Modulatory Effects of Regulated Cell Death: An Innovative Preventive Approach for the Control of Mastitis
Abstract
1. Introduction
2. Apoptosis
2.1. Overview of the Core Mechanisms of Apoptosis
2.2. Apoptosis in Mastitis
2.3. Potential Therapeutic Applications of Apoptosis in Mastitis
3. Autophagy
3.1. Overview of the Core Mechanisms of Autophagy
3.2. Autophagy in Mastitis
3.3. Potential Therapeutic Applications of Autophagy in Mastitis
4. Pyroptosis
4.1. Overview of the Core Mechanisms of Pyroptosis
4.2. Pyroptosis in Mastitis
4.3. Potential Therapeutic Applications of Pyroptosis in Mastitis
5. Ferroptosis
5.1. Overview of the Core Mechanisms of Ferroptosis
5.2. Ferroptosis in Mastitis
5.3. Potential Therapeutic Applications of Ferroptosis in Mastitis
6. Necroptosis
6.1. Overview of the Core Mechanisms of Necroptosis
6.2. Necroptosis in Mastitis
6.3. Potential Therapeutic Applications of Necroptosis in Mastitis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RCD | regulated cell death |
| PCD | programmed cell death |
| ROS | reactive oxygen species |
| LPS | lipopolysaccharide |
| BMECs | bovine mammary epithelial cells |
| GMECs | goat mammary epithelial cells |
| Cytc | cytochrome C |
| TNF | tumor necrosis factor |
| PVL | Panton–Valentine leukocidin |
| MAPK4 | mitogen-activated protein kinase 4 |
| MSCs | mesenchymal stem cells |
| ACE2 | Angiotensin-converting enzyme 2 |
| ATGs | autophagy-associated genes |
| CMA | chaperone-mediated autophagy |
| AMPK | adenosine monophosphate-activated protein kinase |
| NLRP3 | NOD-like receptor protein 3 |
| NETs | neutrophil extracellular traps |
| PUFAs | polyunsaturated fatty acids |
| FTH1 | ferritin heavy chain 1 |
| HMOX1 | heme oxygenase-1 |
| DAMPs | damage-associated molecular patterns |
| TRAIL | TNF-related apoptosis-inducing ligand receptor |
| TRADD | TNF receptor-related death domain |
| Keap1 | Kelch-like epichlorohydrin-related protein 1 |
| SCC | somatic cell count |
| AOB | antioxidants of bamboo leaf |
| MMP | mitochondrial membrane potential |
| PIK3C3 | phosphatidylinositol 3-kinase catalytic subunit type 3 |
References
- Shangraw, E.M.; McFadden, T.B. Graduate Student Literature Review: Systemic mediators of inflammation during mastitis and the search for mechanisms underlying impaired lactation. J. Dairy Sci. 2022, 105, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Cote-Gravel, J.; Malouin, F. Symposium review: Features of Staphylococcus aureus mastitis pathogenesis that guide vaccine development strategies. J. Dairy Sci. 2019, 102, 4727–4740. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Sanchez, J.R.; Castro-Del, C.N.; Medrano-Felix, J.A.; Martinez-Torres, A.O.; Chaidez, C.; Querol-Audi, J.; Castro-Del, C.N. Genomic insights of S. aureus associated with bovine mastitis in a high livestock activity region of Mexico. J. Vet. Sci. 2024, 25, e42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xu, Y.; Lu, J.; Liu, M.; Bin, D.; Miao, J.; Yin, Y. Variant innate immune responses of mammary epithelial cells to challenge by Staphylococcus aureus, Escherichia coli and the regulating effect of taurine on these bioprocesses. Free. Radic. Biol. Med. 2016, 96, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Tang, Y.; Chen, Q.; Chen, S.; Li, W.; Mi, S.; Yu, Y. The role of RNA epigenetic modification-related genes in the immune response of cattle to mastitis induced by Staphylococcus aureus. Anim. Biosci. 2024, 37, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Sheng, S.-Y.; Li, J.-M.; Hu, X.-Y.; Wang, Y. Regulated cell death pathways in cardiomyopathy. Acta Pharmacol. Sin. 2023, 44, 1521–1535. [Google Scholar] [CrossRef]
- Sun, D.; Lei, L.; Zhang, F. Cell Death Inhibitor Drugs and Their Application in Disease Treatment. Prog. Veterrinary Med. 2022, 43, 117–121. [Google Scholar]
- Xia, X.; Li, J.; Yu, J.; Ren, P.; Liu, M.; Hou, J.; Teng, Z.; Wang, L.; Zhang, X.; Bai, Y. Modulatory effects of necroptosis: A potential preventive approach to control diseases in fish. Fish Shellfish. Immunol. 2024, 152, 109802. [Google Scholar] [CrossRef]
- Baker, P.H.; Enger, K.M.; Jacobi, S.K.; Akers, R.M.; Enger, B.D. Cellular proliferation and apoptosis in Staphylococcus aureus-infected heifer mammary glands experiencing rapid mammary gland growth. J. Dairy Sci. 2023, 106, 2642–2650. [Google Scholar] [CrossRef]
- Boutet, P.; Boulanger, D.; Gillet, L.; Vanderplasschen, A.; Closset, R.; Bureau, F.; Lekeux, P. Delayed neutrophil apoptosis in bovine subclinical mastitis. J. Dairy Sci. 2004, 87, 4104–4114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lan, R.; Xu, Y.; Zuo, J.; Han, X.; Phouthapane, V.; Luo, Z.; Miao, J. Taurine alleviates Streptococcus uberis-induced inflammation by activating autophagy in mammary epithelial cells. Front. Immunol. 2021, 12, 631113. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xie, N.; Aleem, M.T.; Ji, X.; Zhang, C.; Cao, X.; Zhang, Y. Overexpression of angiotensin-converting enzyme 2 contributes to the amelioration of Streptococcus uberis-induced inflammatory injury in mammary epithelial cells. Vet. Microbiol. 2022, 268, 109398. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Chen, C.; Li, Z.; Chen, Y.; Zhang, X.; Wang, L.; Zhou, J. Polyphyllin ⅲ-induced ferroptosis in MDA-MB-231 triple-negative breast cancer cells can be protected against by KLF4-mediated upregulation of xCT. Front. Pharmacol. 2021, 12, 670224. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Jiang, L.; Chang, W.; Yang, C.; Chen, K.; Wang, H.; Zhong, J.; Xu, Y.; Xing, G. Necroptosis of mammary epithelial cells in bovine mammary glands with mastitis. J. Nanjing Agric. Univ. 2020, 43, 151–156. [Google Scholar]
- Van Oostveldt, K.; Tomita, G.M.; Paape, M.J.; Capuco, A.V.; Burvenich, C. Apoptosis of bovine neutrophils during mastitis experimentally induced with Escherichia coli or endotoxin. Am. J. Vet. Res. 2002, 63, 448–453. [Google Scholar] [CrossRef]
- Sladek, Z.; Rysanek, D.; Ryznarova, H.; Faldyna, M. Neutrophil apoptosis during experimentally induced Staphylococcus aureus mastitis. Vet. Res. 2005, 36, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Zhang, L.; Gu, X.; Liu, G.; Shahid, M.; Gao, J.; Ali, T.; Han, B. Nocardia cyriacigeogica from bovine mastitis induced in vitro apoptosis of bovine mammary epithelial cells via activation of mitochondrial-caspase pathway. Front. Cell. Infect. Microbiol. 2017, 7, 194. [Google Scholar] [CrossRef]
- Martelli, A.M.; Zweyer, M.; Ochs, R.L.; Tazzari, P.L.; Tabellini, G.; Narducci, P.; Bortul, R. Nuclear apoptotic changes: An overview. J. Cell. Biochem. 2001, 82, 634–646. [Google Scholar] [CrossRef]
- Jia, F.; Ma, W.; Zhang, X.; Wang, D.; Zhou, X. Matrine and baicalin inhibit apoptosis induced by panton-valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 2731–2742. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, C.; Ji, X.; Wu, G.; Huang, X.; Zhang, Y.; Zhang, Y. MSC-ACE2 ameliorates Streptococcus uberis-induced inflammatory injury in mammary epithelial cells by upregulating the IL-10/STAT3/SOCS3 pathway. Front. Immunol. 2022, 13, 870780. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J.; Zou, Y. Map of enteropathogenic Escherichia coli targets mitochondria and triggers drp-1-mediated mitochondrial fission and cell apoptosis in bovine mastitis. Int. J. Mol. Sci. 2022, 23, 4907. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huo, W.; Chen, X.; Barkema, H.W.; De Buck, J.; Shahid, M.; Han, B.; Gao, J. ESBL-Producing Escherichia coli from Bovine Mastitis Induced Apoptosis of Bovine Mammary Epithelial Cells Via Alteration of ROS/MMP/bax/bcl-2 Signaling Pathway. Pak. Vet. J. 2020, 40, 307–312. [Google Scholar]
- Zhao, Y.; Tang, J.; Yang, D.; Tang, C.; Chen, J. Staphylococcal enterotoxin m induced inflammation and impairment of bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 8350–8359. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, C.; Li, T.; Lin, C.; Hao, Z.; Zhang, H.; Zhao, G.; Chen, Y.; Guo, A.; Hu, C. Gambogic acid alleviates inflammation and apoptosis and protects the blood-milk barrier in mastitis induced by LPS. Int. Immunopharmacol. 2020, 86, 106697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Zhou, J.; Lu, L.; Wang, X.; Liang, Y.; Loor, J.J.; Gou, D.; Xu, H.; Yang, Z. Tea tree oil prevents mastitis-associated inflammation in lipopolysaccharide-stimulated bovine mammary epithelial cells. Front. Vet. Sci. 2020, 7, 496. [Google Scholar] [CrossRef]
- Yan, S.; Ye, P.; Aleem, M.T.; Chen, X.; Xie, N.; Zhang, Y. Mesenchymal stem cells overexpressing ace2 favorably ameliorate lps-induced inflammatory injury in mammary epithelial cells. Front. Immunol. 2021, 12, 796744. [Google Scholar] [CrossRef]
- Ali, I.; Li, C.; Kuang, M.; Shah, A.U.; Shafiq, M.; Ahmad, M.A.; Abdalmegeed, D.; Li, L.; Wang, G. Nrf2 activation and NF-Kb & caspase/bax signaling inhibition by sodium butyrate alleviates LPS-induced cell injury in bovine mammary epithelial cells. Mol. Immunol. 2022, 148, 54–67. [Google Scholar]
- Wang, H.; Hao, W.; Yang, L.; Yan, P.; Wei, S. Preconditioning with procyanidin b2 protects mac-t cells against heat exposure-induced mitochondrial dysfunction and inflammation. Mol. Immunol. 2022, 147, 126–135. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Ma, H.; Guo, W.; Wang, J.; Kan, X.; Li, Y.; Gong, Q.; Cao, Y.; Cheng, J.; et al. Schisandrin a protects against lipopolysaccharide-induced mastitis through activating nrf2 signaling pathway and inducing autophagy. Int. Immunopharmacol. 2020, 78, 105983. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Li, W.; Ma, H.; Gong, Q.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Niacin alleviates dairy cow mastitis by regulating the GPR109A/AMPK/NRF2 signaling pathway. Int. J. Mol. Sci. 2020, 21, 3321. [Google Scholar] [CrossRef]
- Guo, W.; Li, W.; Su, Y.; Liu, S.; Kan, X.; Ran, X.; Cao, Y.; Fu, S.; Liu, J. Gpr109a alleviate mastitis and enhances the blood milk barrier by activating AMPK/NRF2 and autophagy. Int. J. Biol. Sci. 2021, 17, 4271–4284. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Qian, S.; Li, J.; Zhou, Y.; Zhu, Q.; Cui, L.; Meng, X.; Zhu, G.; Wang, H. The effect of selenium on the autophagy of macrophage infected by Staphylococcus aureus. Int. Immunopharmacol. 2020, 83, 106406. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, W.; Jia, Y.; Ye, B.; Liu, S.; Fu, S.; Liu, J.; Hu, G. Menthol targeting ampk alleviates the inflammatory response of bovine mammary epithelial cells and restores the synthesis of milk fat and milk protein. Front. Immunol. 2021, 12, 782989. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Yan, Z.; Yang, Y.; Hu, G.; Liu, J.; Hou, S.; Guo, W.; Kan, X.; Fu, S. Dioscin improves pyroptosis in lps-induced mice mastitis by activating ampk/nrf2 and inhibiting the nf-kappab signaling pathway. Oxidative Med. Cell. Longev. 2020, 2020, 8845521. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Y.; Xu, J.; Liu, X.; Duan, C.; Wang, M.; Wang, J. Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli-induced activation of nlrp3 and nlrc4 inflammasomes with differential requirement for asc. Front. Microbiol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Li, R. The Antioxidant Effect of Curcumin on Bovine Mammary Epithelial Cells and its Anticancer Mechanism. Ph.D. Dissertation, Jilin University, Changchun, China, 2021. [Google Scholar]
- Zhou, D.; Sun, L.; Li, J.; Yang, Y. Schisandrin B inhibits inflammation and ferroptosis in S. aureus-induced mastitis through regulating SIRT1/p53/SLC7A11 signaling pathway. Int. Immunopharmacol. 2024, 137, 112430. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Z.; Yang, S.; Zheng, X.; Bao, Y.; Lin, W.; Huang, C.; Qiu, L. Taurine inhibits necroptosis helps to alleviate inflammatory and injury induced by klebsiella infection. Vet. Immunol. Immunopathol. 2022, 250, 110444. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Zhou, X.J.; Nath, S.K.; Sun, C.; Wang, Y.N.; Hou, P.; Mu, R.; Li, C.; Guo, J.P.; Li, Z. A rare variant (rs933717) at FBXO31-MAP1LC3B in chinese is associated with systemic lupus erythematosus. Arthritis Rheumatol. 2018, 70, 287–297. [Google Scholar] [CrossRef]
- Geng, N.; Liu, K.; Lu, J.; Xu, Y.; Wang, X.; Wang, R.; Liu, J.; Liu, Y.; Han, B. Autophagy of bovine mammary epithelial cell induced by intracellular Staphylococcus aureus. J. Microbiol. 2020, 58, 320–329. [Google Scholar] [CrossRef]
- Geng, N.; Wang, X.; Yu, X.; Wang, R.; Zhu, Y.; Zhang, M.; Liu, J.; Liu, Y. Staphylococcus aureus avoids autophagy clearance of bovine mammary epithelial cells by impairing lysosomal function. Front. Immunol. 2020, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Ji, C.; Bi, Z.-G.; Lu, C.-C.; Wang, R.; Gu, B.; Cheng, L. Deguelin induces both apoptosis and autophagy in cultured head and neck squamous cell carcinoma cells. PLoS ONE 2013, 8, e54736. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Tomaz, M.; de Souza, I.; Rocha, C.R.R.; Gomes, L.R. The role of chaperone-mediated autophagy in cell cycle control and its implications in cancer. Cells 2020, 9, 2140. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Bharti, P.S.; Rafat, S.; Bhatt, D.; Goyal, Y.; Pandey, K.K.; Ranjan, S.; Almatroodi, S.A.; Alsahli, M.A.; Rahmani, A.H.; et al. Autophagy paradox of cancer: Role, regulation, and duality. Oxidative Med. Cell. Longev. 2021, 2021, 8832541. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Jia, J.; Claude-Taupin, A.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; et al. Cellular and molecular mechanism for secretory autophagy. Autophagy 2017, 13, 1084–1085. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Fu, Z. Role of autophagy in lung diseases and ageing. Eur. Respir. Rev. 2022, 31, 220134. [Google Scholar] [CrossRef]
- Shi, Y.; Norberg, E.; Vakifahmetoglu-Norberg, H. Mutant p53 as a regulator and target of autophagy. Front. Oncol. 2020, 10, 607149. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Yu, X.; Xu, S.; Luo, J. Autophagy and cardiac diseases: Therapeutic potential of natural products. Med. Res. Rev. 2021, 41, 314–341. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Bi, C.; Zhu, Q.; Cui, L.; Meng, X.; Zhu, G.; Li, J. Effect of selenium on autophagy and intracellular bacterial proliferation in bovine mammary epithelial cells induced by S. aureus. Chin. J. Vet. Sci. 2020, 40, 91–96. [Google Scholar] [CrossRef]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.K.; Lee, S.-J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial dna mediated by the nalp3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sugimoto, Y. Variant in the 5’ untranslated region of insulin-like growth factor 1 receptor is associated with susceptibility to mastitis in cattle. G3 Genes Genomes Genet. 2012, 2, 1077–1084. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Y.; Ma, N.; Dai, H.; Roy, A.C.; Chang, G.; Shi, X.; Shen, X. Sodium valproate attenuates the ie-dap induced inflammatory response by inhibiting the nod1-nf-kappab pathway and histone modifications in bovine mammary epithelial cells. Int. Immunopharmacol. 2020, 83, 106392. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Geng, H.; Geng, N.; Cui, Y.; Qi, C.; Cheng, G.; Song, K.; Hu, L.; Liu, Y.; Liu, J.; et al. Streptococcus agalactiae-induced autophagy of bovine mammary epithelial cell via pi3k/akt/mtor pathway. J. Dairy Res. 2022, 2022, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, L.; Chen, K.; Wang, Y.; Yang, F.; Wang, G. Ufl1 alleviates lipopolysaccharide-induced cell damage and inflammation via regulation of the tlr4/nf-kappab pathway in bovine mammary epithelial cells. Oxidative Med. Cell. Longev. 2019, 2019, 6505373. [Google Scholar]
- Wang, H.; Zhou, Y.; Zhu, Q.; Zang, H.; Cai, J.; Wang, J.; Cui, L.; Meng, X.; Zhu, G.; Li, J. Staphylococcus aureus induces autophagy in bovine mammary epithelial cells and the formation of autophagosomes facilitates intracellular replication of Staph. aureus. J. Dairy Sci. 2019, 102, 8264–8272. [Google Scholar] [CrossRef] [PubMed]
- Schnaith, A.; Kashkar, H.; Leggio, S.A.; Addicks, K.; Krönke, M.; Krut, O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 2007, 282, 2695–2706. [Google Scholar] [CrossRef] [PubMed]
- Mestre, M.B.; Fader, C.M.; Sola, C.; Colombo, M.I. Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy 2010, 6, 110–125. [Google Scholar] [CrossRef]
- Chen, P.; Yang, J.; Wu, N.; Han, B.; Kastelic, J.P.; Gao, J. Streptococcus lutetiensis induces autophagy via oxidative stress in bovine mammary epithelial cells. Oxidative Med. Cell. Longev. 2022, 2022, 2549772. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, K.; Li, J.; Cui, L.; Dong, J.; Li, J.; Meng, X.; Zhu, G.; Wang, H. Pink1/parkin-mediated mitophagy enhances the survival of Staphylococcus aureus in bovine macrophages. J. Cell Mol. Med. 2023, 27, 412–421. [Google Scholar] [CrossRef]
- Xu, D.; Hu, G.; Luo, J.; Cheng, J.; Wu, D.; Cheng, L.; Huang, X.; Fu, S.; Liu, J. Staphylococcus aureus induces mitophagy to promote its survival within bovine mammary epithelial cells. Vet. Microbiol. 2023, 280, 109697. [Google Scholar] [CrossRef]
- Santos, E.M.S.; Almeida, A.C.; Santos, H.O.; Cangussu, A.R.; Costa, K.S.; Alves, J.N.; Barbosa, L.C.B.; Aguiar, R.W.S. Mechanism of brassica oleracea performance in bovine infectious mastitis by bioinformatic analysis. Microb. Pathog. 2019, 129, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of gsdmd by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.A.; Dixit, V.M.; Power, C. Fiery cell death: Pyroptosis in the central nervous system. Trends Neurosci. 2020, 43, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, X.; Cheng, Z.; Qin, W.; Lei, L.; Jiang, J.; Hu, J. The role of pyroptosis in cancer: Pro-cancer or pro-”host”? Cell Death Dis. 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R.; et al. Caspase-8 induces cleavage of gasdermin d to elicit pyroptosis during yersinia infection. Proc. Natl. Acad. Sci. USA 2018, 115, E10888-97. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, X.; Xu, J.; Li, S.; Wang, S.; Zhu, Y.; Wang, J. Antimicrobial effect of zophobas morio hemolymph against bovine mastitis pathogens. Microorganisms 2020, 8, 1488. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Wang, Y.; Wang, C.; Liu, X.; Han, Z.; Fu, Y.; Yang, Z. Effects of neutrophil extracellular traps on bovine mammary epithelial cells in vitro. Front. Immunol. 2019, 10, 1003. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Geng, N.; Du, Y.; Li, Z.; Gao, X.; Han, B.; Liu, J.; Liu, Y. Staphylococcus aureus mediates pyroptosis in bovine mammary epithelial cell via activation of nlrp3 inflammasome. Vet. Res. 2022, 53, 10. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Kuang, M.; Li, L.; Wang, Y.; Yang, F.; Wang, G. Ufl1 modulates nlrp3 inflammasome activation and protects against pyroptosis in lps-stimulated bovine mammary epithelial cells. Mol. Immunol. 2019, 112, 1–9. [Google Scholar] [CrossRef]
- Bao, L.; Zhao, Y.; Duan, S.; Wu, K.; Shan, R.; Liu, Y.; Yang, Y.; Chen, Q.; Song, C.; Li, W. Ferroptosis is involved in Staphylococcus aureus-induced mastitis through autophagy activation by endoplasmic reticulum stress. Int. Immunopharmacol. 2024, 140, 112818. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Cheng, H.; Su, J.; Wang, X.; Wang, Q.; Chu, J.; Li, Q. Gastrodin protects against glutamate-induced ferroptosis in ht-22 cells through nrf2/ho-1 signaling pathway. Toxicol. Vitr. 2020, 62, 104715. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Aabed, N.; Shah, Y.M. Reactive oxygen species and ferroptosis at the nexus of inflammation and colon cancer. Antioxid. Redox Signaling 2023, 39, 551–568. [Google Scholar] [CrossRef]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of ferroptosis and relations with regulated cell death: A review. Front. Physiol. 2019, 10, 139. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by gpx4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Park, E.; Chung, S.W. Ros-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, J.J. The emerging roles of ferroptosis in vascular cognitive impairment. Front. Neurosci. 2019, 13, 811. [Google Scholar] [CrossRef]
- Xia, X.; Cheng, Z.; He, B.; Liu, H.; Liu, M.; Hu, J.; Lei, L.; Wang, L.; Bai, Y. Ferroptosis in aquaculture research. Aquaculture 2021, 541, 736760. [Google Scholar] [CrossRef]
- Russell-Jones, R. Air pollution in the uk: Better ways to solve the problem. BMJ 2017, 357, j2713. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Lin, T.; Wang, X.; Zhang, B.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X. Hmox1 promotes ferroptosis in mammary epithelial cells via fth1 and is involved in the development of clinical mastitis in dairy cows. Antioxidants 2022, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, Y.; Liu, X.; Zhang, J.; Khan, M.Z.; Mi, S.; Wang, C.; Yu, Y. Characterization of peripheral white blood cells transcriptome to unravel the regulatory signatures of bovine subclinical mastitis resistance. Front. Genet. 2022, 13, 949850. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [PubMed]
- Wang, L.; Du, F.; Wang, X. Tnf-alpha induces two distinct caspase-8 activation pathways. Cell 2008, 133, 693–703. [Google Scholar] [CrossRef]
- Shan, B.; Pan, H.; Najafov, A.; Yuan, J. Necroptosis in development and diseases. Genes Dev. 2018, 32, 327–340. [Google Scholar] [CrossRef]
- Samson, A.L.; Garnish, S.E.; Hildebrand, J.M.; Murphy, J.M. Location, location, location: A compartmentalized view of tnf-induced necroptotic signaling. Sci. Signal. 2021, 14, eabc6178. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’En, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of rip1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef]
- Xia, X.; Hou, J.; Ren, P.; Liu, M.; Wang, L.; Wei, X.; Teng, Z.; Kasianenko, O.; Cheng, L.; Hu, J. Coexpression analysis of lncRNAs and mRNAs identifies potential regulatory long noncoding RNAs involved in the inflammatory effects of lipopolysaccharide on bovine mammary epithelial cells. BMC Vet. Res. 2023, 19, 209. [Google Scholar] [CrossRef]
- Zhan, J.; Shen, Y.; Li, X.; Zhang, H.; Niu, H.; Fang, L.; Xiong, B.; Tong, J.; Jiang, L. Microbiome and metabolic changes of milk in response to dietary supplementation with bamboo leaf extract in dairy cows. Front. Nutr. 2021, 8, 723446. [Google Scholar]
- Zhou, H.; Zhu, P.; Guo, J.; Hu, N.; Wang, S.; Li, D.; Hu, S.; Ren, J.; Cao, F.; Chen, Y. Ripk3 induces mitochondrial apoptosis via inhibition of fundc1 mitophagy in cardiac ir injury. Redox Biol. 2017, 13, 498–507. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Berleth, N.; Deitersen, J.; Wallot-Hieke, N.; Böhler, P.; Schlütermann, D.; Stuhldreie, F.; Cox, J.; Schmitz, K.; et al. The autophagy-initiating kinase ulk1 controls ripk1-mediated cell death. Cell Rep. 2020, 31, 107547. [Google Scholar] [CrossRef]
- Skouta, R.; Dixon, S.J.; Wang, J.; Dunn, D.E.; Orman, M.; Shimada, K.; Rosenberg, P.A.; Lo, D.C.; Weinberg, J.M.; Linkermann, A.; et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014, 136, 4551–4556. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Kesavardhana, S.; Kanneganti, T.-D. ZBP1 and TAK1, master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis). Front. Cell. Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhao, J.; Zhang, Q.; Zhu, Z.; Zhang, J.; Liu, C.; Zheng, X.; Wang, F.; Su, J.; Ma, X.; et al. Generation of Anti-Mastitis Gene-Edited Dairy Goats with Enhancing Lysozyme Expression by Inflammatory Regulatory Sequence using ISDra2-TnpB System. Adv. Sci. 2024, 5, e2404408. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Zhang, W.; Chen, S.; Wen, X.; Ran, X.; Wang, H.; Zhao, J.; Qi, Y.; Xue, N. Prevalence of subclinical mastitis among dairy cattle and associated risks factors in china during 2012–2021: A systematic review and meta-analysis. Res. Vet. Sci. 2022, 148, 65–73. [Google Scholar] [CrossRef]
- Bronzo, V.; Lopreiato, V.; Riva, F.; Amadori, M.; Curone, G.; Addis, M.F.; Cremonesi, P.; Moroni, P.; Trevisi, E.; Castiglioni, B. The role of innate immune response and microbiome in resilience of dairy cattle to disease: The mastitis model. Animals 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
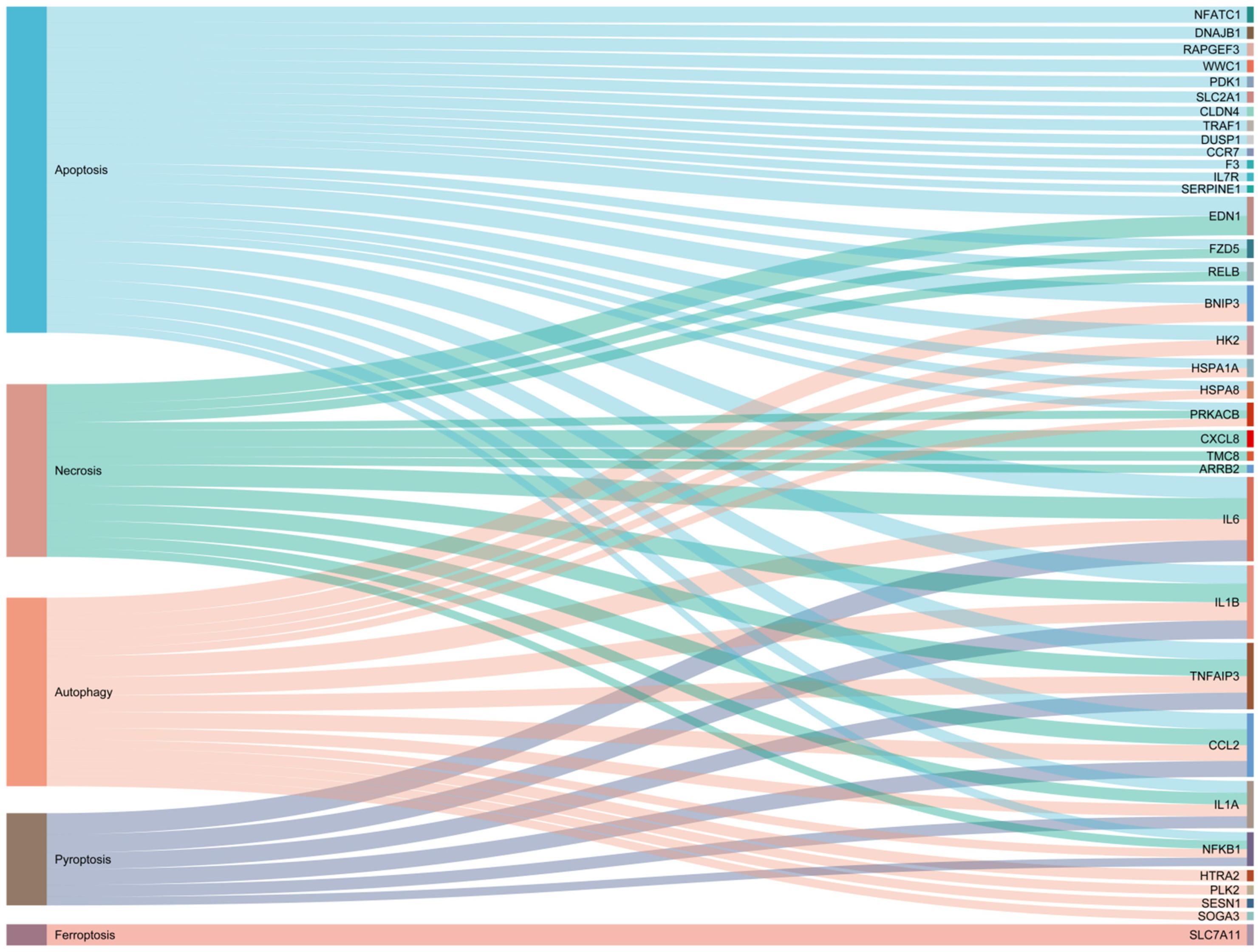

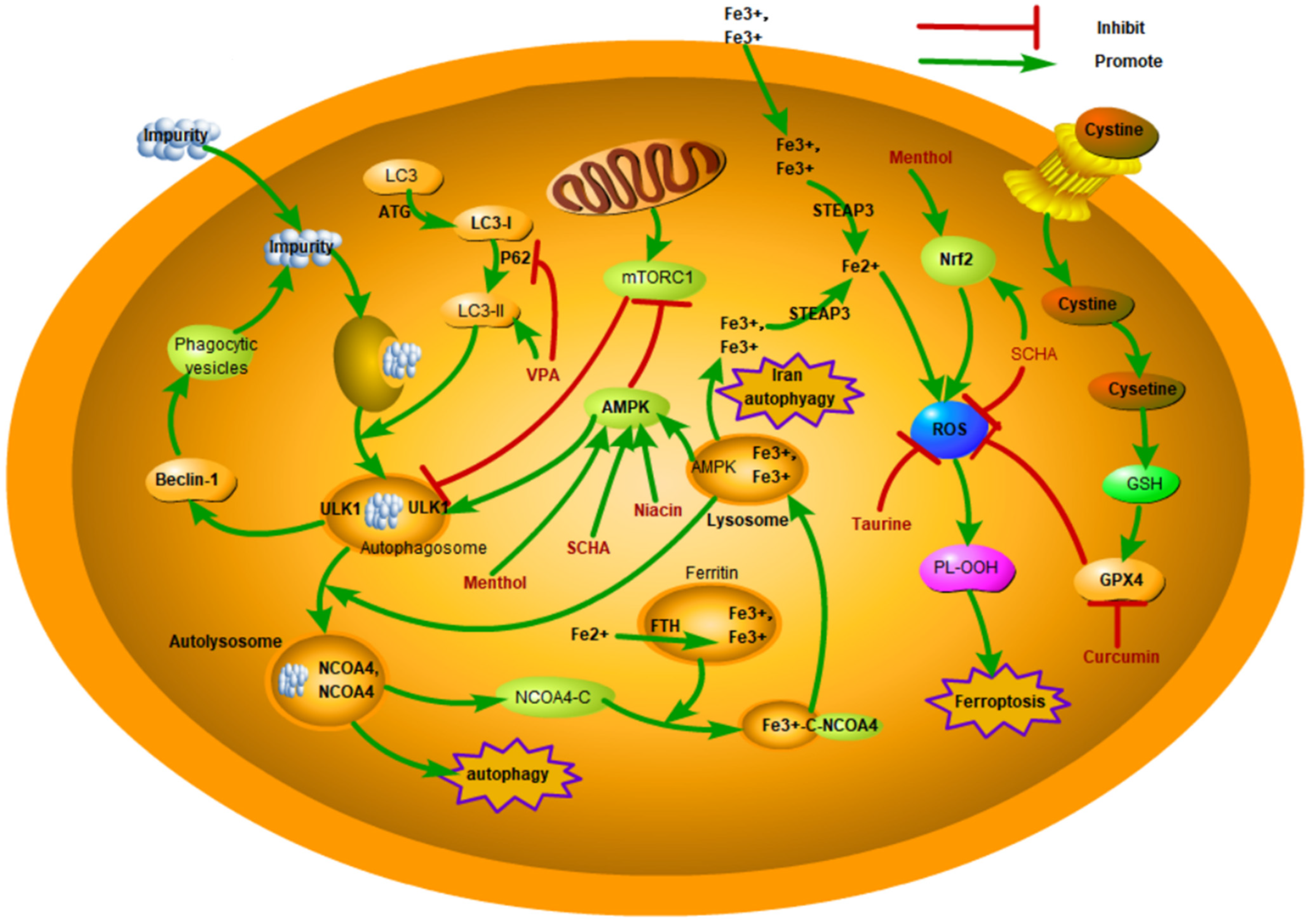
| RCD Item | Drugs | Immune Response | Animal Model/Cell Lines | Functional Mechanism | Clinical Application Value | References |
|---|---|---|---|---|---|---|
| Apoptosis | Gambogic acid (GA) | Not clear | Primary and HC11 murine mammary epithelial cells | GA inhibits apoptosis via Bcl-2, caspase-3, and 9. | GA has potential benefits as a potential treatment for mastitis. | [25] |
| Matrine | Matrine has immune-enhancing effects | BMEC cell line (MAC-T) | Matrine downregulated the levels of cleaved caspase-3, cleaved caspase-8, and cleaved caspase-9. | Matrine has potential value against cow mastitis caused by the toxin PVL. | [20] | |
| Baicalin | Baicalin has immune-enhancing effects | BMEC cell line (MAC-T) | Baicalin downregulated the expression of cleaved caspase-9. | Baicalin has potential value against cow mastitis caused by the toxin PVL. | [20] | |
| Tea tree oil (TTO) | Not clear | Isolated bovine mammary epithelial cells | TTO inhibits the production of caspase-3. | TTO has a protective effect against LPS-induced mastitis. | [26] | |
| MSC-ACE2 | MSC-ACE2 has immune regulatory activity | EpH4-Ev cells (Mouse mammary epithelial cells) | MSC-ACE2 reversed the LPS-induced down-regulation of Bcl2 and the up-regulation of Bax and caspase-3 | MSCs overexpressing ACE2 are expected to serve as a potential strategy for mastitis treatment. | [21,27] | |
| Sodium butyrate (SB) | Not clear | BMEC cell line (MAC-T) | SB reduced LPS-induced apoptosis by inhibiting the NF-kB and caspase/Bax signaling pathways. | SB may be used as a therapeutic agent for mastitis. | [28] | |
| Procyanidin B2 (PB2) | Not clear | BMEC cell line (MAC-T) | PB2 increased expressions of Bax, Bcl-2, Bax/Bcl-2, and Cyto-c while decreasing expressions of cleaved caspase-3. | PB2 is an antioxidant to improve HS-induced mitochondrial dysfunction and inflammation in BMEC. | [29] | |
| Autophagy | Schisandrin A (Sch A) | Not clear | C57BL/6 mice and mouse mammary epithelial cells (mMECs) | Sch A induces autophagy by suppressing the mTOR signaling pathway and activating the AMPK-ULK1 signaling pathway. | Sch A is promising for use in the treatment of mastitis. | [30] |
| Niacin | Niacin increases innate immunity | Lactating dairy cows and primary bovine mammary epithelial cells (BMECs) | Niacin might promote autophagy by activating the GPR109A/AMPK/NRF-2 signaling pathway. | Niacin alleviates mastitis in dairy cows. | [31,32] | |
| Selenium | Selenium regulates immunity | RAW264.7 macrophages | Selenium improves autophagy by modulating the expression of LC3 II and p62. | Not clear | [33] | |
| Menthol | Not clear | Primary bovine mammary gland epithelial cells (BMECs) | Mnthol activates the AMPK-ULK1 pathway to initiate the onset of autophagy and maintains the level of autophagy through the AMPK-Nrf-2 pathway. | Not clear | [34] | |
| Taurine | Taurine regulates innate immunity | BMEC cell line (MAC-T) | Taurine activates autophagy in an mTOR-dependent manner. | It provides theoretical support for the development of prophylactic strategies for pathogen | [12] | |
| Pyroptosis | Dioscin | Not clear | BALB/c mice | Dioscin reduced pyroptosis by triggering AMPK/NRF2 and suppressing the NF-κB signal pathway. | It provides a new potential therapy of dioscin for the treatment and prevention of mastitis. | [35] |
| Lactobacillus rhamnosus GR-1 | L. rhamnosus GR-1regulates the immune response | BMEC cell line (MAC-T) | L. rhamnosus GR-1 suppresses E. coli-induced pyroptosis through attenuation of NLRC4 inflammasome and non-canonical caspase-4 activation, independent of ASC. | L. rhamnosus GR-1 represents a potentially promising therapeutic agent in E. coli-associated mastitis. | [36] | |
| Ferroptosis | Curcumin | Not clear | BMEC cell line (MAC-T) | Curcumin induces ferroptosis by upregulating HMOX1 and downregulating GPX4 expression. | Curcumin has potential benefits as a potential treatment for mastitis. | [37] |
| Schisandrin B (SCB) | Not clear | Balb/c mice | SCB attenuates S. aureus-induced ferroptosis via up-regulating SIRT1/p53/SLC7A11 signaling pathway. | SCB shows great potential to resist inflammation in S. aureus-induced mastitis. | [38] | |
| Necroptosis | Taurine | Taurine regulates the innate immune | EpH4-Ev cells (mouse mammary epithelial cells) | Taurine could suppress the RIPK1/RIPK3/MLKL signaling pathway and subsequently relieve necroptosis caused by Klebsiella infection. | It provides a basis for using Taurine to prevent Klebsiella infection and the development of novel prophylactic strategies. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Ren, P.; Bai, Y.; Li, J.; Zhang, H.; Wang, L.; Hu, J.; Li, X.; Ding, K. Modulatory Effects of Regulated Cell Death: An Innovative Preventive Approach for the Control of Mastitis. Cells 2024, 13, 1699. https://doi.org/10.3390/cells13201699
Xia X, Ren P, Bai Y, Li J, Zhang H, Wang L, Hu J, Li X, Ding K. Modulatory Effects of Regulated Cell Death: An Innovative Preventive Approach for the Control of Mastitis. Cells. 2024; 13(20):1699. https://doi.org/10.3390/cells13201699
Chicago/Turabian StyleXia, Xiaojing, Pengfei Ren, Yilin Bai, Jingjing Li, Huihui Zhang, Lei Wang, Jianhe Hu, Xinwei Li, and Ke Ding. 2024. "Modulatory Effects of Regulated Cell Death: An Innovative Preventive Approach for the Control of Mastitis" Cells 13, no. 20: 1699. https://doi.org/10.3390/cells13201699
APA StyleXia, X., Ren, P., Bai, Y., Li, J., Zhang, H., Wang, L., Hu, J., Li, X., & Ding, K. (2024). Modulatory Effects of Regulated Cell Death: An Innovative Preventive Approach for the Control of Mastitis. Cells, 13(20), 1699. https://doi.org/10.3390/cells13201699







