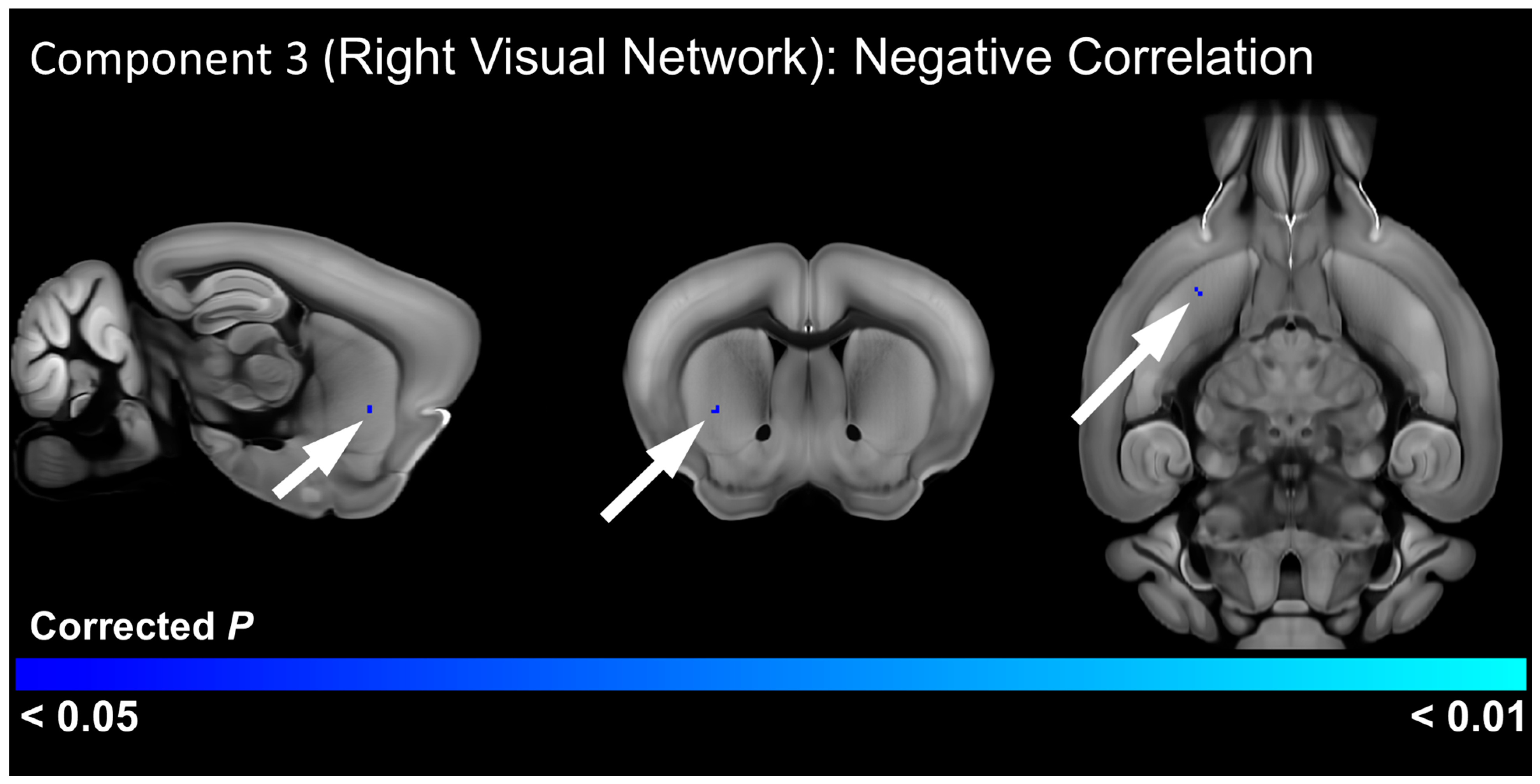
3.4. Relationship of RRB to Functional Connectivity
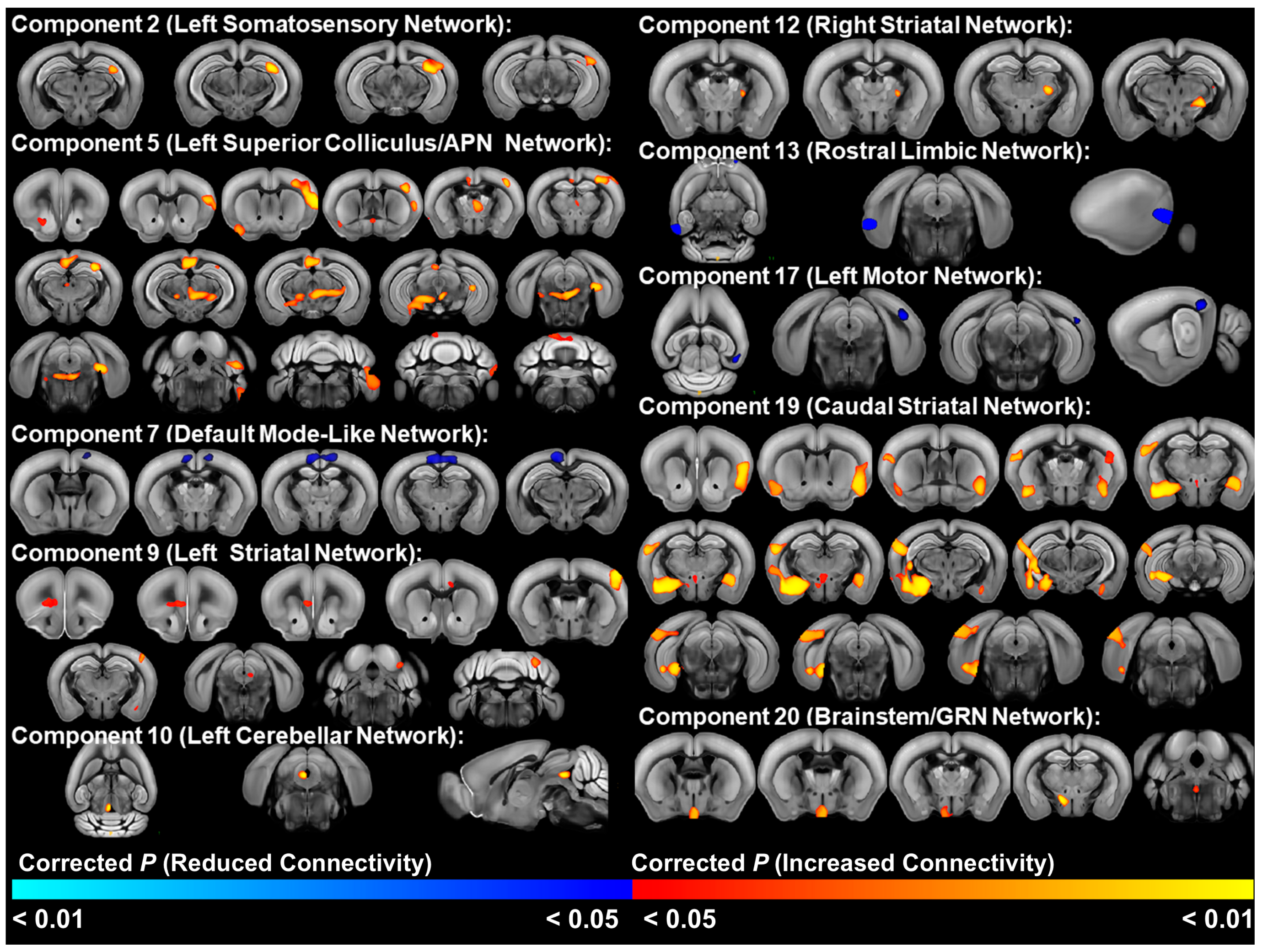
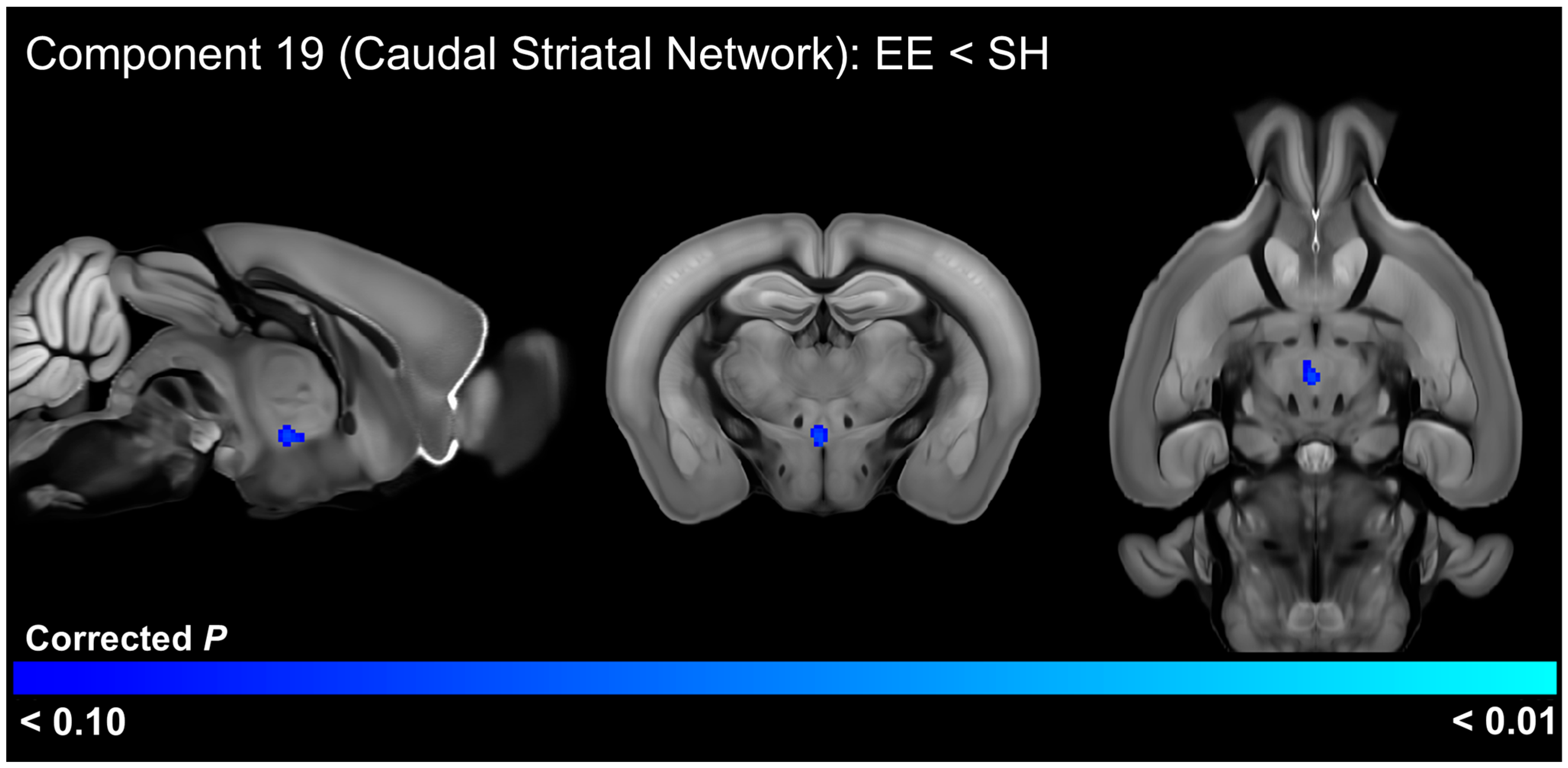
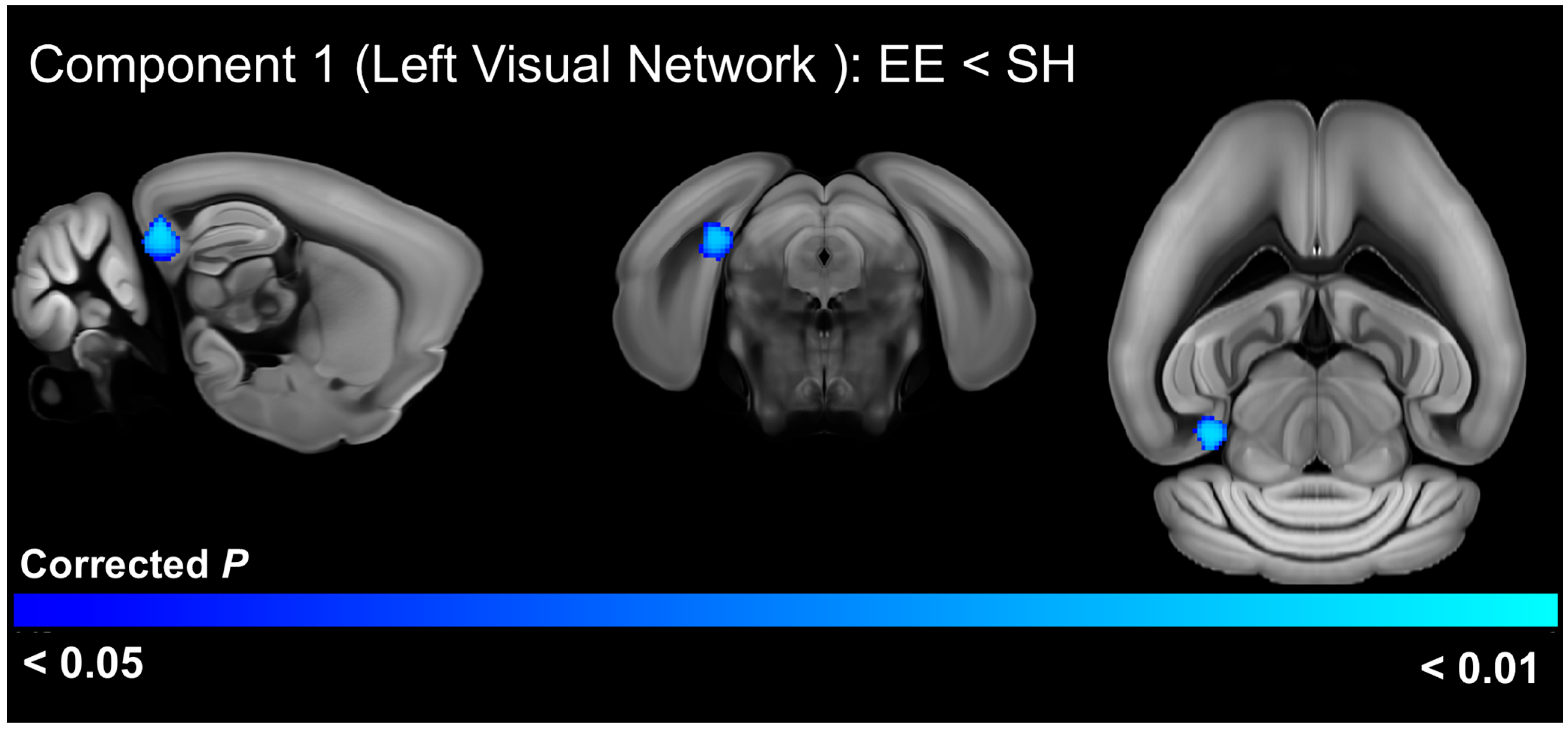
3.5. EE Effects on Functional Connectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodfish, J.W.; Symons, F.J.; Parker, D.E.; Lewis, M.H. Varieties of repetitive behavior in autism: Comparisons to mental retardation. J. Autism Dev. Disord. 2000, 30, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Turner, M. Annotation: Repetitive behaviour in autism: A review of psychological research. J. Child Psychol. Psychiatry 1999, 40, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; Kim, S.J. The pathophysiology of restricted repetitive behavior. J. Neurodev. Disord. 2009, 1, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, B.J.; Lewis, M.H. The neural circuitry of restricted repetitive behavior: Magnetic resonance imaging in neurodevelopmental disorders and animal models. Neurosci. Biobehav. Rev. 2018, 92, 152–171. [Google Scholar] [CrossRef]
- Tian, J.; Gao, X.; Yang, L. Repetitive restricted behaviors in autism spectrum disorder: From mechanism to development of therapeutics. Front. Neurosci. 2022, 16, 780407. [Google Scholar] [CrossRef]
- Farmer, A.L.; Lewis, M.H. Reduction of restricted repetitive behavior by environmental enrichment: Potential neurobiological mechanisms. Neurosci. Biobehav. Rev. 2023, 152, 105291. [Google Scholar] [CrossRef]
- Forbes, T.A.; Gallo, V. All wrapped up: Environmental effects on myelination. Trends Neurosci. 2017, 40, 572–587. [Google Scholar] [CrossRef]
- Hannan, A.J. Environmental enrichment and brain repair: Harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol. Appl. Neurobiol. 2014, 40, 13–25. [Google Scholar] [CrossRef]
- Hirase, H.; Shinohara, Y. Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 2014, 280, 282–298. [Google Scholar] [CrossRef]
- Canario, E.; Chen, D.; Biswal, B. A review of resting-state fMRI and its use to examine psychiatric disorders. Psychoradiology 2021, 1, 42–53. [Google Scholar] [CrossRef]
- Delmonte, S.; Gallagher, L.; O’hanlon, E.; McGrath, J.; Balsters, J.H. Functional and structural connectivity of frontostriatal circuitry in Autism Spectrum Disorder. Front. Hum. Neurosci. 2013, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Supekar, K.; Lynch, C.J.; Khouzam, A.; Phillips, J.; Feinstein, C.; Ryali, S.; Menon, V. Salience network–based classification and prediction of symptom severity in children with autism. JAMA Psychiatry 2013, 70, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.J.; Wiggins, J.L.; Peltier, S.J.; Carrasco, M.; Risi, S.; Lord, C.; Monk, C.S. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010, 1313, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Sforazzini, F.; Bertero, A.; Dodero, L.; David, G.; Galbusera, A.; Scattoni, M.L.; Pasqualetti, M.; Gozzi, A. Altered functional connectivity networks in acallosal and socially impaired BTBR mice. Brain Struct. Funct. 2016, 221, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.H.; Lindenmaier, Z.; Boswell, K.; Edington, G.; King, M.A.; Muehlmann, A.M. Subthalamic nucleus pathology contributes to repetitive behavior expression and is reversed by environmental enrichment. Genes Brain Behav. 2018, 17, e12468. [Google Scholar] [CrossRef]
- Muehlmann, A.M.; Edington, G.; Mihalik, A.C.; Buchwald, Z.; Koppuzha, D.; Korah, M.; Lewis, M.H. Further characterization of repetitive behavior in C58 mice: Developmental trajectory and effects of environmental enrichment. Behav. Brain Res. 2012, 235, 143–149. [Google Scholar] [CrossRef]
- Curry-Pochy, L.S.; Kravetz, Z.; Feinstein, J.; Yaffe, B.; Tanios, V.; Makar, J.; Lewis, M.H. Differential consequences of habitual responding in a mouse model of repetitive behavior. Behav. Neurosci. 2020, 134, 21–33. [Google Scholar] [CrossRef]
- Whitehouse, C.M.; Curry-Pochy, L.S.; Shafer, R.; Rudy, J.; Lewis, M.H. Reversal learning in C58 mice: Modeling higher order repetitive behavior. Behav. Brain Res. 2017, 332, 372–378. [Google Scholar] [CrossRef]
- Bechard, A.R.; Bliznyuk, N.; Lewis, M.H. The development of repetitive motor behaviors in deer mice: Effects of environmental enrichment, repeated testing, and differential mediation by indirect basal ganglia pathway activation. Dev. Psychobiol. 2017, 59, 390–399. [Google Scholar] [CrossRef]
- Bechard, A.R.; Cacodcar, N.; King, M.A.; Lewis, M.H. How does environmental enrichment reduce repetitive motor behaviors? Neuronal activation and dendritic morphology in the indirect basal ganglia pathway of a mouse model. Behav. Brain Res. 2016, 299, 122–131. [Google Scholar] [CrossRef]
- Tanimura, Y.; Vaziri, S.; Lewis, M.H. Indirect basal ganglia pathway mediation of repetitive behavior: Attenuation by adenosine receptor agonists. Behav. Brain Res. 2010, 210, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.A.; Lewis, M.H.; King, M.A. Environmental enrichment: Effects on stereotyped behavior and dendritic morphology. Dev. Psychobiol. 2003, 43, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.A.; Yang, M.C.; Lewis, M.H. Environmental enrichment: Effects on stereotyped behavior and regional neuronal metabolic activity. Brain Res. 2002, 938, 15–21. [Google Scholar] [CrossRef]
- Farmer, A.L.; Febo, M.; Wilkes, B.J.; Lewis, M.H. Environmental enrichment reduces restricted repetitive behavior by altering gray matter microstructure. PLoS ONE 2024, 19, e0307290. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, T.; Pompilus, M.; Fu, Y.; Zhu, J.; Arjona, K.; Arja, R.D.; Grudny, M.M.; Plant, H.D.; Bose, P.; et al. Compensatory functional connectome changes in a rat model of traumatic brain injury. Brain Commun. 2021, 3, fcab244. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Klein, A.; Andersson, J.; Ardekani, B.A.; Ashburner, J.; Avants, B.; Chiang, M.C.; Christensen, G.E.; Collins, L.; Gee, J.; Hellier, P.; et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009, 46, 786–802. [Google Scholar] [CrossRef]
- Chou, N.; Wu, J.; Bingren, J.B.; Qiu, A.; Chuang, K.H. Robust automatic rodent brain extraction using 3-D pulse-coupled neural networks (PCNN). IEEE Trans. Image Process. 2011, 20, 2554–2564. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.F.; DeLuca, M.; Devlin, J.T.; Smith, S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.F.; Mackay, C.E.; Filippini, N.; Smith, S.M. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage 2009, 47, S148. [Google Scholar] [CrossRef]
- Jonckers, E.; Van Audekerke, J.; De Visscher, G.; Van der Linden, A.; Verhoye, M. Functional connectivity fMRI of the rodent brain: Comparison of functional connectivity networks in rat and mouse. PLoS ONE 2011, 6, e18876. [Google Scholar] [CrossRef] [PubMed]
- Mechling, A.E.; Hübner, N.S.; Lee, H.L.; Hennig, J.; von Elverfeldt, D.; Harsan, L.A. Fine-grained mapping of mouse brain functional connectivity with resting-state fMRI. Neuroimage 2014, 96, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Sforazzini, F.; Schwarz, A.J.; Galbusera, A.; Bifone, A.; Gozzi, A. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage 2014, 87, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, V.; Grandjean, J.; Rudin, M.; Wenderoth, N. Mapping the mouse brain with rs-fMRI: An optimized pipeline for functional network identification. Neuroimage 2015, 123, 11–21. [Google Scholar] [CrossRef]
- Stafford, J.M.; Jarrett, B.R.; Miranda-Dominguez, O.; Mills, B.D.; Cain, N.; Mihalas, S.; Lahvis, G.P.; Lattal, K.M.; Mitchell, S.H.; David, S.V.; et al. Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. USA 2014, 111, 18745–18750. [Google Scholar] [CrossRef]
- Mandino, F.; Vrooman, R.M.; Foo, H.E.; Yeow, L.Y.; Bolton, T.A.; Salvan, P.; Teoh, C.L.; Lee, C.Y.; Beauchamp, A.; Luo, S.; et al. A triple-network organization for the mouse brain. Mol. Psychiatry 2022, 27, 865–872. [Google Scholar] [CrossRef]
- Fanselow, M.S. Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev. 1994, 1, 429–438. [Google Scholar] [CrossRef]
- Genaro, K.; Prado, W.A. The role of the anterior pretectal nucleus in pain modulation: A comprehensive review. Eur. J. Neurosci. 2021, 54, 4358–4380. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Liu, N.; Zhang, Z.; Liu, X.; Tang, Y.; He, X.; Wu, B.; Zhou, Z.; Liu, Y.; Li, J.; et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 2015, 6, 6756. [Google Scholar] [CrossRef] [PubMed]
- Akiti, K.; Tsutsui-Kimura, I.; Xie, Y.; Mathis, A.; Markowitz, J.E.; Anyoha, R.; Datta, S.R.; Mathis, M.W.; Uchida, N.; Watabe-Uchida, M. Striatal dopamine explains novelty-induced behavioral dynamics and individual variability in threat prediction. Neuron 2022, 110, 3789–3804. [Google Scholar] [CrossRef]
- Hunnicutt, B.J.; Jongbloets, B.C.; Birdsong, W.T.; Gertz, K.J.; Zhong, H.; Mao, T. A comprehensive excitatory input map of the striatum reveals novel functional organization. eLife 2016, 5, e19103. [Google Scholar] [CrossRef] [PubMed]
- Valjent, E.; Gangarossa, G. The tail of the striatum: From anatomy to connectivity and function. Trends Neurosci. 2021, 44, 203–214. [Google Scholar] [CrossRef]
- Menegas, W.; Akiti, K.; Amo, R.; Uchida, N.; Watabe-Uchida, M. Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat. Neurosci. 2018, 21, 1421–1430. [Google Scholar] [CrossRef]
- Krüttner, S.; Falasconi, A.; Valbuena, S.; Galimberti, I.; Bouwmeester, T.; Arber, S.; Caroni, P. Absence of familiarity triggers hallmarks of autism in mouse model through aberrant tail-of-striatum and prelimbic cortex signaling. Neuron 2022, 110, 1468–1482. [Google Scholar] [CrossRef]
- Ramanathan, K.R.; Jin, J.; Giustino, T.F.; Payne, M.R.; Maren, S. Prefrontal projections to the thalamic nucleus reuniens mediate fear extinction. Nat. Commun. 2018, 9, 4527. [Google Scholar] [CrossRef]
- Guo, F.; Du, Y.; Qu, F.H.; Lin, S.D.; Chen, Z.; Zhang, S.H. Dissecting the neural circuitry for pain modulation and chronic pain: Insights from optogenetics. Neurosci. Bull. 2022, 38, 440–452. [Google Scholar] [CrossRef]
- Rees, H.; Roberts, M.H.T. The anterior pretectal nucleus: A proposed role in sensory processing. Pain 1993, 53, 121–135. [Google Scholar] [CrossRef]
- Hess, A.; Sergejeva, M.; Budinsky, L.; Zeilhofer, H.U.; Brune, K. Imaging of hyperalgesia in rats by functional MRI. Eur. J. Pain 2007, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.J. Pain perception and response: Central nervous system mechanisms. Can. J. Neurol. Sci. 2000, 27, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Peyron, R.; Laurent, B.; Garcia-Larrea, L. Functional imaging of brain responses to pain. A review and meta-analysis. Clin. Neurophysiol. 2000, 30, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Genaro, K.; Prado, W.A. Neural correlates of the antinociceptive effects of stimulating the anterior pretectal nucleus in rats. J. Pain 2016, 17, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Rossaneis, A.C.; Reis, G.M.; Prado, W.A. Stimulation of the occipital or retrosplenial cortex reduces incision pain in rats. Pharmacol. Biochem. Behav. 2011, 100, 220–227. [Google Scholar] [CrossRef]
- Giber, K.; Slézia, A.; Bokor, H.; Bodor, Á.L.; Ludányi, A.; Katona, I.; Acsády, L. Heterogeneous output pathways link the anterior pretectal nucleus with the zona incerta and the thalamus in rat. J. Comp. Neurol. 2008, 506, 122–140. [Google Scholar] [CrossRef]
- Groh, A.; Krieger, P.; Mease, R.A.; Henderson, L. Acute and chronic pain processing in the thalamocortical system of humans and animal models. Neuroscience 2018, 387, 58–71. [Google Scholar] [CrossRef]
- Potes, C.S.; Neto, F.L.; Castro-Lopes, J.M. Inhibition of pain behavior by GABAB receptors in the thalamic ventrobasal complex: Effect on normal rats subjected to the formalin test of nociception. Brain Res. 2006, 1115, 37–47. [Google Scholar] [CrossRef]
- Wang, L.H.; Ding, W.Q.; Sun, Y.G. Spinal ascending pathways for somatosensory information processing. Trends Neurosci. 2022, 45, 594–607. [Google Scholar] [CrossRef]
- Liu, P.F.; Wang, Y.; Xu, L.; Xiang, A.F.; Liu, M.Z.; Zhu, Y.B.; Jia, X.; Zhang, R.; Li, J.-B.; Zhang, L.; et al. Modulation of itch and pain signals processing in ventrobasal thalamus by thalamic reticular nucleus. Iscience 2022, 25, 103625. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, R.X.; Zhang, Y.; Wang, J.; Liu, F.Y.; Cai, J.; Liao, F.; Xu, F.; Yi, M.; Wan, Y. Reduced GABAergic transmission in the ventrobasal thalamus contributes to thermal hyperalgesia in chronic inflammatory pain. Sci. Rep. 2017, 7, 41439. [Google Scholar] [CrossRef]
- Isa, K.; Sooksawate, T.; Kobayashi, K.; Kobayashi, K.; Redgrave, P.; Isa, T. Dissecting the tectal output channels for orienting and defense responses. ENeuro 2020, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Feldheim, D.A. The mouse superior colliculus: An emerging model for studying circuit formation and function. Front. Neural Circuits 2018, 12, 10. [Google Scholar] [CrossRef]
- Wheatcroft, T.; Saleem, A.B.; Solomon, S.G. Functional organisation of the mouse superior colliculus. Front. Neural Circuits 2022, 16, 792959. [Google Scholar] [CrossRef]
- Almada, R.C.; Genewsky, A.J.; Heinz, D.E.; Kaplick, P.M.; Coimbra, N.C.; Wotjak, C.T. Stimulation of the nigrotectal pathway at the level of the superior colliculus reduces threat recognition and causes a shift from avoidance to approach behavior. Front. Neural Circuits 2018, 12, 36. [Google Scholar] [CrossRef]
- Evans, D.A.; Stempel, A.V.; Vale, R.; Ruehle, S.; Lefler, Y.; Branco, T. A synaptic threshold mechanism for computing escape decisions. Nature 2018, 558, 590–594. [Google Scholar] [CrossRef]
- Hormigo, S.; Vega-Flores, G.; Rovira, V.; Castro-Alamancos, M.A. Circuits that mediate expression of signaled active avoidance converge in the pedunculopontine tegmentum. J. Neurosci. 2019, 39, 4576–4594. [Google Scholar] [CrossRef]
- Raam, T.; Hong, W. Organization of neural circuits underlying social behavior: A consideration of the medial amygdala. Curr. Opin. Neurol. 2021, 68, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Wei, D.; Song, S.C.; Lim, B.; Tritsch, N.X.; Lin, D. Posterior amygdala regulates sexual and aggressive behaviors in male mice. Nat. Neurosci. 2020, 23, 1111–1124. [Google Scholar] [CrossRef]
- Hong, W.; Kim, D.W.; Anderson, D.J. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 2014, 158, 1348–1361. [Google Scholar] [CrossRef]
- Tsutsui-Kimura, I.; Natsubori, A.; Mori, M.; Kobayashi, K.; Drew, M.R.; de Kerchove d’Exaerde, A.; Mimura, M.; Tanaka, K.F. Distinct roles of ventromedial versus ventrolateral striatal medium spiny neurons in reward-oriented behavior. Curr. Biol. 2017, 27, 3042–3048. [Google Scholar] [CrossRef] [PubMed]
- Chartoff, E.H.; Marck, B.T.; Matsumoto, A.M.; Dorsa, D.M.; Palmiter, R.D. Induction of stereotypy in dopamine-deficient mice requires striatal D1 receptor activation. Proc. Natl. Acad. Sci. USA 2001, 98, 10451–10456. [Google Scholar] [CrossRef] [PubMed]
- Delfs, J.M.; Kelley, A.E. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience 1990, 39, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yeghiayan, S.K.; Kelley, A.E. Serotonergic stimulation of the ventrolateral striatum induces orofacial stereotypy. Pharmacol. Biochem. Behav. 1995, 52, 493–501. [Google Scholar] [CrossRef]
- Natsubori, A.; Miyazawa, M.; Kojima, T.; Honda, M. Region-specific involvement of ventral striatal dopamine D2 receptor-expressing medium spiny neurons in nociception. Neurosci. Res. 2023, 191, 48–56. [Google Scholar] [CrossRef]
- Mukherjee, D.; Gonzales, B.J.; Ashwal-Fluss, R.; Turm, H.; Groysman, M.; Citri, A. Egr2 induction in spiny projection neurons of the ventrolateral striatum contributes to cocaine place preference in mice. eLife 2021, 10, e65228. [Google Scholar] [CrossRef]
- Thapa, R.; Gruber, A.J. Lesions of ventrolateral striatum eliminate lose-shift but not win-stay behaviour in rats. Neurobiol. Learn. Mem. 2018, 155, 446–451. [Google Scholar] [CrossRef]
- Yawata, Y.; Shikano, Y.; Ogasawara, J.; Makino, K.; Kashima, T.; Ihara, K.; Yoshimoto, A.; Morikawa, S.; Yagishita, S.; Tanaka, K.F.; et al. Mesolimbic dopamine release precedes actively sought aversive stimuli in mice. Nat. Commun. 2023, 14, 2433. [Google Scholar] [CrossRef]
- Gogolla, N.; Takesian, A.E.; Feng, G.; Fagiolini, M.; Hensch, T.K. Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron 2014, 83, 894–905. [Google Scholar] [CrossRef]
- Schulz, S.E.; Stevenson, R.A. Sensory hypersensitivity predicts repetitive behaviours in autistic and typically-developing children. Autism 2019, 23, 1028–1041. [Google Scholar] [CrossRef]
- Wang, L.; Almeida, L.E.; Nettleton, M.; Khaibullina, A.; Albani, S.; Kamimura, S.; Nouraie, M.; Quezado, Z.M. Altered nocifensive behavior in animal models of autism spectrum disorder: The role of the nicotinic cholinergic system. Neuropharmacology 2016, 111, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.J.; Swanson, M.R.; Elison, J.T.; Gerig, G.; Pruett, J.R.; Styner, M.A.; Vachet, C.; Botteron, K.N.; Dager, S.R.; Estes, A.M.; et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol. Autism 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Monday, H.R.; Wang, H.C.; Feldman, D.E. Circuit-level theories for sensory dysfunction in autism: Convergence across mouse models. Front. Neurol. 2023, 14, 1254297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Rodgers, J.; McConachie, H. Restricted and repetitive behaviours, sensory processing and cognitive style in children with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Gabriels, R.L.; Agnew, J.A.; Miller, L.J.; Gralla, J.; Pan, Z.; Goldson, E.; Ledbetter, J.C.; Dinkins, J.P.; Hooks, E. Is there a relationship between restricted, repetitive, stereotyped behaviors and interests and abnormal sensory response in children with autism spectrum disorders? Res. Autism Spectr. Disord. 2008, 2, 660–670. [Google Scholar] [CrossRef]
- Manor-Binyamini, I.; Schreiber-Divon, M. Repetitive behaviors: Listening to the voice of people with high-functioning autism spectrum disorder. Res. Autism Spectr. Disord. 2019, 64, 23–30. [Google Scholar] [CrossRef]
- Wang, L.; Almeida, L.E.; Spornick, N.A.; Kenyon, N.; Kamimura, S.; Khaibullina, A.; Nouraie, M.; Quezado, Z.M. Modulation of social deficits and repetitive behaviors in a mouse model of autism: The role of the nicotinic cholinergic system. Psychopharmacology 2015, 232, 4303–4316. [Google Scholar] [CrossRef]
- Gandhi, T.; Lee, C.C. Neural mechanisms underlying repetitive behaviors in rodent models of autism spectrum disorders. Front. Cell. Neurosci. 2021, 14, 592710. [Google Scholar] [CrossRef] [PubMed]
- Muehlmann, A.M.; Maletz, S.; King, M.A.; Lewis, M.H. Pharmacological targeting of striatal indirect pathway neurons improves subthalamic nucleus dysfunction and reduces repetitive behaviors in C58 mice. Behav. Brain Res. 2020, 391, 112708. [Google Scholar] [CrossRef]
- Wilkes, B.J.; Bass, C.; Korah, H.; Febo, M.; Lewis, M.H. Volumetric magnetic resonance and diffusion tensor imaging of C58/J mice: Neural correlates of repetitive behavior. Brain Imaging Behav. 2020, 14, 2084–2096. [Google Scholar] [CrossRef]
- Langen, M.; Kas, M.J.; Staal, W.G.; van Engeland, H.; Durston, S. The neurobiology of repetitive behavior: Of mice…. Neurosci. Biobehav. Rev. 2011, 35, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Reig, R.; Silberberg, G. Multisensory integration in the mouse striatum. Neuron 2014, 83, 1200–1212. [Google Scholar] [CrossRef]
- Chang, A.D.; Berges, V.A.; Chung, S.J.; Fridman, G.Y.; Baraban, J.M.; Reti, I.M. High-frequency stimulation at the subthalamic nucleus suppresses excessive self-grooming in autism-like mouse models. Neuropsychopharmacology 2016, 41, 1813–1821. [Google Scholar] [CrossRef]
- Bellistri, E.; Aguilar, J.; Brotons-Mas, J.R.; Foffani, G.; De la Prida, L.M. Basic properties of somatosensory-evoked responses in the dorsal hippocampus of the rat. J. Physiol. 2013, 591, 2667–2686. [Google Scholar] [CrossRef]
- Ferguson, A.V.; Latchford, K.J.; Samson, W.K. The paraventricular nucleus of the hypothalamus–a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets 2008, 12, 717–727. [Google Scholar] [CrossRef]
- Salay, L.D.; Ishiko, N.; Huberman, A.D. A midline thalamic circuit determines reactions to visual threat. Nature 2018, 557, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hu, J.M.; Zhang, S.Y.; Xiang, X.J.; Chen, S.Q.; Ding, S.L. Rodent area prostriata converges multimodal hierarchical inputs and projects to the structures important for visuomotor behaviors. Front. Neurosci. 2021, 15, 772016. [Google Scholar] [CrossRef] [PubMed]
- Mikellidou, K.; Kurzawski, J.W.; Frijia, F.; Montanaro, D.; Greco, V.; Burr, D.C.; Morrone, M.C. Area prostriata in the human brain. Curr. Biol. 2017, 27, 3056–3060. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, F.; Mandolesi, L.; Serra, L.; Sorrentino, G.; Caltagirone, C. The neuroprotective effects of experience on cognitive functions: Evidence from animal studies on the neurobiological bases of brain reserve. Neuroscience 2018, 370, 218–235. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef]
- Begenisic, T.; Spolidoro, M.; Braschi, C.; Baroncelli, L.; Milanese, M.; Pietra, G.; Fabbri, M.E.; Bonanno, G.; Cionni, G.; Maffei, L.; et al. Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome. Front. Cell Neurosci. 2011, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Engineer, N.D.; Percaccio, C.R.; Pandya, P.K.; Moucha, R.; Rathbun, D.L.; Kilgard, M.P. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J. Neurophysiol. 2004, 92, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, H.; Zheng, Q.; Fok, A.H.K.; Li, X.; Lau, C.G.; Lai, C.S.W. Environmental enrichment or selective activation of parvalbumin-expressing interneurons ameliorates synaptic and behavioral deficits in animal models with schizophrenia-like behaviors during adolescence. Mol. Psychiatry 2021, 26, 2533–2552. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.F.; Paoletti, G.; Della Seta, D.; Panelli, R.; Marcus, M.A.; Farabollini, F.; Giancarlo, C.; Joosten, E.A. Enriched environment and the recovery from inflammatory pain: Social versus physical aspects and their interaction. Behav. Brain Res. 2010, 208, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Turczak, J.; Przewłocki, R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: Issues for a therapeutic approach in autism. Neuropsychopharmacology 2006, 31, 36–46. [Google Scholar] [CrossRef]
- Vachon, P.; Millecamps, M.; Low, L.; Thompsosn, S.J.; Pailleux, F.; Beaudry, F.; Bushnell, C.M.; Stone, L.S. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav. Brain Funct. 2013, 9, 22. [Google Scholar] [CrossRef]
- Clipperton-Allen, A.E.; Zhang, A.; Cohen, O.S.; Page, D.T. Environmental enrichment rescues social behavioral deficits and synaptic abnormalities in pten haploinsufficient mice. Genes 2021, 12, 1366. [Google Scholar] [CrossRef]


| Age Group | Housing Type | C58 Females | C58 Males | C57 Females | C57 Males |
|---|---|---|---|---|---|
| 6 weeks post-weaning | EE | 8 (8) | 12 (10) | 12 (12) | 9 (9) |
| SH | 11 (11) | 12 (10) | 11 (11) | 7 (7) | |
| 3 weeks post-weaning | EE | 6 (6) | 4 (4) | Not included | Not included |
| SH | 6 (5) | 6 (3) | Not included | Not included |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farmer, A.L.; Febo, M.; Wilkes, B.J.; Lewis, M.H. Environmental Enrichment Attenuates Repetitive Behavior and Alters the Functional Connectivity of Pain and Sensory Pathways in C58 Mice. Cells 2024, 13, 1933. https://doi.org/10.3390/cells13231933
Farmer AL, Febo M, Wilkes BJ, Lewis MH. Environmental Enrichment Attenuates Repetitive Behavior and Alters the Functional Connectivity of Pain and Sensory Pathways in C58 Mice. Cells. 2024; 13(23):1933. https://doi.org/10.3390/cells13231933
Chicago/Turabian StyleFarmer, Anna L., Marcelo Febo, Bradley J. Wilkes, and Mark H. Lewis. 2024. "Environmental Enrichment Attenuates Repetitive Behavior and Alters the Functional Connectivity of Pain and Sensory Pathways in C58 Mice" Cells 13, no. 23: 1933. https://doi.org/10.3390/cells13231933
APA StyleFarmer, A. L., Febo, M., Wilkes, B. J., & Lewis, M. H. (2024). Environmental Enrichment Attenuates Repetitive Behavior and Alters the Functional Connectivity of Pain and Sensory Pathways in C58 Mice. Cells, 13(23), 1933. https://doi.org/10.3390/cells13231933








