H3K27-Altered Diffuse Midline Glioma of the Brainstem: From Molecular Mechanisms to Targeted Interventions
Abstract
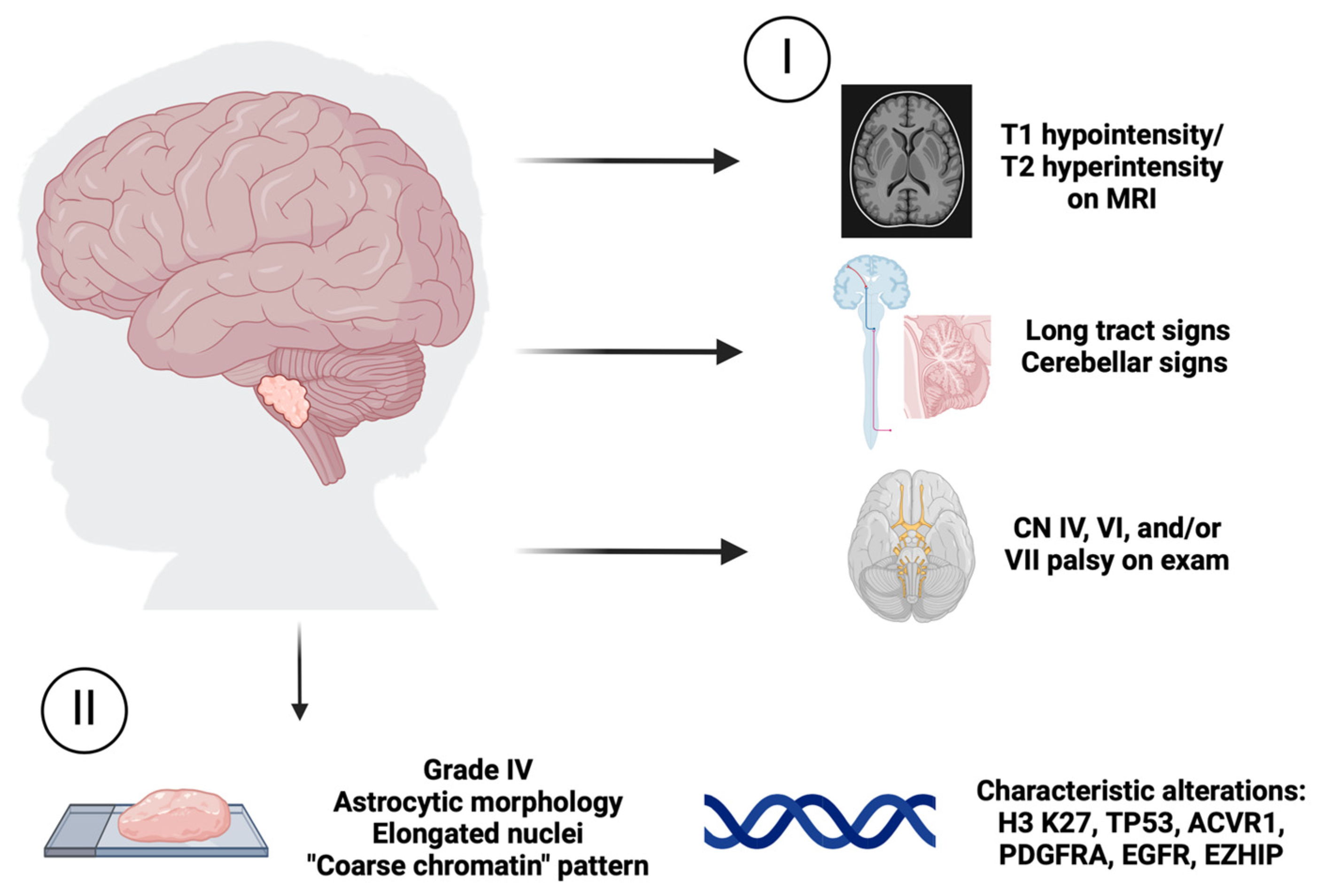
1. Introduction
2. Mechanisms Driving Tumorigenesis of H3 K27-Altered DMG
3. Treatment Strategies in H3 K27-Altered DMG: Translational Advances and Challenges
4. Beyond Chemotherapy—Targeted Therapy in H3 K27-Altered DMG
4.1. Pathway Inhibitors
4.2. ONC201
4.3. Immunotherapies
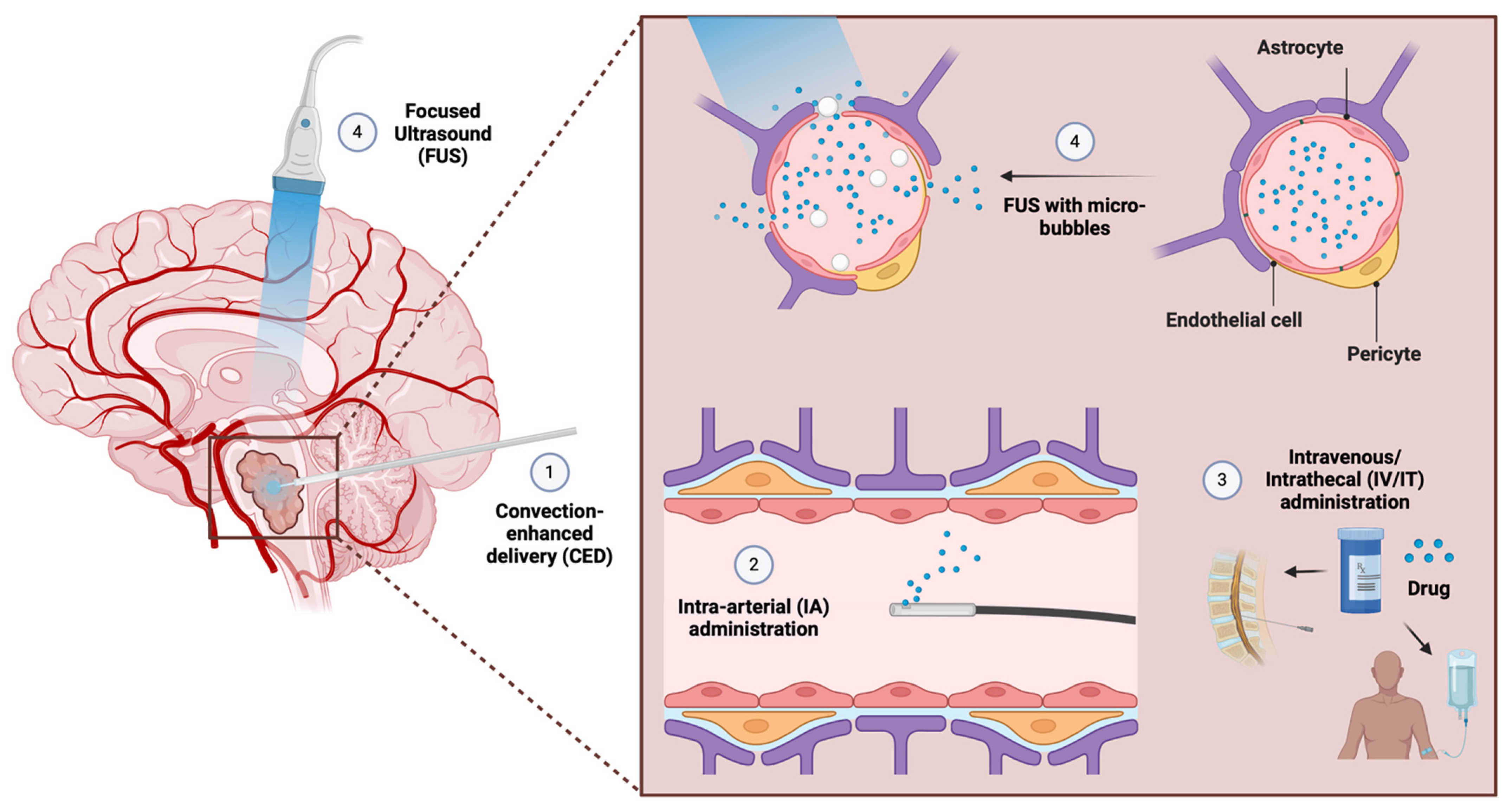
5. Improving Intratumoral Drug Delivery in H3 K27-Altered DMG
5.1. Targeted Drug Delivery Systems
5.2. Nanoparticles
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Ryan, K.; Edelson, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2022, 24, iii1–iii38. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Johnson, K.J.; Cullen, J.; Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Langer, C.E.; Turner, M.C.; McKean-Cowdin, R.; Fisher, J.L.; Lupo, P.J.; Partap, S.; et al. Childhood Brain Tumor Epidemiology: A Brain Tumor Epidemiology Consortium Review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2716–2736. [Google Scholar] [CrossRef]
- Patil, N.; Kelly, M.E.; Yeboa, D.N.; Buerki, R.A.; Cioffi, G.; Balaji, S.; Ostrom, Q.T.; Kruchko, C.; Barnholtz-Sloan, J.S. Epidemiology of brainstem high-grade gliomas in children and adolescents in the United States, 2000–2017. Neuro-Oncology 2020, 23, 990–998. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Mendez, F.M.; Núñez, F.J.; Garcia-Fabiani, M.B.; Haase, S.; Carney, S.; Gauss, J.C.; Becher, O.J.; Lowenstein, P.R.; Castro, M.G. Epigenetic reprogramming and chromatin accessibility in pediatric diffuse intrinsic pontine gliomas: A neural developmental disease. Neuro-Oncology 2019, 22, 195–206. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Veldhuijzen van Zanten, S.E.M.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C.; et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963–1972. [Google Scholar] [CrossRef]
- Dmetrichuk, J.M.; Pendleton, C.; Jallo, G.I.; Quiñones-Hinojosa, A. Father of neurosurgery: Harvey Cushing’s early experience with a pediatric brainstem glioma at the Johns Hopkins Hospital: Historical vignette. J. Neurosurg. Pediatr. PED 2011, 8, 337–341. [Google Scholar] [CrossRef]
- Tosi, U.; Souweidane, M. Fifty years of DIPG: Looking at the future with hope. Childs Nerv. Syst. 2023, 39, 2675–2686. [Google Scholar] [CrossRef]
- Gwak, H.-S.; Park, H.J. Developing chemotherapy for diffuse pontine intrinsic gliomas (DIPG). Crit. Rev. Oncol./Hematol. 2017, 120, 111–119. [Google Scholar] [CrossRef]
- Bailey, S.; Howman, A.; Wheatley, K.; Wherton, D.; Boota, N.; Pizer, B.; Fisher, D.; Kearns, P.; Picton, S.; Saran, F.; et al. Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy—Results of a United Kingdom phase II trial (CNS 2007 04). Eur. J. Cancer 2013, 49, 3856–3862. [Google Scholar] [CrossRef]
- Puget, S.; Beccaria, K.; Blauwblomme, T.; Roujeau, T.; James, S.; Grill, J.; Zerah, M.; Varlet, P.; Sainte-Rose, C. Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Child’s Nerv. Syst. 2015, 31, 1773–1780. [Google Scholar] [CrossRef]
- Pfaff, E.; El Damaty, A.; Balasubramanian, G.P.; Blattner-Johnson, M.; Worst, B.C.; Stark, S.; Witt, H.; Pajtler, K.W.; van Tilburg, C.M.; Witt, R.; et al. Brainstem biopsy in pediatric diffuse intrinsic pontine glioma in the era of precision medicine: The INFORM study experience. Eur. J. Cancer 2019, 114, 27–35. [Google Scholar] [CrossRef]
- Liu, I.; Jiang, L.; Samuelsson, E.R.; Marco Salas, S.; Beck, A.; Hack, O.A.; Jeong, D.; Shaw, M.L.; Englinger, B.; LaBelle, J.; et al. The landscape of tumor cell states and spatial organization in H3-K27M mutant diffuse midline glioma across age and location. Nat. Genet. 2022, 54, 1881–1894. [Google Scholar] [CrossRef]
- Tosi, U.; Souweidane, M. Convection Enhanced Delivery for Diffuse Intrinsic Pontine Glioma: Review of a Single Institution Experience. Pharmaceutics 2020, 12, 660. [Google Scholar] [CrossRef]
- Solomon, D.A.; Wood, M.D.; Tihan, T.; Bollen, A.W.; Gupta, N.; Phillips, J.J.; Perry, A. Diffuse Midline Gliomas with Histone H3-K27M Mutation: A Series of 47 Cases Assessing the Spectrum of Morphologic Variation and Associated Genetic Alterations. Brain Pathol. 2016, 26, 569–580. [Google Scholar] [CrossRef]
- Williams, J.R.; Young, C.C.; Vitanza, N.A.; McGrath, M.; Feroze, A.H.; Browd, S.R.; Hauptman, J.S. Progress in diffuse intrinsic pontine glioma: Advocating for stereotactic biopsy in the standard of care. Neurosurg. Focus 2020, 48, E4. [Google Scholar] [CrossRef]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.Y.; Jones, D.T.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.A.; Tönjes, M.; et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Wu, G.; Broniscer, A.; McEachron, T.A.; Lu, C.; Paugh, B.S.; Becksfort, J.; Qu, C.; Ding, L.; Huether, R.; Parker, M.; et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012, 44, 251–253. [Google Scholar] [CrossRef]
- Castel, D.; Philippe, C.; Calmon, R.; Le Dret, L.; Truffaux, N.; Boddaert, N.; Pagès, M.; Taylor, K.R.; Saulnier, P.; Lacroix, L.; et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015, 130, 815–827. [Google Scholar] [CrossRef]
- Chan, K.M.; Fang, D.; Gan, H.; Hashizume, R.; Yu, C.; Schroeder, M.; Gupta, N.; Mueller, S.; James, C.D.; Jenkins, R.; et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013, 27, 985–990. [Google Scholar] [CrossRef]
- Kfoury-Beaumont, N.; Prakasam, R.; Pondugula, S.; Lagas, J.S.; Matkovich, S.; Gontarz, P.; Yang, L.; Yano, H.; Kim, A.H.; Rubin, J.B.; et al. The H3K27M mutation alters stem cell growth, epigenetic regulation, and differentiation potential. BMC Biol. 2022, 20, 124. [Google Scholar] [CrossRef]
- Bender, S.; Tang, Y.; Lindroth, A.M.; Hovestadt, V.; Jones, D.T.; Kool, M.; Zapatka, M.; Northcott, P.A.; Sturm, D.; Wang, W.; et al. Reduced H3K27me3 and DNA Hypomethylation Are Major Drivers of Gene Expression in K27M Mutant Pediatric High-Grade Gliomas. Cancer Cell 2013, 24, 660–672. [Google Scholar] [CrossRef]
- Herz, H.-M.; Morgan, M.; Gao, X.; Jackson, J.; Rickels, R.; Swanson, S.K.; Florens, L.; Washburn, M.P.; Eissenberg, J.C.; Shilatifard, A. Histone H3 lysine-to-methionine mutants as a paradigm to study chromatin signaling. Science 2014, 345, 1065–1070. [Google Scholar] [CrossRef]
- Paugh, B.S.; Zhu, X.; Qu, C.; Endersby, R.; Diaz, A.K.; Zhang, J.; Bax, D.A.; Carvalho, D.; Reis, R.M.; Onar-Thomas, A.; et al. Novel Oncogenic PDGFRA Mutations in Pediatric High-Grade Gliomas. Cancer Res. 2013, 73, 6219–6229. [Google Scholar] [CrossRef]
- Larson, J.D.; Kasper, L.H.; Paugh, B.S.; Jin, H.; Wu, G.; Kwon, C.-H.; Fan, Y.; Shaw, T.I.; Silveira, A.B.; Qu, C.; et al. Histone H3.3 K27M Accelerates Spontaneous Brainstem Glioma and Drives Restricted Changes in Bivalent Gene Expression. Cancer Cell 2019, 35, 140–155.e147. [Google Scholar] [CrossRef]
- Damodharan, S.; Abbott, A.; Kellar, K.; Zhao, Q.; Dey, M. Molecular Characterization and Treatment Approaches for Pediatric H3 K27-Altered Diffuse Midline Glioma: Integrated Systematic Review of Individual Clinical Trial Participant Data. Cancers 2023, 15, 3478. [Google Scholar] [CrossRef]
- Roberts, H.J.; Ji, S.; Picca, A.; Sanson, M.; Garcia, M.; Snuderl, M.; Schüller, U.; Picart, T.; Ducray, F.; Green, A.L.; et al. Clinical, genomic, and epigenomic analyses of H3K27M-mutant diffuse midline glioma long-term survivors reveal a distinct group of tumors with MAPK pathway alterations. Acta Neuropathol. 2023, 146, 849–852. [Google Scholar] [CrossRef]
- Gianno, F.; Giovannoni, I.; Cafferata, B.; Diomedi-Camassei, F.; Minasi, S.; Barresi, S.; Buttarelli, F.R.; Alesi, V.; Cardoni, A.; Antonelli, M.; et al. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO Classification. Pathologica 2022, 114, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.G.; Tirosh, I.; Hovestadt, V.; Shaw, M.L.; Escalante, L.E.; Mathewson, N.D.; Neftel, C.; Frank, N.; Pelton, K.; Hebert, C.M.; et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science 2018, 360, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Mitra, S.S.; Freret, M.E.; Raveh, T.B.; Kim, J.; Masek, M.; Attema, J.L.; Li, G.; Haddix, T.; Edwards, M.S.B.; et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl. Acad. Sci. USA 2011, 108, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Bandopadhayay, P.; Jenkins, M.R. Towards Immunotherapy for Pediatric Brain Tumors. Trends Immunol. 2019, 40, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Subashi, E.; Cordero, F.J.; Halvorson, K.G.; Qi, Y.; Nouls, J.C.; Becher, O.J.; Allan Johnson, G. Tumor location, but not H3.3K27M, significantly influences the blood–brain-barrier permeability in a genetic mouse model of pediatric high-grade glioma. J. Neuro-Oncol. 2016, 126, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zhou, Z.; Haque, S.; Zanzonico, P.; Carrasquillo, J.A.; Lyashchenko, S.K.; Thakur, S.B.; Donzelli, M.; et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018, 19, 1040–1050. [Google Scholar] [CrossRef]
- Englander, Z.K.; Wei, H.J.; Pouliopoulos, A.N.; Bendau, E.; Upadhyayula, P.; Jan, C.I.; Spinazzi, E.F.; Yoh, N.; Tazhibi, M.; McQuillan, N.M.; et al. Focused ultrasound mediated blood-brain barrier opening is safe and feasible in a murine pontine glioma model. Sci. Rep. 2021, 11, 6521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, D.; Yang, L.; Yue, Y.; Sultan, D.; Pacia, C.P.; Pang, H.; Detering, L.; Heo, G.S.; Luehmann, H.; et al. Magnetic Resonance Imaging-Guided Focused Ultrasound-Based Delivery of Radiolabeled Copper Nanoclusters to Diffuse Intrinsic Pontine Glioma. ACS Appl. Nano Mater. 2020, 3, 11129–11134. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Baker, J.N.; Tagen, M.; Onar-Thomas, A.; Gilbertson, R.J.; Davidoff, A.M.; Pai Panandiker, A.S.; Leung, W.; Chin, T.K.; Stewart, C.F.; et al. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J. Clin. Oncol. 2010, 28, 4762–4768. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Gill, R.; Bull, K.S. Does a Bevacizumab-based regime have a role in the treatment of children with diffuse intrinsic pontine glioma? A systematic review. Neuro-Oncol. Adv. 2022, 4, vdac100. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.R.; Salloum, R.; Drissi, R.; Kumar, S.; Sobo, M.; Goldman, S.; Pai, A.; Leach, J.; Lane, A.; Pruitt, D.; et al. A pilot study of bevacizumab-based therapy in patients with newly diagnosed high-grade gliomas and diffuse intrinsic pontine gliomas. J. Neuro-Oncol. 2016, 127, 53–61. [Google Scholar] [CrossRef]
- Gururangan, S.; Chi, S.N.; Young Poussaint, T.; Onar-Thomas, A.; Gilbertson, R.J.; Vajapeyam, S.; Friedman, H.S.; Packer, R.J.; Rood, B.N.; Boyett, J.M.; et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: A Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 2010, 28, 3069–3075. [Google Scholar] [CrossRef]
- El-Khouly, F.E.; Veldhuijzen van Zanten, S.E.M.; Jansen, M.H.A.; Bakker, D.P.; Sanchez Aliaga, E.; Hendrikse, N.H.; Vandertop, W.P.; van Vuurden, D.G.; Kaspers, G.J.L. A phase I/II study of bevacizumab, irinotecan and erlotinib in children with progressive diffuse intrinsic pontine glioma. J. Neuro-Oncol. 2021, 153, 263–271. [Google Scholar] [CrossRef]
- McCrea, H.J.; Ivanidze, J.; O’Connor, A.; Hersh, E.H.; Boockvar, J.A.; Gobin, Y.P.; Knopman, J.; Greenfield, J.P. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: Results of a phase I trial. J. Neurosurg. Pediatr. 2021, 28, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Truffaux, N.; Philippe, C.; Paulsson, J.; Andreiuolo, F.; Guerrini-Rousseau, L.; Cornilleau, G.; Le Dret, L.; Richon, C.; Lacroix, L.; Puget, S.; et al. Preclinical evaluation of dasatinib alone and in combination with cabozantinib for the treatment of diffuse intrinsic pontine glioma. Neuro-Oncology 2015, 17, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Jia, S.; Mandrell, B.; Hamideh, D.; Huang, J.; Onar-Thomas, A.; Gajjar, A.; Raimondi, S.C.; Tatevossian, R.G.; Stewart, C.F. Phase 1 trial, pharmacokinetics, and pharmacodynamics of dasatinib combined with crizotinib in children with recurrent or progressive high-grade and diffuse intrinsic pontine glioma. Pediatr. Blood Cancer 2018, 65, e27035. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Baker, S.D.; Wetmore, C.; Pai Panandiker, A.S.; Huang, J.; Davidoff, A.M.; Onar-Thomas, A.; Panetta, J.C.; Chin, T.K.; Merchant, T.E.; et al. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin. Cancer Res. 2013, 19, 3050–3058. [Google Scholar] [CrossRef]
- Hoeman, C.; Shen, C.; Becher, O.J. CDK4/6 and PDGFRA Signaling as Therapeutic Targets in Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Zuo, P.; Li, Y.; Wang, T.; Lin, X.; Wu, Z.; Zhang, J.; Liao, X.; Zhang, L. A novel CDK4/6 inhibitor combined with irradiation demonstrates potent anti-tumor efficacy in diffuse midline glioma. J. Neuro-Oncol. 2023, 163, 159–171. [Google Scholar] [CrossRef] [PubMed]
- DeWire, M.; Fuller, C.; Hummel, T.R.; Chow, L.M.L.; Salloum, R.; de Blank, P.; Pater, L.; Lawson, S.; Zhu, X.; Dexheimer, P.; et al. A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J. Neuro-Oncol. 2020, 149, 511–522. [Google Scholar] [CrossRef]
- Nduom, E.K.; Glod, J.; Brown, D.A.; Fagan, M.; Dalmage, M.; Heiss, J.; Steinberg, S.M.; Peer, C.; Figg, W.D.; Jackson, S. Clinical protocol: Feasibility of evaluating abemaciclib neuropharmacokinetics of diffuse midline glioma using intratumoral microdialysis. PLoS ONE 2023, 18, e0291068. [Google Scholar] [CrossRef]
- Grasso, C.S.; Tang, Y.; Truffaux, N.; Berlow, N.E.; Liu, L.; Debily, M.-A.; Quist, M.J.; Davis, L.E.; Huang, E.C.; Woo, P.J.; et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat. Med. 2015, 21, 555–559. [Google Scholar] [CrossRef]
- Ehteda, A.; Simon, S.; Franshaw, L.; Giorgi, F.M.; Liu, J.; Joshi, S.; Rouaen, J.R.C.; Pang, C.N.I.; Pandher, R.; Mayoh, C.; et al. Dual targeting of the epigenome via FACT complex and histone deacetylase is a potent treatment strategy for DIPG. Cell Rep. 2021, 35, 108994. [Google Scholar] [CrossRef]
- Hennika, T.; Hu, G.; Olaciregui, N.G.; Barton, K.L.; Ehteda, A.; Chitranjan, A.; Chang, C.; Gifford, A.J.; Tsoli, M.; Ziegler, D.S.; et al. Pre-Clinical Study of Panobinostat in Xenograft and Genetically Engineered Murine Diffuse Intrinsic Pontine Glioma Models. PLoS ONE 2017, 12, e0169485. [Google Scholar] [CrossRef] [PubMed]
- Vitanza, N.A.; Biery, M.C.; Myers, C.; Ferguson, E.; Zheng, Y.; Girard, E.J.; Przystal, J.M.; Park, G.; Noll, A.; Pakiam, F.; et al. Optimal therapeutic targeting by HDAC inhibition in biopsy-derived treatment-naïve diffuse midline glioma models. Neuro-Oncology 2021, 23, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; Wilson, K.M.; Ceribelli, M.; Stanton, B.Z.; Woo, P.J.; Kreimer, S.; Qin, E.Y.; Zhang, X.; Lennon, J.; Nagaraja, S.; et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci. Transl. Med. 2019, 11, eaaw0064. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Cooney, T.; Glod, J.; Huang, J.; Peer, C.J.; Faury, D.; Baxter, P.; Kramer, K.; Lenzen, A.; Robison, N.J.; et al. Phase I trial of panobinostat in children with diffuse intrinsic pontine glioma: A report from the Pediatric Brain Tumor Consortium (PBTC-047). Neuro-Oncology 2023, 25, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Rechberger, J.S.; Bouchal, S.M.; Power, E.A.; Nonnenbroich, L.F.; Nesvick, C.L.; Daniels, D.J. Bench-to-bedside investigations of H3 K27-altered diffuse midline glioma: Drug targets and potential pharmacotherapies. Expert Opin. Ther. Targets 2023, 27, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Rahal, F.; Capdevielle, C.; Rousseau, B.; Izotte, J.; Dupuy, J.W.; Cappellen, D.; Chotard, G.; Ménard, M.; Charpentier, J.; Jecko, V.; et al. An EZH2 blocker sensitizes histone mutated diffuse midline glioma to cholesterol metabolism inhibitors through an off-target effect. Neuro-Oncol. Adv. 2022, 4, vdac018. [Google Scholar] [CrossRef]
- Dhar, S.; Gadd, S.; Patel, P.; Vaynshteyn, J.; Raju, G.P.; Hashizume, R.; Brat, D.J.; Becher, O.J. A tumor suppressor role for EZH2 in diffuse midline glioma pathogenesis. Acta Neuropathol. Commun. 2022, 10, 47. [Google Scholar] [CrossRef]
- Brown, E.J.; Balaguer-Lluna, L.; Cribbs, A.P.; Philpott, M.; Campo, L.; Browne, M.; Wong, J.F.; Oppermann, U.; Carcaboso, Á.M.; Bullock, A.N.; et al. PRMT5 inhibition shows in vitro efficacy against H3K27M-altered diffuse midline glioma, but does not extend survival in vivo. Sci. Rep. 2024, 14, 328. [Google Scholar] [CrossRef]
- Przystal, J.M.; Cianciolo Cosentino, C.; Yadavilli, S.; Zhang, J.; Laternser, S.; Bonner, E.R.; Prasad, R.; Dawood, A.A.; Lobeto, N.; Chin Chong, W.; et al. Imipridones affect tumor bioenergetics and promote cell lineage differentiation in diffuse midline gliomas. Neuro-Oncology 2022, 24, 1438–1451. [Google Scholar] [CrossRef]
- Hall, M.D.; Odia, Y.; Allen, J.E.; Tarapore, R.; Khatib, Z.; Niazi, T.N.; Daghistani, D.; Schalop, L.; Chi, A.S.; Oster, W.; et al. First clinical experience with DRD2/3 antagonist ONC201 in H3 K27M–mutant pediatric diffuse intrinsic pontine glioma: A case report. J. Neurosurg. Pediatr. 2019, 23, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.S.; Tarapore, R.S.; Hall, M.D.; Shonka, N.; Gardner, S.; Umemura, Y.; Sumrall, A.; Khatib, Z.; Mueller, S.; Kline, C.; et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J. Neuro-Oncol. 2019, 145, 97–105. [Google Scholar] [CrossRef]
- Duchatel, R.J.; Mannan, A.; Woldu, A.S.; Hawtrey, T.; Hindley, P.A.; Douglas, A.M.; Jackson, E.R.; Findlay, I.J.; Germon, Z.P.; Staudt, D.; et al. Preclinical and clinical evaluation of German-sourced ONC201 for the treatment of H3K27M-mutant diffuse intrinsic pontine glioma. Neuro-Oncol. Adv. 2021, 3, vdab169. [Google Scholar] [CrossRef]
- Tanrıkulu, B.; Yaşar, A.H.; Canpolat, C.; Çorapçıoğlu, F.; Tezcanli, E.; Abacioglu, U.; Danyeli, A.E.; Özek, M.M. Preliminary findings of German-sourced ONC201 treatment in H3K27 altered pediatric pontine diffuse midline gliomas. J. Neuro-Oncol. 2023, 163, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Venneti, S.; Garimella, M.T.; Sullivan, L.M.; Martinez, D.; Huse, J.T.; Heguy, A.; Santi, M.; Thompson, C.B.; Judkins, A.R. Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol. 2013, 23, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.L.; Tarapore, R.S.; Allen, J.; McGovern, S.L.; Zaky, W.; Odia, Y.; Daghistani, D.; Diaz, Z.; Hall, M.D.; Khatib, Z.; et al. Phase I dose escalation and expansion trial of single agent ONC201 in pediatric diffuse midline gliomas following radiotherapy. Neuro-Oncol. Adv. 2022, 4, vdac143. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; Hoffman, S.E.; Kappel, A.D.; Valdes, P.A.; Essayed, W.; Klinger, N.V.; Kang, K.D.; Totsch, S.K.; Olsen, H.E.; Schlappi, C.W.; et al. Immunotherapy approaches for the treatment of diffuse midline gliomas. Oncoimmunology 2022, 11, 2124058. [Google Scholar] [CrossRef]
- Mount, C.W.; Majzner, R.G.; Sundaresh, S.; Arnold, E.P.; Kadapakkam, M.; Haile, S.; Labanieh, L.; Hulleman, E.; Woo, P.J.; Rietberg, S.P.; et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat. Med. 2018, 24, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Vitanza, N.A.; Wilson, A.L.; Huang, W.; Seidel, K.; Brown, C.; Gustafson, J.A.; Yokoyama, J.K.; Johnson, A.J.; Baxter, B.A.; Koning, R.W.; et al. Intraventricular B7-H3 CAR T Cells for Diffuse Intrinsic Pontine Glioma: Preliminary First-in-Human Bioactivity and Safety. Cancer Discov. 2023, 13, 114–131. [Google Scholar] [CrossRef]
- Wang, S.S.; Davenport, A.J.; Iliopoulos, M.; Hughes-Parry, H.E.; Watson, K.A.; Arcucci, V.; Mulazzani, M.; Eisenstat, D.D.; Hansford, J.R.; Cross, R.S.; et al. HER2 chimeric antigen receptor T cell immunotherapy is an effective treatment for diffuse intrinsic pontine glioma. Neuro-Oncol. Adv. 2023, 5, vdad024. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.; Liu, S.J.; Duriseti, S.; Banerjee, A.; Nicolaides, T.; Raber, S.; Gupta, N.; Haas-Kogan, D.; Braunstein, S.; Mueller, S. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: A single-institution experience. J. Neuro-Oncol. 2018, 140, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Rechberger, J.S.; Porath, K.A.; Zhang, L.; Nesvick, C.L.; Schrecengost, R.S.; Sarkaria, J.N.; Daniels, D.J. IL-13Rα2 Status Predicts GB-13 (IL13.E13K-PE4E) Efficacy in High-Grade Glioma. Pharmaceutics 2022, 14, 922. [Google Scholar] [CrossRef] [PubMed]
- Stine, C.A.; Munson, J.M. Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow. Front. Oncol. 2019, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Ochs, K.; Ott, M.; Bunse, T.; Sahm, F.; Bunse, L.; Deumelandt, K.; Sonner, J.K.; Keil, M.; von Deimling, A.; Wick, W.; et al. K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology 2017, 6, e1328340. [Google Scholar] [CrossRef] [PubMed]
- Grassl, N.; Poschke, I.; Lindner, K.; Bunse, L.; Mildenberger, I.; Boschert, T.; Jähne, K.; Green, E.W.; Hülsmeyer, I.; Jünger, S.; et al. A H3K27M-targeted vaccine in adults with diffuse midline glioma. Nat. Med. 2023, 29, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Grassl, N.; Sahm, K.; Süße, H.; Poschke, I.; Bunse, L.; Bunse, T.; Boschert, T.; Mildenberger, I.; Rupp, A.K.; Ewinger, M.P.; et al. INTERCEPT H3: A multicenter phase I peptide vaccine trial for the treatment of H3-mutated diffuse midline gliomas. Neurol Res. Pract. 2023, 5, 55. [Google Scholar] [CrossRef]
- Power, E.A.; Rechberger, J.S.; Gupta, S.; Schwartz, J.D.; Daniels, D.J.; Khatua, S. Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors—An update. Adv. Drug Deliv. Rev. 2022, 185, 114303. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Sun, H.; Dai, H.; Shaik, N.; Elmquist, W.F. Drug efflux transporters in the CNS. Adv. Drug Deliv. Rev. 2003, 55, 83–105. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef]
- Luo, H.; Shusta, E.V. Blood-Brain Barrier Modulation to Improve Glioma Drug Delivery. Pharmaceutics 2020, 12, 1085. [Google Scholar] [CrossRef]
- Welby, J.P.; Kaptzan, T.; Wohl, A.; Peterson, T.E.; Raghunathan, A.; Brown, D.A.; Gupta, S.K.; Zhang, L.; Daniels, D.J. Current Murine Models and New Developments in H3K27M Diffuse Midline Gliomas. Front. Oncol. 2019, 9, 92. [Google Scholar] [CrossRef]
- Warren, K.E. Beyond the Blood:Brain Barrier: The Importance of Central Nervous System (CNS) Pharmacokinetics for the Treatment of CNS Tumors, Including Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8, 239. [Google Scholar] [CrossRef]
- Loeffen, E.A.H.; Knops, R.R.G.; Boerhof, J.; Feijen, E.; Merks, J.H.M.; Reedijk, A.M.J.; Lieverst, J.A.; Pieters, R.; Boezen, H.M.; Kremer, L.C.M.; et al. Treatment-related mortality in children with cancer: Prevalence and risk factors. Eur. J. Cancer 2019, 121, 113–122. [Google Scholar] [CrossRef]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-Enhanced Delivery for Diffuse Intrinsic Pontine Glioma Treatment. Curr. Neuropharmacol. 2017, 15, 116–128. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Sasaki, T.; Katagi, H.; Goldman, S.; Becher, O.J.; Hashizume, R. Convection-Enhanced Delivery of Enhancer of Zeste Homolog-2 (EZH2) Inhibitor for the Treatment of Diffuse Intrinsic Pontine Glioma. Neurosurgery 2020, 87, E680–E688. [Google Scholar] [CrossRef]
- Chang, R.; Tosi, U.; Voronina, J.; Adeuyan, O.; Wu, L.Y.; Schweitzer, M.E.; Pisapia, D.J.; Becher, O.J.; Souweidane, M.M.; Maachani, U.B. Combined targeting of PI3K and MEK effector pathways via CED for DIPG therapy. Neuro-Oncol. Adv. 2019, 1, vdz004b. [Google Scholar] [CrossRef]
- Rechberger, J.S.; Power, E.A.; Lu, V.M.; Zhang, L.; Sarkaria, J.N.; Daniels, D.J. Evaluating infusate parameters for direct drug delivery to the brainstem: A comparative study of convection-enhanced delivery versus osmotic pump delivery. Neurosurg. Focus 2020, 48, E2. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Pui, C.-H.; Gayon, P.; Childhood Acute Lymphoblastic Leukemia Collaborative Group (CALLCG). Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2013, 60, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Thomas, D.; Cortes, J.; Kantarjian, H.M.; O’Brien, S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia: Current and emerging therapies. Cancer 2010, 116, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Triarico, S.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Capozza, M.A.; Ruggiero, A. Improving the Brain Delivery of Chemotherapeutic Drugs in Childhood Brain Tumors. Cancers 2019, 11, 824. [Google Scholar] [CrossRef]
- Partap, S.; Murphy, P.A.; Vogel, H.; Barnes, P.D.; Edwards, M.S.; Fisher, P.G. Liposomal cytarabine for central nervous system embryonal tumors in children and young adults. J. Neuro-Oncol. 2011, 103, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, D.I.; Yu, B.; Patel, R.; Hagan, J.; Miesner, E.; Sabin, J.; Smith, S.; Fletcher, S.; Shah, M.N.; Sirianni, R.W.; et al. Infusion of 5-Azacytidine (5-AZA) into the fourth ventricle or resection cavity in children with recurrent posterior Fossa Ependymoma: A pilot clinical trial. J. Neuro-Oncol. 2019, 141, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020, 165–166, 77–95. [Google Scholar] [CrossRef]
- Joshi, S.; Ellis, J.A.; Ornstein, E.; Bruce, J.N. Intraarterial drug delivery for glioblastoma mutiforme. J. Neuro-Oncol. 2015, 124, 333–343. [Google Scholar] [CrossRef]
- Kroll, R.A.; Neuwelt, E.A. Outwitting the blood-brain barrier for therapeutic purposes: Osmotic opening and other means. Neurosurgery 1998, 42, 1083–1099; discussion 1099–1100. [Google Scholar] [CrossRef]
- Uluc, K.; Ambady, P.; McIntyre, M.K.; Tabb, J.P.; Kersch, C.N.; Nerison, C.S.; Huddleston, A.; Liu, J.J.; Dogan, A.; Priest, R.A.; et al. Safety of intra-arterial chemotherapy with or without osmotic blood-brain barrier disruption for the treatment of patients with brain tumors. Neuro-Oncol. Adv. 2022, 4, vdac104. [Google Scholar] [CrossRef]
- Uluc, K.; Siler, D.A.; Lopez, R.; Varallyay, C.; Netto, J.P.; Firkins, J.; Lacy, C.; Huddleston, A.; Ambady, P.; Neuwelt, E.A. Long-Term Outcomes of Intra-Arterial Chemotherapy for Progressive or Unresectable Pilocytic Astrocytomas: Case Studies. Neurosurgery 2021, 88, E336–E342. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Meel, M.H.; Breur, M.; Bugiani, M.; Hulleman, E.; Phoenix, T.N. Defining tumor-associated vascular heterogeneity in pediatric high-grade and diffuse midline gliomas. Acta Neuropathol. Commun. 2021, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Matsukado, K.; Inamura, T.; Nakano, S.; Fukui, M.; Bartus, R.T.; Black, K.L. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery 1996, 39, 125–133; discussion 133–134. [Google Scholar] [CrossRef] [PubMed]
- Ningaraj, N.S.; Rao, M.; Hashizume, K.; Asotra, K.; Black, K.L. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2002, 301, 838–851. [Google Scholar] [CrossRef]
- Prados, M.D.; Schold, S.C., Jr.; Fine, H.A.; Jaeckle, K.; Hochberg, F.; Mechtler, L.; Fetell, M.R.; Phuphanich, S.; Feun, L.; Janus, T.J.; et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro-Oncology 2003, 5, 96–103. [Google Scholar] [CrossRef]
- Hynynen, K.; Jolesz, F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998, 24, 275–283. [Google Scholar] [CrossRef]
- Bonosi, L.; Marino, S.; Benigno, U.E.; Musso, S.; Buscemi, F.; Giardina, K.; Gerardi, R.; Brunasso, L.; Costanzo, R.; Iacopino, D.G.; et al. Sonodynamic therapy and magnetic resonance-guided focused ultrasound: New therapeutic strategy in glioblastoma. J. Neuro-Oncol. 2023, 163, 219–238. [Google Scholar] [CrossRef]
- Meng, Y.; Reilly, R.M.; Pezo, R.C.; Trudeau, M.; Sahgal, A.; Singnurkar, A.; Perry, J.; Myrehaug, S.; Pople, C.B.; Davidson, B.; et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021, 13, eabj4011. [Google Scholar] [CrossRef]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef]
- Ishida, J.; Alli, S.; Bondoc, A.; Golbourn, B.; Sabha, N.; Mikloska, K.; Krumholtz, S.; Srikanthan, D.; Fujita, N.; Luck, A.; et al. MRI-guided focused ultrasound enhances drug delivery in experimental diffuse intrinsic pontine glioma. J. Control. Release 2021, 330, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Sultan, D.; Zhang, X.; Yue, Y.; Heo, G.S.; Kothapalli, S.; Luehmann, H.; Tai, Y.C.; Rubin, J.B.; Liu, Y.; et al. Focused ultrasound-enabled delivery of radiolabeled nanoclusters to the pons. J. Control. Release 2018, 283, 143–150. [Google Scholar] [CrossRef]
- Martinez, P.; Nault, G.; Steiner, J.; Wempe, M.F.; Pierce, A.; Brunt, B.; Slade, M.; Song, J.J.; Mongin, A.; Song, K.H.; et al. MRI-guided focused ultrasound blood-brain barrier opening increases drug delivery and efficacy in a diffuse midline glioma mouse model. Neuro-Oncol. Adv. 2023, 5, vdad111. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Vanbilloen, W.; Rechberger, J.; Anderson, J.; Nonnenbroich, L.; Zhang, L.; Daniels, D. Nanoparticle Strategies to Improve the Delivery of Anticancer Drugs across the Blood–Brain Barrier to Treat Brain Tumors. Pharmaceutics 2023, 15, 1804. [Google Scholar] [CrossRef] [PubMed]
- Bredlau, L.A.; Dixit, S.; Chen, C.; Broome, A.-M. Nanotechnology Applications for Diffuse Intrinsic Pontine Glioma. Curr. Neuropharmacol. 2017, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Cavalli, R.; Panciani, P.P.; Battaglia, L. Overcoming the Blood-Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours. Int. J. Nanomed. 2020, 15, 2999–3022. [Google Scholar] [CrossRef]
- Heravi Shargh, V.; Luckett, J.; Bouzinab, K.; Paisey, S.; Turyanska, L.; Singleton, W.G.B.; Lowis, S.; Gershkovich, P.; Bradshaw, T.D.; Stevens, M.F.G.; et al. Chemosensitization of Temozolomide-Resistant Pediatric Diffuse Midline Glioma Using Potent Nanoencapsulated Forms of a N(3)-Propargyl Analogue. ACS Appl. Mater. Interfaces 2021, 13, 35266–35280. [Google Scholar] [CrossRef]
- Ung, C.; Tsoli, M.; Liu, J.; Cassano, D.; Pocoví-Martínez, S.; Upton, D.H.; Ehteda, A.; Mansfeld, F.M.; Failes, T.W.; Farfalla, A.; et al. Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma. Cancers 2021, 13, 1278. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.J.; Green, A.L.; Borden, M.A. Targeting diffuse midline gliomas: The promise of focused ultrasound-mediated blood-brain barrier opening. J. Control. Release 2024, 365, 412–421. [Google Scholar] [CrossRef]
- Lim, C.; Dismuke, T.; Malawsky, D.; Ramsey, J.D.; Hwang, D.; Godfrey, V.L.; Kabanov, A.V.; Gershon, T.R.; Sokolsky-Papkov, M. Enhancing CDK4/6 inhibitor therapy for medulloblastoma using nanoparticle delivery and scRNA-seq-guided combination with sapanisertib. Sci. Adv. 2022, 8, eabl5838. [Google Scholar] [CrossRef] [PubMed]
- Tylawsky, D.E.; Kiguchi, H.; Vaynshteyn, J.; Gerwin, J.; Shah, J.; Islam, T.; Boyer, J.A.; Boué, D.R.; Snuderl, M.; Greenblatt, M.B.; et al. P-selectin-targeted nanocarriers induce active crossing of the blood-brain barrier via caveolin-1-dependent transcytosis. Nat. Mater. 2023, 22, 391–399. [Google Scholar] [CrossRef]
- King, A.R.; Corso, C.D.; Chen, E.M.; Song, E.; Bongiorni, P.; Chen, Z.; Sundaram, R.K.; Bindra, R.S.; Saltzman, W.M. Local DNA Repair Inhibition for Sustained Radiosensitization of High-Grade Gliomas. Mol. Cancer Ther. 2017, 16, 1456–1469. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, J.; He, X.; Katagi, H.; Suri, A.; Ishi, Y.; Abe, K.; Natsumeda, M.; Frey, W.H.; Zhang, P.; et al. Intranasal delivery of nanoliposomal SN-38 for treatment of diffuse midline glioma. J. Neurosurg. 2022, 138, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Rechberger, J.S.; Lu, V.M.; Zhang, L.; Power, E.A.; Daniels, D.J. Clinical trials for diffuse intrinsic pontine glioma: The current state of affairs. Childs Nerv. Syst. 2020, 36, 39–46. [Google Scholar] [CrossRef]
- Noon, A.; Galban, S. Therapeutic avenues for targeting treatment challenges of diffuse midline gliomas. Neoplasia 2023, 40, 100899. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonnenbroich, L.F.; Bouchal, S.M.; Millesi, E.; Rechberger, J.S.; Khatua, S.; Daniels, D.J. H3K27-Altered Diffuse Midline Glioma of the Brainstem: From Molecular Mechanisms to Targeted Interventions. Cells 2024, 13, 1122. https://doi.org/10.3390/cells13131122
Nonnenbroich LF, Bouchal SM, Millesi E, Rechberger JS, Khatua S, Daniels DJ. H3K27-Altered Diffuse Midline Glioma of the Brainstem: From Molecular Mechanisms to Targeted Interventions. Cells. 2024; 13(13):1122. https://doi.org/10.3390/cells13131122
Chicago/Turabian StyleNonnenbroich, Leo F., Samantha M. Bouchal, Elena Millesi, Julian S. Rechberger, Soumen Khatua, and David J. Daniels. 2024. "H3K27-Altered Diffuse Midline Glioma of the Brainstem: From Molecular Mechanisms to Targeted Interventions" Cells 13, no. 13: 1122. https://doi.org/10.3390/cells13131122
APA StyleNonnenbroich, L. F., Bouchal, S. M., Millesi, E., Rechberger, J. S., Khatua, S., & Daniels, D. J. (2024). H3K27-Altered Diffuse Midline Glioma of the Brainstem: From Molecular Mechanisms to Targeted Interventions. Cells, 13(13), 1122. https://doi.org/10.3390/cells13131122




