Characterization of Tumor-Infiltrating Lymphocyte-Derived Atypical TCRs Recognizing Breast Cancer in an MR1-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines
2.3. Generation of HLA-E Knockout MCF7 Cells
2.4. Construction of TCR Expression Vectors
2.5. Retrovirus Production
2.6. Retroviral Transduction of TCRs into Primary T Cells
2.7. Tetramer Staining of BW hCD8⍺β+ Cells Expressing HLA Class-I-Unrestricted TCRs
2.8. RNA Extraction and RT-PCR
2.9. Detection of MR1 Surface Expression
2.10. Detection of HLA-E and CD1s Expression
2.11. ELISA of Secreted Human IFN-γ
2.12. ELISA to Evaluate the Stimulation of TCR-Expressing BW Cells
2.13. Overexpression of MR1 in MCF7 Cells
2.14. Generation of K43A-Mutated MR1-Expressing MCF7 Cells
2.15. Cytotoxicity Assay
2.16. Statistical Analysis
3. Results
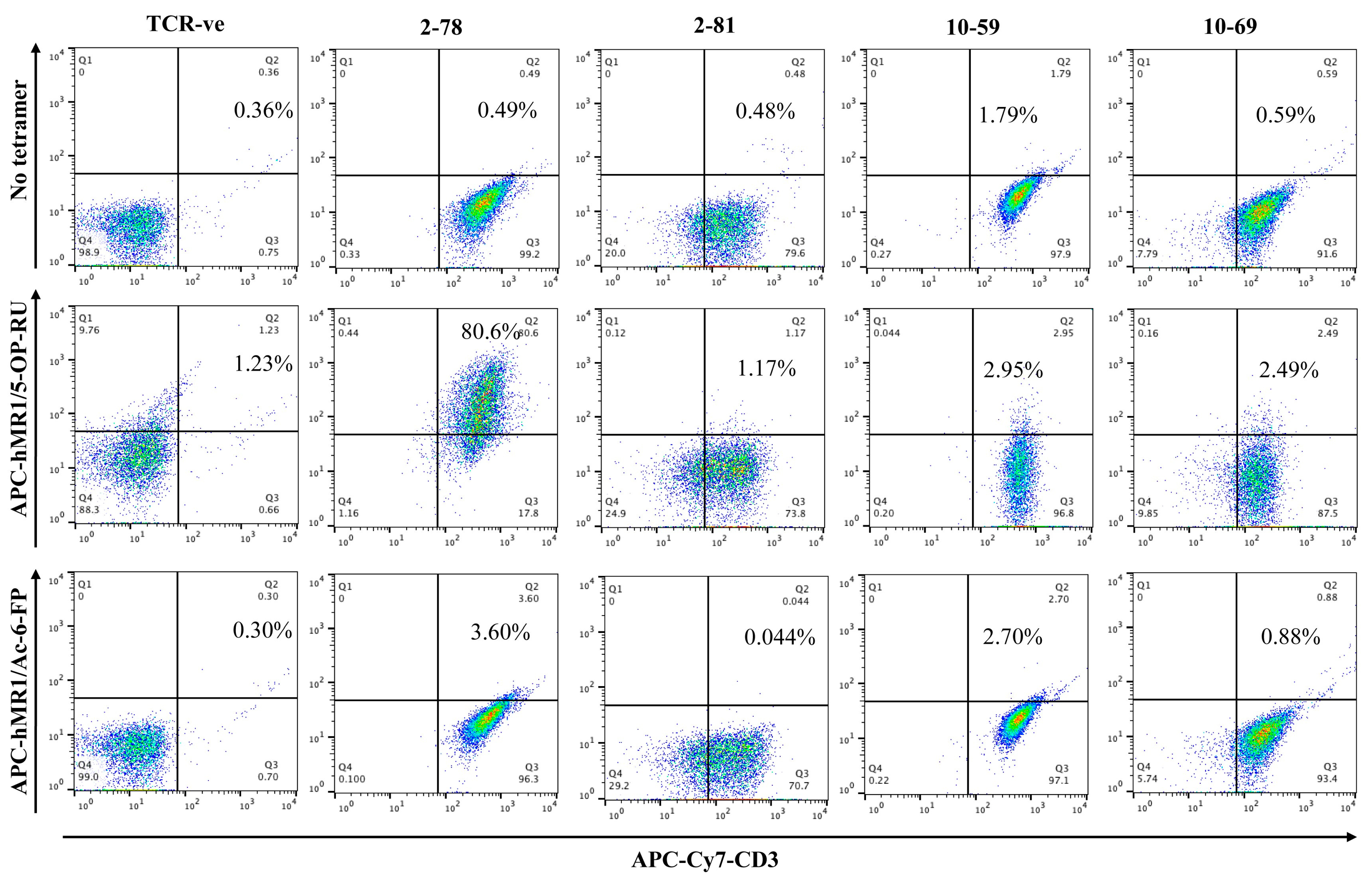
3.1. MR1/5-OP-RU Tetramer Binding
3.2. Characterization of Unconventional HLA-Class I-like Molecules in Various Cell Lines (MR1, CD1, and HLA-E)
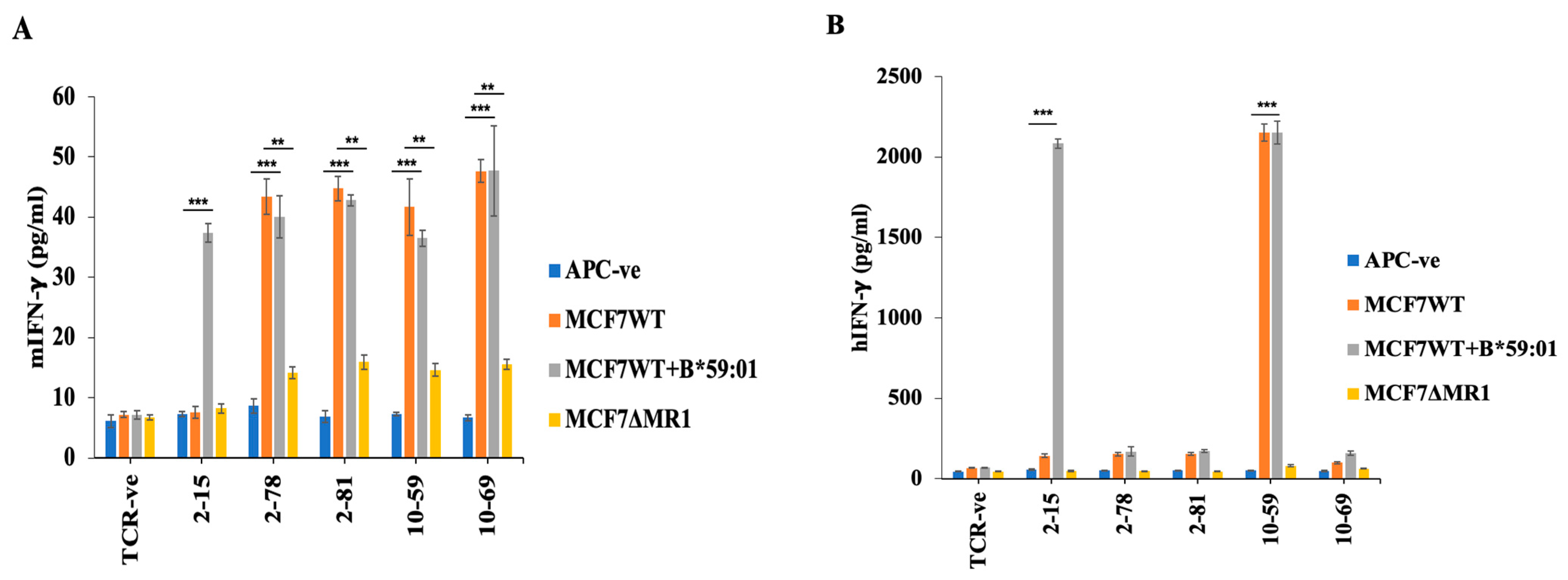
3.3. Reactivity of HLA-Unrestricted TCR-Expressing Mouse Spleen T Cells and Human PBMCs to MCF7 Cells
3.4. Reactivity of TCR10-59 to MCF7 KO Cells
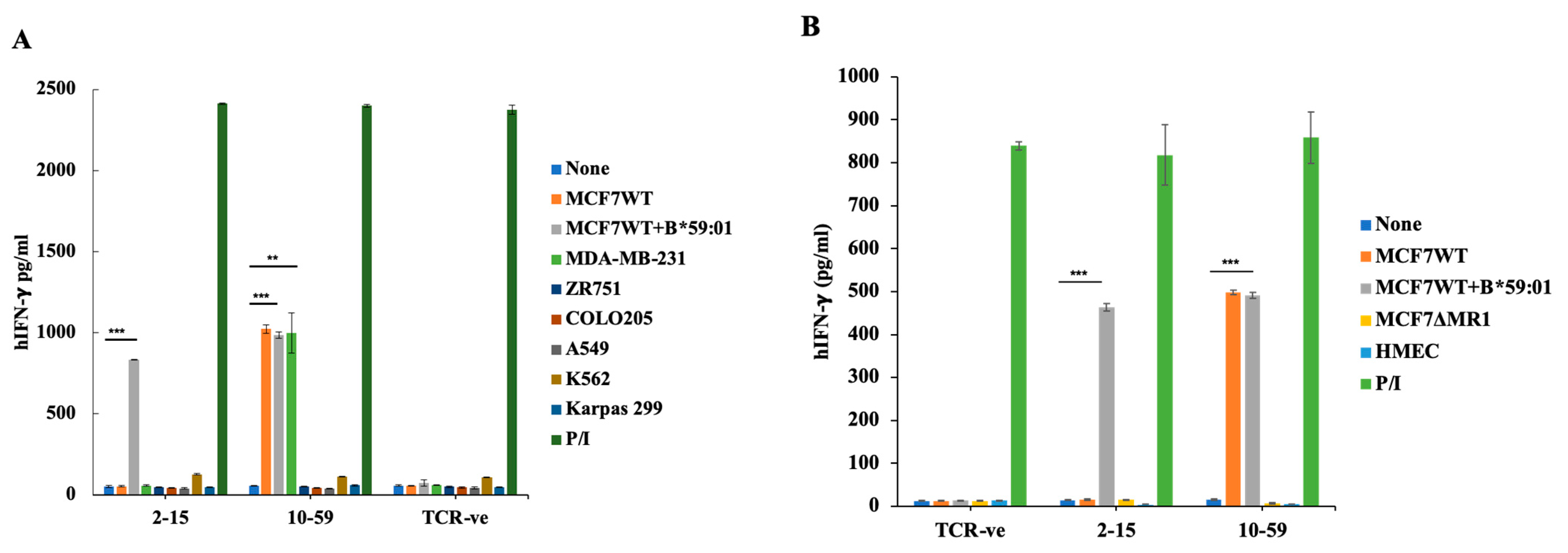
3.5. Reactivity of TCR10-59 to Breast Cancer Cells but Not to Other Cancer Cells
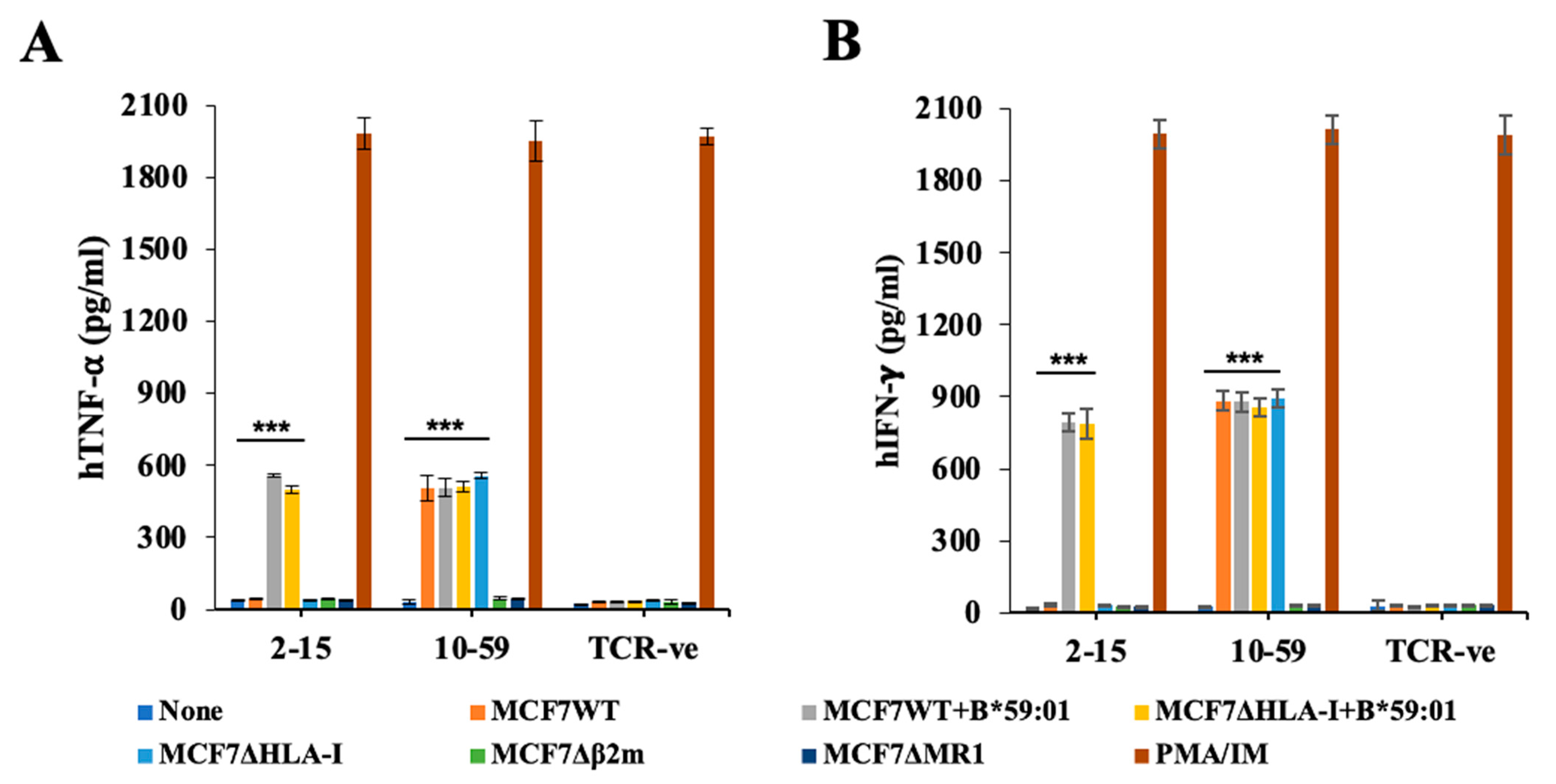
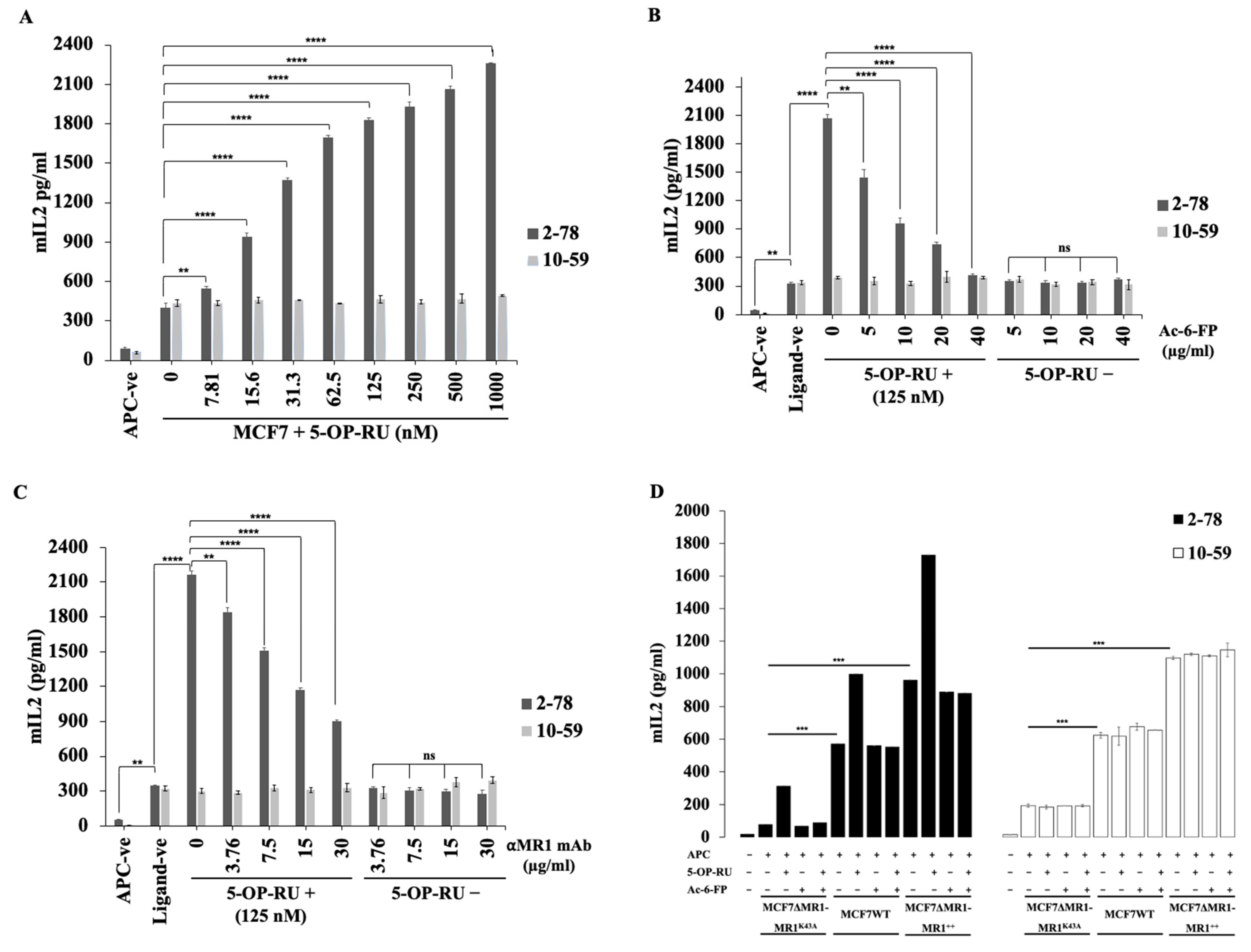
3.6. Ac-6-FP and MR1 mAb Did Not Inhibit the Reactivity of MR1-Restricted T Cells to MCF7 Cells
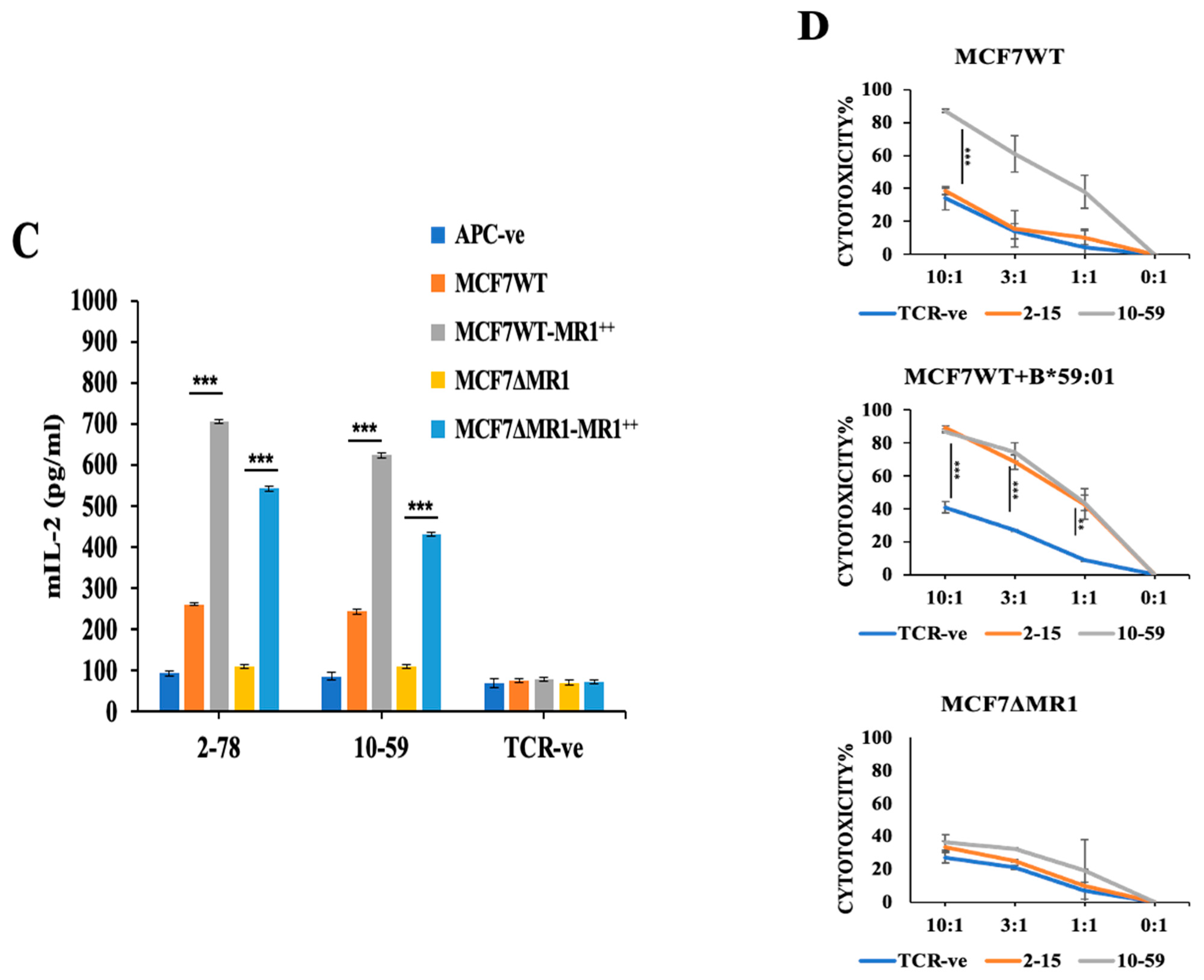
3.7. Reactivity to Breast Cancer Cells Is Dependent on K43 Residue of MR1 Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfrey, D.I.; Uldrich, A.P.; McCluskey, J.; Rossjohn, J.; Moody, D.B. The burgeoning family of unconventional T cells. Nat. Immunol. 2015, 16, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Huang, S.; Arnold, P.L.; Fremont, D.H. Patterns of nonclassical MHC antigen presentation. Nat. Immunol. 2007, 8, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Hirai, M.; Kurosawa, Y. A Gene Outside the Human MHC Related to Classical HLA Class I Genes. Science 1995, 269, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Riegert, P.; Warner, V.; Bahram, S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J. Immunol. 1998, 161, 4066–4077. [Google Scholar] [CrossRef]
- Lamichhane, R.; Ussher, J.E. Expression and trafficking of MR1. Immunology 2017, 151, 270–279. [Google Scholar] [CrossRef]
- McWilliam, H.E.G.; Eckle, S.B.G.; Theodossis, A.; Liu, L.; Chen, Z.; Wubben, J.M.; Fairlie, D.P.; Strugnell, R.A.; Mintern, J.D.; McCluskey, J.; et al. The intracellular pathway for the presentation of vitamin B–related antigens by the antigen-presenting molecule MR1. Nat. Immunol. 2016, 17, 531–537. [Google Scholar] [CrossRef]
- Lepore, M.; Kalinichenko, A.; Colone, A.; Paleja, B.; Singhal, A.; Tschumi, A.; Lee, B.; Poidinger, M.; Zolezzi, F.; Quagliata, L.; et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat. Commun. 2014, 5, 3866. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.J.; Awad, W.; Wang, H.; Chen, Z. Antigen Recognition by MR1-Reactive T Cells; MAIT Cells, Metabolites, and Remaining Mysteries. Front. Immunol. 2020, 11, 1961. [Google Scholar] [CrossRef]
- Gherardin, N.A.; Keller, A.N.; Woolley, R.E.; Le Nours, J.; Ritchie, D.S.; Neeson, P.J.; Birkinshaw, R.W.; Eckle, S.B.G.; Waddington, J.N.; Liu, L.; et al. Diversity of T Cells Restricted by the MHC Class I-Related Molecule MR1 Facilitates Differential Antigen Recognition. Immunity 2016, 44, 32–45. [Google Scholar] [CrossRef]
- Lepore, M.; Kalinichenko, A.; Calogero, S.; Kumar, P.; Paleja, B.; Schmaler, M.; Narang, V.; Zolezzi, F.; Poidinger, M.; Mori, L.; et al. Functionally diverse human T cells recognize non-microbial antigens presented by MR1. eLife 2017, 6, e24476. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.D.; Dolton, G.; Legut, M.; Caillaud, M.E.; Lloyd, A.; Attaf, M.; Galloway, S.A.E.; Rius, C.; Farrell, C.P.; Szomolay, B.; et al. Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat. Immunol. 2020, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.D.; Sewell, A.K. The burgeoning role of MR1-restricted T-cells in infection, cancer and autoimmune disease. Curr. Opin. Immunol. 2021, 69, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Motozono, C.; Nagae, M.; Shimizu, T.; Ishikawa, E.; Motooka, D.; Okuzaki, D.; Izumi, Y.; Takahashi, M.; Fujimori, N.; et al. Symbiotic bacteria-dependent expansion of MR1-reactive T cells causes autoimmunity in the absence of Bcl11b. Nat. Commun. 2022, 13, 6948. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hamana, H.; Shitaoka, K.; Sukegawa, K.; Nagata, T.; Hayee, A.; Kobayashi, E.; Ozawa, T.; Fujii, T.; Muraguchi, A.; et al. TCR function analysis using a novel system reveals the multiple unconventional tumor-reactive T cells in human breast cancer-infiltrating lymphocytes. Eur. J. Immunol. 2021, 51, 2306–2316. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar]
- Shitaoka, K.; Hamana, H.; Kishi, H.; Hayakawa, Y.; Kobayashi, E.; Sukegawa, K. Identification of Tumoricidal TCRs from Tumor-Infiltrating Lymphocytes by Single-Cell Analysis. Cancer Immunol. Res. 2018, 6, 378–388. [Google Scholar] [CrossRef]
- Seliger, B.; Jasinski-Bergner, S.; Quandt, D.; Stoehr, C.; Bukur, J.; Wach, S.; Legal, W.; Taubert, H.; Wullich, B.; Hartmann, A.; et al. HLA-E expression and its clinical relevance in human renal cell carcinoma. Oncotarget 2016, 7, 67360–67372. [Google Scholar] [CrossRef] [PubMed]
- Salio, M.; Awad, W.; Veerapen, N.; Gonzalez-Lopez, C.; Kulicke, C.; Waithe, D.; Martens, A.W.J.; Lewinsohn, D.M.; Hobrath, J.V.; Cox, L.R.; et al. Ligand-dependent downregulation of MR1 cell surface expression. Proc. Natl. Acad. Sci. USA 2020, 117, 10465–10475. [Google Scholar] [CrossRef]
- Eckle, S.B.G.; Birkinshaw, R.W.; Kostenko, L.; Corbett, A.J.; McWilliam, H.E.G.; Reantragoon, R.; Chen, Z.; Gherardin, N.A.; Beddoe, T.; Liu, L.; et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J. Exp. Med. 2014, 211, 1585–1600. [Google Scholar] [CrossRef]
- Souter, M.N.T.; Awad, W.; Li, S.; Pediongco, T.J.; Meehan, B.S.; Meehan, L.J.; Tian, Z.; Zhao, Z.; Wang, H.; Nelson, A.; et al. CD8 coreceptor engagement of MR1 enhances antigen responsiveness by human MAIT and other MR1-reactive T cells. J. Exp. Med. 2022, 219, e20210828. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.J.; Eckle, S.B.G.; Birkinshaw, R.W.; Liu, L.; Patel, O.; Mahony, J.; Chen, Z.; Reantragoon, R.; Meehan, B.; Cao, H.; et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Seneviratna, R.; Redmond, S.; Mcwilliam, H.; Reantragoon, J.; Villadangos, J.; Mccluskey, J.; Godfrey, D.I.; Gherardin, N.A. Differential antigenic requirements by diverse MR1-restricted T cells. Immunol. Cell Biol. 2022, 100, 112–126. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, H.E.G.; Villadangos, J.A. How MR1 Presents a Pathogen Metabolic Signature to Mucosal-Associated Invariant T (MAIT) Cells. Trends Immunol. 2017, 38, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Cornforth, T.V.; Moyo, N.; Cole, S.; Lam, E.; Lobry, T.; Wolchinsky, R.; Lloyd, A.; Ward, K.; Denham, E.M.; Besra, G.S.; et al. Conserved allomorphs of MR1 drive specificity of MR1-restricted TCRs. bioRxiv 2023. [Google Scholar] [CrossRef]
- Reantragoon, R.; Kjer-Nielsen, L.; Patel, O.; Chen, Z.; Illing, P.T.; Bhati, M.; Kostenko, L.; Bharadwaj, M.; Meehan, B.; Hansen, T.H.; et al. Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. J. Exp. Med. 2012, 209, 761–774. [Google Scholar] [CrossRef]
- Rozemuller, E.; Eckle, S.B.G.; McLaughlin, I.; Penning, M.; Mulder, W.; de Bruin, H.; van Wageningen, S. MR1 encompasses at least six allele groups with coding region alterations. HLA 2021, 98, 509–516. [Google Scholar] [CrossRef]
- Howson, L.J.; Awad, W.; von Borstel, A.; Lim, H.J.; McWilliam, H.E.G.; Sandoval-Romero, M.L.; Majumdar, S.; Hamzeh, A.R.; Andrews, T.D.; Mcdermott, D.H.; et al. Absence of mucosal-associated invariant T cells in a person with a homozygous point mutation in MR1. Sci. Immunol. 2020, 5, eabc9492. [Google Scholar] [CrossRef]
- Vacchini, A.; Chancellor, A.; Yang, Q.; Colombo, R.; Spagnuolo, J.; Berloffa, G.; Joss, D.; Øyås, O.; Lecchi, C.; De Simone, G.; et al. Nucleobase adducts bind MR1 and stimulate MR1-restricted T cells. Sci. Immunol. 2024, 9, eadn0126. [Google Scholar] [CrossRef]
| TCR | TRAV CDR3ɑ TRAJ | TRBV CDR3β TRAJ |
|---|---|---|
| 2-78 | 1-2 CAAIDSNYQLIW 33 | 6-1 CASKERSGSGDGEQYF 2-7 |
| 2-81 | 19 CALPSRLMF 31 | 2 CASSLTSIYEQFF 2-1 |
| 10-59 | 26-1 HRQSRSR#YGGSQGNLIF 42 | 5-4 CASSFYGSETQYF 2-5 |
| 10-69 | 13-1 CAASMGNTPLVF 29 | 20-1 CSARVEKLFF 1-4 |
| Cells | Mean Fluorescence Intensity | ||
|---|---|---|---|
| Isotype | Without Ac-6-FP | With Ac-6-FP | |
| MCF7 | 4.7 | 5.4 | 20.4 |
| MDA-MB-231 | 3.2 | 3.7 | 6.7 |
| MDA-MB-453 | 2.8 | 4.1 | 30.1 |
| MDA-MB-468 | 2.8 | 5.1 | 10.0 |
| BT474 | 3.9 | 4.6 | 8.9 |
| ZR-75-1 | 2.6 | 4.8 | 34.5 |
| A549 | 2.6 | 4.5 | 5.7 |
| COLO205 | 2.0 | 2.6 | 12.2 |
| Jurkat | 5.2 | 12.9 | 84.0 |
| Karpas299 | 4.3 | 7.5 | 39.1 |
| K562 | 3.1 | 6.8 | 51.1 |
| Cells | Mean Fluorescence Intensity | ||
|---|---|---|---|
| Control | IFN-γ - | IFN-γ + | |
| MCF7 | 5.23 | 6.28 | 62.9 |
| MDA-MB-231 | 5.09 | 6.09 | 8.23 |
| MDA-MB-453 | 15.2 | 18.9 | 51.3 |
| MDA-MB-468 | 13.6 | 14.8 | 15.2 |
| BT474 | 10.2 | 12.1 | 14.4 |
| ZR-75-1 | 7.00 | 8.66 | 45.5 |
| A549 | 5.5 | 11.6 | 25.6 |
| COLO205 | 3.5 | 22.0 | 86.4 |
| Jurkat | 3.4 | 4.0 | 15.8 |
| Karpas299 | 3.63 | 18.5 | 15.1 |
| K562 | 5.63 | 7.93 | 9.84 |
| Cells | Mean Fluorescence Intensity | ||||
|---|---|---|---|---|---|
| Control | CD1a | CD1b | CD1c | CD1d | |
| MCF7 | 6.61 | 7.68 | 5.74 | 6.23 | 5.92 |
| MDA-MB-231 | 5.61 | 5.63 | 5.34 | 5.87 | 5.62 |
| MDA-MB-453 | 8.50 | 8.53 | 8.49 | 8.42 | 7.71 |
| MDA-MB-468 | 8.60 | 8.65 | 8.11 | 8.50 | 9.94 |
| BT474 | 11.0 | 11.3 | 9.81 | 10.4 | 11.4 |
| ZR-75-1 | 11.4 | 11.6 | 11.3 | 12.0 | 14.4 |
| A549 | 11.8 | 13.4 | 12.0 | 11.4 | 8.01 |
| COLO205 | 8.30 | 9.34 | 8.42 | 8.63 | 9.28 |
| Jurkat | 8.0 | 440 | 231 | 366 | 916 |
| Karpas299 | 3.63 | 3.81 | 3.96 | 3.78 | 13.5 |
| K562 | 5.63 | 7.13 | 4.53 | 4.72 | 4.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayee, A.; Kobayashi, E.; Motozono, C.; Hamana, H.; My, H.T.V.; Okada, T.; Toyooka, N.; Yamaguchi, S.; Ozawa, T.; Kishi, H. Characterization of Tumor-Infiltrating Lymphocyte-Derived Atypical TCRs Recognizing Breast Cancer in an MR1-Dependent Manner. Cells 2024, 13, 1711. https://doi.org/10.3390/cells13201711
Hayee A, Kobayashi E, Motozono C, Hamana H, My HTV, Okada T, Toyooka N, Yamaguchi S, Ozawa T, Kishi H. Characterization of Tumor-Infiltrating Lymphocyte-Derived Atypical TCRs Recognizing Breast Cancer in an MR1-Dependent Manner. Cells. 2024; 13(20):1711. https://doi.org/10.3390/cells13201711
Chicago/Turabian StyleHayee, Abdul, Eiji Kobayashi, Chihiro Motozono, Hiroshi Hamana, Ha Thi Viet My, Takuya Okada, Naoki Toyooka, Satoshi Yamaguchi, Tatsuhiko Ozawa, and Hiroyuki Kishi. 2024. "Characterization of Tumor-Infiltrating Lymphocyte-Derived Atypical TCRs Recognizing Breast Cancer in an MR1-Dependent Manner" Cells 13, no. 20: 1711. https://doi.org/10.3390/cells13201711
APA StyleHayee, A., Kobayashi, E., Motozono, C., Hamana, H., My, H. T. V., Okada, T., Toyooka, N., Yamaguchi, S., Ozawa, T., & Kishi, H. (2024). Characterization of Tumor-Infiltrating Lymphocyte-Derived Atypical TCRs Recognizing Breast Cancer in an MR1-Dependent Manner. Cells, 13(20), 1711. https://doi.org/10.3390/cells13201711






