The Role of Plzf in Spermatogonial Stem Cell Maintenance and Differentiation: Mapping the Transcriptional Dynamics and Key Interactions
Abstract
1. Introduction
2. Material and Methods
2.1. Testis Digestion and Culture of Testicular Cells
2.2. Electron Microscopy
2.3. Tissue Processing and Immunostaining Steps
2.4. Gene Expression Analyses on the Fluidigm Biomark System
2.5. Microarray Data Analysis and Data Normalization
2.6. Protein–Protein Interaction (PPI) Networks Construction and Modularity Analysis
2.7. Enrichment Analysis
2.8. AI Tools
2.9. Statistical Analysis
3. Results
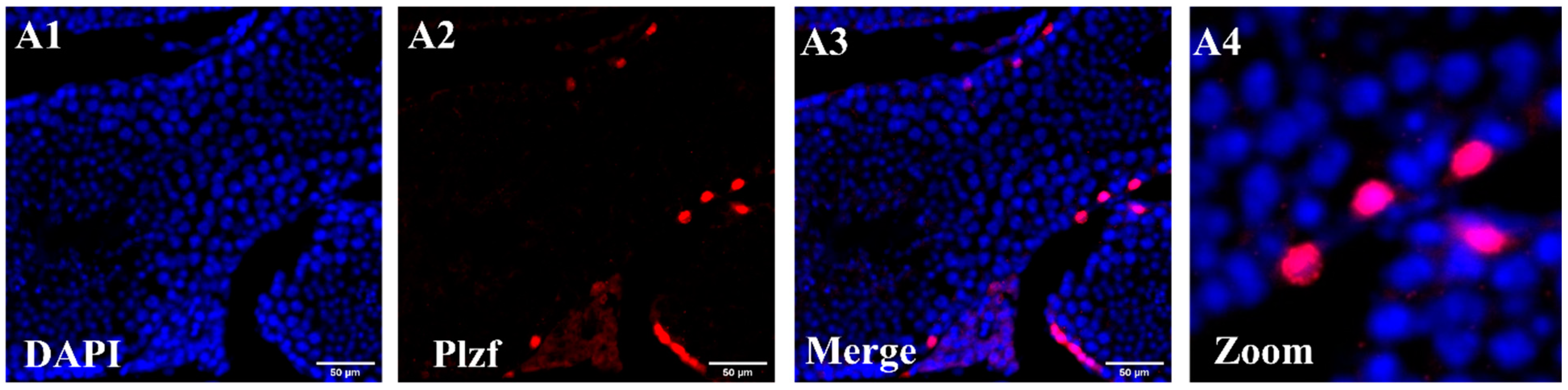
3.1. In Vivo Gene Expression Across Seminiferous Tubules by Immunohistochemistry Test
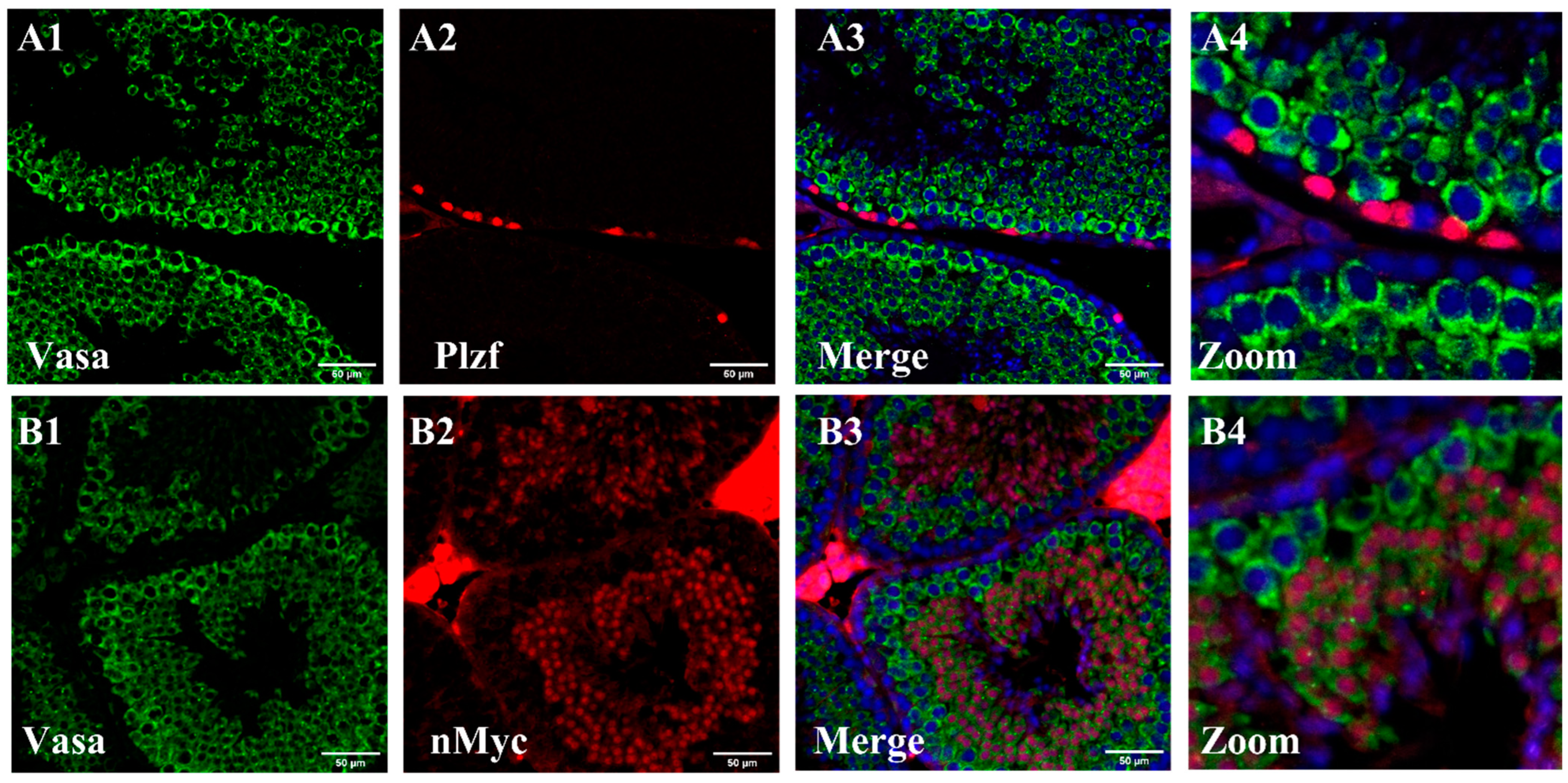
3.2. In Vitro Gene Expression of Spermatogonial Stem Cells by Immunocytochemistry Test
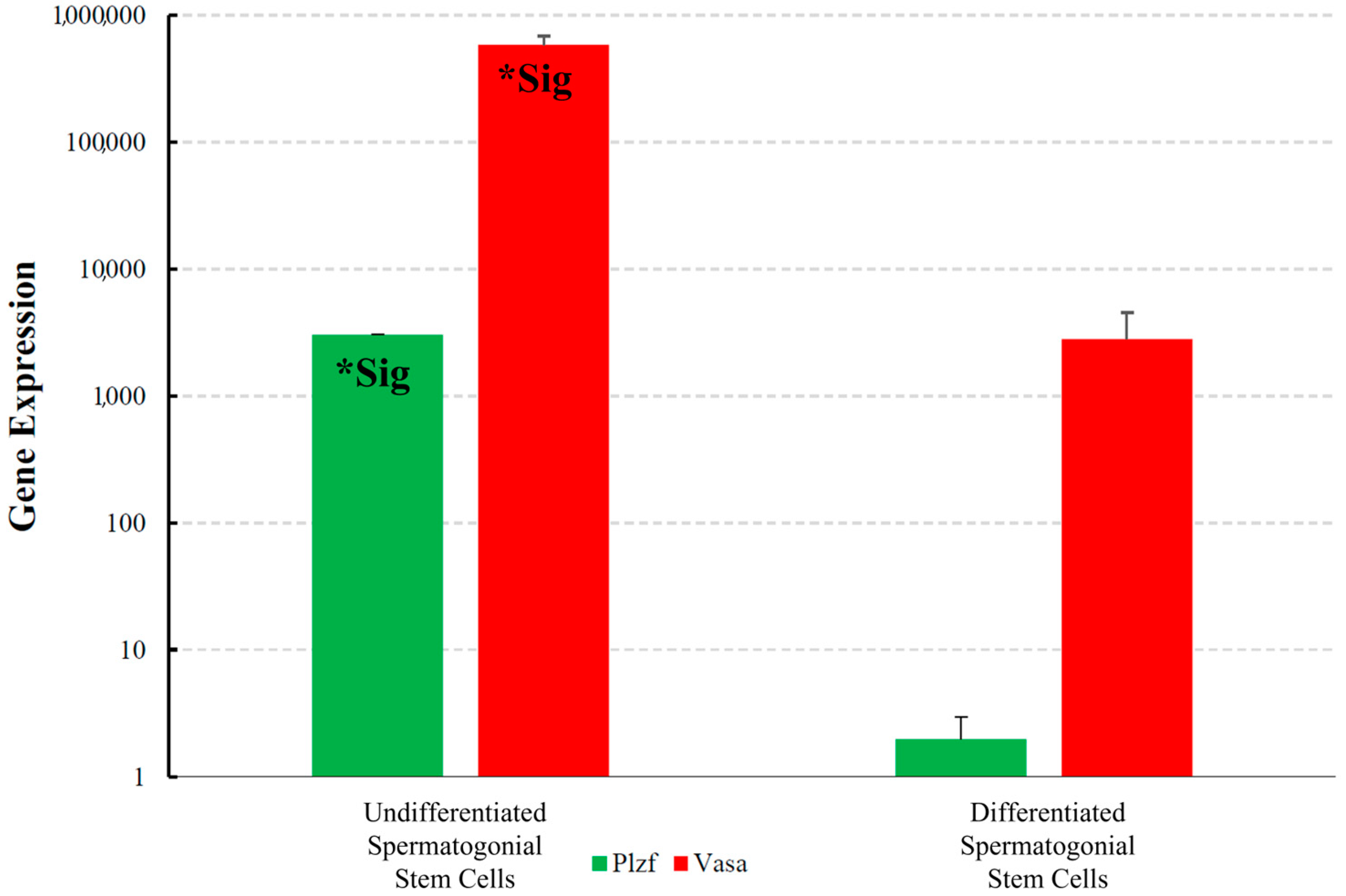
3.3. Analyses of Genes Expression by Fluidigm Biomark System Between Spermatogonial Stem Cells
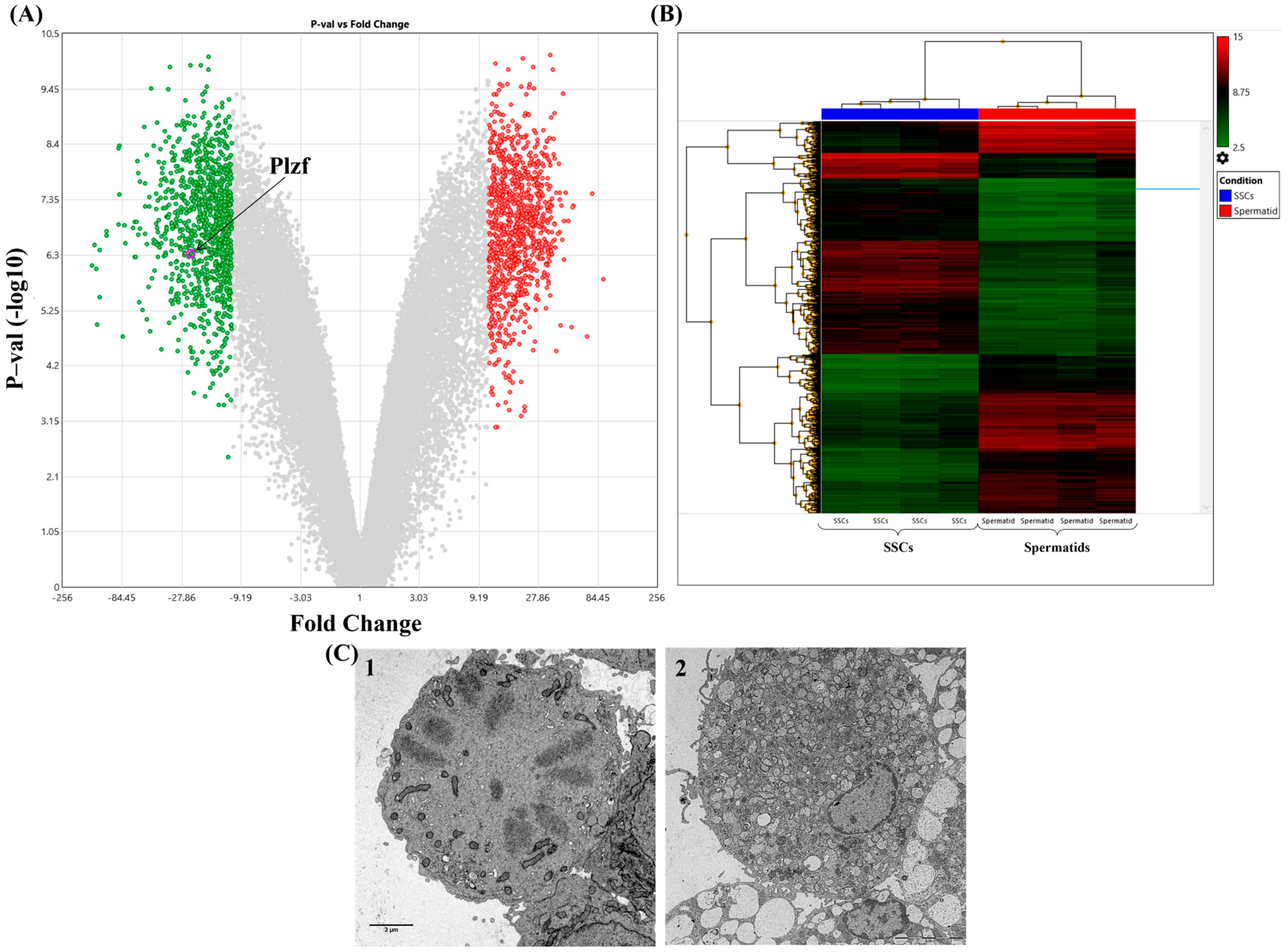
3.4. Microarray Analysis and Identification of Differentially Expressed Genes in the Spermatogenesis Process
3.5. Protein–Protein Interaction (PPI) Networks Construction and Gene Clustering Analysis
3.6. Enrichment and Biological Pathways Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danial Hashemi, K.; Hossein, A. Undifferentiated and Differentiated Spermatogonial Stem Cells. In Advances in Pluripotent Stem Cells; Leisheng, Z., Ed.; IntechOpen: Rijeka, Croatia, 2023; Chapter 2. [Google Scholar]
- Luca, G.; Arato, I.; Sorci, G.; Cameron, D.; Hansen, B.; Baroni, T.; Donato, R.; White, D.; Calafiore, R. Sertoli cells for cell transplantation: Pre-clinical studies and future perspectives. Andrology 2018, 6, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Hamano, K.-I.; Sugimoto, R.; Takahashi, H.; Tsujii, H. Spermatogenesis in immature mammals. Reprod. Med. Biol. 2007, 6, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Lombó, M.; Fernández-Díez, C.; González-Rojo, S.; Herráez, M.P. Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: From spermatozoa to the progeny. Sci. Rep. 2019, 9, 18029. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; DeFalco, T. Steroid hormone signaling: Multifaceted support of testicular function. Front. Cell Dev. Biol. 2023, 11, 1339385. [Google Scholar] [CrossRef]
- Maclean, J.A., 2nd; Wilkinson, M.F. Gene regulation in spermatogenesis. Curr. Top. Dev. Biol. 2005, 71, 131–197. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J. Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 55–65. [Google Scholar] [CrossRef]
- Walker, W.H.; Cooke, P.S. Functions of Steroid Hormones in the Male Reproductive Tract as Revealed by Mouse Models. Int. J. Mol. Sci. 2023, 24, 2748. [Google Scholar] [CrossRef]
- Lui, W.Y.; Cheng, C.Y. Transcriptional regulation of cell adhesion at the blood-testis barrier and spermatogenesis in the testis. Adv. Exp. Med. Biol. 2012, 763, 281–294. [Google Scholar] [CrossRef]
- Sánchez-Jasso, D.E.; López-Guzmán, S.F.; Bermúdez-Cruz, R.M.; Oviedo, N. Novel Aspects of cAMP-Response Element Modulator (CREM) Role in Spermatogenesis and Male Fertility. Int. J. Mol. Sci. 2023, 24, 12558. [Google Scholar] [CrossRef]
- Bernardo, M.V.; Yelo, E.; Gimeno, L.; Campillo, J.A.; Parrado, A. Identification of apoptosis-related PLZF target genes. Biochem. Biophys. Res. Commun. 2007, 359, 317–322. [Google Scholar] [CrossRef]
- Sadler, A.J.; Rossello, F.J.; Yu, L.; Deane, J.A.; Yuan, X.; Wang, D.; Irving, A.T.; Kaparakis-Liaskos, M.; Gantier, M.P.; Ying, H.; et al. BTB-ZF transcriptional regulator PLZF modifies chromatin to restrain inflammatory signaling programs. Proc. Natl. Acad. Sci. USA 2015, 112, 1535–1540. [Google Scholar] [CrossRef]
- Song, W.; Shi, X.; Xia, Q.; Yuan, M.; Liu, J.; Hao, K.; Qian, Y.; Zhao, X.; Zou, K. PLZF suppresses differentiation of mouse spermatogonial progenitor cells via binding of differentiation associated genes. J. Cell. Physiol. 2020, 235, 3033–3042. [Google Scholar] [CrossRef]
- Shamhari, A.; Jefferi, N.E.; Abd Hamid, Z.; Budin, S.B.; Idris, M.H.; Taib, I.S. The Role of Promyelocytic Leukemia Zinc Finger (PLZF) and Glial-Derived Neurotrophic Factor Family Receptor Alpha 1 (GFRα1) in the Cryopreservation of Spermatogonia Stem Cells. Int. J. Mol. Sci. 2023, 24, 1945. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, S.; Qin, W.; Wang, X.; Tang, Y.; Yuan, S. Epigenetic Regulation of Spermatogonial Stem Cell Homeostasis: From DNA Methylation to Histone Modification. Stem Cell Rev. Rep. 2021, 17, 562–580. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Azizi, H.; Skutella, T. Unraveling the Significance of Nanog in the Generation of Embryonic Stem-like Cells from Spermatogonia Stem Cells: A Combined In Silico Analysis and In Vitro Experimental Approach. Int. J. Mol. Sci. 2024, 25, 4833. [Google Scholar] [CrossRef] [PubMed]
- Getun, I.V.; Wu, Z.; Fallahi, M.; Ouizem, S.; Liu, Q.; Li, W.; Costi, R.; Roush, W.R.; Cleveland, J.L.; Bois, P.R.J. Functional Roles of Acetylated Histone Marks at Mouse Meiotic Recombination Hot Spots. Mol. Cell. Biol. 2017, 37, e00942-15. [Google Scholar] [CrossRef] [PubMed]
- Azizi, H.; Conrad, S.; Hinz, U.; Asgari, B.; Nanus, D.; Peterziel, H.; Hajizadeh Moghaddam, A.; Baharvand, H.; Skutella, T. Derivation of Pluripotent Cells from Mouse SSCs Seems to Be Age Dependent. Stem Cells Int. 2016, 2016, 8216312. [Google Scholar] [CrossRef] [PubMed]
- Subash, S.K.; Kumar, P.G. Spermatogonial stem cells: A story of self-renewal and differentiation. Front. Biosci. (Landmark Ed.) 2021, 26, 163–205. [Google Scholar] [CrossRef]
- He, Z.; Kokkinaki, M.; Dym, M. Signaling molecules and pathways regulating the fate of spermatogonial stem cells. Microsc. Res. Tech. 2009, 72, 586–595. [Google Scholar] [CrossRef]
- Yang, C.; Yao, C.; Ji, Z.; Zhao, L.; Chen, H.; Li, P.; Tian, R.; Zhi, E.; Huang, Y.; Han, X.; et al. RNA-binding protein ELAVL2 plays post-transcriptional roles in the regulation of spermatogonia proliferation and apoptosis. Cell Prolif. 2021, 54, e13098. [Google Scholar] [CrossRef]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Orwig, K.E.; Wolgemuth, D.J.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Buaas, F.W.; Kirsh, A.L.; Sharma, M.; McLean, D.J.; Morris, J.L.; Griswold, M.D.; de Rooij, D.G.; Braun, R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004, 36, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Lee, E.H.; Lim, B.; Shyh-Chang, N. Concise Review: Balancing Stem Cell Self-Renewal and Differentiation with PLZF. Stem Cells 2016, 34, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Filipponi, D.; Hobbs, R.M.; Ottolenghi, S.; Rossi, P.; Jannini, E.A.; Pandolfi, P.P.; Dolci, S. Repression of kit expression by Plzf in germ cells. Mol. Cell. Biol. 2007, 27, 6770–6781. [Google Scholar] [CrossRef] [PubMed]
- Goertz, M.J.; Wu, Z.; Gallardo, T.D.; Hamra, F.K.; Castrillon, D.H. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Investig. 2011, 121, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, M.; Gao, Y.; Dong, F.; Cen, C.; Wu, H.; Wang, N.; Cui, X.; Han, C.; Gao, F. The function of Foxo1 in spermatogonia development is independent of PI3K/PTEN signaling. FASEB J. 2022, 36, e22522. [Google Scholar] [CrossRef]
- Mäkelä, J.-A.; Hobbs, R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction 2019, 158, R169–R187. [Google Scholar] [CrossRef]
- Ustianenko, D.; Chiu, H.S.; Treiber, T.; Weyn-Vanhentenryck, S.M.; Treiber, N.; Meister, G.; Sumazin, P.; Zhang, C. LIN28 Selectively Modulates a Subclass of Let-7 MicroRNAs. Mol. Cell 2018, 71, 271–283.e275. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Chakraborty, P.; Buaas, F.W.; Sharma, M.; Snyder, E.; de Rooij, D.G.; Braun, R.E. LIN28A marks the spermatogonial progenitor population and regulates its cyclic expansion. Stem Cells 2014, 32, 860–873. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, X.; Kaestner, K.H.; Wang, P.J. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev. Biol. 2009, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhou, Z.; Li, N.; Zheng, L.; Wu, C.; Niu, B.; Tang, F.; He, X.; Li, G.; Hua, J. Lin28a promotes self-renewal and proliferation of dairy goat spermatogonial stem cells (SSCs) through regulation of mTOR and PI3K/AKT. Sci. Rep. 2016, 6, 38805. [Google Scholar] [CrossRef] [PubMed]
- Khanehzad, M.; Abbaszadeh, R.; Holakuyee, M.; Modarressi, M.H.; Nourashrafeddin, S.M. FSH regulates RA signaling to commit spermatogonia into differentiation pathway and meiosis. Reprod. Biol. Endocrinol. 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Alpaugh, W.F.; Voigt, A.L.; Dardari, R.; Su, L.; Al Khatib, I.; Shin, W.; Goldsmith, T.M.; Coyle, K.M.; Tang, L.A.; Shutt, T.E.; et al. Loss of Ubiquitin Carboxy-Terminal Hydrolase L1 Impairs Long-Term Differentiation Competence and Metabolic Regulation in Murine Spermatogonial Stem Cells. Cells 2021, 10, 2265. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Megee, S.; Dobrinski, I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J. Cell. Physiol. 2009, 220, 460–468. [Google Scholar] [CrossRef]
- Reinicke, A.T.; Laban, K.; Sachs, M.; Kraus, V.; Walden, M.; Damme, M.; Sachs, W.; Reichelt, J.; Schweizer, M.; Janiesch, P.C.; et al. Ubiquitin C-terminal hydrolase L1 (UCH-L1) loss causes neurodegeneration by altering protein turnover in the first postnatal weeks. Proc. Natl. Acad. Sci. USA 2019, 116, 7963–7972. [Google Scholar] [CrossRef]
- Hobbs, R.M.; Seandel, M.; Falciatori, I.; Rafii, S.; Pandolfi, P.P. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell 2010, 142, 468–479. [Google Scholar] [CrossRef]
- Kolesnichenko, M.; Vogt, P.K. Understanding PLZF: Two transcriptional targets, REDD1 and smooth muscle α-actin, define new questions in growth control, senescence, self-renewal and tumor suppression. Cell Cycle 2011, 10, 771–775. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Wang, Z.; Xiong, Z.; Dai, H.; Zou, Z.; Jia, C.; Bai, X.; Chen, Z. mTORC1 Activation Promotes Spermatogonial Differentiation and Causes Subfertility in Mice1. Biol. Reprod. 2016, 95, 140947. [Google Scholar] [CrossRef]
- Hobbs, R.M.; Fagoonee, S.; Papa, A.; Webster, K.; Altruda, F.; Nishinakamura, R.; Chai, L.; Pandolfi, P.P. Functional antagonism between Sall4 and Plzf defines germline progenitors. Cell Stem Cell 2012, 10, 284–298. [Google Scholar] [CrossRef]
- Lovelace, D.L.; Gao, Z.; Mutoji, K.; Song, Y.C.; Ruan, J.; Hermann, B.P. The regulatory repertoire of PLZF and SALL4 in undifferentiated spermatogonia. Development 2016, 143, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.L.; La, H.M.; Legrand, J.M.D.; Mäkelä, J.A.; Eichenlaub, M.; De Seram, M.; Ramialison, M.; Hobbs, R.M. Germline Stem Cell Activity Is Sustained by SALL4-Dependent Silencing of Distinct Tumor Suppressor Genes. Stem Cell Rep. 2017, 9, 956–971. [Google Scholar] [CrossRef] [PubMed]
- Anjum, J.; Mitra, S.; Das, R.; Alam, R.; Mojumder, A.; Emran, T.B.; Islam, F.; Rauf, A.; Hossain, M.J.; Aljohani, A.S.M.; et al. A renewed concept on the MAPK signaling pathway in cancers: Polyphenols as a choice of therapeutics. Pharmacol. Res. 2022, 184, 106398. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Botta, G.; Gao, S.; Li, T.; Van Allen, E.M.; Treacy, D.J.; Cai, C.; He, H.H.; Sweeney, C.J.; Brown, M.; et al. PLZF, a tumor suppressor genetically lost in metastatic castration-resistant prostate cancer, is a mediator of resistance to androgen deprivation therapy. Cancer Res. 2015, 75, 1944–1948. [Google Scholar] [CrossRef]
- Deng, C.Y.; Lv, M.; Luo, B.H.; Zhao, S.Z.; Mo, Z.C.; Xie, Y.J. The Role of the PI3K/AKT/mTOR Signalling Pathway in Male Reproduction. Curr. Mol. Med. 2021, 21, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ma, T.; Jia, S.; Ouyang, Y. AKT3 and related molecules as potential biomarkers responsible for cryptorchidism and cryptorchidism-induced azoospermia. Transl. Pediatr. 2021, 10, 1805–1817. [Google Scholar] [CrossRef]
- Azizi, H.; Koruji, M.; Skutella, T. Comparison of PLZF Gene Expression between Pluripotent Stem Cells and Testicular Germ Cells. Cell J. 2020, 22, 60–65. [Google Scholar] [CrossRef]
- Zhou, W.; Shao, H.; Zhang, D.; Dong, J.; Cheng, W.; Wang, L.; Teng, Y.; Yu, Z. PTEN signaling is required for the maintenance of spermatogonial stem cells in mouse, by regulating the expressions of PLZF and UTF1. Cell Biosci. 2015, 5, 42. [Google Scholar] [CrossRef]
- Azizi, H.; Ranjbar, M.; Rahaiee, S.; Govahi, M.; Skutella, T. Investigation of VASA Gene and Protein Expression in Neonate and Adult Testicular Germ Cells in Mice In Vivo and In Vitro. Cell J. 2020, 22, 171–177. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.; Kostereva, N.; Dym, M.; Hofmann, M.C. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev. Biol. 2007, 304, 34–45. [Google Scholar] [CrossRef]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e1658. [Google Scholar] [CrossRef] [PubMed]
- Dym, M.; Kokkinaki, M.; He, Z. Spermatogonial stem cells: Mouse and human comparisons. Birth Defects Res. C Embryo Today 2009, 87, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Orwig, K.E. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sohni, A.; Tan, K.; Song, H.W.; Burow, D.; de Rooij, D.G.; Laurent, L.; Hsieh, T.C.; Rabah, R.; Hammoud, S.S.; Vicini, E.; et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019, 26, 1501–1517.e1504. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, N.; Azizi, H.; Razavi-Amoli, S.-K.; Skutella, T. The Role of Plzf in Spermatogonial Stem Cell Maintenance and Differentiation: Mapping the Transcriptional Dynamics and Key Interactions. Cells 2024, 13, 1930. https://doi.org/10.3390/cells13231930
Ghasemi N, Azizi H, Razavi-Amoli S-K, Skutella T. The Role of Plzf in Spermatogonial Stem Cell Maintenance and Differentiation: Mapping the Transcriptional Dynamics and Key Interactions. Cells. 2024; 13(23):1930. https://doi.org/10.3390/cells13231930
Chicago/Turabian StyleGhasemi, Nima, Hossein Azizi, Seyedeh-Kiana Razavi-Amoli, and Thomas Skutella. 2024. "The Role of Plzf in Spermatogonial Stem Cell Maintenance and Differentiation: Mapping the Transcriptional Dynamics and Key Interactions" Cells 13, no. 23: 1930. https://doi.org/10.3390/cells13231930
APA StyleGhasemi, N., Azizi, H., Razavi-Amoli, S.-K., & Skutella, T. (2024). The Role of Plzf in Spermatogonial Stem Cell Maintenance and Differentiation: Mapping the Transcriptional Dynamics and Key Interactions. Cells, 13(23), 1930. https://doi.org/10.3390/cells13231930






