Natural Products for Preventing and Managing Anthracycline-Induced Cardiotoxicity: A Comprehensive Review
Abstract
1. Introduction
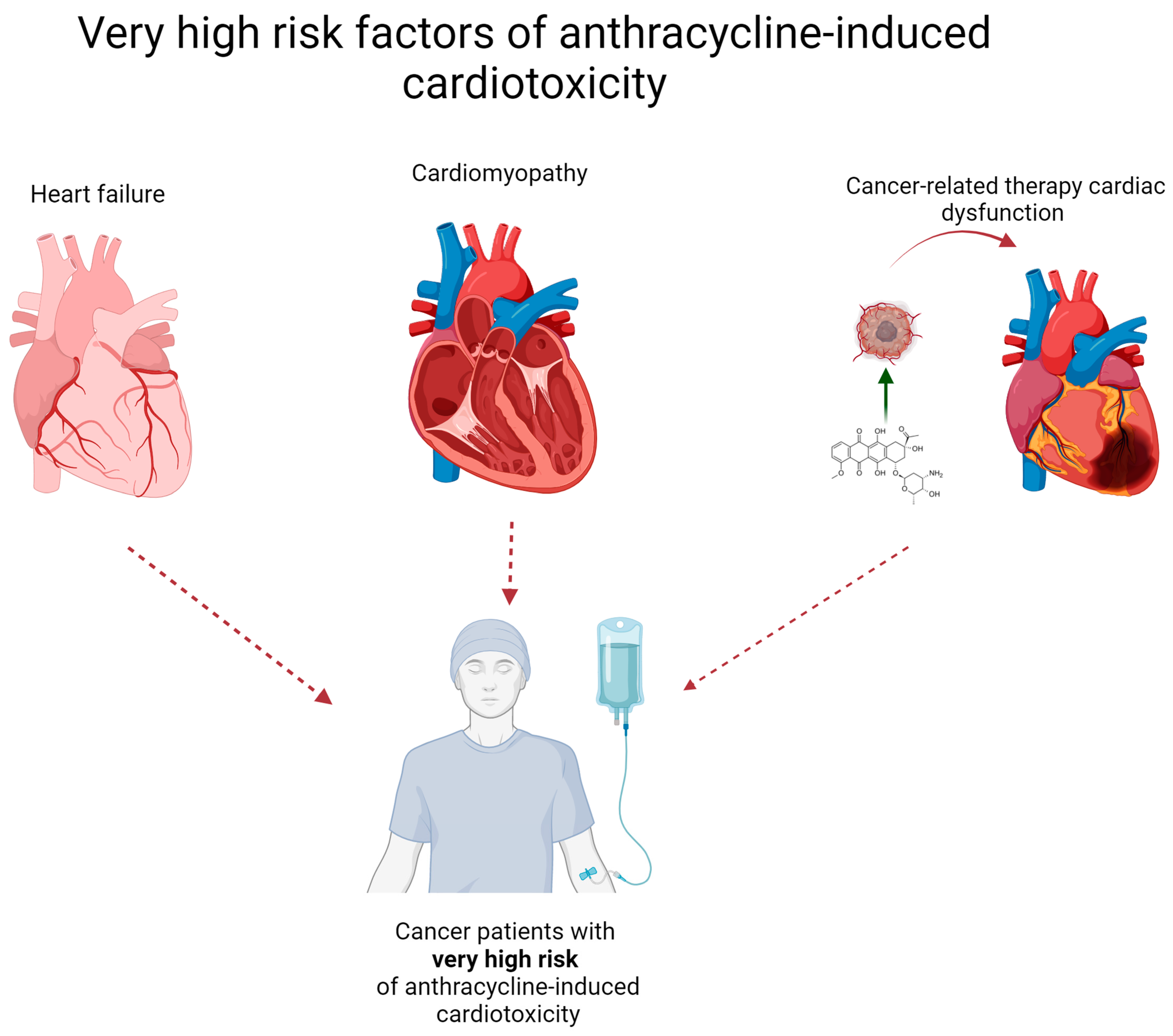
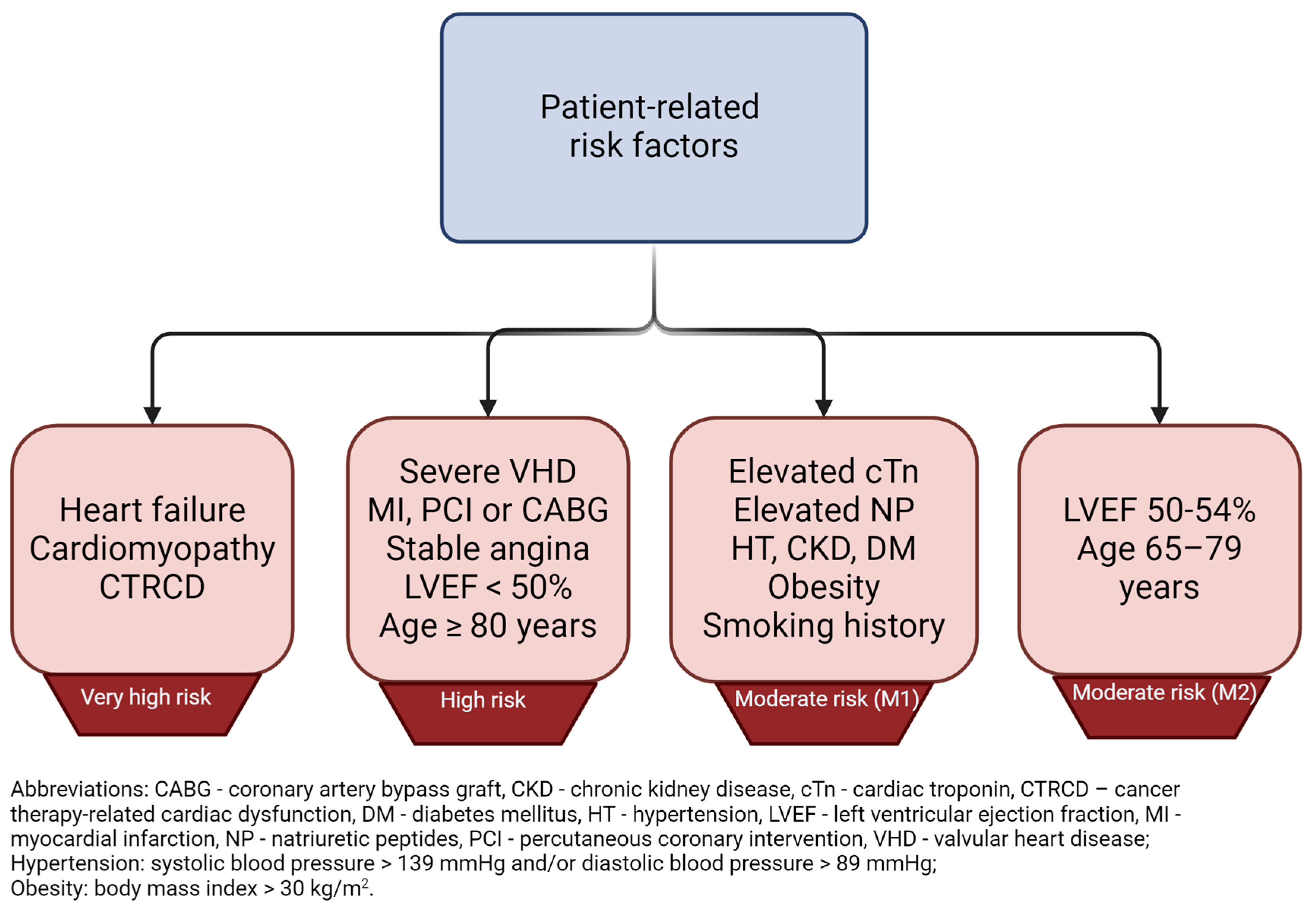
2. What Patient Groups Are Considered to Be at High Risk for Cardiotoxicity?
3. Mechanisms of Anthracycline-Induced Cardiomyopathy
3.1. Oxidative Stress, Mitochondrial Dysfunction and Inflammatory Processes
3.2. Calcium Levels Alterations, Apoptosis and Autophagy
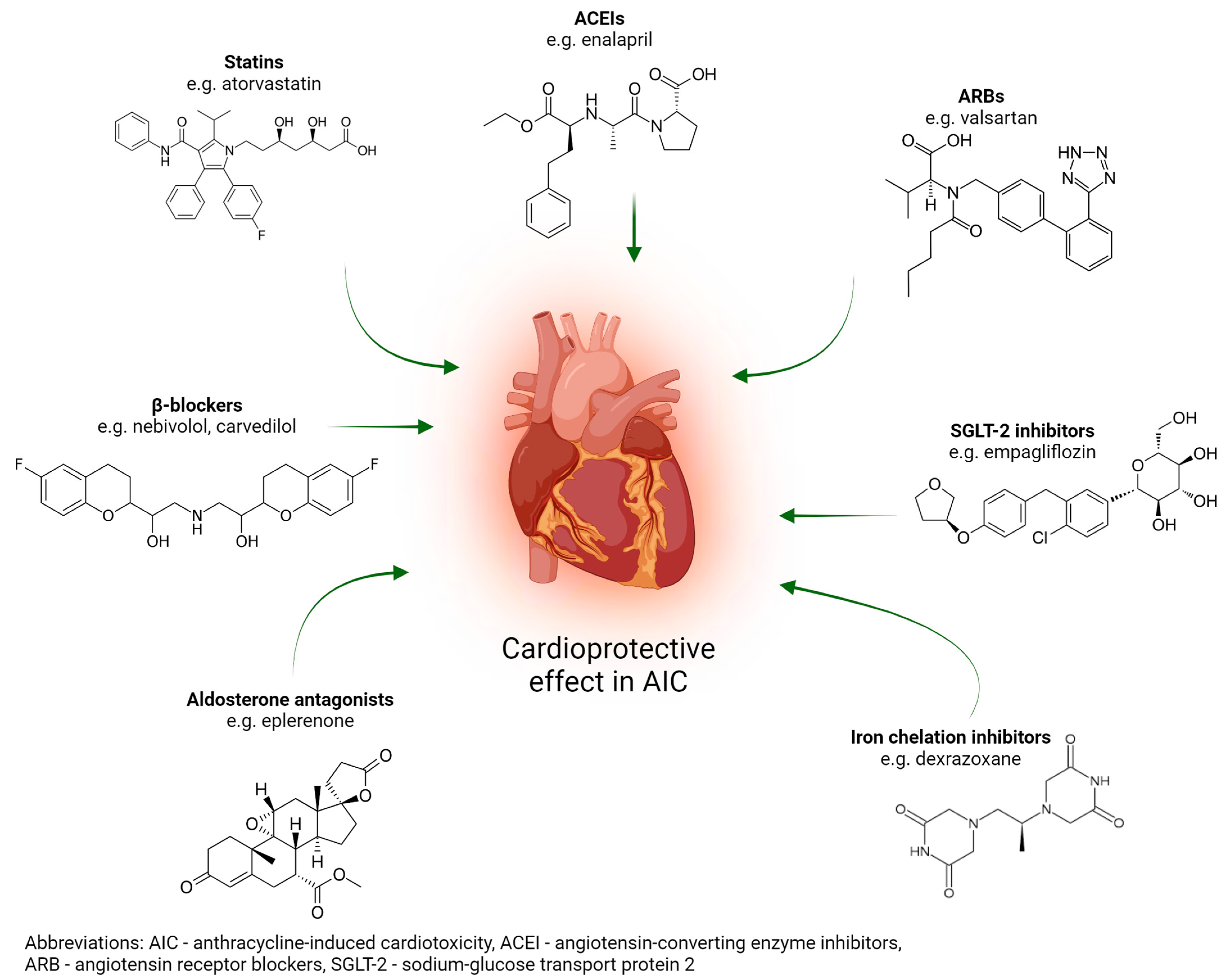
4. Brief Overview of the Most Significant Pharmaceutical Agents for the Prevention of Anthracycline-Induced Cardiotoxicity
4.1. Pharmacological Treatment
4.2. New DOX Delivery Methods and DOX Derivatives
5. Natural Drugs for Preventing Anthracycline-Induced Cardiomyopathy
5.1. Polyphenols
5.2. Alkaloids
5.3. Saponins
5.4. Terpenoids
5.5. Polysaccharides
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ACEIs | angiotensin-converting enzyme inhibitors |
| AE | adverse effects |
| AIC | anthracycline-induced cardiotoxicity |
| ARBs | angiotensin receptor blockers |
| BMI | body mass index |
| BNP | B-type brain natriuretic peptide |
| BP | blood pressure |
| CABG | coronary artery bypass graft |
| CAT | catalase |
| CK | creatine kinase |
| CKD | chronic kidney disease |
| CK-MB | creatine kinase-MB |
| cTn | cardiac troponin |
| CTRCD | cancer therapy-related cardiac dysfunction |
| CV | cardiovascular |
| CVD | cardiovascular disease |
| DM | diabetes mellitus |
| DOX | doxorubicin |
| eNOS | endothelial nitric oxide synthase |
| ESC | European Society of Cardiology |
| FDA | Food and Drug Administration |
| Ft | ferritin |
| GRP78 | glucose-regulated protein 78 |
| HF | heart failure |
| HFA | Heart Failure Association |
| HT | hypertension |
| ICOS | International Cardio-Oncology Society |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| iNOS | induced nitric oxide synthase |
| IRPs | iron regulatory proteins |
| KLF15 | Krüppel-like factor 15 |
| LDH | lactate dehydrogenase |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| MAPK | mitogen-activated protein kinase |
| MDA | lipid peroxidation marker |
| MI | myocardial infarction |
| MMP | matrix metalloproteinase |
| MnSOD | mitochondrial superoxide dismutase |
| NADH | nicotinamide adenine dinucleotide |
| NADPHNOX2 | nicotinamide adenine dinucleotide phosphateNADPH oxidase 2 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLPR3 | nucleotide-binding domain-like receptor protein 3 |
| NP | natriuretic peptides |
| Nrf2 | nuclear factor-erythroid 2-related factor 2 |
| PCI | percutaneous coronary intervention |
| ROS | reactive oxygen species |
| RT | radiotherapy |
| SGLT-2 | sodium glucose transporter protein 2 |
| SLC | solute carrier transporter |
| SNPs | single nucleotide polymorphisms |
| SOD | superoxide dismutase |
| TIF1 | transcription intermediary factor 1 |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor-α |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| VHD | valvular heart disease |
| WGS | whole genome sequencing |
References
- Yu, J.; Wang, C.; Kong, Q.; Wu, X.; Lu, J.-J.; Chen, X. Recent Progress in Doxorubicin-Induced Cardiotoxicity and Protective Potential of Natural Products. Phytomedicine 2018, 40, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Carelle, N.; Piotto, E.; Bellanger, A.; Germanaud, J.; Thuillier, A.; Khayat, D. Changing Patient Perceptions of the Side Effects of Cancer Chemotherapy. Cancer 2002, 95, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, S.; Dai, Y. Research Progress of Therapeutic Drugs for Doxorubicin-Induced Cardiomyopathy. Biomed. Pharmacother. 2022, 156, 113903. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.; Bussom, S.; Guan, F.; Jiang, Z.; Zhang, W.; Gullen, E.A.; Liu, S.-H.; Cheng, Y.-C. Chemotherapy: The Four-Herb Chinese Medicine PHY906 Reduces Chemotherapy-Induced Gastrointestinal Toxicity. Sci. Transl. Med. 2010, 2, 45ra59. [Google Scholar] [CrossRef] [PubMed]
- Osoro, I.; Sharma, A.; Amir, M.; Vohra, M.; Kumar, R.; Kumar, H.; Zargar, A.; Bangar, H. Prevention and Management of Anthracycline Induced Cardiotoxicity: A Review. Health Sci. Rev. 2022, 5, 100070. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to Prevent Anthracycline-Induced Cardiotoxicity in Cancer Survivors. Cardio-Oncol. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Cassinelli, G. The Roots of Modern Oncology: From Discovery of New Antitumor Anthracyclines to Their Clinical Use. Tumori 2016, 102, 226–235. [Google Scholar] [CrossRef]
- WHO Model List of Essential Medicines—23rd List. 2023. Available online: https://www.who.int/publications-detail-redirect/WHO-MHP-HPS-EML-2023.02 (accessed on 3 May 2024).
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the Task Force on Cardio-Oncology of the European Society of Cardiology (ESC). Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Dorresteijn, J.A.; Janssen, A.; Lambrinou, E.; Scherrenberg, M.; Bonnefoy-Cudraz, E.; Cobain, M.; Piepoli, M.F.; Visseren, F.L.; Dendale, P.; et al. Risk Prediction Tools in Cardiovascular Disease Prevention: A Report from the ESC Prevention of CVD Programme Led by the European Association of Preventive Cardiology (EAPC) in Collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur. J. Prev. Cardiol. 2019, 26, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin Cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Feijen, E.A.M.; Leisenring, W.M.; Stratton, K.L.; Ness, K.K.; van der Pal, H.J.H.; van Dalen, E.C.; Armstrong, G.T.; Aune, G.J.; Green, D.M.; Hudson, M.M.; et al. Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity. JAMA Oncol. 2019, 5, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.-M.; Yang, J.-F.; Miao, J.; Xu, J. Association between Genetic Variants of Transmembrane Transporters and Susceptibility to Anthracycline-Induced Cardiotoxicity: Current Understanding and Existing Evidence. Clin. Genet. 2024, 105, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Du, K.; Niu, Y.-J.; Wang, Y.; Xu, X. Genetic Susceptibility and Mechanisms Underlying the Pathogenesis of Anthracycline-Associated Cardiotoxicity. Oxidative Med. Cell. Longev. 2022, 2022, e5818612. [Google Scholar] [CrossRef]
- Magdy, T.; Burmeister, B.T.; Burridge, P.W. Validating the Pharmacogenomics of Chemotherapy-Induced Cardiotoxicity: What Is Missing? Pharmacol. Ther. 2016, 168, 113–125. [Google Scholar] [CrossRef]
- Berkman, A.M.; Hildebrandt, M.A.T.; Landstrom, A.P. The Genetic Underpinnings of Anthracycline-Induced Cardiomyopathy Predisposition. Clin. Genet. 2021, 100, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kingsmore, S.F. Newborn Testing and Screening by Whole-Genome Sequencing. Genet. Med. 2016, 18, 214–216. [Google Scholar] [CrossRef]
- Saraogi, P.; Pillai, K.K.; Singh, B.K.; Dubey, K. Rosiglitazone and Pioglitazone Aggravate Doxorubicin-Induced Cardiomyopathy in Wistar Rats. Biomed. Aging Pathol. 2011, 1, 65–71. [Google Scholar] [CrossRef]
- Doerr, V.; Montalvo, R.N.; Nguyen, B.L.; Boeno, F.P.; Sunshine, M.D.; Bindi, V.E.; Fuller, D.D.; Smuder, A.J. Effects of Hyperbaric Oxygen Preconditioning on Doxorubicin Cardiorespiratory Toxicity. Antioxidants 2022, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Y.; Abdelkarim, O.; Herzallah, K.; Abela, G.S. Anthracycline-Induced Cardiotoxicity: Mechanisms of Action, Incidence, Risk Factors, Prevention, and Treatment. Heart Fail. Rev. 2021, 26, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Scully, R.E.; Stevenson, K.E.; Franco, V.I.; Neuberg, D.S.; Colan, S.D.; Silverman, L.B.; Moslehi, J.J.; Cheng, S.; Sallan, S.E. Hearts Too Small for Body Size after Doxorubicin for Childhood ALL: Grinch Syndrome. J. Clin. Oncol. 2014, 32, 10021. [Google Scholar] [CrossRef]
- Pajović, V.; Kovácsházi, C.; Kosić, M.; Vasić, M.; Đukić, L.; Brenner, G.B.; Giricz, Z.; Bajić, D.; Ferdinandy, P.; Japundžić-Žigon, N. Phenomapping for Classification of Doxorubicin-Induced Cardiomyopathy in Rats. Toxicol. Appl. Pharmacol. 2021, 423, 115579. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, P.; Huang, Y. Anthracycline-Induced Cardiotoxicity: Mechanisms, Monitoring, and Prevention. Front. Cardiovasc. Med. 2023, 10, 1242596. [Google Scholar] [CrossRef]
- Zhu, S.-G.; Kukreja, R.C.; Das, A.; Chen, Q.; Lesnefsky, E.J.; Xi, L. Dietary Nitrate Supplementation Protects against Doxorubicin-Induced Cardiomyopathy by Improving Mitochondrial Function. J. Am. Coll. Cardiol. 2011, 57, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.F.; Batista, R.I.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix Metalloproteinases and Arterial Hypertension: Role of Oxidative Stress and Nitric Oxide in Vascular Functional and Structural Alterations. Biomolecules 2021, 11, 585. [Google Scholar] [CrossRef]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2015, e795602. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Mitochondrial Bioenergetics and Cardiolipin Alterations in Myocardial Ischemia-Reperfusion Injury: Implications for Pharmacological Cardioprotection. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1341–H1352. [Google Scholar] [CrossRef]
- Gambardella, J.; Santulli, G.; Fiordelisi, A.; Cerasuolo, F.A.; Wang, X.; Prevete, N.; Sommella, E.; Avvisato, R.; Buonaiuto, A.; Altobelli, G.G.; et al. Infiltrating Macrophages Amplify Doxorubicin-Induced Cardiac Damage: Role of Catecholamines. Cell. Mol. Life Sci. 2023, 80, 323. [Google Scholar] [CrossRef]
- Gammella, E.; Maccarinelli, F.; Buratti, P.; Recalcati, S.; Cairo, G. The Role of Iron in Anthracycline Cardiotoxicity. Front. Pharmacol. 2014, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.C.; Fidale, T.M.; Pereira, T.C.R.; Lopes, P.R.; Ferreira-Junior, M.D.; Deconte, S.R.; Ferreira-Neto, M.L.; Brito, W.S.; Gomes, R.M.; de Souza, F.R.; et al. Cardioprotective Effects of Leucine Supplementation against Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2024, 24, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Kwon, O.-S.; Smuder, A.J.; Wiggs, M.P.; Sollanek, K.J.; Christou, D.D.; Yoo, J.-K.; Hwang, M.-H.; Szeto, H.H.; Kavazis, A.N.; et al. Increased Mitochondrial Emission of Reactive Oxygen Species and Calpain Activation Are Required for Doxorubicin-Induced Cardiac and Skeletal Muscle Myopathy. J. Physiol. 2015, 593, 2017–2036. [Google Scholar] [CrossRef]
- Zeng, C.; Duan, F.; Hu, J.; Luo, B.; Huang, B.; Lou, X.; Sun, X.; Li, H.; Zhang, X.; Yin, S.; et al. NLRP3 Inflammasome-Mediated Pyroptosis Contributes to the Pathogenesis of Non-Ischemic Dilated Cardiomyopathy. Redox Biol. 2020, 34, 101523. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.-P.; Bultynck, G.; Parys, J.B. A Dual Role for Ca2+ in Autophagy Regulation. Cell Calcium 2011, 50, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Della Sala, A.; Tocchetti, C.G.; Porporato, P.E.; Ghigo, A. Metabolic Aspects of Anthracycline Cardiotoxicity. Curr. Treat. Options Oncol. 2021, 22, 18. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Abdelwahid, E.; Wei, L. Apoptosis in Anthracycline Cardiomyopathy. Curr. Pediatr. Rev. 2011, 7, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yuan, C.; Su, X.; Zhang, J.; Gokulnath, P.; Vulugundam, G.; Li, G.; Yang, X.; An, N.; Liu, C.; et al. Relevance of Ferroptosis to Cardiotoxicity Caused by Anthracyclines: Mechanisms to Target Treatments. Front. Cardiovasc. Med. 2022, 9, 896792. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Brunham, L.R. Regulated Cell Death Pathways in Doxorubicin-Induced Cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Sargent, J.M.; Williamson, C.J.; Yardley, C.; Taylor, C.G.; Hellmann, K. Dexrazoxane Significantly Impairs the Induction of Doxorubicin Resistance in the Human Leukaemia Line, K562. Br. J. Cancer 2001, 84, 959–964. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, F.; Qin, F.; Li, J.; Liu, N.; Li, D.; Li, T.; Xie, H.; Liu, D.; Zhou, S.; et al. Beta-Blockers for the Primary Prevention of Anthracycline-Induced Cardiotoxicity: A Meta-Analysis of Randomized Controlled Trials. BMC Pharmacol. Toxicol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Fu, E.L.; Uijl, A.; Dekker, F.W.; Lund, L.H.; Savarese, G.; Carrero, J.J. Association Between β-Blocker Use and Mortality/Morbidity in Patients With Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction and Advanced Chronic Kidney Disease. Circ. Heart Fail. 2020, 13, e007180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, Y.; Zhang, J.; Ji, T. Cardioprotective Effect of Carvedilol: Inhibition of Apoptosis in H9c2 Cardiomyocytes via the TLR4/NF-κB Pathway Following Ischemia/Reperfusion Injury. Exp. Ther. Med. 2014, 8, 1092–1096. [Google Scholar] [CrossRef]
- Seicean, S.; Seicean, A.; Alan, N.; Plana, J.C.; Budd, G.T.; Marwick, T.H. Cardioprotective Effect of β-Adrenoceptor Blockade in Patients With Breast Cancer Undergoing Chemotherapy. Circ. Heart Fail. 2013, 6, 420–426. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R.; das Dores Cruz, F.; Gonçalves Brandão, S.M.; Rigaud, V.O.C.; Higuchi-dos-Santos, M.H.; Hajjar, L.A.; Kalil Filho, R.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef]
- Cochera, F.; Dinca, D.; Bordejevic, D.A.; Citu, I.M.; Mavrea, A.M.; Andor, M.; Trofenciuc, M.; Tomescu, M.C. Nebivolol Effect on Doxorubicin-Induced Cardiotoxicity in Breast Cancer. Cancer Manag. Res. 2018, 10, 2071–2081. [Google Scholar] [CrossRef]
- Kavalipati, N.; Shah, J.; Ramakrishan, A.; Vasnawala, H. Pleiotropic Effects of Statins. Indian J. Endocrinol. Metab. 2015, 19, 554. [Google Scholar] [CrossRef] [PubMed]
- Sobczuk, P.; Czerwińska, M.; Kleibert, M.; Cudnoch-Jędrzejewska, A. Anthracycline-Induced Cardiotoxicity and Renin-Angiotensin-Aldosterone System—From Molecular Mechanisms to Therapeutic Applications. Heart Fail. Rev. 2022, 27, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Nakamae, H.; Tsumura, K.; Terada, Y.; Nakane, T.; Nakamae, M.; Ohta, K.; Yamane, T.; Hino, M. Notable Effects of Angiotensin II Receptor Blocker, Valsartan, on Acute Cardiotoxic Changes after Standard Chemotherapy with Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone. Cancer 2005, 11, 2492–2498. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.21478 (accessed on 27 May 2024). [CrossRef]
- Hullin, R.; Métrich, M.; Sarre, A.; Basquin, D.; Maillard, M.; Regamey, J.; Martin, D. Diverging Effects of Enalapril or Eplerenone in Primary Prevention against Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Res. 2018, 114, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Lother, A.; Bergemann, S.; Kowalski, J.; Huck, M.; Gilsbach, R.; Bode, C.; Hein, L. Inhibition of the Cardiac Myocyte Mineralocorticoid Receptor Ameliorates Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Res. 2018, 114, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Botti, G.; Maurea, N. SGLT2 Inhibitor Dapagliflozin against Anthracycline and Trastuzumab-Induced Cardiotoxicity: The Role of MYD88, NLRP3, Leukotrienes/Interleukin 6 Axis and mTORC1 /Fox01/3a Mediated Apoptosis. Eur. Heart J. 2020, 41, ehaa946.3253. [Google Scholar] [CrossRef]
- Sabatino, J.; De Rosa, S.; Tammè, L.; Iaconetti, C.; Sorrentino, S.; Polimeni, A.; Mignogna, C.; Amorosi, A.; Spaccarotella, C.; Yasuda, M.; et al. Empagliflozin Prevents Doxorubicin-Induced Myocardial Dysfunction. Cardiovasc. Diabetol. 2020, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yan, T.; Jendrny, C.; Nemecek, A.; Vincetic, M.; Gödtel-Armbrust, U.; Wojnowski, L. Dexrazoxane May Prevent Doxorubicin-Induced DNA Damage via Depleting Both Topoisomerase II Isoforms. BMC Cancer 2014, 14, 842. [Google Scholar] [CrossRef] [PubMed]
- Bertorello, N.; Luksch, R.; Bisogno, G.; Haupt, R.; Spallarossa, P.; Cenna, R.; Fagioli, F. Cardiotoxicity in Children with Cancer Treated with Anthracyclines: A Position Statement on Dexrazoxane. Pediatr. Blood Cancer 2023, 70, e30515. [Google Scholar] [CrossRef]
- Chen, J.J.; Wu, P.-T.; Middlekauff, H.R.; Nguyen, K.-L. Aerobic Exercise in Anthracycline-Induced Cardiotoxicity: A Systematic Review of Current Evidence and Future Directions. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H213–H222. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, M. The Synthesis of Nano-Doxorubicin and Its Anticancer Effect. Anti-Cancer Agents Med. Chem. 2021, 21, 2466–2477. Available online: https://www.eurekaselect.com/article/112781 (accessed on 27 May 2024). [CrossRef] [PubMed]
- Gyöngyösi, M.; Lukovic, D.; Zlabinger, K.; Spannbauer, A.; Gugerell, A.; Pavo, N.; Traxler, D.; Pils, D.; Maurer, G.; Jakab, A.; et al. Liposomal Doxorubicin Attenuates Cardiotoxicity via Induction of Interferon-Related DNA Damage Resistance. Cardiovasc. Res. 2020, 116, 970–982. [Google Scholar] [CrossRef]
- Szmit, S.; Grela-Wojewoda, A.; Talerczyk, M.; Kufel-Grabowska, J.; Streb, J.; Smok-Kalwat, J.; Iżycki, D.; Chmielowska, E.; Wilk, M.; Sosnowska-Pasiarska, B. Predictors of New-Onset Heart Failure and Overall Survival in Metastatic Breast Cancer Patients Treated with Liposomal Doxorubicin. Sci. Rep. 2020, 10, 18481. Available online: https://www.nature.com/articles/s41598-020-75614-4 (accessed on 27 May 2024). [CrossRef]
- Chen, J.; Qian, C.; Ren, P.; Yu, H.; Kong, X.; Huang, C.; Luo, H.; Chen, G. Light-Responsive Micelles Loaded with Doxorubicin for Osteosarcoma Suppression. Front. Pharmacol. 2021, 12, 679610. Available online: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2021.679610/full (accessed on 27 May 2024). [CrossRef] [PubMed]
- Bhattacharya, T.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Akhtar, M.F.; Saleem, A.; Bin-Jumah, M.; Kamel, M.; Abdel-Latif, M.A.; Abdel-Daim, M.M. A Review on Natural Sources Derived Protein Nanoparticles as Anticancer Agents. Curr. Top. Med. Chem. 2021, 21, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, B.; Zuo, S.; Li, X.; Zhou, S.; Li, L.; Luo, C.; Liu, H.; Cheng, M.; Wang, Y.; et al. Trisulfide Bond–Mediated Doxorubicin Dimeric Prodrug Nanoassemblies with High Drug Loading, High Self-Assembly Stability, and High Tumor Selectivity. Sci. Adv. 2020, 6, eabc1725. Available online: https://www.science.org/doi/10.1126/sciadv.abc1725 (accessed on 27 May 2024). [CrossRef] [PubMed]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-Modified Chitosan/Hyaluronic Acid Nanoparticles as a New Avenue for Local Delivery of Doxorubicin to Oral Cancer Cells. Colloids Surf. B Biointerfaces 2020, 196, 111279. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in Vaccine Delivery: Recent Progress and Advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef]
- Singh, V.; Kesharwani, P. Dendrimer as a Promising Nanocarrier for the Delivery of Doxorubicin as an Anticancer Therapeutics. J. Biomater. Sci. Polym. Ed. 2021, 32, 1882–1909. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.P.; Ganjoo, K.N.; Schuetze, S.; Papai, Z.; Van Tine, B.A.; Choy, E.; Liebner, D.A.; Agulnik, M.; Chawla, S.; Wieland, S.; et al. Phase III Study of Aldoxorubicin vs Investigators’ Choice as Treatment for Relapsed/Refractory Soft Tissue Sarcomas. J. Clin. Oncol. 2017, 35, 11000. [Google Scholar] [CrossRef]
- Dempke, W.C.M.; Zielinski, R.; Winkler, C.; Silberman, S.; Reuther, S.; Priebe, W. Anthracycline-Induced Cardiotoxicity—Are We about to Clear This Hurdle? Eur. J. Cancer 2023, 185, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Parikh, M.; Shah, H.; Gandhi, T. Modulation of Nrf2 by Quercetin in Doxorubicin-Treated Rats. Heliyon 2020, 6, e03803. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Zhao, L.; Li, S.; Li, K. Quercetin Attenuates the Cardiotoxicity of Doxorubicin–Cyclophosphamide Regimen and Potentiates Its Chemotherapeutic Effect against Triple-Negative Breast Cancer. Phytother. Res. 2022, 36, 551–561. [Google Scholar] [CrossRef]
- Aziz, T.A. Cardioprotective Effect of Quercetin and Sitagliptin in Doxorubicin-Induced Cardiac Toxicity in Rats. Cancer Manag. Res. 2021, 13, 2349–2357. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Cheng, W.-T.; Cheng, H.-C.; Chou, W.-C.; Chen, H.-I.; Ou, H.-C.; Tsai, K.-L. Baicalin Enhances Chemosensitivity to Doxorubicin in Breast Cancer Cells via Upregulation of Oxidative Stress-Mediated Mitochondria-Dependent Apoptosis. Antioxidants 2021, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kumar, J.M.; Kuncha, M.; Borkar, R.M.; Srinivas, R.; Sistla, R. Baicalein Alleviates Doxorubicin-Induced Cardiotoxicity via Suppression of Myocardial Oxidative Stress and Apoptosis in Mice. Life Sci. 2016, 144, 8–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Liu, C.; Wei, F. Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity by Modulating the PHLPP1/AKT/Bcl-2 Signalling Pathway. PeerJ 2020, 8, e8845. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.F.R.; Rakhshan, K.; Aboutaleb, N.; Nikbakht, F.; Naderi, N.; Bakhshesh, M.; Azizi, Y. Apigenin Attenuates Doxorubicin Induced Cardiotoxicity via Reducing Oxidative Stress and Apoptosis in Male Rats. Life Sci. 2019, 232, 116623. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, F.; Shen, M.; Sun, C.; Hao, W.; Wu, C.; Xie, Y.; Zhang, S.; Gao, H.; Yang, J.; et al. Luteolin Prevents Cardiac Dysfunction and Improves the Chemotherapeutic Efficacy of Doxorubicin in Breast Cancer. Front. Cardiovasc. Med. 2021, 8, 750186. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, W.; Sun, S.; Li, C.; Zhang, Y.; Ren, J. Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity Through Promoting Mitochondrial Autophagy. Front. Physiol. 2020, 11, 113. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Todorovic, V.; Sobajic, S.; Mahajna, J.; Gerić, M.; Tur, J.A.; Bartoszek, A. Natural Products Counteracting Cardiotoxicity during Cancer Chemotherapy: The Special Case of Doxorubicin, a Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 10037. [Google Scholar] [CrossRef]
- Yao, Y.; Liang, S.; Li, X.; Liu, S.; Chen, J.; Zhang, X.; Jia, K.; Jiang, B.; Xie, X.; Munier, S.; et al. Assessment and Simulation of Global Terrestrial Latent Heat Flux by Synthesis of CMIP5 Climate Models and Surface Eddy Covariance Observations. Agric. For. Meteorol. 2016, 223, 151–167. [Google Scholar] [CrossRef]
- Saleh Ahmed, A.S. Potential Protective Effect of Catechin on Doxorubicin-Induced Cardiotoxicity in Adult Male Albino Rats. Toxicol. Mech. Methods 2022, 32, 97–105. [Google Scholar] [CrossRef]
- Ozyurt, H.; Ozyurt, B.; Koca, K.; Ozgocmen, S. Caffeic Acid Phenethyl Ester (CAPE) Protects Rat Skeletal Muscle against Ischemia–Reperfusion-Induced Oxidative Stress. Vasc. Pharmacol. 2007, 47, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lei, Y.; Lei, S.; Gong, X. Cardioprotective Effects of Corilagin on Doxorubicin Induced Cardiotoxicity via P13K/Akt and NF-κB Signaling Pathways in a Rat Model. Toxicol. Mech. Methods 2022, 32, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Timm, K.N.; Tyler, D.J. The Role of AMPK Activation for Cardioprotection in Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Drugs Ther. 2020, 34, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Yang, L.; Liu, Y.; He, J.; Yang, L.; Zhang, Q.; Liu, F.; Li, J.; Liu, J.; Sumi, S.; et al. Resveratrol Attenuates Doxorubicin-Induced Cardiotoxicity in Rats by up-Regulation of Vascular Endothelial Growth Factor B. J. Nutr. Biochem. 2020, 79, 108132. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.B.; Nascimento, L.V.M.; Moura, D.J.; Saffi, J. Cardioprotective Effect of Maternal Supplementation with Resveratrol on Toxicity Induced by Doxorubicin in Offspring Cardiomyocytes. Arq. Bras. Cardiol. 2021, 117, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Maayah, Z.H.; Alam, A.S.; Takahara, S.; Soni, S.; Ferdaoussi, M.; Matsumura, N.; Zordoky, B.N.; Eisenstat, D.D.; Dyck, J.R.B. Resveratrol Reduces Cardiac NLRP3-Inflammasome Activation and Systemic Inflammation to Lessen Doxorubicin-Induced Cardiotoxicity in Juvenile Mice. FEBS Lett. 2021, 595, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, W.; Song, Z.; Chen, Y.; Zhang, D.; Wang, C. Resveratrol-Induced Autophagy Promotes Survival and Attenuates Doxorubicin-Induced Cardiotoxicity. Int. Immunopharmacol. 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Qu, S.; Wei, X.; Zhu, H.; Luo, Q.; Liu, M.; Chen, G.; Xiao, X. Resveratrol Attenuates Doxorubicin-Induced Cardiomyocyte Apoptosis in Mice through SIRT1-Mediated Deacetylation of P53. Cardiovasc. Res. 2011, 90, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Aswar, U.; Mahajan, U.; Kandhare, A.; Aswar, M. Ferulic Acid Ameliorates Doxorubicin-Induced Cardiac Toxicity in Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 659–668. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. The Effects of Bergamot Polyphenolic Fraction, Cynara Cardunculus, and Olea Europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients 2021, 13, 2158. [Google Scholar] [CrossRef]
- Patil, P.P.; Khanal, P.; Patil, V.S.; Charla, R.; Harish, D.R.; Patil, B.M.; Roy, S. Effect of Theobroma Cacao L. on the Efficacy and Toxicity of Doxorubicin in Mice Bearing Ehrlich Ascites Carcinoma. Antioxidants 2022, 11, 1094. [Google Scholar] [CrossRef]
- Pepe, G.; Salviati, E.; Rapa, S.F.; Ostacolo, C.; Cascioferro, S.; Manfra, M.; Autore, G.; Marzocco, S.; Campiglia, P. Citrus Sinensis and Vitis Vinifera Protect Cardiomyocytes from Doxorubicin-Induced Oxidative Stress: Evaluation of Onconutraceutical Potential of Vegetable Smoothies. Antioxidants 2020, 9, 378. [Google Scholar] [CrossRef]
- Fouad, A.A.; Yacoubi, M.T. Mechanisms Underlying the Protective Effect of Eugenol in Rats with Acute Doxorubicin Cardiotoxicity. Arch. Pharm. Res. 2011, 34, 821–828. [Google Scholar] [CrossRef] [PubMed]
- El-Bakly, W.M.; Louka, M.L.; El-Halawany, A.M.; Schaalan, M.F. 6-Gingerol Ameliorated Doxorubicin-Induced Cardiotoxicity: Role of Nuclear Factor Kappa B and Protein Glycation. Cancer Chemother. Pharmacol. 2012, 70, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.P.; Kushwaha, S.; Tripathi, D.N.; Jena, G.B. Cardioprotective Effects of Hesperetin against Doxorubicin-Induced Oxidative Stress and DNA Damage in Rat. Cardiovasc. Toxicol. 2011, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, G.; Meng, X.; Wang, H.; Luo, Y.; Qin, M.; Ma, B.; Wang, M.; Cai, D.; Guo, P.; et al. Isorhamnetin Protects against Doxorubicin-Induced Cardiotoxicity In Vivo and In Vitro. PLoS ONE 2013, 8, e64526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Sun, G.-B.; Sun, B.; Wu, Y.; He, L.; Wang, X.; Chen, R.-C.; Cao, L.; Ren, X.-Y.; Sun, X.-B. Kaempferol Protects against Doxorubicin-Induced Cardiotoxicity In Vivo and In Vitro. Toxicology 2012, 292, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, L.; Li, M.; Wu, W.; Yang, M.; Wang, J.; Guo, D. Salvianolic Acids Prevent Acute Doxorubicin Cardiotoxicity in Mice through Suppression of Oxidative Stress. Food Chem. Toxicol. 2008, 46, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Psotová, J.; Chlopčíková, Š.; Grambal, F.; Šimánek, V.; Ulrichová, J. Influence of Silymarin and Its Flavonolignans on Doxorubicin-Iron Induced Lipid Peroxidation in Rat Heart Microsomes and Mitochondria in Comparison with Quercetin. Phytother. Res. 2002, 16, 63–67. [Google Scholar] [CrossRef]
- Xiong, C.; Wu, Y.-Z.; Zhang, Y.; Wu, Z.-X.; Chen, X.-Y.; Jiang, P.; Guo, H.-C.; Xie, K.-R.; Wang, K.-X.; Su, S.-W. Protective Effect of Berberine on Acute Cardiomyopathy Associated with Doxorubicin Treatment. Oncol. Lett. 2018, 15, 5721–5729. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Zhang, L.; Wu, Z.-X.; Shan, T.; Xiong, C. Berberine Ameliorates Doxorubicin-Induced Cardiotoxicity via a SIRT1/p66Shc-Mediated Pathway. Oxidative Med. Cell. Longev. 2019, 2019, e2150394. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Tong, N.; Liao, X.; Wang, E.; Li, Z.; Luo, Y.; Zuo, H. Berberine Attenuates Doxorubicin-Induced Cardiotoxicity in Mice. J. Int. Med. Res. 2011, 39, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yu, X.; Wang, Y.; Wang, F.; Li, H.; Wang, Y.; Lu, D.; Qi, R.; Wang, H. Berberine Inhibits Doxorubicin-Triggered Cardiomyocyte Apoptosis via Attenuating Mitochondrial Dysfunction and Increasing Bcl-2 Expression. PLoS ONE 2012, 7, e47351. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Wei, W.; Zhang, N.; Wu, H.; Ma, Z.; Li, L.; Deng, W.; Tang, Q. Matrine Attenuates Oxidative Stress and Cardiomyocyte Apoptosis in Doxorubicin-Induced Cardiotoxicity via Maintaining AMPKα/UCP2 Pathway. Acta Pharm. Sin. B 2019, 9, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C.; Zhang, N.; Wei, W.; Li, L.; Wu, H.; Ma, Z.; Tang, Q. Matrine Attenuates Pathological Cardiac Fibrosis via RPS5/P38 in Mice. Acta Pharmacol. Sin. 2021, 42, 573–584. [Google Scholar] [CrossRef]
- Xu, Z.-M.; Li, C.-B.; Liu, Q.-L.; Li, P.; Yang, H. Ginsenoside Rg1 Prevents Doxorubicin-Induced Cardiotoxicity through the Inhibition of Autophagy and Endoplasmic Reticulum Stress in Mice. Int. J. Mol. Sci. 2018, 19, 3658. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Gao, F.; Yin, Z.; Zhang, R. Ginsenoside RG1 Augments Doxorubicin-Induced Apoptotic Cell Death in MDA-MB-231 Breast Cancer Cell Lines. J. Biochem. Mol. Toxicol. 2022, 36, e22945. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, P.; Gou, H.; Zhang, J.; Zhu, M.; Wang, Z.-H.; Tian, J.-W.; Jiang, Y.-T.; Fu, F.-H. Cardioprotective Effects of 20(S)-Ginsenoside Rh2 against Doxorubicin-Induced Cardiotoxicity in Vitro and in Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 506214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wang, T.; Jiang, X.; Zhang, H.; Li, P.; Lv, B.; Gao, X. Ginsenoside Rg3 Antagonizes Adriamycin-Induced Cardiotoxicity by Improving Endothelial Dysfunction from Oxidative Stress via Upregulating the Nrf2-ARE Pathway through the Activation of Akt. Phytomedicine 2015, 22, 875–884. [Google Scholar] [CrossRef]
- Luo, L.-F.; Guan, P.; Qin, L.-Y.; Wang, J.-X.; Wang, N.; Ji, E.-S. Astragaloside IV Inhibits Adriamycin-Induced Cardiac Ferroptosis by Enhancing Nrf2 Signaling. Mol. Cell. Biochem. 2021, 476, 2603–2611. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, J.; Le, Y.; Pan, J.; Liu, Y.; Liu, Z.; Wang, C.; Dou, X.; Lu, D. Salidroside Inhibits Doxorubicin-Induced Cardiomyopathy by Modulating a Ferroptosis-Dependent Pathway. Phytomedicine 2022, 99, 153964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, Q.; Huo, C.; Li, Y.; He, L.; Ran, B.; Chen, J.; Li, Y.; Liu, W. Ferroptosis: A Novel Mechanism of Artemisinin and Its Derivatives in Cancer Therapy. Curr. Med. Chem. 2021, 28, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Wang, D.; Zou, L.; Fu, C.; Zhang, J.; Leung, G.P.-H. Oridonin Synergistically Enhances the Anti-Tumor Efficacy of Doxorubicin against Aggressive Breast Cancer via pro-Apoptotic and Anti-Angiogenic Effects. Pharmacol. Res. 2019, 146, 104313. [Google Scholar] [CrossRef]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef]
- Deng, J.; Huang, M.; Wu, H. Protective Effect of Limonin against Doxorubicin-Induced Cardiotoxicity via Activating Nuclear Factor—Like 2 and Sirtuin 2 Signaling Pathways. Bioengineered 2021, 12, 7975–7984. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Azimullah, S.; Mamoudh, H.H.; Sharma, C.; Kumar, S.; Goyal, S.N.; Ojha, S. Nerolidol, a Sesquiterpene from the Essential Oils of Aromatic Plants, Attenuates Doxorubicin-Induced Chronic Cardiotoxicity in Rats. J. Agric. Food Chem. 2021, 69, 7334–7343. [Google Scholar] [CrossRef]
- Cao, Y.; Ruan, Y.; Shen, T.; Huang, X.; Li, M.; Yu, W.; Zhu, Y.; Man, Y.; Wang, S.; Li, J. Astragalus Polysaccharide Suppresses Doxorubicin-Induced Cardiotoxicity by Regulating the PI3k/Akt and p38MAPK Pathways. Oxidative Med. Cell. Longev. 2014, 2014, e674219. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, T.; Huang, X.; Lin, Y.; Chen, B.; Pang, J.; Li, G.; Wang, Q.; Zohrabian, S.; Duan, C.; et al. Astragalus Polysaccharide Restores Autophagic Flux and Improves Cardiomyocyte Function in Doxorubicin-Induced Cardiotoxicity. Oncotarget 2016, 8, 4837–4848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; Wu, H.; Zhang, H. Delivery of Astragalus Polysaccharide by Ultrasound Microbubbles Attenuate Doxorubicin-Induced Cardiomyopathy in Rodent Animals. Bioengineered 2022, 13, 8419–8431. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Ding, X.; Wang, H.; Tan, G. HILIC-MS-Based Metabolomics Reveal That Astragalus Polysaccharide Alleviates Doxorubicin-Induced Cardiomyopathy by Regulating Sphingolipid and Glycerophospholipid Homeostasis. J. Pharm. Biomed. Anal. 2021, 203, 114177. [Google Scholar] [CrossRef]
- Amgalan, D.; Garner, T.P.; Pekson, R.; Jia, X.F.; Yanamandala, M.; Paulino, V.; Liang, F.G.; Corbalan, J.J.; Lee, J.; Chen, Y.; et al. A Small-Molecule Allosteric Inhibitor of BAX Protects against Doxorubicin-Induced Cardiomyopathy. Nat. Cancer 2020, 1, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, L.; Rossi, F.; Ragni, M.; Ruocco, C.; Brunetti, D.; Carruba, M.O.; Torrente, Y.; Valerio, A.; Nisoli, E. A Special Amino-Acid Formula Tailored to Boosting Cell Respiration Prevents Mitochondrial Dysfunction and Oxidative Stress Caused by Doxorubicin in Mouse Cardiomyocytes. Nutrients 2020, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Basilicata, M.G.; Pepe, G.; De Anseris, R.; Di Mauro, A.; Scognamiglio, G.; Palma, G.; Vestuto, V.; Buccolo, S.; Luciano, A.; et al. Combination of Spirulina Platensis, Ganoderma Lucidum and Moringa Oleifera Improves Cardiac Functions and Reduces Pro-Inflammatory Biomarkers in Preclinical Models of Short-Term Doxorubicin-Mediated Cardiotoxicity: New Frontiers in Cardioncology? J. Cardiovasc. Dev. Dis. 2022, 9, 423. [Google Scholar] [CrossRef] [PubMed]

| Doxorubicin | Epirubicin | Daunorubicin | Idarubicin | Mitoxantrone | |
|---|---|---|---|---|---|
| CVD dose ratio | 1 | 0.8 | 0.6 | NE | 10.5 |
| Drug | Molecule | Clinical Trial Phase | Target/Effect | Ref. |
|---|---|---|---|---|
| Aldoxorubicin | Hydrazone derivative of doxorubicin | III | Relapsed/refractory, soft-tissue sarcoma; The results of the phase III study did not demonstrate a beneficial effect on median progression-free survival or median overall survival in the entire patient population. | [68] |
| Camsirubicin | 13-deoxy-5-imino analogue of doxorubicin | II | Soft-tissue sarcoma; Significant myelosuppression | [69] |
| Annamycin | Iodine sugar derivative and liposomal formulation | I/II | Soft-tissue sarcoma, acute myelogenous leukemia, pancreatin carcinoma; 30-fold enrichment in lungs targeting; no cardiotoxicity (FDA-certified) | [69] |
| DTS-201 | tetrapeptide pro-drug | I | Solid tumors | [69] |
| Compound | Model | Result | Mechanism | References |
|---|---|---|---|---|
| Eugenol | Sprague Dawley rats | Improved myocardial injury and protected heart function. | Decreased myocardial oxidative stress and Ca2+ accumulation. | [94] |
| 6-gingerol | Albino rats | Improved myocardial injury. | Decreased myocardial oxidative stress and inhibited apoptosis. | [95] |
| Hesperetin | Wistar rats | Improved myocardial injury and apoptosis. | Decreased myocardial oxidative stress and DNA damage. | [96] |
| Isorhamnetin | Sprague Dawley rats H9c2 cells | Improved myocardial injury and histopathological damage. | Decreased myocardial oxidative stress and inhibited mitochondrial dysfunction-mediated cardiomyocyte apoptosis. | [97] |
| Kaempferol | Sprague Dawley rats H9c2 cells | Improved myocardial injury, improved body weight, heart weight, survival rate and cardiac function. | Inhibited activation of p53-mediated, mitochondrion-dependent apoptotic pathway. | [98] |
| Salvianolic acids | Kunming mice | Improved myocardial injury and histopathological damage, protected heart function. | Decreased myocardial oxidative stress. | [99] |
| Silybinin | Wistar rats microsomes and mitochondria | Improved myocardial, liver injury, histopathological and heart membrane damage, protected heart and liver function. | Not mentioned. | [100] |
| Silychristin | Wistar rats microsomes and mitochondria | Improved heart membrane damage. | Decreased myocardial oxidative stress. | [100] |
| Silydianin | Wistar rats microsomes and mitochondria | Improved heart membrane damage. | Decreased myocardial oxidative stress. | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szponar, J.; Niziński, P.; Dudka, J.; Kasprzak-Drozd, K.; Oniszczuk, A. Natural Products for Preventing and Managing Anthracycline-Induced Cardiotoxicity: A Comprehensive Review. Cells 2024, 13, 1151. https://doi.org/10.3390/cells13131151
Szponar J, Niziński P, Dudka J, Kasprzak-Drozd K, Oniszczuk A. Natural Products for Preventing and Managing Anthracycline-Induced Cardiotoxicity: A Comprehensive Review. Cells. 2024; 13(13):1151. https://doi.org/10.3390/cells13131151
Chicago/Turabian StyleSzponar, Jarosław, Przemysław Niziński, Jarosław Dudka, Kamila Kasprzak-Drozd, and Anna Oniszczuk. 2024. "Natural Products for Preventing and Managing Anthracycline-Induced Cardiotoxicity: A Comprehensive Review" Cells 13, no. 13: 1151. https://doi.org/10.3390/cells13131151
APA StyleSzponar, J., Niziński, P., Dudka, J., Kasprzak-Drozd, K., & Oniszczuk, A. (2024). Natural Products for Preventing and Managing Anthracycline-Induced Cardiotoxicity: A Comprehensive Review. Cells, 13(13), 1151. https://doi.org/10.3390/cells13131151











