The Protective Effect of a Human Umbilical Cord Mesenchymal Stem Cell Supernatant on UVB-Induced Skin Photodamage
Abstract
1. Introduction
2. Methods and Materials
2.1. Isolation and Identification of HUCMSCs
2.2. CHS Collection and Treatment
2.3. UVB-Damage Cell Model Establishment and Cell Viability Measurement
2.4. Wound-Healing Measurement
2.5. Cell Cycle Measurement
2.6. SA-β-Gal, AO/EB and Monodansylcadaverine (MDC) Staining
2.7. Western Blotting
2.8. Animal Study
2.9. Skin Histology Examinations
3. Result
3.1. Identification of HUCMSCs and Preparation of CHS
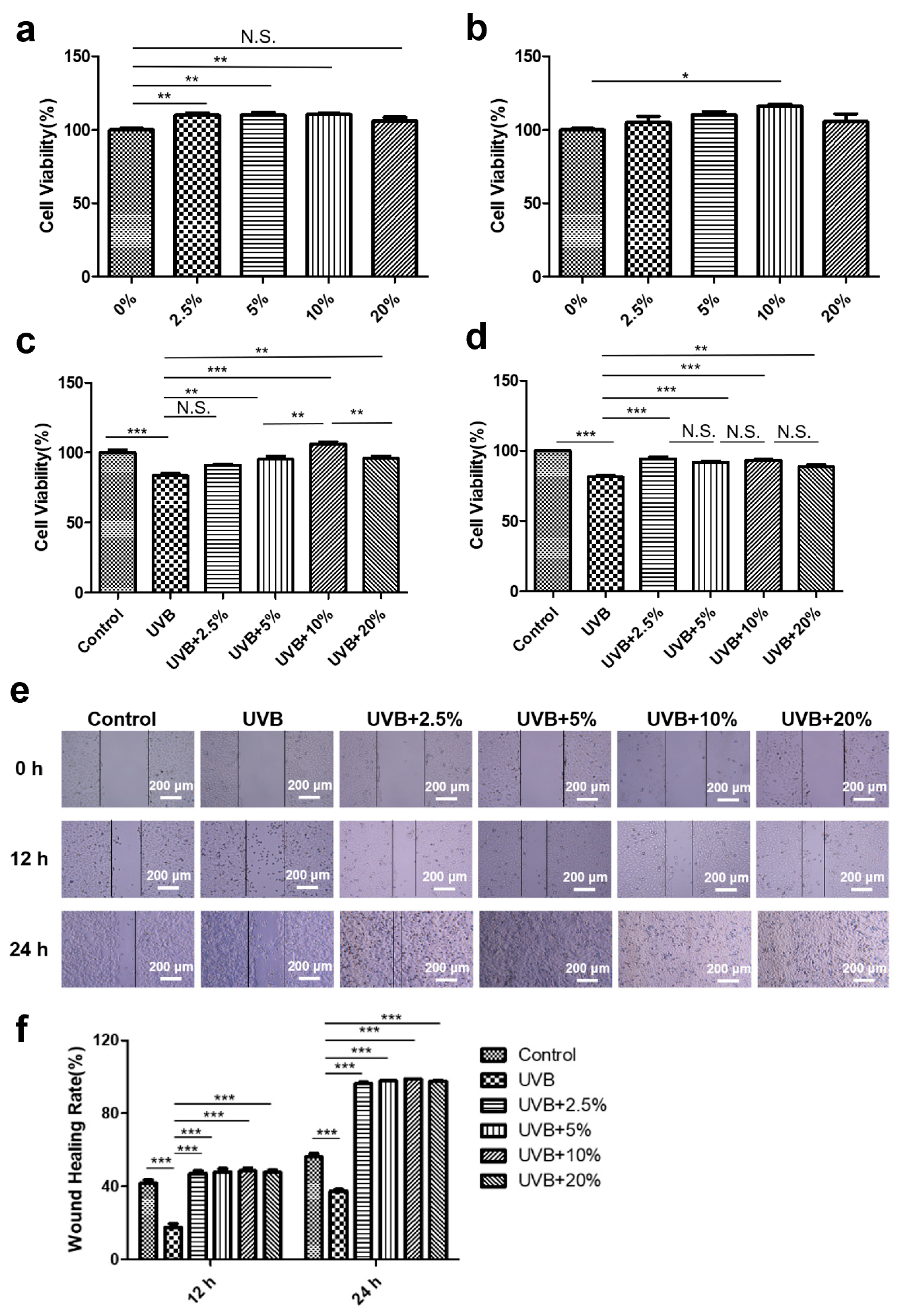
3.2. CHS Improved UVB Irradiation-Induced Decrease in Cell Viability and Migration
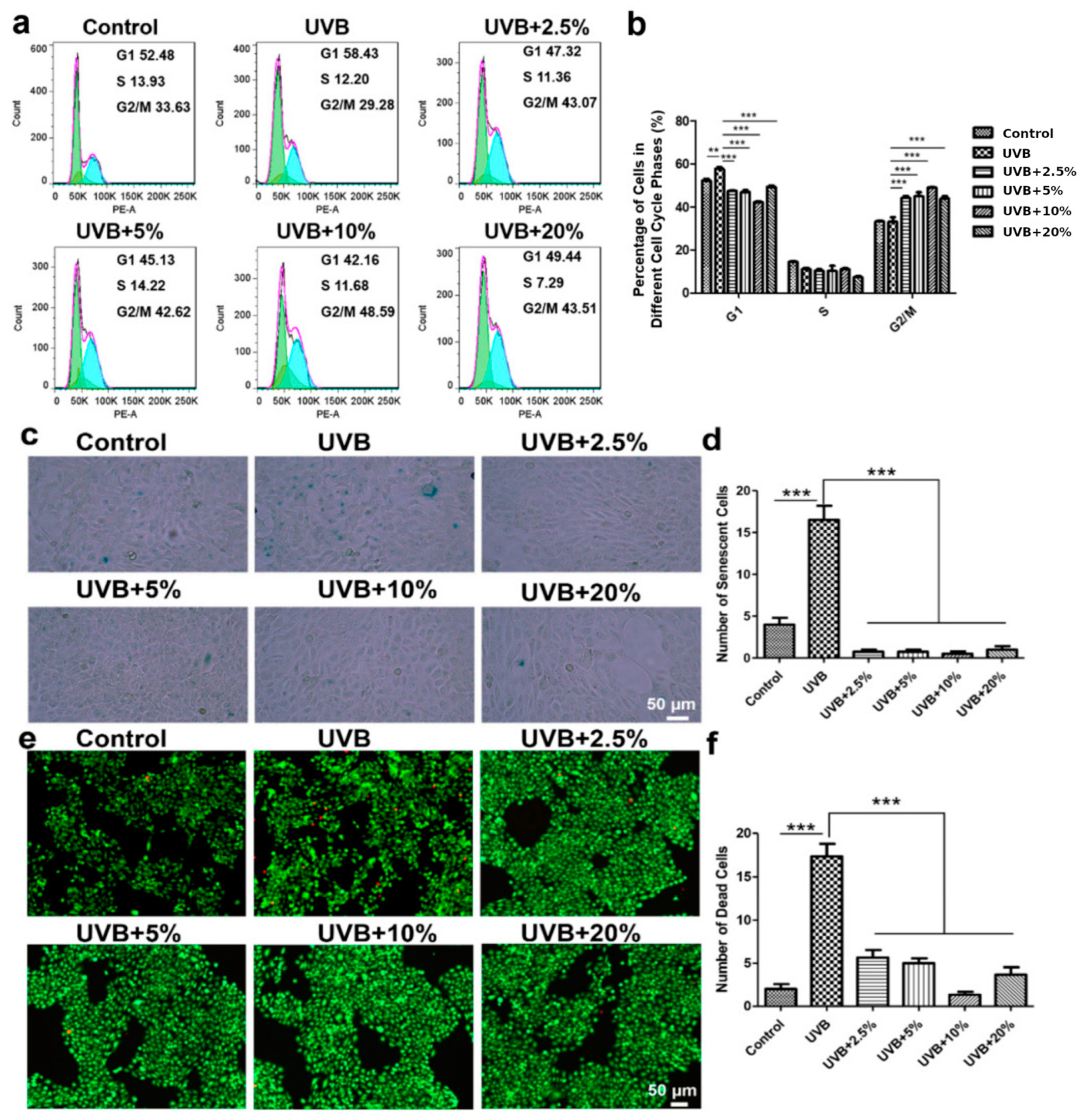
3.3. CHS Rescues UVB Irradiation-Induced G1 Phase Arrest in Cell Cycle
3.4. CHS Reverses UVB Irradiation-Induced Cellular Senescence and Apoptosis
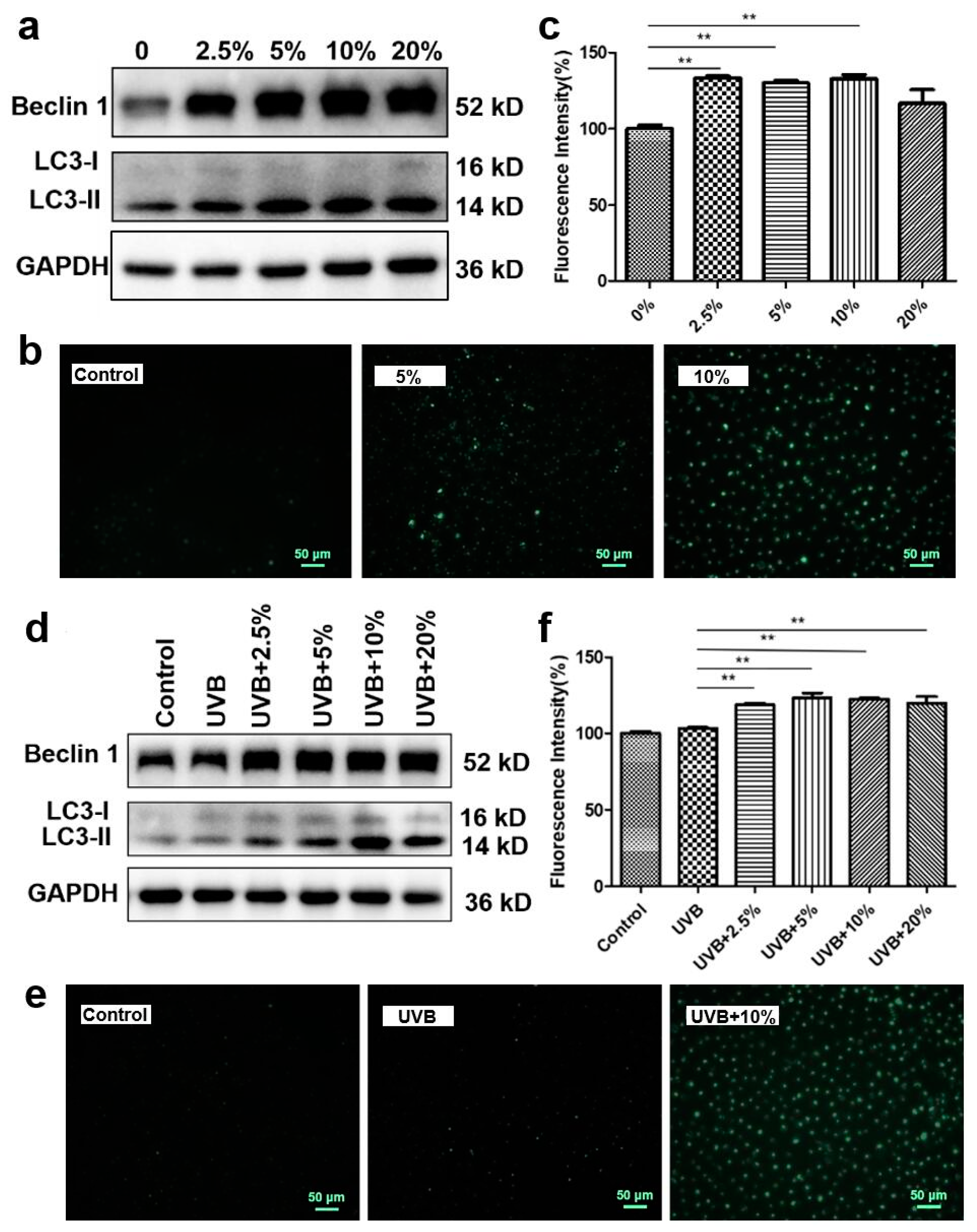
3.5. CHS-Induced Autophagy Activation after UVB Exposure
3.6. CHS Protects UVB Irradiation-Induced Skin Damage and Inflammatory Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Sinha, R.P.; Hader, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Abada, A.; Elazar, Z. Getting ready for building: Signaling and autophagosome biogenesis. EMBO Rep. 2014, 15, 839–852. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Hsu, W.L.; Lu, J.H.; Noda, M.; Wu, C.Y.; Liu, J.D.; Sakakibara, M.; Tsai, M.H.; Yu, H.S.; Lin, M.W.; Huang, Y.B.; et al. Derinat Protects Skin against Ultraviolet-B (UVB)-Induced Cellular Damage. Molecules 2015, 20, 20297–20311. [Google Scholar] [CrossRef]
- Hao, D.; Wen, X.; Liu, L.; Wang, L.; Zhou, X.; Li, Y.; Zeng, X.; He, G.; Jiang, X. Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophagy. Cell Death Dis. 2019, 10, 19. [Google Scholar] [CrossRef]
- Ke, B.; Tian, M.; Li, J.; Liu, B.; He, G. Targeting Programmed Cell Death Using Small-Molecule Compounds to Improve Potential Cancer Therapy. Med. Res. Rev. 2016, 36, 983–1035. [Google Scholar] [CrossRef]
- Lamore, S.D.; Wondrak, G.T. Autophagic-lysosomal dysregulation downstream of cathepsin B inactivation in human skin fibroblasts exposed to UVA. Photochem. Photobiol. Sci. 2012, 11, 163–172. [Google Scholar] [CrossRef]
- Markiewicz, E.; Idowu, O.C. DNA damage in human skin and the capacities of natural compounds to modulate the bystander signalling. Open Biol. 2019, 9, 190208. [Google Scholar] [CrossRef]
- Suman, S.; Domingues, A.; Ratajczak, J.; Ratajczak, M.Z. Potential Clinical Applications of Stem Cells in Regenerative Medicine. Adv. Exp. Med. Biol. 2019, 1201, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; De Maria, R.; Kroemer, G.; Galluzzi, L. DNA Damage in Stem Cells. Mol. Cell 2017, 66, 306–319. [Google Scholar] [CrossRef]
- Jo, H.; Brito, S.; Kwak, B.M.; Park, S.; Lee, M.G.; Bin, B.H. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 2410. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018, 93, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xia, M.; Gao, Y.; Chen, Y.; Xu, Y. Human umbilical cord mesenchymal stem cells: An overview of their potential in cell-based therapy. Expert. Opin. Biol. Ther. 2015, 15, 1293–1306. [Google Scholar] [CrossRef]
- Liu, S.J.; Meng, M.Y.; Han, S.; Gao, H.; Zhao, Y.Y.; Yang, Y.; Lin, Z.Y.; Yang, L.R.; Zhu, K.; Han, R.; et al. Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Ameliorate HaCaT Cell Photo-Aging. Rejuvenation Res. 2021, 24, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, S.; Lv, M.; Lv, J.; Sui, Y.; Guo, S. Protective roles of mesenchymal stem cells on skin photoaging: A narrative review. Tissue Cell 2022, 76, 101746. [Google Scholar] [CrossRef]
- Hwang, S.M.; See, C.J.; Choi, J.; Kim, S.Y.; Choi, Q.; Kim, J.A.; Kwon, J.; Park, S.N.; Im, K.; Oh, I.H.; et al. The application of an in situ karyotyping technique for mesenchymal stromal cells: A validation and comparison study with classical G-banding. Exp. Mol. Med. 2013, 45, e68. [Google Scholar] [CrossRef]
- Han, B.; Wang, K.; Tu, Y.; Tan, L.; He, C. Low-Dose Berberine Attenuates the Anti-Breast Cancer Activity of Chemotherapeutic Agents via Induction of Autophagy and Antioxidation. Dose Response 2020, 18, 1559325820939751. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Chen, Y.Q.; Kopp, J.B.; Fisher, L.; Brown, D.B.; Hahn, P.J.; Robey, F.A.; Lakkakorpi, J.; Uitto, J. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J. Am. Acad. Dermatol. 1996, 34, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Csekes, E.; Rackova, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Lowry, W.E. Its written all over your face: The molecular and physiological consequences of aging skin. Mech. Ageing Dev. 2020, 190, 111315. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Seo, D.H.; Lee, S.H.; Lee, S.H.; An, G.H.; Ahn, H.J.; Kwon, D.; Seo, K.W.; Kang, K.S. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 2018, 16, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef]
- Young, A.R.; Narita, M.; Ferreira, M.; Kirschner, K.; Sadaie, M.; Darot, J.F.; Tavare, S.; Arakawa, S.; Shimizu, S.; Watt, F.M.; et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009, 23, 798–803. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, X.; Wan, J.; Chang, L.; Hu, W.; Bing, Z.; Zhang, S.; Li, J.; He, J.; Wang, J.; et al. Radiation-induced cellular senescence results from a slippage of long-term G2 arrested cells into G1 phase. Cell Cycle 2013, 12, 1424–1432. [Google Scholar] [CrossRef][Green Version]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.M. Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, L.; Fan, M.; Chen, Z.; Yan, L.; Lu, H.; Jia, M.; Wu, H.; Shan, L. Human Umbilical Cord-Derived Mesenchymal Stem Cells Ameliorate Skin Aging of Nude Mice Through Autophagy-Mediated Anti-Senescent Mechanism. Stem Cell Rev. Rep. 2022, 18, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Qomaladewi, N.P.; Lee, J.; Park, S.H.; Cho, J.Y. The Role of Autophagy in Skin Fibroblasts, Keratinocytes, Melanocytes, and Epidermal Stem Cells. J. Investig. Dermatol. 2020, 140, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Gu, H. The signaling involved in autophagy machinery in keratinocytes and therapeutic approaches for skin diseases. Oncotarget 2016, 7, 50682–50697. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Brown, S.; Gorenc, T.; Rodriguez, J.; Fuchs, E.; Steller, H. Sept4/ARTS regulates stem cell apoptosis and skin regeneration. Science 2013, 341, 286–289. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, J.W.; Jun, H.S.; Roh, J.Y.; Lee, H.Y.; Hong, I.S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef]
- Luo, X.; Yin, J.; Cai, Y.; Lin, S.; Tong, C.; Sui, H.; Ye, M.; Long, Y.; Lin, P.; Lan, T. Cytoplasm or Supernatant: Where Is the Treasury of the Bioactive Antiaging Factor from Mesenchymal Stem Cells? Stem Cells Dev. 2022, 31, 529–540. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Zhang, Q.; Zhang, Y. Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells. Int. J. Stem Cells 2022, 15, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Wu, J.; Gao, M.; Wang, Q.; Zhao, Y.Y.; Shan, C.; Shen, Y.; Chen, G. Multilineage-differentiating stress-enduring cells alleviate atopic dermatitis-associated behaviors in mice. Stem Cell Res. Ther. 2021, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; Pavlopoulou, A.; Louka, M.; Nikitaki, Z.; Vorgias, C.E.; Bagos, P.G.; Michalopoulos, I. Emerging molecular networks common in ionizing radiation, immune and inflammatory responses by employing bioinformatics approaches. Cancer Lett. 2015, 368, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, Z.W.; Zhao, W. Therapeutic implications of transplanted-cell death. Nat. Biomed. Eng. 2021, 5, 379–384. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Liu, J.; Wang, Q.; Hu, H.; Zhou, L. The Protective Effect of a Human Umbilical Cord Mesenchymal Stem Cell Supernatant on UVB-Induced Skin Photodamage. Cells 2024, 13, 156. https://doi.org/10.3390/cells13020156
Cheng L, Liu J, Wang Q, Hu H, Zhou L. The Protective Effect of a Human Umbilical Cord Mesenchymal Stem Cell Supernatant on UVB-Induced Skin Photodamage. Cells. 2024; 13(2):156. https://doi.org/10.3390/cells13020156
Chicago/Turabian StyleCheng, Lin, Jiaqi Liu, Qi Wang, Huozhen Hu, and Liming Zhou. 2024. "The Protective Effect of a Human Umbilical Cord Mesenchymal Stem Cell Supernatant on UVB-Induced Skin Photodamage" Cells 13, no. 2: 156. https://doi.org/10.3390/cells13020156
APA StyleCheng, L., Liu, J., Wang, Q., Hu, H., & Zhou, L. (2024). The Protective Effect of a Human Umbilical Cord Mesenchymal Stem Cell Supernatant on UVB-Induced Skin Photodamage. Cells, 13(2), 156. https://doi.org/10.3390/cells13020156




