In Vitro Safety Study on the Use of Cold Atmospheric Plasma in the Upper Respiratory Tract
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture Conditions
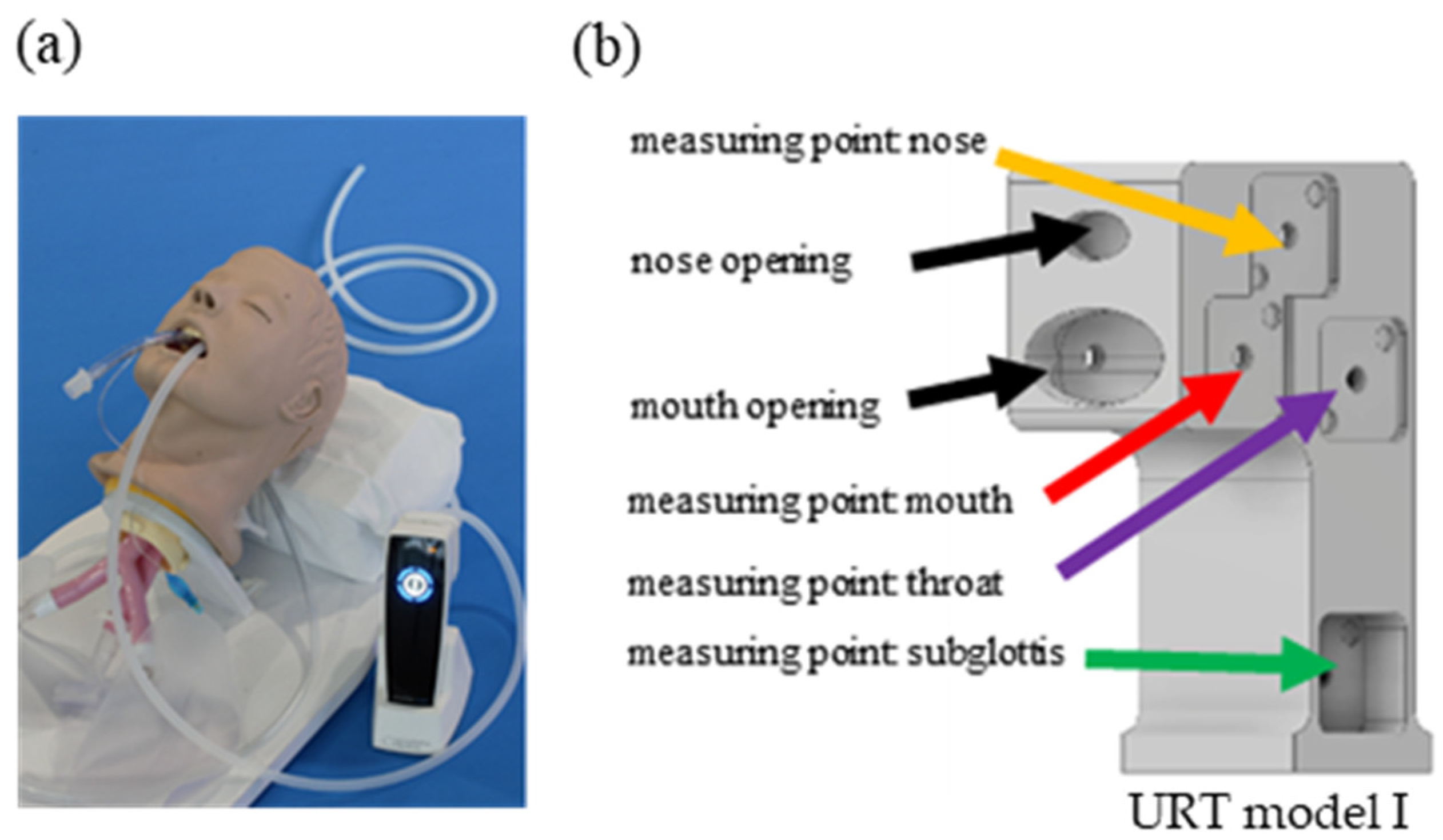
2.2. Plasma Intensive Care Device and the Upper Respiratory Tract Model I
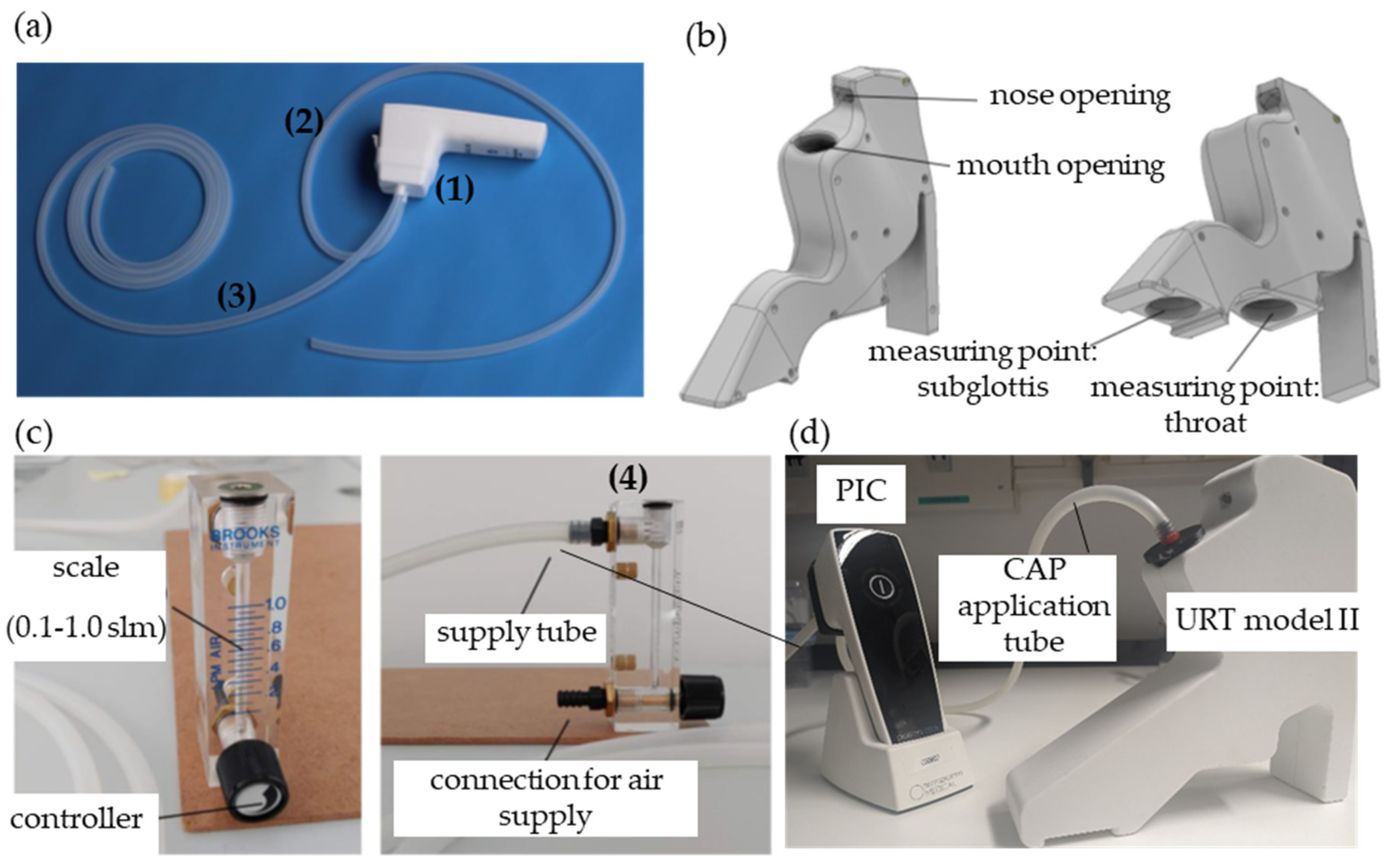
2.3. Treatment of Bacteria and Cells with the Plasma Intensive Care Device in the Upper Respiratory Tract Model II
2.4. Quantification of Bacteria Inactivation after Plasma Intensive Care Treatment
2.5. Assessment of H2O2 and NO2−/NO3− in DPBS
2.6. Morphology of the Cells
2.7. Viability of the Cells
2.8. Immunofluorescence Analysis
2.9. Measurement of Cell Apoptosis and Necrosis
2.10. Migration/Proliferation Analysis
2.11. Isolation of Ribonucleic Acid (RNA) and Reverse Transcription
2.12. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.13. Statistical Analysis
3. Results
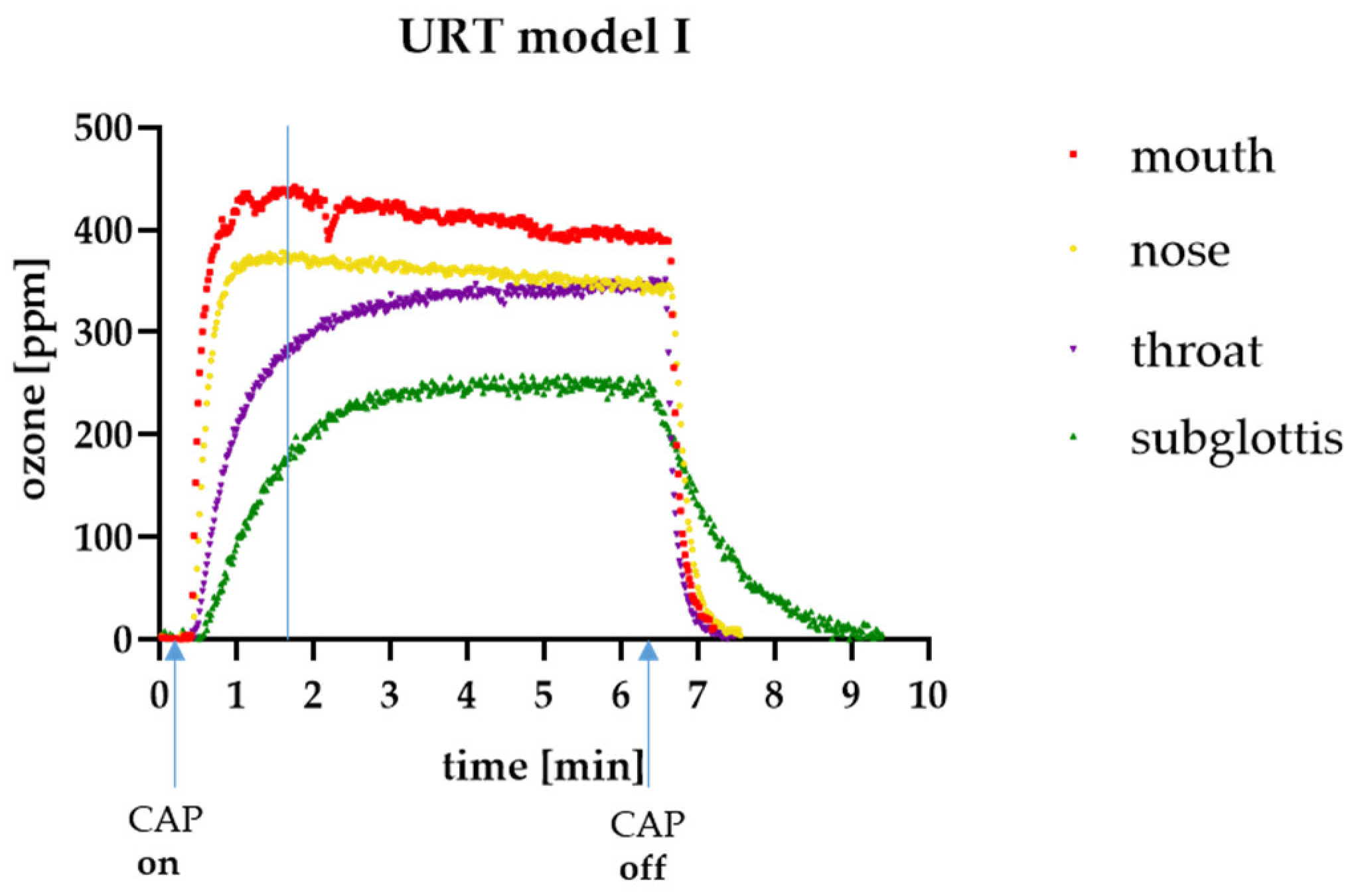
3.1. Ozone Measurements in the URT Model I
3.2. Antibacterial Efficacy of the PIC Device
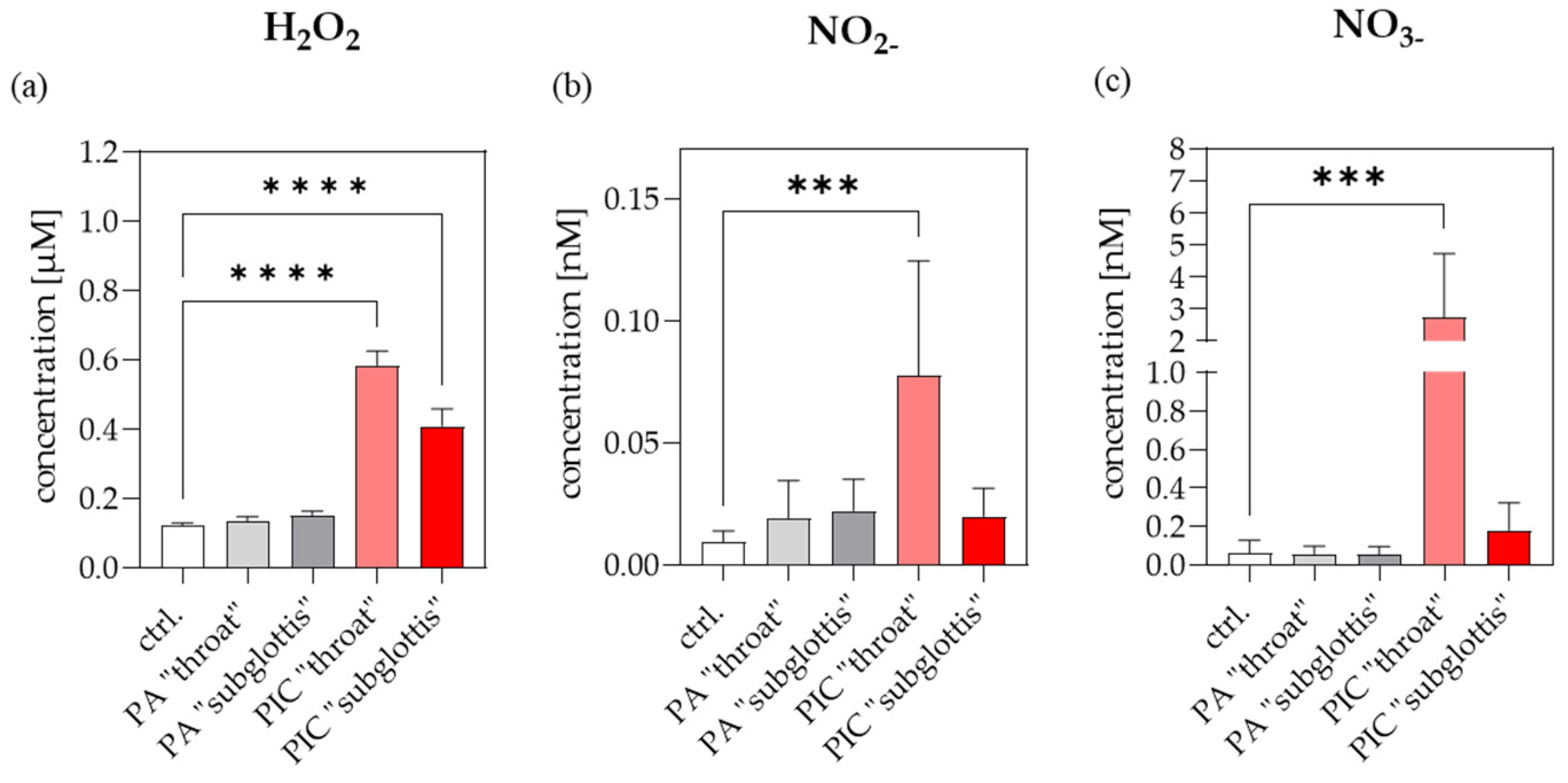
3.3. Reactive Species-Measurements in the URT Model II
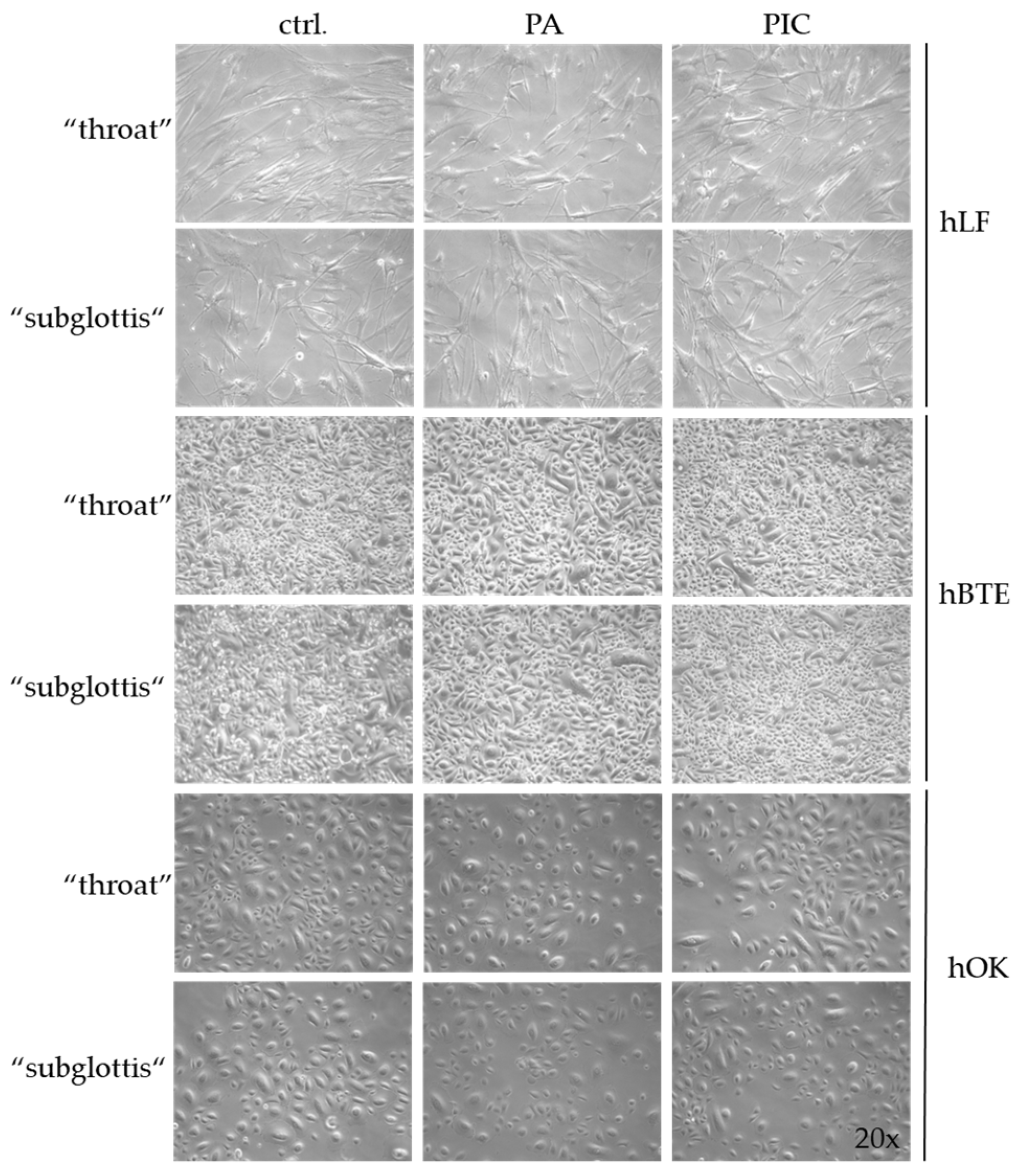
3.4. Cell Morphology Is Not Changed after PIC Treatment
3.5. Cell Viability Is Not Influenced after PIC Treatment
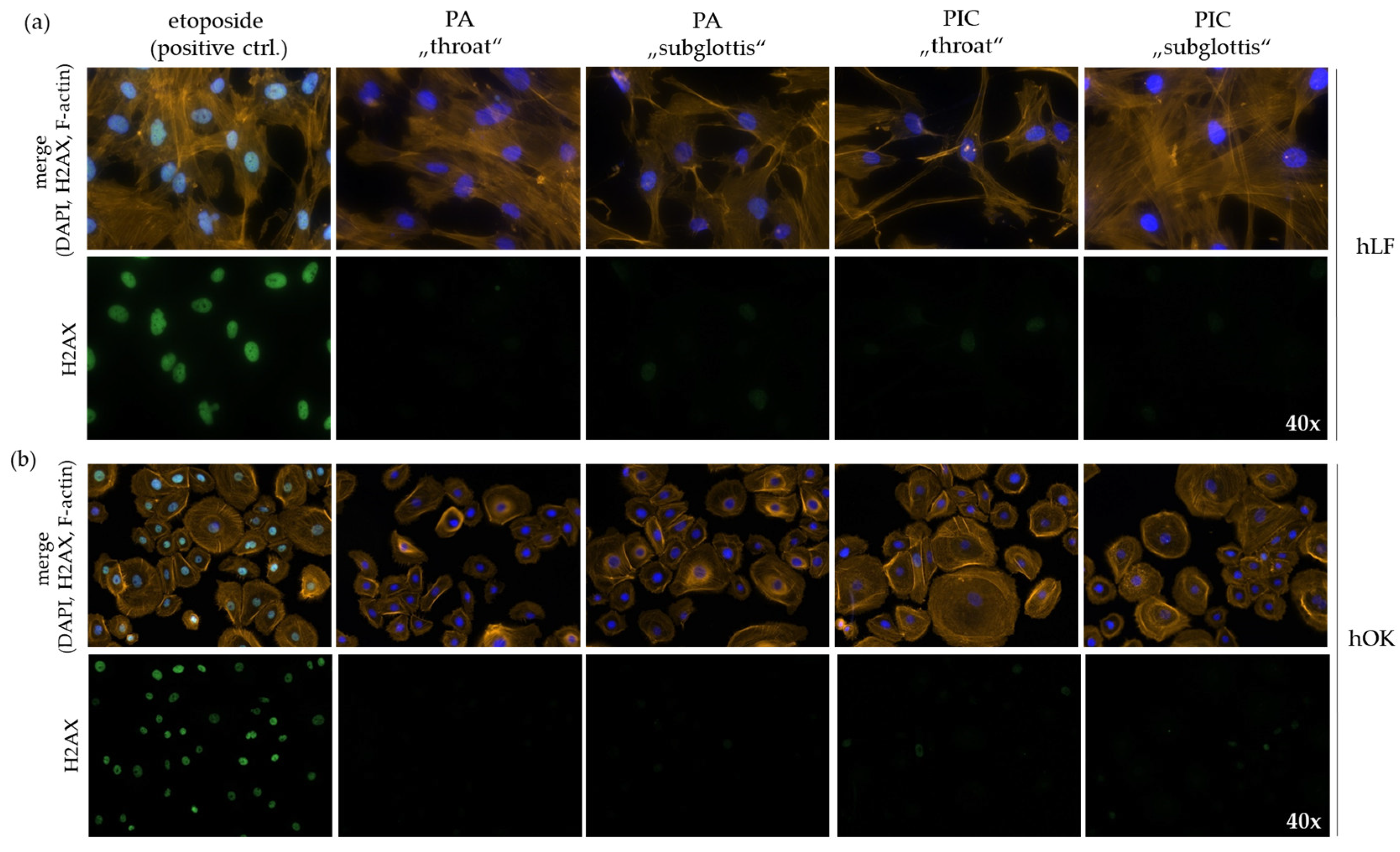
3.6. DNA Is Not Damaged after PIC Treatment
3.7. Cell Apoptosis Is Not Affected after PIC Treatment
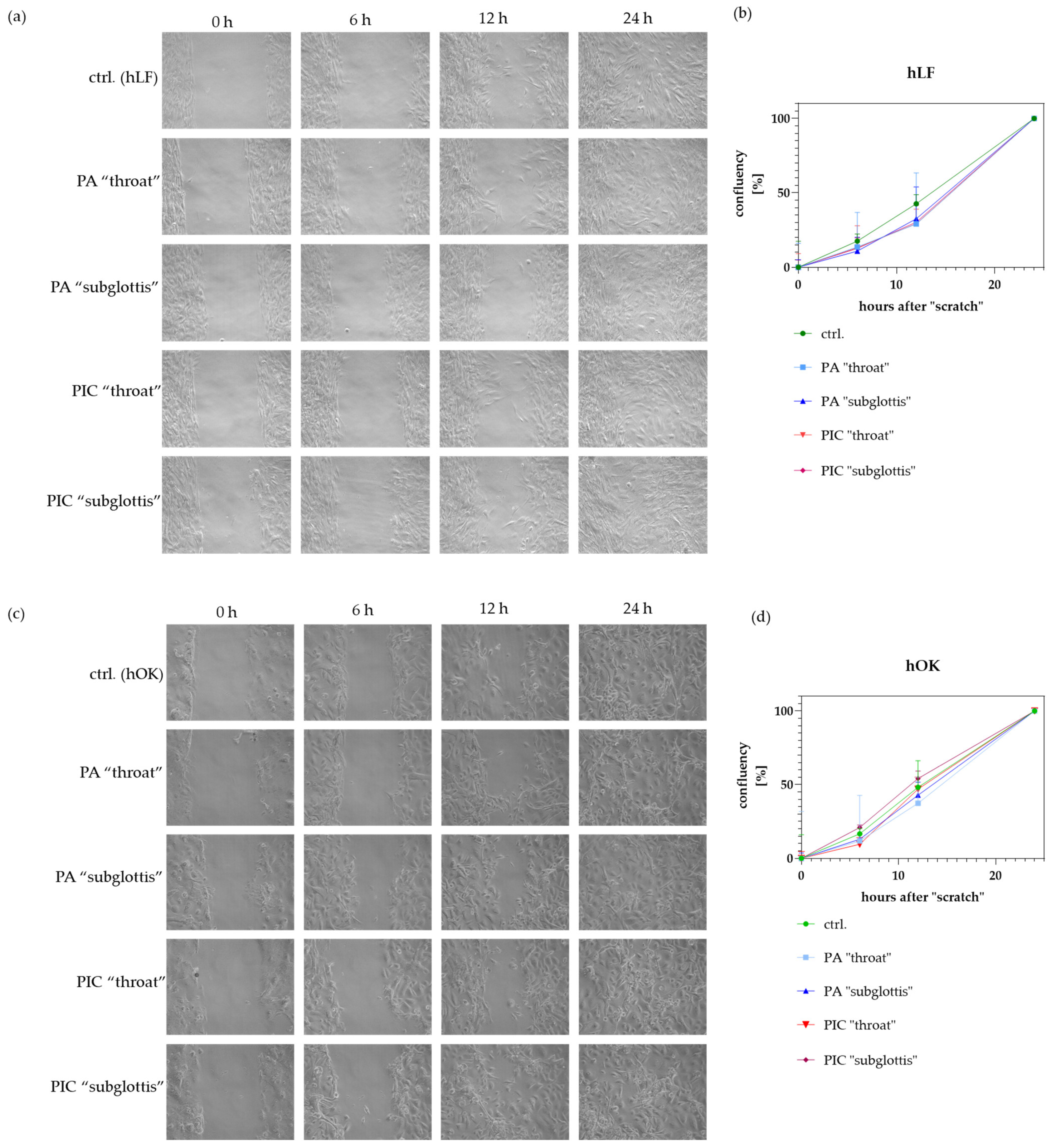
3.8. Cell Migration/Proliferation Is Not Significantly Modified after PIC Treatment
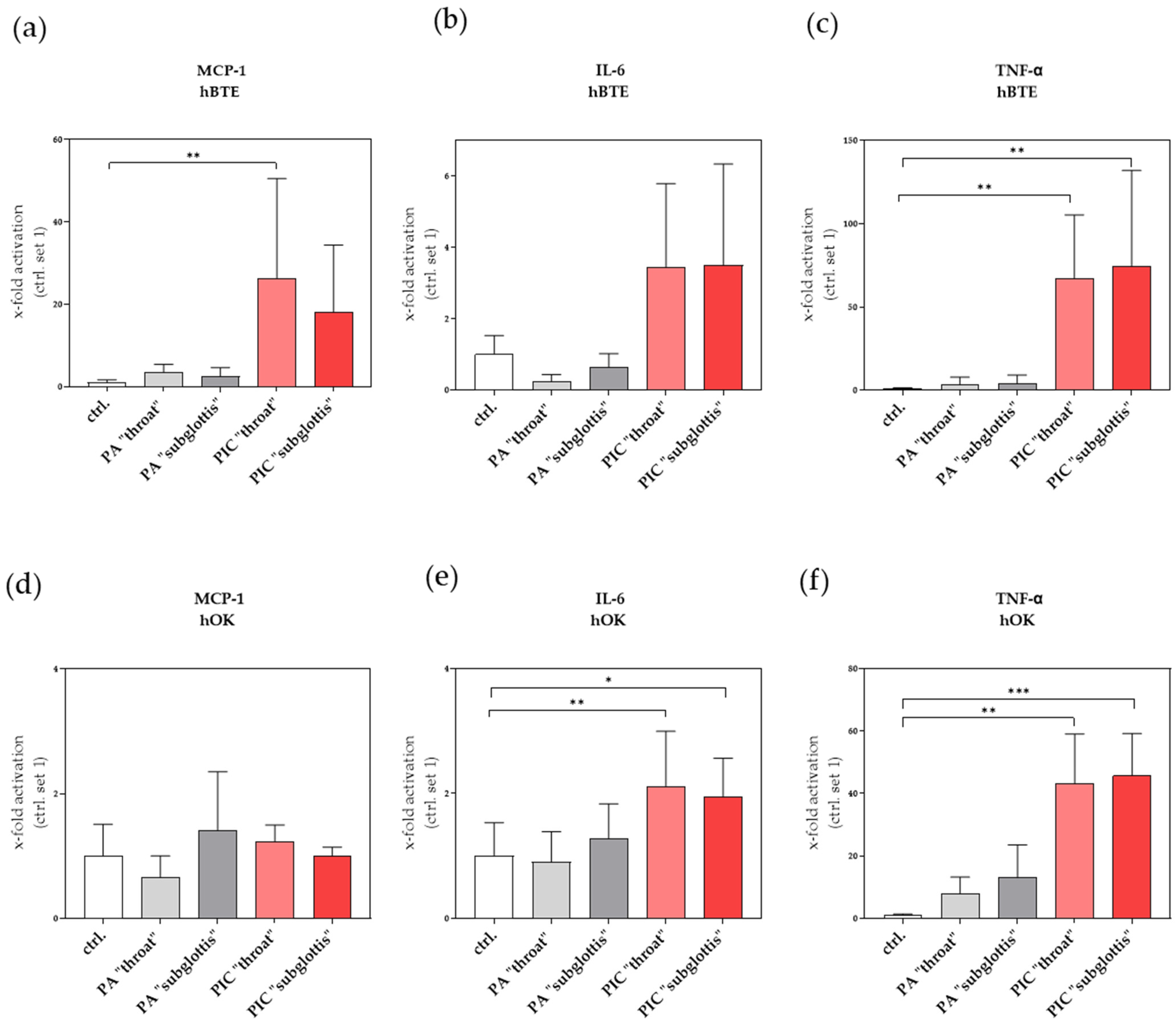
3.9. Immunomodulatory Effects Are Recognized after PIC Treatment
3.9.1. Induction of Pro-Inflammatory Factors in hBTE and hOK Cells after PIC Treatment
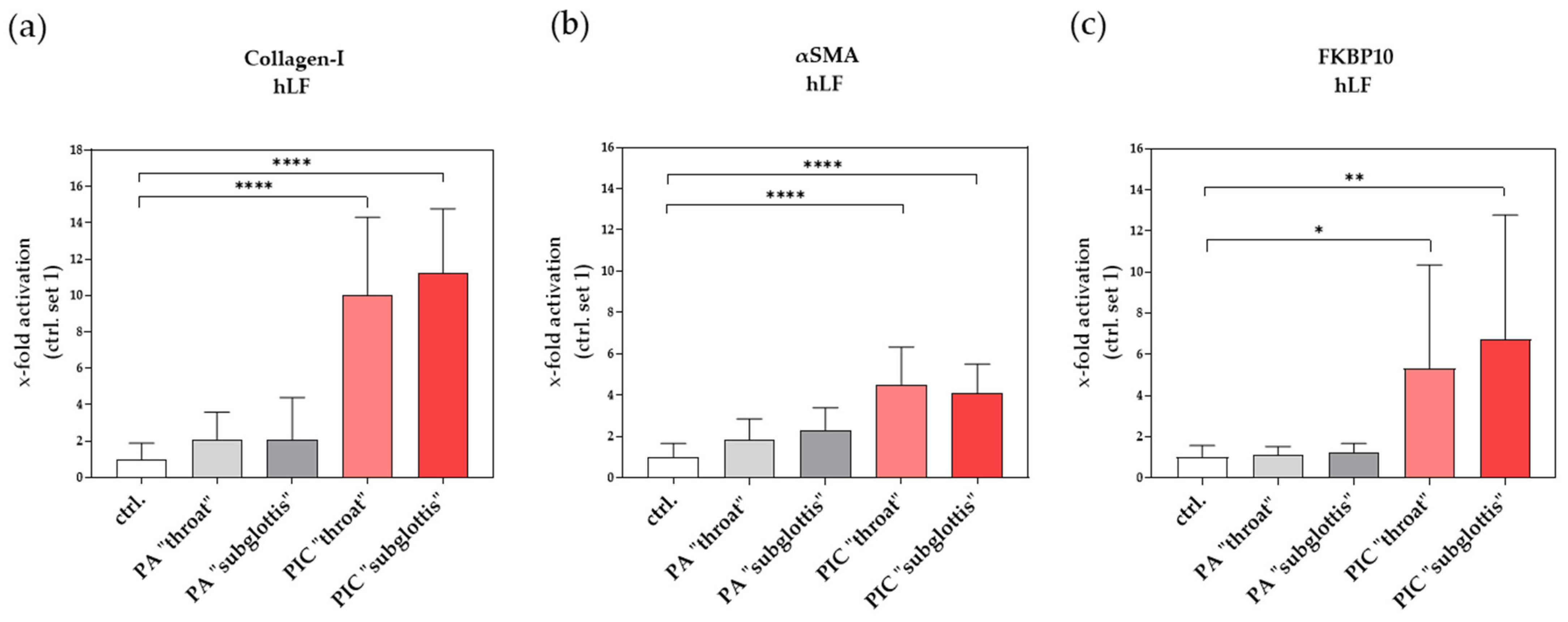
3.9.2. Induction of Profibrotic Molecules in hLF after PIC Treatment
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Li, Y.F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S. aureus and E. coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS ONE 2012, 7, e34610. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Theinkom, F.; Singer, L.; Cieplik, F.; Cantzler, S.; Weilemann, H.; Cantzler, M.; Hiller, K.A.; Maisch, T.; Zimmermann, J.L. Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. PLoS ONE 2019, 14, e0223925. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bai, F.; Jonas, S.J.; Wirz, R.E. Cold atmospheric plasma for addressing the COVID-19 pandemic. Plasma Process. 2022, 19, 2200012. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Welz, C.; Becker, S.; Li, Y.-F.; Shimizu, T.; Jeon, J.; Schwenk-Zieger, S.; Thomas, H.M.; Isbary, G.; Morfill, G.E.; Harréus, U. Effects of cold atmospheric plasma on mucosal tissue culture. J. Phys. D Appl. Phys. 2012, 46, 045401. [Google Scholar] [CrossRef]
- Hasse, S.; Hahn, O.; Kindler, S.; von Woedtke, T.; Metelmann, H.-R.; Masur, K. Atmospheric pressure plasma jet application on human oral mucosa modulates tissue regeneration. Plasma Med. 2014, 4, 117–129. [Google Scholar] [CrossRef]
- Becker, S.; Zimmermann, J.L.; Baumeister, P.; Brunner, T.F.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Harréus, U.; Welz, C. Effects of cold atmospheric plasma (CAP) on bacteria and mucosa of the upper aerodigestive tract. Auris Nasus Larynx 2019, 46, 294–301. [Google Scholar] [CrossRef]
- Sun, T.; Yu, S.; Song, X.; Zhang, J.; Bao, Q.; Mei, Q.; Shen, Q.; Wang, D.; Ni, G. Cold plasma irradiation regulates inflammation and oxidative stress in human bronchial epithelial cells and human non-small cell lung carcinoma. Radiat. Res. 2022, 197, 166–174. [Google Scholar] [CrossRef]
- Golubitskaya, E.; Troitskaya, O.; Yelak, E.; Gugin, P.; Richter, V.; Schweigert, I.; Zakrevsky, D.; Koval, O. Cold physical plasma decreases the viability of lung adenocarcinoma cells. Acta Naturae 2019, 11, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mang, X.; Li, X.; Cai, Z.; Tan, F. Cold atmospheric plasma induces apoptosis in human colon and lung cancer cells through modulating mitochondrial pathway. Front. Cell Dev. Biol. 2022, 10, 915785. [Google Scholar] [CrossRef]
- Yazdani, Z.; Mehrabanjoubani, P.; Biparva, P.; Rafiei, A. Cytotoxicity effect of cold atmospheric plasma on melanoma (B16-F10), breast (MCF-7) and lung (A549) cancer cell lines compared with normal cells. J. Maz. Univ. Med. Sci. 2020, 30, 38–48. [Google Scholar]
- Kim, S.J.; Chung, T. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Masur, K. Cold Plasma Based Wound Healing Application. In Plasma Biosciences and Medicine; Springer: Berlin/Heidelberg, Germany, 2023; pp. 93–109. [Google Scholar]
- Abu Rached, N.; Kley, S.; Storck, M.; Meyer, T.; Stücker, M. Cold Plasma Therapy in Chronic Wounds—A Multicenter, Randomized Controlled Clinical Trial (Plasma on Chronic Wounds for Epidermal Regeneration Study): Preliminary Results. J. Clin. Med. 2023, 12, 5121. [Google Scholar] [CrossRef]
- de Morais Gouvêa Lima, G.; Carta, C.F.L.; Borges, A.C.; Nishime, T.M.C.; da Silva, C.A.V.; Caliari, M.V.; Mayer, M.P.A.; Kostov, K.G.; Koga-Ito, C.Y. Cold Atmospheric Pressure Plasma Is Effective against P. gingivalis (HW24D-1) Mature Biofilms and Non-Genotoxic to Oral Cells. Appl. Sci. 2022, 12, 7247. [Google Scholar] [CrossRef]
- Jahandideh, A.; Amini, M.; Porbagher, H.; Amini, M. Evaluating the effect of cold plasma on the healing of gingival wound. J. Diabetes Metab. Disord. 2021, 20, 741–745. [Google Scholar] [CrossRef]
- Kleineidam, B.; Nokhbehsaim, M.; Deschner, J.; Wahl, G. Effect of cold plasma on periodontal wound healing—An in vitro study. Clin. Oral Investig. 2019, 23, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.d.M.G.; Borges, A.C.; Nishime, T.M.C.; Santana-Melo, G.d.F.; Kostov, K.G.; Mayer, M.P.A.; Koga-Ito, C.Y. Cold atmospheric plasma jet as a possible adjuvant therapy for periodontal disease. Molecules 2021, 26, 5590. [Google Scholar] [CrossRef] [PubMed]
- Mietto, C.; Pinciroli, R.; Patel, N.; Berra, L. Ventilator associated pneumonia: Evolving definitions and preventive StrategiesDiscussion. Respir. Care 2013, 58, 990–1007. [Google Scholar] [CrossRef]
- Arndt, S.; Wacker, E.; Li, Y.F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- Xu, D.; Luo, X.; Xu, Y.; Cui, Q.; Yang, Y.; Liu, D.; Chen, H.; Kong, M.G. The effects of cold atmospheric plasma on cell adhesion, differentiation, migration, apoptosis and drug sensitivity of multiple myeloma. Biochem. Biophys. Res. Commun. 2016, 473, 1125–1132. [Google Scholar] [CrossRef]
- Stoffels, E.; Roks, A.J.; Deelman, L.E. Delayed effects of cold atmospheric plasma on vascular cells. Plasma Process. Polym. 2008, 5, 599–605. [Google Scholar] [CrossRef]
- Kieft, I.E.; Darios, D.; Roks, A.J.; Stoffels, E. Plasma treatment of mammalian vascular cells: A quantitative description. IEEE Trans. Plasma Sci. 2005, 33, 771–775. [Google Scholar] [CrossRef]
- Kieft, I.E.; Kurdi, M.; Stoffels, E. Reattachment and apoptosis after plasma-needle treatment of cultured cells. IEEE Trans. Plasma Sci. 2006, 34, 1331–1336. [Google Scholar] [CrossRef]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.F.; Morfill, G.E.; Bosserhoff, A.K.; Karrer, S. Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS ONE 2015, 10, e0120041. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Kramer, A.; Weltmann, K.-D.; Masur, K. Cold physical plasma treatment alters redox balance in human immune cells. Plasma Med. 2013, 3, 267–278. [Google Scholar] [CrossRef]
- Kupke, L.S.; Arndt, S.; Lenzer, S.; Metz, S.; Unger, P.; Zimmermann, J.L.; Bosserhoff, A.-K.; Gruber, M.; Karrer, S. Cold atmospheric plasma promotes the immunoreactivity of granulocytes in vitro. Biomolecules 2021, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Bosserhoff, A.-K.; Berneburg, M.; Karrer, S. The anti-fibrotic effect of cold atmospheric plasma on localized scleroderma in vitro and in vivo. Biomedicines 2021, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.U.; Kim, Y.S.; Kim, Y.E.; Park, J.-K.; Lee, Y.S.; Kang, H.Y.; Jang, J.W.; Ryeo, J.B.; Lee, Y.; Shin, Y.S. Opposite effects of non-thermal plasma on cell migration and collagen production in keloid and normal fibroblasts. PLoS ONE 2017, 12, e0187978. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Dempke, W. Interleukin-6: A masterplayer in the cytokine network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNFα in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef]
- Selman, M.; King, T.E., Jr.; Pardo, A. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 2001, 134, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Montorfano, I.; Becerra, A.; Cerro, R.; Echeverría, C.; Sáez, E.; Morales, M.G.; Fernández, R.; Cabello-Verrugio, C.; Simon, F. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-β1 and TGF-β2-dependent pathway. Lab. Investig. 2014, 94, 1068–1082. [Google Scholar] [CrossRef]
- Annett, S.; Moore, G.; Robson, T. FK506 binding proteins and inflammation related signalling pathways; basic biology, current status and future prospects for pharmacological intervention. J. Pharmacol. Ther. 2020, 215, 107623. [Google Scholar] [CrossRef]
- Knüppel, L.; Heinzelmann, K.; Lindner, M.; Hatz, R.; Behr, J.; Eickelberg, O.; Staab-Weijnitz, C.A. FK506-binding protein 10 (FKBP10) regulates lung fibroblast migration via collagen VI synthesis. Respir. Res. 2018, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, J.; Wang, Y.; Chen, L.; Wang, D.; Ni, S.; Liu, H. The effects of three cold plasma treatments on the osteogenic activity and antibacterial property of PEEK. Dent. Mater. 2021, 37, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Bunz, O.; Mese, K.; Funk, C.; Wulf, M.; Bailer, S.M.; Piwowarczyk, A.; Ehrhardt, A. Cold atmospheric plasma as antiviral therapy–effect on human herpes simplex virus type 1. J. Gen. Virol. 2020, 101, 208–215. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Environ. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells—A review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17. [Google Scholar] [CrossRef]
- Evert, K.; Kocher, T.; Schindler, A.; Müller, M.; Müller, K.; Pink, C.; Holtfreter, B.; Schmidt, A.; Dombrowski, F.; Schubert, A. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci. Rep. 2021, 11, 20672. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Moritz, J.; Helfrich, I.; Boeckmann, L.; Weltmann, K.-D.; Emmert, S.; Metelmann, H.-R.; Stoffels, I.; von Woedtke, T. Ex vivo exposure of human melanoma tissue to cold physical plasma elicits apoptosis and modulates inflammation. Appl. Sci. 2020, 10, 1971. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Jablonowski, H.; Barton, A.; Weltmann, K.-D.; Wende, K. Role of ambient gas composition on cold physical plasma-elicited cell signaling in keratinocytes. Biophys. J. 2017, 112, 2397–2407. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Ombrello, M.J.; Schulert, G.S. COVID-19 and cytokine storm syndrome: Are there lessons from macrophage activation syndrome? Transl. Res. 2021, 232, 1–12. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Forward Primer 5′→3′ | Reverse Primer 5′→3′ | Condition 1 (Annealing, Melting) |
|---|---|---|---|
| β-actin | CTACGTCGCCCTGGACTTCGAGC | GATGGAGCCGCCGATCCACACGG | ann. 60 °C, melt. 85 °C |
| Coll I | CGGCTCCTGCTCCTCTT | GGGGCAGTTCTTGGTCTC | ann. 60 °C, melt. 86 °C |
| αSMA | GGCCGAGATCTCACTGACTAC | TTCATGGATGCCAGCAGA | ann. 58 °C, melt. 84 °C |
| FKBP10 | TGCGGATGTGGTGGAAATCA | CCGTAGTCATGCGAGGTGAA | ann. 60 °C, melt. 81 °C |
| IL-6 | GGTACATCCTCGACGGCATCT | GTGCCTCTTTGCTGCTTTCAC | ann. 60 °C, melt. 79 °C |
| TNF-α | ATCCTGGGGGACCCAATCTA | AAAAGAAGGCACAGAGGCCA | ann. 60 °C, melt. 81 °C |
| MCP-1 | AATCAATGCCCCAGTCACCT | GGGTCAGCACAGATCTCCTT | ann. 60 °C, melt. 82 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karrer, S.; Unger, P.; Gruber, M.; Gebhardt, L.; Schober, R.; Berneburg, M.; Bosserhoff, A.K.; Arndt, S. In Vitro Safety Study on the Use of Cold Atmospheric Plasma in the Upper Respiratory Tract. Cells 2024, 13, 1411. https://doi.org/10.3390/cells13171411
Karrer S, Unger P, Gruber M, Gebhardt L, Schober R, Berneburg M, Bosserhoff AK, Arndt S. In Vitro Safety Study on the Use of Cold Atmospheric Plasma in the Upper Respiratory Tract. Cells. 2024; 13(17):1411. https://doi.org/10.3390/cells13171411
Chicago/Turabian StyleKarrer, Sigrid, Petra Unger, Michael Gruber, Lisa Gebhardt, Robert Schober, Mark Berneburg, Anja Katrin Bosserhoff, and Stephanie Arndt. 2024. "In Vitro Safety Study on the Use of Cold Atmospheric Plasma in the Upper Respiratory Tract" Cells 13, no. 17: 1411. https://doi.org/10.3390/cells13171411
APA StyleKarrer, S., Unger, P., Gruber, M., Gebhardt, L., Schober, R., Berneburg, M., Bosserhoff, A. K., & Arndt, S. (2024). In Vitro Safety Study on the Use of Cold Atmospheric Plasma in the Upper Respiratory Tract. Cells, 13(17), 1411. https://doi.org/10.3390/cells13171411






