Targeting Hdm2 and Hdm4 in Anticancer Drug Discovery: Implications for Checkpoint Inhibitor Immunotherapy
Abstract
1. Introduction
2. The Genomic Structure of Hdm2, Hdm4, and p53
3. Structural Features of the Proteins and Mechanisms of Interaction
4. Comparative Evaluation of the Chemical Classes
| Class | Drug Candidates | MoA | Comments | References |
|---|---|---|---|---|
| Imidazolidines/pyrrolidines | Nutlin3a (idasanutlin), also known as RG7388, RG7112 | Mimic p53 Box1 peptide | Advanced to Phase III. Enhanced potency, selectivity, and bioavailability. Solid and hematological tumors. Nutlin3a is a poor antagonist of Hdm4. Severe side effects led to the abandonment of Phase III clinical trials due to futility. | [25,29,33,51,52,53] |
| WK298 * (also known as Novartis-101) | Inhibits Hdm4-p53 interaction. Binds both Hdm2 and Hdm4 | Hdm4 inhibitor. | [54,55] | |
| Pyrazolylidine | SJ-172550 * | Prevents the formation of p53-Hdm4 complex by changing the conformation of Hdm4 | Pre-clinical. Chemically and thermally stable. Needs further optimization. | [22,36] |
| Spiro-oxindoles | MI-63 | Poor PK and poor oral bioavailability. | [54] | |
| MI-219 | Poor PK and improved oral bioavailability and minimal toxicity in mice. | [54] | ||
| MI-147 | Highly potent, highly desirable PK, better oral bioavailability than MI-219, and low toxicity. | [56] | ||
| MI-188 | Highly potent with excellent oral PK profile and bioavailability, efficacious with minimal toxicity. | [56] | ||
| MI-1061 | Good oral bioavailability and chemical stability in mice. | [57] | ||
| Piperidinone | AMG-232 (navtemadlin) | Inhibits p53-Hdm2 interaction | Phase I orally bioavailable. | [58,59] |
| Stapled peptides (peptidomimetic) | ALRN-6924 * | Hdm2. Mimics the a-helical peptide N-terminal peptide of p53 and binds with high affinity to Mdm2 and Mdm4 | Phase I solid and hematological cancers. | [60] |
| Benzodiazepines | TDP521252 | Inhibit p53-Hdm2 complex | [61] | |
| TDP665759 | Inhibit p53-Hdm2 complex | [61] | ||
| New drugs Azetidine-2-one | ↓ Ezetimibe | Inhibits p53-Hdm2 | Pre-clinical. | [27,28] |
| Spiro-oxindole | APG-115 ( alrizomadlin) | [62,63,64] | ||
| SAR405838 (MI-773) | Phase I. Stable with favorable PK in mice, rats, and dogs. |
5. The Potential p53 Influence on Checkpoint Inhibitors
6. Challenges and Future Directions
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cressey, R.; Pimpa, S.; Tontrong, W.; Watananupong, O.; Leartprasertsuke, N. Expression of Cyclooxygenase-2 in Colorectal Adenocarcinoma Is Associated with P53 Accumulation and Hdm2 Overexpression. Cancer Lett. 2006, 233, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Shiina, H.; Igawa, M.; Shigeno, K.; Yamasaki, Y.; Urakami, S.; Yoneda, T.; Wada, Y.; Honda, S.; Nagasaki, M. Clinical Significance of Mdm2 and P53 Expression in Bladder Cancer. Oncology 1999, 56, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Momand, J.; David, J.; Wilczynski, W.; Niland, J. The MDM2 Gene Amplification Database. Nucleic Acids Res. 1998, 26, 3453–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, Z.; Li, Y.; Gong, Y.; Lyu, X.; Lou, J.; Zhang, D.; Meng, X.; Zhao, Y. Structure-Based Discovery of MDM2/4 Dual Inhibitors That Exert Antitumor Activities against MDM4-Overexpressing Cancer Cells. J. Med. Chem. 2022, 65, 6207–6230. [Google Scholar] [CrossRef] [PubMed]
- Pant, V.; Larsson, C.A.; Aryal, N.; Xiong, S.; You, M.J.; Quintas-Cardama, A.; Lozano, G. Tumorigenesis Promotes Mdm4-S Overexpression. Oncotarget 2017, 8, 25837–25847. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.R.; Peng, B.; Cherry, J.C.; Hurt, E.M.; Fox, S.D.; Kelley, J.A.; Munroe, D.J.; Farrar, W.L. Interleukin 6 Supports the Maintenance of P53 Tumor Suppressor Gene Promoter Methylation. Cancer Res. 2005, 65, 4673–4682. [Google Scholar] [CrossRef] [PubMed]
- Singavi, A.K.; Menon, S.; Kilari, D.; Alqwasmi, A.; Ritch, P.S.; Thomas, J.P.; Martin, A.L.; Oxencis, C.; Ali, S.; George, B. Predictive Biomarkers for Hyper-Progression (HP) in Response to Immune Checkpoint Inhibitors (ICI)—Analysis of Somatic Alterations (SAs). Ann. Oncol. 2017, 28, v405. [Google Scholar] [CrossRef]
- Oliner, J.D.; Kinzler, K.W.; Meltzer, P.S.; George, D.L.; Vogelstein, B. Amplification of a Gene Encoding a P53-Associated Protein in Human Sarcomas. Nature 1992, 358, 80–83. [Google Scholar] [CrossRef]
- Mendoza, M.; Mandani, G.; Momand, J. The MDM2 Gene Family. Biomol. Concepts 2014, 5, 9–19. [Google Scholar] [CrossRef]
- Stad, R.; Little, N.A.; Xirodimas, D.P.; Frenk, R.; van der Eb, A.J.; Lane, D.P.; Saville, M.K.; Jochemsen, A.G. Mdmx Stabilizes P53 and Mdm2 via Two Distinct Mechanisms. EMBO Rep. 2001, 2, 1029–1034. [Google Scholar] [CrossRef]
- Sharp, D.A.; Kratowicz, S.A.; Sank, M.J.; George, D.L. Stabilization of the MDM2 Oncoprotein by Interaction with the Structurally Related MDMX Protein. J. Biol. Chem. 1999, 274, 38189–38196. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; de Queiroz, R.M.; Venkatesh, D.; Prives, C. The Roles and Regulation of MDM2 and MDMX: It Is Not Just about P53. Genes. Dev. 2021, 35, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Parant, J.M.; Reinke, V.; Mims, B.; Lozano, G. Organization, Expression, and Localization of the Murine Mdmx Gene and Pseudogene. Gene 2001, 270, 277–283. [Google Scholar] [CrossRef]
- Shvarts, A.; Steegenga, W.T.; Riteco, N.; van Laar, T.; Dekker, P.; Bazuine, M.; van Ham, R.C.; van der Houven van Oordt, W.; Hateboer, G.; van der Eb, A.J.; et al. MDMX: A Novel P53-Binding Protein with Some Functional Properties of MDM2. EMBO J. 1996, 15, 5349–5357. [Google Scholar] [CrossRef] [PubMed]
- Biderman, L.; Poyurovsky, M.V.; Assia, Y.; Manley, J.L.; Prives, C. MdmX Is Required for P53 Interaction with and Full Induction of the Mdm2 Promoter after Cellular Stress. Mol. Cell Biol. 2012, 32, 1214–1225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shadfan, M.; Lopez-Pajares, V.; Yuan, Z.-M. MDM2 and MDMX: Alone and Together in Regulation of P53. Transl. Cancer Res. 2012, 1, 88–89. [Google Scholar] [PubMed]
- Tackmann, N.R.; Zhang, Y. Mouse Modelling of the MDM2/MDMX−p53 Signalling Axis. J. Mol. Cell Biol. 2017, 9, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Marine, J.-C.; Francoz, S.; Maetens, M.; Wahl, G.; Toledo, F.; Lozano, G. Keeping P53 in Check: Essential and Synergistic Functions of Mdm2 and Mdm4. Cell Death Differ. 2006, 13, 927–934. [Google Scholar] [CrossRef]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the P53 Network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Jiang, L.; Zawacka-Pankau, J. The P53/MDM2/MDMX-Targeted Therapies—A Clinical Synopsis. Cell Death Dis. 2020, 11, 237. [Google Scholar] [CrossRef]
- Toledo, F.; Wahl, G.M. MDM2 and MDM4: P53 Regulators as Targets in Anticancer Therapy. Int. J. Biochem. Cell Biol. 2007, 39, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Bista, M.; Smithson, D.; Pecak, A.; Salinas, G.; Pustelny, K.; Min, J.; Pirog, A.; Finch, K.; Zdzalik, M.; Waddell, B.; et al. On the Mechanism of Action of SJ-172550 in Inhibiting the Interaction of MDM4 and P53. PLoS ONE 2012, 7, e37518. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, M.M.J.; Law, F.V.; Chow, W.A.; Kaiser, P. Small-Molecule Correctors and Stabilizers to Target P53. Trends Pharmacol. Sci. 2023, 44, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Wong, E.T.; Tang, M.; Stommel, J.M.; Wahl, G.M. Hdmx Modulates the Outcome of P53 Activation in Human Tumor Cells. J. Biol. Chem. 2006, 281, 33036–33044. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Gilkes, D.M.; Farooqi, B.; Sebti, S.M.; Chen, J. MDMX Overexpression Prevents P53 Activation by the MDM2 Inhibitor Nutlin. J. Biol. Chem. 2006, 281, 33030–33035. [Google Scholar] [CrossRef] [PubMed]
- Perdrix, A.; Najem, A.; Saussez, S.; Awada, A.; Journe, F.; Ghanem, G.; Krayem, M. PRIMA-1 and PRIMA-1Met (APR-246): From Mutant/Wild Type P53 Reactivation to Unexpected Mechanisms Underlying Their Potent Anti-Tumor Effect in Combinatorial Therapies. Cancers 2017, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Ntwasa, M. Ezetimibe and Curcumin for Use in Cancer Treatment. US20220387383A1, 8 December 2022. [Google Scholar]
- Ntwasa, M.; Twala, C. Modified Ezetimibe Drug for Cancer Treatment. US20230295082A1, 21 September 2023. [Google Scholar]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In Vivo Activation of the P53 Pathway by Small-Molecule Antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Vu, B.; Wovkulich, P.; Pizzolato, G.; Lovey, A.; Ding, Q.; Jiang, N.; Liu, J.-J.; Zhao, C.; Glenn, K.; Wen, Y.; et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med. Chem. Lett. 2013, 4, 466–469. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.-E. Small-Molecule MDM2 Inhibitors in Clinical Trials for Cancer Therapy. Eur. J. Med. Chem. 2022, 236, 114334. [Google Scholar] [CrossRef]
- Andreeff, M.; Kelly, K.R.; Yee, K.; Assouline, S.; Strair, R.; Popplewell, L.; Bowen, D.; Martinelli, G.; Drummond, M.W.; Vyas, P.; et al. Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia. Clin. Cancer Res. 2016, 22, 868–876. [Google Scholar] [CrossRef]
- Konopleva, M.Y.; Röllig, C.; Cavenagh, J.; Deeren, D.; Girshova, L.; Krauter, J.; Martinelli, G.; Montesinos, P.; Schäfer, J.A.; Ottmann, O.G.; et al. Idasanutlin Plus Cytarabine in Relapsed or Refractory Acute Myeloid Leukemia: Results of the MIRROS Trial. Blood Adv. 2022, 6, 4147–4156. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, P.; Beckermann, B.M.; Catalani, O.; Esteve, J.; Gamel, K.; Konopleva, M.Y.; Martinelli, G.; Monnet, A.; Papayannidis, C.; Park, A.; et al. MIRROS: A Randomized, Placebo-Controlled, Phase III Trial of Cytarabine ± Idasanutlin in Relapsed or Refractory Acute Myeloid Leukemia. Future Oncol. 2020, 16, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, Y.; He, Y.; Liu, Y.; Guo, Z.; He, J.; Han, X.; Hu, Y.; Li, M.; Jiang, R.; et al. Discovery of Novel P53-MDM2 Inhibitor (RG7388)-Conjugated Platinum IV Complexes as Potent Antitumor Agents. J. Med. Chem. 2024, 67, 9645–9661. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.; Shen, Y.; Shelat, A.A.; Arnold, L.A.; Ferreira, A.M.; Zhu, F.; Mills, N.; Smithson, D.C.; Regni, C.A.; Bashford, D.; et al. Identification and Characterization of the First Small Molecule Inhibitor of MDMX. J. Biol. Chem. 2010, 285, 10786–10796. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, A.; Cho, S.; Jun, Y.; Na, D.; Lee, A.; Jang, G.; Kwon, J.Y.; Kim, J.; Lee, S.; et al. Genome-scale CRISPR Screening Identifies Cell Cycle and Protein Ubiquitination Processes as Druggable Targets for Erlotinib-resistant Lung Cancer. Mol. Oncol. 2021, 15, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liao, G.; Yu, B. Small-Molecule MDM2/X Inhibitors and PROTAC Degraders for Cancer Therapy: Advances and Perspectives. Acta Pharm. Sin. B 2020, 10, 1253–1278. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Wang, S. Therapeutic Strategies to Activate P53. Pharmaceuticals 2022, 16, 24. [Google Scholar] [CrossRef]
- Kallen, J.; Izaac, A.; Chau, S.; Wirth, E.; Schoepfer, J.; Mah, R.; Schlapbach, A.; Stutz, S.; Vaupel, A.; Guagnano, V.; et al. Structural States of Hdm2 and HdmX: X-ray Elucidation of Adaptations and Binding Interactions for Different Chemical Compound Classes. ChemMedChem 2019, 14, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lou, J.; Li, Y.; Zhou, F.; Yan, Z.; Lyu, X.; Zhao, Y. Recent Progress and Clinical Development of Inhibitors That Block MDM4/P53 Protein–Protein Interactions. J. Med. Chem. 2021, 64, 10621–10640. [Google Scholar] [CrossRef]
- Essmann, F.; Schulze-Osthoff, K. Translational Approaches Targeting the P53 Pathway for Anti-cancer Therapy. Br. J. Pharmacol. 2012, 165, 328–344. [Google Scholar] [CrossRef]
- Canner, J.A.; Sobo, M.; Ball, S.; Hutzen, B.; DeAngelis, S.; Willis, W.; Studebaker, A.W.; Ding, K.; Wang, S.; Yang, D.; et al. MI-63: A Novel Small-Molecule Inhibitor Targets MDM2 and Induces Apoptosis in Embryonal and Alveolar Rhabdomyosarcoma Cells with Wild-Type P53. Br. J. Cancer 2009, 101, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; King, K.; Qiu, S.; Liu, M.; Nikolovska-Coleska, Z.; McEachern, D.; Miller, R.; Lu, Y.; Yu, S.; Wang, G.; et al. Preclinical Characterization of MI-219, A Novel, Potent and Orally Active Small Molecule Inhibitor of the MDM2-P53 Interaction. Cancer Res. 2007, 67, LB-365. [Google Scholar]
- Sosin, A.M.; Burger, A.M.; Siddiqi, A.; Abrams, J.; Mohammad, R.M.; Al-Katib, A.M. HDM2 Antagonist MI-219 (Spiro-Oxindole), but Not Nutlin-3 (Cis-Imidazoline), Regulates P53 through Enhanced HDM2 Autoubiquitination and Degradation in Human Malignant B-Cell Lymphomas. J. Hematol. Oncol. 2012, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, W.; Zhao, Y.; McEachern, D.; Meaux, I.; Barrière, C.; Stuckey, J.A.; Meagher, J.L.; Bai, L.; Liu, L.; et al. SAR405838: An Optimized Inhibitor of MDM2–P53 Interaction That Induces Complete and Durable Tumor Regression. Cancer Res. 2014, 74, 5855–5865. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, M.; de Weger, V.A.; Dickson, M.A.; Langenberg, M.; Le Cesne, A.; Wagner, A.J.; Hsu, K.; Zheng, W.; Macé, S.; Tuffal, G.; et al. A Phase I Study of SAR405838, a Novel Human Double Minute 2 (HDM2) Antagonist, in Patients with Solid Tumours. Eur. J. Cancer 2017, 76, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ma, D.J.; Calligaris, D.; Zhang, S.; Feathers, R.W.; Vaubel, R.A.; Meaux, I.; Mladek, A.C.; Parrish, K.E.; Jin, F.; et al. Efficacy of the MDM2 Inhibitor SAR405838 in Glioblastoma Is Limited by Poor Distribution Across the Blood–Brain Barrier. Mol. Cancer Ther. 2018, 17, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- de Weger, V.A.; de Jonge, M.; Langenberg, M.H.G.; Schellens, J.H.M.; Lolkema, M.; Varga, A.; Demers, B.; Thomas, K.; Hsu, K.; Tuffal, G.; et al. A Phase I Study of the HDM2 Antagonist SAR405838 Combined with the MEK Inhibitor Pimasertib in Patients with Advanced Solid Tumours. Br. J. Cancer 2019, 120, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Bill, K.L.J.; Garnett, J.; Meaux, I.; Ma, X.; Creighton, C.J.; Bolshakov, S.; Barriere, C.; Debussche, L.; Lazar, A.J.; Prudner, B.C.; et al. SAR405838: A Novel and Potent Inhibitor of the MDM2:P53 Axis for the Treatment of Dedifferentiated Liposarcoma. Clin. Cancer Res. 2016, 22, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Ntwasa, M. Drugs That Target P53-Mdm2 Interaction. Biomed. J. Sci. Tech. Res. 2021, 37. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Liu, J.-J.; Jiang, N.; Zhang, J.; Ross, T.M.; Chu, X.-J.; Bartkovitz, D.; Podlaski, F.; Janson, C.; et al. Discovery of RG7388, a Potent and Selective P53–MDM2 Inhibitor in Clinical Development. J. Med. Chem. 2013, 56, 5979–5983. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Q.; Li, Z.; Zhang, H. MDM2: Current Research Status and Prospects of Tumor Treatment. Cancer Cell Int. 2024, 24, 170. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, G.M.; Czarna, A.; Wolf, S.; Wang, K.; Wang, W.; Dömling, A.; Holak, T.A. Structures of Low Molecular Weight Inhibitors Bound to MDMX and MDM2 Reveal New Approaches for P53-MDMX/MDM2 Antagonist Drug Discovery. Cell Cycle 2010, 9, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Ortiz, N.; Neochoritis, C.G.; Dömling, A. How To Design a Successful P53–MDM2/X Interaction Inhibitor: A Thorough Overview Based on Crystal Structures. ChemMedChem 2016, 11, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising Scaffolds for Anticancer Agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Albadari, N.; Du, Y.; Fowler, J.F.; Sang, H.T.; Xian, W.; McKeon, F.; Li, W.; Zhou, J.; Zhang, R. MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future. Pharmacol. Rev. 2024, 76, 414–453. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, Z.; Rew, Y.; Gribble, M.; Bartberger, M.D.; Beck, H.P.; Canon, J.; Chen, A.; Chen, X.; Chow, D.; et al. Discovery of AMG 232, a Potent, Selective, and Orally Bioavailable MDM2–P53 Inhibitor in Clinical Development. J. Med. Chem. 2014, 57, 1454–1472. [Google Scholar] [CrossRef] [PubMed]
- Canon, J.; Osgood, T.; Olson, S.H.; Saiki, A.Y.; Robertson, R.; Yu, D.; Eksterowicz, J.; Ye, Q.; Jin, L.; Chen, A.; et al. The MDM2 Inhibitor AMG 232 Demonstrates Robust Antitumor Efficacy and Potentiates the Activity of P53-Inducing Cytotoxic Agents. Mol. Cancer Ther. 2015, 14, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.A.; Neriah, D.B.; Senecal, A.; Benard, L.; Thiruthuvanathan, V.; Yatsenko, T.; Narayanagari, S.-R.; Wheat, J.C.; Todorova, T.I.; Mitchell, K.; et al. Dual Inhibition of MDMX and MDM2 as a Therapeutic Strategy in Leukemia. Sci. Transl. Med. 2018, 10, eaao3003. [Google Scholar] [CrossRef]
- Koblish, H.K.; Zhao, S.; Franks, C.F.; Donatelli, R.R.; Tominovich, R.M.; LaFrance, L.V.; Leonard, K.A.; Gushue, J.M.; Parks, D.J.; Calvo, R.R.; et al. Benzodiazepinedione Inhibitors of the Hdm2:P53 Complex Suppress Human Tumor Cell Proliferation in Vitro and Sensitize Tumors to Doxorubicin in Vivo. Mol. Cancer Ther. 2006, 5, 160–169. [Google Scholar] [CrossRef]
- Aguilar, A.; Lu, J.; Liu, L.; Du, D.; Bernard, D.; McEachern, D.; Przybranowski, S.; Li, X.; Luo, R.; Wen, B.; et al. Discovery of 4-((3′ R, 4′ S, 5′ R)-6″-Chloro-4′-(3-Chloro-2-Fluorophenyl)-1′-Ethyl-2″-Oxodispiro [Cyclohexane-1,2′-Pyrrolidine-3′,3″-Indoline]-5′-Carboxamido)Bicyclo [2.2.2]Octane-1-Carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J. Med. Chem. 2017, 60, 2819–2839. [Google Scholar] [CrossRef]
- Tang, Q.; Fang, D.D.; Yu, H.; Wu, B.; Yin, Y.; Yang, D.; Zhai, Y. Abstract 2998: Inhibition of MDM2-P53 Interaction by Alrizomadlin (APG-115) Induces Pyroptotic Cell Death in Gasdermin E (GSDME)-Expressing Cancer Cells. Cancer Res 2022, 82, 2998. [Google Scholar] [CrossRef]
- Fang, D.D.; Tang, Q.; Kong, Y.; Rong, T.; Wang, Q.; Li, N.; Fang, X.; Gu, J.; Xiong, D.; Yin, Y.; et al. MDM2 Inhibitor APG-115 Exerts Potent Antitumor Activity and Synergizes with Standard-of-Care Agents in Preclinical Acute Myeloid Leukemia Models. Cell Death Discov. 2021, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Xiang, C.; Zhang, C.-X.; Xie, Y.-Y.; Chen, L.; Zhang, G.-X.; Lu, Y.; Liu, G. P53 in the Myeloid Lineage Modulates an Inflammatory Microenvironment Limiting Initiation and Invasion of Intestinal Tumors. Cell Rep. 2015, 13, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ng, D.S.W.; Mah, W.-C.; Almeida, F.F.; Rahmat, S.A.; Rao, V.K.; Leow, S.C.; Laudisi, F.; Peh, M.T.; Goh, A.M.; et al. A Unique Role for P53 in the Regulation of M2 Macrophage Polarization. Cell Death Differ. 2015, 22, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Lujambio, A.; Akkari, L.; Simon, J.; Grace, D.; Tschaharganeh, D.F.; Bolden, J.E.; Zhao, Z.; Thapar, V.; Joyce, J.A.; Krizhanovsky, V.; et al. Non-Cell-Autonomous Tumor Suppression by P53. Cell 2013, 153, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Luster, A.D. Chemokines in Cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Richmond, A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Uehara, I.; Tanaka, N. Role of P53 in the Regulation of the Inflammatory Tumor Microenvironment and Tumor Suppression. Cancers 2018, 10, 219. [Google Scholar] [CrossRef]
- Blagih, J.; Buck, M.D.; Vousden, K.H. P53, Cancer and the Immune Response. J. Cell Sci. 2020, 133, jcs237453. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Mañes, S.; Mira, E.; Colomer, R.; Montero, S.; Real, L.M.; Gómez-Moutón, C.; Jiménez-Baranda, S.; Garzón, A.; Lacalle, R.A.; Harshman, K.; et al. CCR5 Expression Influences the Progression of Human Breast Cancer in a P53-Dependent Manner. J. Exp. Med. 2003, 198, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Fingerle-Rowson, G.; Petrenko, O.; Metz, C.N.; Forsthuber, T.G.; Mitchell, R.; Huss, R.; Moll, U.; Müller, W.; Bucala, R. The P53-Dependent Effects of Macrophage Migration Inhibitory Factor Revealed by Gene Targeting. Proc. Natl. Acad. Sci. USA 2003, 100, 9354–9359. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Reeves, J.A.; McKean, M.; Chmielowski, B.; Beck, J.T.; Shaheen, M.F.; Somaiah, N.; Wilson, M.; Spira, A.I.; Drabick, J.J.; et al. Preliminary Results of a Phase II Study of Alrizomadlin (APG-115), a Novel, Small-Molecule MDM2 Inhibitor, in Combination with Pembrolizumab in Patients (Pts) with Unresectable or Metastatic Melanoma or Advanced Solid Tumors That Have Failed Immuno-Oncologic (I-O) Drugs. J. Clin. Oncol. 2021, 39, 2506. [Google Scholar] [CrossRef]
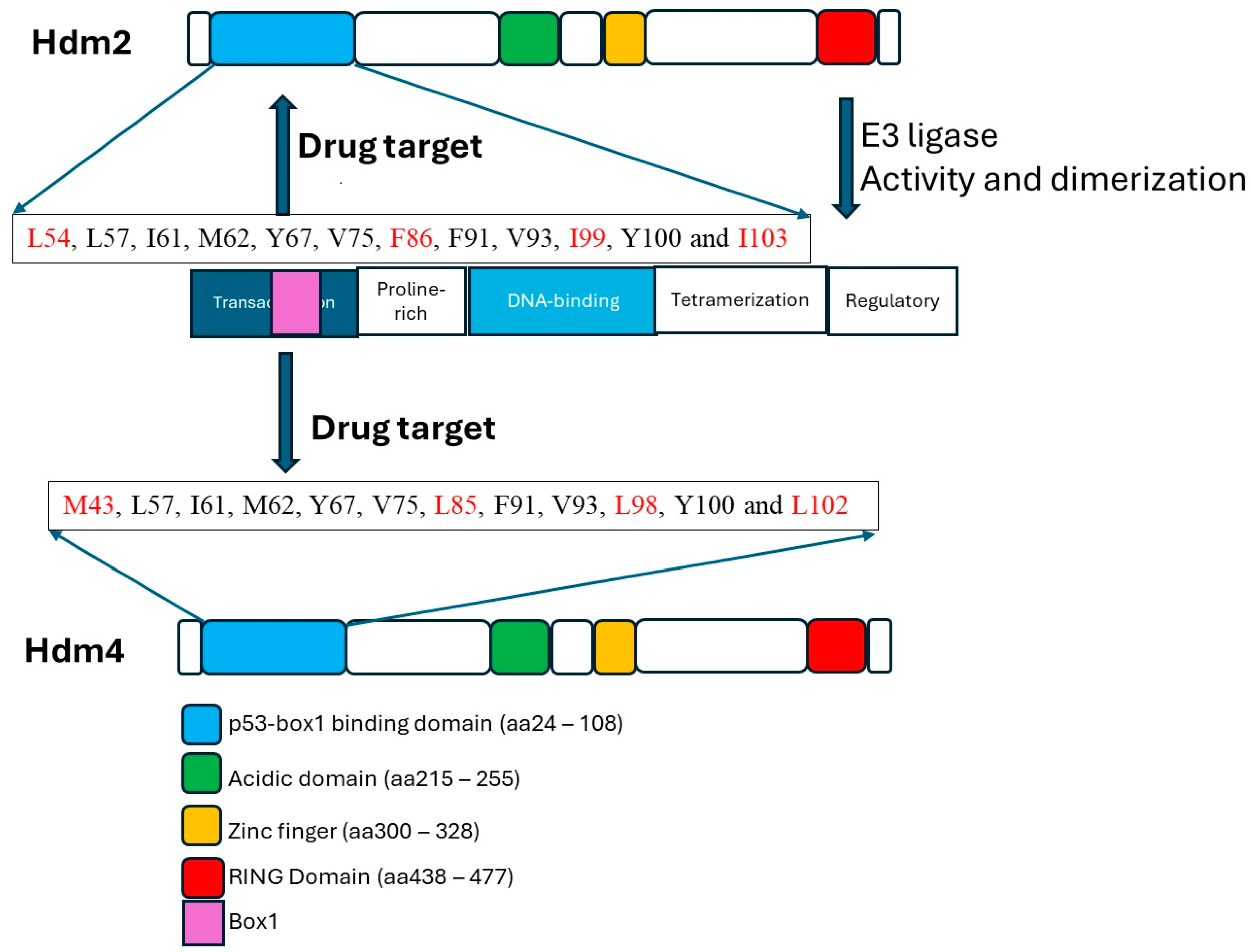
| Hdm2 Residues | Hdm4 | p53 Transactivation Domain Residues | Comments |
|---|---|---|---|
| L54 ** | M43 | F19 * (binding pocket) | L54 forms hydrogen bond with p53 W23 |
| L57 | + | W23 ** (binding pocket) | |
| I61 | + | N29 *** | |
| M62 | + | L26 (binding pocket) | |
| Y67 | + | ||
| Q72 * | + | Forms hydrogen bond with p53 F19 | |
| V75 | + | ||
| F86 | L85 | ||
| F91 | + | ||
| V93 | + | ||
| I99 | L98 | ||
| Y100 *** | + | Forms hydrogen bond with p53 N29 | |
| I103 | L102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntwasa, M. Targeting Hdm2 and Hdm4 in Anticancer Drug Discovery: Implications for Checkpoint Inhibitor Immunotherapy. Cells 2024, 13, 1124. https://doi.org/10.3390/cells13131124
Ntwasa M. Targeting Hdm2 and Hdm4 in Anticancer Drug Discovery: Implications for Checkpoint Inhibitor Immunotherapy. Cells. 2024; 13(13):1124. https://doi.org/10.3390/cells13131124
Chicago/Turabian StyleNtwasa, Monde. 2024. "Targeting Hdm2 and Hdm4 in Anticancer Drug Discovery: Implications for Checkpoint Inhibitor Immunotherapy" Cells 13, no. 13: 1124. https://doi.org/10.3390/cells13131124
APA StyleNtwasa, M. (2024). Targeting Hdm2 and Hdm4 in Anticancer Drug Discovery: Implications for Checkpoint Inhibitor Immunotherapy. Cells, 13(13), 1124. https://doi.org/10.3390/cells13131124







