Comprehensive Review of Uterine Leiomyosarcoma: Pathogenesis, Diagnosis, Prognosis, and Targeted Therapy
Abstract
1. Introduction: Biology and Pathogenesis of Uterine Leiomyosarcoma
2. Differential Diagnosis of Uterine Leiomyosarcoma and Leiomyoma
2.1. Challenges with Differentiating Uterine Leiomyoma from Uterine Leiomyosarcoma
2.2. Comparison between Uterine Leiomyosarcoma and Leiomyoma
2.2.1. Clinicopathologic Features
2.2.2. Tissue Origin and Driver Mutations
2.2.3. Morphology
2.2.4. Behavior, Onset, and Incidence
2.2.5. Genetic Mutation, Molecular Prognostic Biomarkers, and Transcriptional Difference
3. Role of Biological Pathways in uLMS
3.1. DNA Repair Pathways
3.2. Hedgehog Pathway
3.3. VEGF/VEGFR
3.4. Immune Checkpoint Blockade
3.5. Protein Kinases and Intracellular Signaling Pathways
3.6. Wnt/β-Catenin Pathways
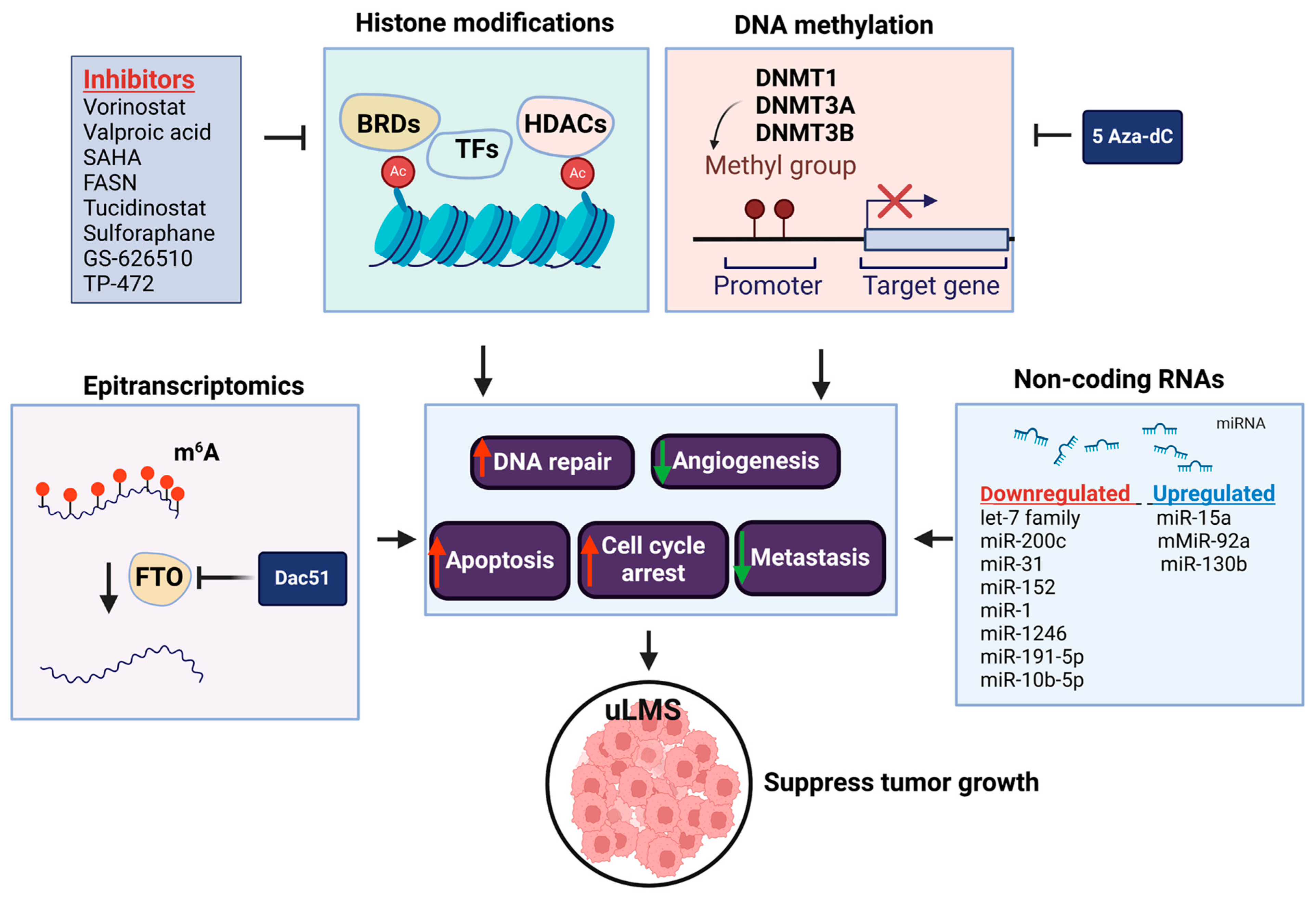
4. The Role of Epigenetics in the Pathogenesis of Leiomyosarcoma
4.1. DNA Methylation
4.2. Histone Modification
4.3. Non-Coding RNA
4.4. RNA Methylation
5. Treatment of Uterine Leiomyosarcoma
5.1. Surgery Management
5.2. Adjuvant Therapy
5.3. Primary Systemic Treatment
5.4. Systemic Treatment for Recurrent Disease
6. Future Perspective
6.1. Clinical Diagnosis between Malignant Uterine Leiomyosarcoma and Benign Leiomyoma
6.1.1. Serum Biomarkers
6.1.2. Advanced Imaging—Artificial Intelligence and Machine Learning
6.1.3. Shear Wave Elastography
6.2. Prevention
| Drugs/Factors | Epigenetic Targets | Biological Samples | Effectors | Biological Effect | Approach | Publication Time | Refs. |
|---|---|---|---|---|---|---|---|
| Vorinostat | HDACs | MES-SA | p21 | apoptosis, tumor growth inhibition | in vitro and in vivo | 2010 | [131] |
| Valproic acid | HDACs | MES-SA | NA | cytotoxic effect | in vitro | 2013 | [130] |
| SAHA, LY294002, rapamycin | HDACs | MES-SA | AKT, mTOR/p70S6K | growth inhibition | in vitro | 2014 | [104] |
| FASN | H3K9me3, H3K27ac | SK-UT-1 | CRISP1 | proliferation, migration, cellular motion | in vitro | 2017 | [227] |
| GS-626510 | BET bromodomain | uLMS PDX model | NA | tumor growth inhibition | in vivo | 2021 | [59] |
| Tucidinostat, sulforaphane | HDACs | SK-UT-1 | PCNA, CDK members | cell proliferation, | in vitro | 2022 | [126] |
| TP-472 | BRD9 | SK-UT-1 | H3K4me3, YTHDC1, YTHDF2 | cell cycle, cell proliferation, apoptosis | in vitro | 2022 | [142] |
| MiRNAs | Biological Samples | Expression | Targets | Biological Events | Year Published | Refs. |
|---|---|---|---|---|---|---|
| let-7s | LMS tissues, SK-LMS-1, SK-UT-1, and SK-UT-1B cell lines | downregulated | HMGA2 | cell proliferation | 2008 | [228] |
| 72 miRNAs | LMS tissues | deregulated (32 up-Mir, 40 down-Mir) | NA | differentiation, neoplastic transformation | 2010 | [146] |
| miR-200c | SK-LMS-1 | downregulated | IKBKB, IL8, CDK2, and CCNE2 | inflammatory response, angiogenesis, cell cycle, migration | 2014 | [149] |
| miR-31 | metastatic and primary LMS tissues, SK-LMS-1 | downregulated in metastatic LMS tissues | MAPK signaling, Wnt signaling | metastasis | 2016 | [159] |
| miR-15a, miR- 92a | metastatic and primary LMS tissues | upregulated in metastatic LMS tissues | Wnt signaling | metastasis | 2016 | [159] |
| 13 miRNAs | LMS, MM, LMS-derived cell line | deregulated (8 up-Mir, 5 down-Mir) | BCL2, EGFR, VEGFA, IGF1R, EGF-R, MET, MYCN | tumor apoptosis, angiogenesis, proliferation | 2017 | [147] |
| miR-152 | LMS tissues, SK-LMS-1 | downregulated | MET, KIT, PI3K/AKT (transcription factors) | cell proliferation, apoptosis, cell cycle | 2017 | [229] |
| miR-1 | LMS tissues | downregulated | NA | disrupted tumor suppression | 2018 | [154] |
| let-7 family | LMS tissues | downregulated | NA | PFS, OS | 2019 | [151] |
| miR-1246, miR-191-5p | serum from LMS patients | downregulated | NA | diagnostic biomarker | 2019 | [230] |
| miR-10b-5p | LMS tissues, SK-UT-1, SK-LMS-1 | downregulated | G1/S checkpoint, MYC-mediated apoptosis, epithelial–mesenchymal transition | cell proliferation, cell cycle | 2023 | [145] |
| miR-130b | LMS, MM tissues, SK-LMS-1, SK-UT-1 | upregulated | TSC1 | tumor proliferation and metastasis | 2023 | [150] |
| Drug(s) and Clinical Trial Identifier | Study Design | Indication | Grade 3 and 4 Toxicities | Efficacy | Year | Refs. |
|---|---|---|---|---|---|---|
| Intensified doxorubicin plus ifosfamide NCT00061984 EORTC 62012 (complete) | EORTC phase 3 randomized study evaluating OS of intensified doxorubicin plus ifosfamide vs. doxorubicin use as first-line treatment | locally advanced, unresectable, or metastatic high-grade soft tissue sarcoma and no prior systemic cytotoxic treatment (but adjuvant chemo allowed) | ebrile neutropenia (46%) leukopenia (43%) neutropenia (42%) anemia (35%) thrombocytopenia (33%) | mPFS: 7.4 mo vs. 4.6 mo HR 0.74 p = 0.003 mOS: 14.3 mo vs. 12.8 mo HR 0.83 p = 0.076 ORR: 26% (60/227) | 2014 | [193] |
| Fixed-dose rate gemcitabine plus docetaxel | GOG phase 2 study evaluating PFS of fixed-dose rate gemcitabine plus docetaxel use as first-line treatment | metastatic unresectable uLMS and no prior systemic cytotoxic treatment | anemia (23.8%) thrombocytopenia (19%) neutropenia (16.7%) fatigue (16.7%) metabolic toxicities (16.7%) leukopenia (14.3%) GI toxicity (14.3%) | mPFS: 4.4 mo mOS: 16.1+ mo ORR: 35.7% (15/42) | 2008 | [194] |
| Doxorubicin plus trabectedin NCT02997358 (Complete) | randomized phase 3 study evaluating PFS of doxorubicin and trabectedin use vs. doxorubicin alone as first-line treatment | metastatic or relapsed unresectable LMS without prior systemic treatment | neutropenia (80%) leukopenia (75%) thrombocytopenia (57%) ALT increase (42%) anemia (31%) renal creatinine clearance decreases (31%) febrile neutropenia (28%) fatigue (11%) | mPFS: 12.2 mo vs. 6.2 mo HR 0.41, p < 0.0001 ORR: 36% (27/74) vs. 13% (10/74) uLMS ORR: 36% (12/33) vs. 15% (5/34) | 2022 | [196] |
| Fixed-dose rate gemcitabine with docetaxel | GOG phase 2 study evaluating efficacy of fixed-dose gemcitabine with docetaxel | advanced or recurrent uLMS progressed after at least one prior line excluding gemcitabine or docetaxel use | thrombocytopenia (39.5%) anemia (25%) leukopenia (23%) neutropenia (20.8%) | mPFS: 6.7+ mo mOS: 14.7 mo ORR: 27% (13/48) | 2008 | [194] |
| Trabectedin NCT01343277 (Complete) | randomized phase 3 study evaluating OS of trabectedin use compared to dacarbazine use | advanced liposarcoma or LMS after at least two prior lines with at least one containing anthracycline | neutropenia (37%) ALT elevation (26%) thrombocytopenia (17%) anemia (14%) AST elevation (13%) | mPFS: 4.2 mo vs. 1.5 mo HR 0.55, p < 0.001 mOS: 12.4 mo vs. 12.9 mo HR 0.87, p = 0.37 ORR: 9.9% (34/345) vs. 6.9% (12/173), p = 0.33 | 2016 | [200] |
| Pazopanib NCT00753688 (Complete) | randomized phase 3 study evaluating PFS of pazopanib use compared to placebo | progressive metastatic soft tissue sarcoma with at least one prior line containing anthracycline, up to four prior lines | fatigue (14%) hypertension (7%) anorexia (6%) | mPFS: 4.6 mo vs. 1.6 mo HR 0.31, p < 0.0001 mOS: 12.5 mo vs. 10.7 mo HR 0.86, p = 0.2514 ORR: 14/246 (6%) vs. 0/123 (0%) | 2012 | [201] |
| Eribulin NCT01327885 (Complete) | randomized phase 3 study evaluating OS of eribulin compared to dacarbazine use | Intermediate- or high-grade advanced-stage liposarcoma or LMS with at least two prior lines including anthracycline use | neutropenia (35%) leukopenia (10%) anemia (7%) | mPFS: 2.2 mo vs. 2.6 mo HR 1.07, p = 0.58 mOS: 13.5 mo vs. 11.5 mo HR 0.77, p = 0.0169 LMS subgroup mPFS: 1.4 vs. 2.6 mo, HR 1.57 mOS: 12.7 mo vs. 13.0 mo HR 0.93 ORR 9/228 (4%) vs. 11/224 (5%) | 2016 | [202] |
| Olaparib and temozolomide NCT03880019 (Complete) | phase 2 single-arm open-label study evaluating olaparib and temozolomide | advanced and unresectable or metastatic uterine LMS patients | neutropenia (75%) thrombocytopenia (32%) leukopenia (22%) | mPFS 11.2 mo in HR-deficient patients vs. mPFS 5.4 mo in HR-proficient patients p = 0.05 ORR 6/22 (27%) | 2023 | [205] |
| Olaparib and temozolomide NCT05432791 (Active) | randomized phase 2/3 study evaluating PFS and OS of olaparib plus temozolomide compared to investigator’s choice | advanced and unresectable or metastatic uLMS patients who received two or more prior lines including anthracycline use | NA | NA | ongoing | NA |
| Lurbinectedin and doxorubicin NCT05099666 (Active) | phase Ib/2 study exploring safety and efficacy of lurbinectedin and doxorubicin | Phase Ib: advanced or metastatic soft-tissue sarcoma with no more than two prior lines, and no prior anthracycline or trabectedin use. Phase 2: advanced or metastatic LMS with no more than one prior line and no prior anthracycline or trabectedin use. | NA | NA | ongoing | NA |
| Gemcitabine, dacarbazine, and HIPEC NCT04727242 (Active) | phase 2 study evaluating the use of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) with gemcitabine followed by systemic adjuvant chemotherapy with dacarbazine | locally recurrent uLMS without extra-abdominal disease, and no prior gemcitabine or dacarbazine use | NA | NA | ongoing | NA |
6.3. Future Clinical Trials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reichert, V.M.C.; Alwafai, Z.; Zygmunt, M.T.; Vollmer, M.; Köhler, G. Accidental Morcellation of Uterine Leiomyosarcoma Influences Relapse Free Survival but Does Not Negatively Influence Overall Survival. J. Clin. Med. 2023, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Dann, A.M.; Keung, E.Z.; Nakazawa, M.S.; Haddad, E.F.N. The Future of Targeted Therapy for Leiomyosarcoma. Cancers 2024, 16, 938. [Google Scholar] [CrossRef] [PubMed]
- Chibon, F.; Lesluyes, T.; Valentin, T.; Le Guellec, S. CINSARC signature as a prognostic marker for clinical outcome in sarcomas and beyond. Genes Chromosomes Cancer 2018, 58, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Croce, S.; Chibon, F. Molecular prognostication of uterine smooth muscle neoplasms: From CGH array to CINSARC signature and beyond. Genes Chromosomes Cancer 2021, 60, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sefah, N.; Ndebele, S.; Prince, L.; Korasare, E.; Agbleke, M.; Nkansah, A.; Thompson, H.; Al-Hendy, A.; Agbleke, A.A. Uterine fibroids—Causes, impact, treatment, and lens to the African perspective. Front. Pharmacol. 2023, 13, 1045783. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Aynardi, J.T.; Chu, C.S. Uterine leiomyosarcoma: A review of the literature and update on management options. Gynecol Oncol. 2018, 151, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, H.; Ward, A.; Maclean, A.; Lane, S.; Adishesh, M.; Taylor, S.; DeCruze, S.B.; Hapangama, D.K. Developing a Preoperative Algorithm for the Diagnosis of Uterine Leiomyosarcoma. Diagnostics 2020, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.-C.; Horng, H.-C.; Wang, P.-H.; Chen, Y.-J.; Yen, M.-S.; Ng, H.-T. Uterine sarcoma Part I—Uterine leiomyosarcoma: The Topic Advisory Group systematic review. Taiwan. J. Obstet. Gynecol. 2016, 55, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Perri, T.; Korach, J.; Sadetzki, S.; Oberman, B.; Fridman, E.; Ben-Baruch, G. Terine leiomyosarcoma: Does the primary surgical procedure matter? Int. J. Gynecol. Cancer 2009, 19, 257–260. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, J.; Tong, J. Potential Markers to Differentiate Uterine Leiomyosarcomas from Leiomyomas. Int. J. Med. Sci. 2024, 21, 1227–1240. [Google Scholar] [CrossRef]
- Tanos, V.; Berry, K.E. Benign and malignant pathology of the uterus. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 12–30. [Google Scholar] [CrossRef]
- Pérez-Fidalgo, J.A.; Ortega, E.; Ponce, J.; Redondo, A.; Sevilla, I.; Valverde, C.; Verdum, J.I.; de Alava, E.; López, M.G.; Marquina, G.; et al. Uterine sarcomas: Clinical practice guidelines for diagnosis, treatment, and follow-up, by Spanish group for research on sarcomas (GEIS). Ther. Adv. Med. Oncol. 2023, 15, 17588359231157645. [Google Scholar] [CrossRef] [PubMed]
- Tanos, V.; Berry, K. Corrigendum to “Benign and malignant pathology of the uterus” [Best Pract Res Clin Obstet Gynaecol 46 (2018) 12–30]. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 127. [Google Scholar] [CrossRef] [PubMed]
- Mbatani, N.; Olawaiye, A.B.; Prat, J. Uterine sarcomas. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 51–58. [Google Scholar] [CrossRef]
- Chapel, D.B.; Nucci, M.R.; Quade, B.J.; Parra-Herran, C. Epithelioid Leiomyosarcoma of the Uterus: Modern Outcome-based Appraisal of Diagnostic Criteria in a Large Institutional Series. Am. J. Surg. Pathol. 2022, 46, 464–475. [Google Scholar] [CrossRef]
- Sun, S.; Bonaffini, P.; Nougaret, S.; Fournier, L.; Dohan, A.; Chong, J.; Smith, J.; Addley, H.; Reinhold, C. How to differentiate uterine leiomyosarcoma from leiomyoma with imaging. Diagn. Interv. Imaging 2019, 100, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Herzog, T.J.; Burke, W.; Cohen, C.J.; Wright, J.D. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol. Oncol. 2008, 110, 43–48. [Google Scholar] [CrossRef]
- Kho, R.M.; Desai, V.B.; Schwartz, P.E.; Wright, J.D.; Gross, C.P.; Hutchison, L.M.; Boscoe, F.P.; Lin, H.; Xu, X. Endometrial Sampling for Preoperative Diagnosis of Uterine Leiomyosarcoma. J. Minim. Invasive Gynecol. 2022, 29, 119–127. [Google Scholar] [CrossRef]
- Lakhman, Y.; Veeraraghavan, H.; Chaim, J.; Feier, D.; Goldman, D.A.; Moskowitz, C.S.; Nougaret, S.; Sosa, R.E.; Vargas, H.A.; Soslow, R.A.; et al. Differentiation of Uterine Leiomyosarcoma from Atypical Leiomyoma: Diagnostic Accuracy of Qualitative MR Imaging Features and Feasibility of Texture Analysis. Eur. Radiol. 2016, 27, 2903–2915. [Google Scholar] [CrossRef]
- Li, D.; Yin, N.; Du, G.; Wang, S.; Xiao, Z.; Chen, J.; Chen, W. A Real-World Study on Diagnosis and Treatment of Uterine Sarcoma in Western China. Int. J. Biol. Sci. 2020, 16, 388–395. [Google Scholar] [CrossRef]
- Nagai, T.; Takai, Y.; Akahori, T.; Ishida, H.; Hanaoka, T.; Uotani, T.; Sato, S.; Matsunaga, S.; Baba, K.; Seki, H. Highly improved accuracy of the revised PREoperative sarcoma score (rPRESS) in the decision of performing surgery for patients presenting with a uterine mass. SpringerPlus 2015, 4, 520. [Google Scholar] [CrossRef]
- Wojdat, R.; Malanowska, E. An evaluation of a Myomscore in the preoperative assessment of uterus myomatosus: A new diagnostic standard? The experience at the Mathilden Hospital in Herford, Germany. Gynecol. Surg. 2020, 17, 9. [Google Scholar] [CrossRef]
- Suh, D.S.; Song, Y.J.; Roh, H.J.; Lee, S.H.; Jeong, D.H.; Lee, T.H.; Choi, K.U.; Kim, K.H. Preoperative Blood Inflammatory Markers for the Differentiation of Uterine Leiomyosarcoma from Leiomyoma. Cancer Manag Res. 2021, 13, 5001–5011. [Google Scholar] [CrossRef]
- Cohen Rassier, S.L.; Stewart, A.E. Finding the needle in the haystack. Fertil. Steril. 2021, 115, 87–88. [Google Scholar] [CrossRef]
- Giuntoli, R.L., 2nd; Metzinger, D.S.; DiMarco, C.S.; Cha, S.S.; Sloan, J.A.; Keeney, G.L.; Gostout, B.S. Retrospective review of 208 patients with leiomyosarcoma of the uterus: Prognostic indicators, surgical management, and adjuvant therapy. Gynecol. Oncol. 2003, 89, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.A.; Oliva, E.A.; Fuller, A.F., Jr.; Lee, H.; Goodman, A. The treatment of uterine leiomyosarcoma. Results from a 10-year experience (1990–1999) at the Massachusetts General Hospital. Gynecol. Oncol. 2004, 92, 648–652. [Google Scholar] [CrossRef]
- Brooks, S.E.; Zhan, M.; Cote, T.; Baquet, C.R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol. Oncol. 2004, 93, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, S.; Berek, J.S. Uterine Sarcoma: Classification, Epidemiology, Clinical Manifestations, and Diagnosis; Chakrabarti, A., Ed.; UpToDate: Waltham, MA, USA, 2021; Available online: https://www.uptodate.com (accessed on 12 June 2024).
- Abel, M.K.; Liao, C.I.; Chan, C.; Lee, D.; Rohatgi, A.; Darcy, K.M.; Tian, C.; Mann, A.K.; Maxwell, G.L.; Kapp, D.S.; et al. Racial disparities in high-risk uterine cancer histologic subtypes: A United States Cancer Statistics study. Gynecol. Oncol. 2021, 161, 470–476. [Google Scholar] [CrossRef]
- Robboy, S.J.; Bentley, R.C.; Butnor, K.; Anderson, M.C. Pathology and pathophysiology of uterine smooth-muscle tumors. Environ. Health Perspect. 2000, 108 (Suppl. S5), 779–784. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Fan, Q.; Wang, Y.; Li, Y. Benign metastasizing uterine leiomyoma with lymphatic and pulmonary metastases: A case report and literature review. BMC Womens Health 2023, 23, 154. [Google Scholar] [CrossRef]
- Bell, S.W.; Kempson, R.L.; Hendrickson, M.R. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am. J. Surg. Pathol. 1994, 18, 535–558. [Google Scholar] [CrossRef] [PubMed]
- Kempson, R.L.; Hendrickson, M.R. Smooth muscle, endometrial stromal, and mixed Mullerian tumors of the uterus. Mod. Pathol. 2000, 13, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Zannoni, G.F. Uterine smooth muscle tumors of unknown malignant potential: A challenging question. Gynecol. Oncol. 2019, 154, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; International Agency for Research on Cancer; World Health Organization. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; World Health Organization Classification of Tumours; International Agency for Research on Cancer: Lyon, France, 2014; 307p. [Google Scholar]
- Peters, W.A., 3rd; Howard, D.R.; Andersen, W.A.; Figge, D.C. Uterine smooth-muscle tumors of uncertain malignant potential. Obstet. Gynecol. 1994, 83, 1015–1020. [Google Scholar] [CrossRef]
- Guntupalli, S.R.; Ramirez, P.T.; Anderson, M.L.; Milam, M.R.; Bodurka, D.C.; Malpica, A. Uterine smooth muscle tumor of uncertain malignant potential: A retrospective analysis. Gynecol. Oncol. 2009, 113, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Laury, A.L.; Nucci, M.R.; Quade, B.J. Predictors of adverse outcome in uterine smooth muscle tumours of uncertain malignant potential (STUMP): A clinicopathological analysis of 22 cases with a proposal for the inclusion of additional histological parameters. Histopathology 2018, 73, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, I.C.; Barbetakis, N.; Asteriou, C.G.; Voutsas, M.G. Uterine smooth muscle tumor of uncertain malignant potential: A rare cause of multiple pulmonary nodules. Indian J. Med. Paediatr. Oncol. 2012, 33, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef]
- Mas, A.; Nair, S.; Laknaur, A.; Simón, C.; Diamond, M.P.; Al-Hendy, A. Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil. Steril. 2015, 104, 225–234.e3. [Google Scholar] [CrossRef]
- Ono, M.; Qiang, W.; Serna, V.A.; Yin, P.; Coon, J.S.; Navarro, A.; Monsivais, D.; Kakinuma, T.; Dyson, M.; Druschitz, S.; et al. Role of Stem Cells in Human Uterine Leiomyoma Growth. PLoS ONE 2012, 7, e36935. [Google Scholar] [CrossRef]
- Yang, Q.; Ali, M.; Treviño, L.S.; Mas, A.; Al-Hendy, A. Developmental reprogramming of myometrial stem cells by endocrine disruptor linking to risk of uterine fibroids. Cell. Mol. Life Sci. 2023, 80, 274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ali, M.; Bariani, M.V.; Vafaei, S.; Al-Hendy, A. Endocrine-disrupting chemicals and epigenetic reprogramming in developmental origin of uterine fibroids. Sci. Prog. 2023, 106, 368504231215601. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2021, 43, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Mehine, M.; Mäkinen, N.; Heinonen, H.R.; Aaltonen, L.A.; Vahteristo, P. Genomics of uterine leiomyomas: Insights from high-throughput sequencing. Fertil. Steril. 2014, 102, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M. Smooth muscle tumors. In Modern Soft Tissue Pathology, 1st ed.; Cambridge University Press: New York, NY, USA, 2010; pp. 460–490. [Google Scholar]
- Chen, L.; Li, J.; Wu, X.; Zheng, Z. Identification of Somatic Genetic Alterations Using Whole-Exome Sequencing of Uterine Leiomyosarcoma Tumors. Front. Oncol. 2021, 11, 687899. [Google Scholar] [CrossRef] [PubMed]
- Al Ansari, A.A.; Al Hail, F.A.; Abboud, E. Malignant transformation of uterine leiomyoma. Qatar Med. J. 2012, 2012, 71–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolan, C.; Caserta, M.P. MR imaging of atypical fibroids. Abdom. Imaging 2016, 41, 2332–2349. [Google Scholar] [CrossRef] [PubMed]
- Major, F.J.; Blessing, J.A.; Silverberg, S.G.; Morrow, C.P.; Creasman, W.T.; Currie, J.L.; Yordan, E.; Brady, M.F. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group Study. Cancer 1993, 71 (Suppl. S4), 1702–1709. [Google Scholar] [CrossRef]
- Shalaby, S.; Khater, M.; Laknaur, A.; Arbab, A.; Al-Hendy, A. Molecular Bio-Imaging Probe for Non-Invasive Differentiation Between Human Leiomyoma Versus Leiomyosarcoma. Reprod. Sci. 2020, 27, 644–654. [Google Scholar] [CrossRef]
- Bharambe, B.M.; Deshpande, K.A.; Surase, S.G.; Ajmera, A.P. Malignant transformation of leiomyoma of uterus to leiomyosarcoma with metastasis to ovary. J. Obstet. Gynaecol. India 2014, 64, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Piper, I.; Woliovitch, I.; Glezerman, M. Age-related prevalence of sonographicaly confirmed uterine myomas. J. Obstet. Gynaecol. 2005, 25, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Machado-Lopez, A.; Alonso, R.; Lago, V.; Jimenez-Almazan, J.; Garcia, M.; Monleon, J.; Lopez, S.; Barcelo, F.; Torroba, A.; Ortiz, S.; et al. Integrative Genomic and Transcriptomic Profiling Reveals a Differential Molecular Signature in Uterine Leiomyoma versus Leiomyosarcoma. Int. J. Mol. Sci. 2022, 23, 2190. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Latif, N.A.; Ko, E.M.; Cory, L.; Kim, S.H.; Martin, L.; Simpkins, F.; Giuntoli, R. Next generation sequencing reveals a high prevalence of pathogenic mutations in homologous recombination DNA damage repair genes among patients with uterine sarcoma. Gynecol. Oncol. 2023, 177, 14–19. [Google Scholar] [CrossRef]
- Choi, J.; Manzano, A.; Dong, W.; Bellone, S.; Bonazzoli, E.; Zammataro, L.; Yao, X.; Deshpande, A.; Zaidi, S.; Guglielmi, A.; et al. Integrated mutational landscape analysis of uterine leiomyosarcomas. Proc. Natl. Acad. Sci. USA 2021, 118, e2025182118. [Google Scholar] [CrossRef]
- D’Agostino, E.; Mastrodomenico, L.; Ponzoni, O.; Baldessari, C.; Piombino, C.; Pipitone, S.; Vitale, M.G.; Sabbatini, R.; Dominici, M.; Toss, A. Molecular characterization as new driver in prognostic signatures and therapeutic strategies for endometrial cancer. Cancer Treat. Rev. 2024, 126, 102723. [Google Scholar] [CrossRef] [PubMed]
- Dermawan, J.K.; Kelly, C.; Gao, Z.; Smith, S.; Jadeja, B.; Singer, S.; Tap, W.D.; Chi, P.; Antonescu, C.R. Novel Genomic Risk Stratification Model for Primary Gastrointestinal Stromal Tumors (GIST) in the Adjuvant Therapy Era. Clin. Cancer Res. 2023, 29, 3974–3985. [Google Scholar] [CrossRef]
- Dermawan, J.K.; Chiang, S.; Singer, S.; Jadeja, B.; Hensley, M.L.; Tap, W.D.; Movva, S.; Maki, R.G.; Antonescu, C.R. Developing Novel Genomic Risk Stratification Models in Soft Tissue and Uterine Leiomyosarcoma. Clin. Cancer Res. 2024, 30, 2260–2271. [Google Scholar] [CrossRef]
- Li, X.; Heyer, W.-D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef]
- Seligson, N.D.; Kautto, E.A.; Passen, E.N.; Stets, C.; Toland, A.E.; Millis, S.Z.; Meyer, C.F.; Hays, J.L.; Chen, J.L. BRCA1/2 Functional Loss Defines a Targetable Subset in Leiomyosarcoma. Oncologist 2019, 24, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Huh, M.S.; Ivanochko, D.; E Hashem, L.; Curtin, M.; Delorme, M.; Goodall, E.; Yan, K.; Picketts, D.J. Stalled replication forks within heterochromatin require ATRX for protection. Cell Death Dis. 2016, 7, e2220. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Chavan, S.S.; Solit, D.B.; Murali, R.; Soslow, R.; Chiang, S.; Jungbluth, A.A.; Bandlamudi, C.; Srinivasan, P.; Tap, W.D.; et al. Genomic Landscape of Uterine Sarcomas Defined Through Prospective Clinical Sequencing. Clin. Cancer Res. 2020, 26, 3881–3888. [Google Scholar] [CrossRef] [PubMed]
- Harold, J.; Bellone, S.; Manavella, D.D.; Mutlu, L.; McNamara, B.; Hartwich, T.M.P.; Zipponi, M.; Yang-Hartwich, Y.; Demirkiran, C.; Verzosa, M.S.; et al. Elimusertib (BAY1895344), a novel ATR inhibitor, demonstrates in vivo activity in ATRX mutated models of uterine leiomyosarcoma. Gynecol. Oncol. 2023, 168, 157–165. [Google Scholar] [CrossRef]
- Dall, G.; Vandenberg, C.J.; Nesic, K.; Ratnayake, G.; Zhu, W.; Vissers, J.H.A.; Bedő, J.; Penington, J.; Wakefield, M.J.; Kee, D.; et al. Targeting homologous recombination deficiency in uterine leiomyosarcoma. J. Exp. Clin. Cancer Res. 2023, 42, 112. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Genes Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef] [PubMed]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, T.; Lin, J.; Huang, X.; Ke, Q.; Wu, Y.; Fang, C.; Hu, C. Curcumin inhibits the invasion and metastasis of triple negative breast cancer via Hedgehog/Gli1 signaling pathway. J. Ethnopharmacol. 2022, 283, 114689. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; Bozzini, N.; Baiocchi, G.; Da Cunha, I.W.; Maciel, G.A.; Junior, J.M.S.; Soares, F.A.; Baracat, E.C.; Carvalho, K.C. May Sonic Hedgehog proteins be markers for malignancy in uterine smooth muscle tumors? Hum. Pathol. 2016, 50, 43–50. [Google Scholar] [CrossRef]
- Zhao, H.; Li, N.; Pang, Y.; Zhao, J.; Wu, X. Gli affects the stemness and prognosis of epithelial ovarian cancer via homeobox protein NANOG. Mol. Med. Rep. 2020, 23, 1. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Kaylani, S.Z.; Edrees, N.; Li, C.; Talwelkar, S.S.; Xu, J.; Palle, K.; Pressey, J.G.; Athar, M. GLI inhibitor GANT-61 diminishes embryonal and alveolar rhabdomyosarcoma growth by inhibiting Shh/AKT-mTOR axis. Oncotarget 2014, 5, 12151–12165. [Google Scholar] [CrossRef] [PubMed]
- Mullard, M.; Cadé, M.; Morice, S.; Dupuy, M.; Danieau, G.; Amiaud, J.; Renault, S.; Lézot, F.; Brion, R.; Thepault, R.A.; et al. Sonic Hedgehog Signature in Pediatric Primary Bone Tumors: Effects of the GLI Antagonist GANT61 on Ewing’s Sarcoma Tumor Growth. Cancers 2020, 12, 3438. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; Ulin, M.; Ali, M.; Al-Hendy, A.; Carvalho, K.C.; Yang, Q. Evaluation of Hedgehog Pathway Inhibitors as a Therapeutic Option for Uterine Leiomyosarcoma Using the Xenograft Model. Reprod. Sci. 2022, 29, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; Al-Hendy, A.; Baracat, E.C.; Carvalho, K.C.; Yang, Q. Targeting Hedgehog Pathway and DNA Methyltransferases in Uterine Leiomyosarcoma Cells. Cells 2020, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; Ulin, M.; Al-Hendy, A.; Yang, Q. The Role of Hedgehog Pathway in Female Cancers. J. Cancer Sci. Clin. Ther. 2020, 4, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Patni, A.P.; Harishankar, M.K.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma—Clinical implications. Cell. Oncol. 2021, 44, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Sánchez, A.; Suárez-Martínez, E.; Sánchez-Díaz, L.; Carnero, A. Therapeutic Targeting of Signaling Pathways Related to Cancer Stemness. Front. Oncol. 2020, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Po, A.; Silvano, M.; Miele, E.; Capalbo, C.; Eramo, A.; Salvati, V.; Todaro, M.; Besharat, Z.M.; Catanzaro, G.; Cucchi, D.; et al. Noncanonical GLI1 signaling promotes stemness features and in vivo growth in lung adenocarcinoma. Oncogene 2017, 36, 4641–4652. [Google Scholar] [CrossRef]
- Kogerman, P.; Grimm, T.; Kogerman, L.; Krause, D.; Undén, A.B.; Sandstedt, B.; Toftgård, R.; Zaphiropoulos, P.G. Mammalian Suppressor-of-Fused modulates nuclear–cytoplasmic shuttling of GLI-1. Nature 1999, 1, 312–319. [Google Scholar] [CrossRef]
- Humke, E.W.; Dorn, K.V.; Milenkovic, L.; Scott, M.P.; Rohatgi, R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010, 24, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Tukachinsky, H.; Lopez, L.V.; Salic, A. A mechanism for vertebrate Hedgehog signaling: Recruitment to cilia and dissociation of SuFu-Gli protein complexes. J. Cell Biol. 2010, 191, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.; Cho, J. Hedgehog Pathway Inhibitors as Targeted Cancer Therapy and Strategies to Overcome Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1733. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, M.; Masuelli, L.; De Smaele, E.; Fantini, M.; Mattera, R.; Cucchi, D.; Bonanno, E.; Di Stefano, E.; Frajese, G.V.; Orlandi, A.; et al. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget 2016, 7, 9250–9270. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, V.; Boos, J.; Lanvers-Kaminsky, C. Targeting hedgehog signaling pathway in pediatric tumors: In vitro evaluation of SMO and GLI inhibitors. Cancer Chemother. Pharmacol. 2016, 77, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-H.; Huang, R.-L.; Lai, H.-C.; Chen, L.-Y.; Weng, Y.-C.; Wang, C.-C.; Wu, C.-C. NKX6-1 mediates cancer stem-like properties and regulates sonic hedgehog signaling in leiomyosarcoma. J. Biomed. Sci. 2021, 28, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, S.; Tian, Y.; Salwen, H.R.; Chlenski, A.; Weinstein, J.; Cohn, S.L. Methylation-associated silencing of the thrombospondin-1 gene in human neuroblastoma. Cancer Res. 2003, 63, 6299–6310. [Google Scholar] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A.; Trucco, M.M.; Subhawong, T.K.; Florou, V.; Park, W.; Kwon, D.; Wieder, E.D.; Kolonias, D.; Rosenberg, A.E.; Kerr, D.A.; et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: A single-centre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Mackay, H.; Buckanovich, R.; Hirte, H.; Correa, R.; Hoskins, P.; Biagi, J.; Martin, L.; Fleming, G.; Morgan, R.; Wang, L.; et al. A phase II study single agent of aflibercept (VEGF Trap) in patients with recurrent or metastatic gynecologic carcinosarcomas and uterine leiomyosarcoma. A trial of the Princess Margaret Hospital, Chicago and California Cancer Phase II Consortia. Gynecol. Oncol. 2012, 125, 136–140. [Google Scholar] [CrossRef]
- Ren, W.; Korchin, B.; Lahat, G.; Wei, C.; Bolshakov, S.; Nguyen, T.; Merritt, W.; Dicker, A.; Lazar, A.; Sood, A.; et al. Combined Vascular Endothelial Growth Factor Receptor/Epidermal Growth Factor Receptor Blockade with Chemotherapy for Treatment of Local, Uterine, and Metastatic Soft Tissue Sarcoma. Clin. Cancer Res. 2008, 14, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- De Wispelaere, W.; Annibali, D.; Tuyaerts, S.; Lambrechts, D.; Amant, F. Resistance to Immune Checkpoint Blockade in Uterine Leiomyosarcoma: What Can We Learn from Other Cancer Types? Cancers 2021, 13, 2040. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Wei, Y.-Q.; Wei, X.-W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, M.; Bolognesi, M.M.; Antoranz, A.; Mancari, R.; Carinelli, S.; Faretta, M.; Bosisio, F.M.; Cattoretti, G. The Adaptive and Innate Immune Cell Landscape of Uterine Leiomyosarcomas. Sci. Rep. 2020, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, E.; Barysauskas, C.M.; Solomon, S.; Tahlil, K.; Malley, R.; Hohos, M.; Polson, K.; Loucks, M.; Severgnini, M.; Patel, T.; et al. Immunotherapy with Single Agent Nivolumab for Advanced Leiomyosarcoma of the Uterus: Results of a Phase 2 Study. Cancer 2017, 123, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Bhanumathy, K.K.; Balagopal, A.; Vizeacoumar, F.S.; Vizeacoumar, F.J.; Freywald, A.; Giambra, V. Protein Tyrosine Kinases: Their Roles and Their Targeting in Leukemia. Cancers 2021, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yokoi, A.; Yamamoto, T.; Hayashi, Y.; Nakayama, J.; Yokoi, T.; Yoshida, H.; Kato, T.; Kajiyama, H.; Yamamoto, Y. Aberrant Activation of Cell-Cycle-Related Kinases and the Potential Therapeutic Impact of PLK1 or CHEK1 Inhibition in Uterine Leiomyosarcoma. Clin. Cancer Res. 2022, 28, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Kawamura, K.; Sato, W.; Kawamura, N.; Fujimoto, T.; Terada, Y. Inhibition of Uterine Sarcoma Cell Growth through Suppression of Endogenous Tyrosine Kinase B Signaling. PLoS ONE 2012, 7, e41049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raish, M.; Khurshid, M.; Ansari, M.A.; Chaturvedi, P.K.; Bae, S.-M.; Kim, J.H.; Park, E.K.; Park, D.C.; Ahn, W.S. Analysis of molecular cytogenetic alterations in uterine leiomyosarcoma by array-based comparative genomic hybridization. J. Cancer Res. Clin. Oncol. 2012, 138, 1173–1186. [Google Scholar] [CrossRef]
- Cuppens, T.; Moisse, M.; Depreeuw, J.; Annibali, D.; Colas, E.; Gil-Moreno, A.; Huvila, J.; Carpén, O.; Zikán, M.; Matias-Guiu, X.; et al. Integrated genome analysis of uterine leiomyosarcoma to identify novel driver genes and targetable pathways. Int. J. Cancer 2017, 142, 1230–1243. [Google Scholar] [CrossRef]
- Quan, P.; Moinfar, F.; Kufferath, I.; Absenger, M.; Kueznik, T.; Denk, H.; Zatloukal, K.; Haybaeck, J. Effects of Targeting Endometrial Stromal Sarcoma Cells via Histone Deacetylase and PI3K/AKT/mTOR Signaling. Anticancer Res. 2014, 34, 2883–2897. [Google Scholar]
- Benson, C.; Ray-Coquard, I.; Sleijfer, S.; Litière, S.; Blay, J.-Y.; Le Cesne, A.; Papai, Z.; Judson, I.; Schöffski, P.; Chawla, S.; et al. Outcome of uterine sarcoma patients treated with pazopanib: A retrospective analysis based on two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) clinical trials 62043 and 62072. Gynecol. Oncol. 2016, 142, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, I.; Shahzad, M.N.; Hosseinifard, H.; Liu, S.; Sefidan, M.O.; Kahloon, L.E.; Imani, S.; Hua, Z.; Zhang, Y.Q. Evaluation of the efficacy of systemic therapy for advanced uterine leiomyosarcoma: A systematic review, meta-analysis, and meta-regression analysis. Cancer Med. 2023, 12, 13894–13911. [Google Scholar] [CrossRef]
- McLeod, F.; Bossio, A.; Marzo, A.; Ciani, L.; Sibilla, S.; Hannan, S.; Wilson, G.A.; Palomer, E.; Smart, T.G.; Gibb, A.; et al. Wnt Signaling Mediates LTP-Dependent Spine Plasticity and AMPAR Localization through Frizzled-7 Receptors. Cell Rep. 2018, 23, 1060–1071. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, X.; Chen, P.; Liu, L.; Xin, N.; Tian, Y.; Dillin, A. The Mitochondrial Unfolded Protein Response Is Mediated Cell-Non-autonomously by Retromer-Dependent Wnt Signaling. Cell 2018, 174, 870–883.e17. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, M.; Holdsworth-Carson, S.J.; Waldrip, L.; Nevzorova, J.; Martelotto, L.; Vollenhoven, B.J.; Rogers, P.A.W. Aberrant expression and regulation of NR2F2 and CTNNB1 in uterine fibroids. Reproduction 2013, 146, 91–102. [Google Scholar] [CrossRef]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. Activation of beta-Catenin Signaling and its Crosstalk with Estrogen and Histone Deacetylases in Human Uterine Fibroids. J. Clin. Endocrinol. Metab. 2020, 105, e1517-35. [Google Scholar] [CrossRef] [PubMed]
- Kildal, W.; Pradhan, M.; Abeler, V.M.; Kristensen, G.B.; Danielsen, H.E. Beta-catenin expression in uterine sarcomas and its relation to clinicopathological parameters. Eur. J. Cancer 2009, 45, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Mineda, A.; Nakagawa, T.; Shinohara, A.; Arakaki, R.; Inui, H.; Noguchi, H.; Yoshida, A.; Kinouchi, R.; Yamamoto, Y.; et al. New treatment strategies for uterine sarcoma using secreted frizzled-related proteins. Exp. Ther. Med. 2024, 27, 231. [Google Scholar] [CrossRef]
- de Almeida, B.C.; dos Anjos, L.G.; Dobroff, A.S.; Baracat, E.C.; Yang, Q.; Al-Hendy, A.; Carvalho, K.C. Epigenetic Features in Uterine Leiomyosarcoma and Endometrial Stromal Sarcomas: An Overview of the Literature. Biomedicines 2022, 10, 2567. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Cancer Genome Atlas Research. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e28. [Google Scholar] [CrossRef] [PubMed]
- Sacdalan, D.B.; Haq, S.U.; Lok, B.H. Plasma Cell-Free Tumor Methylome as a Biomarker in Solid Tumors: Biology and Applications. Curr. Oncol. 2024, 31, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Lopez-Rodas, G.; Franco, L. Histone Post-Translational Modifications and Nucleosome Organisation in Transcriptional Regulation: Some Open Questions. Adv. Exp. Med. Biol. 2017, 966, 65–92. [Google Scholar] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Liu, J. Role of HDACs in normal and malignant hematopoiesis. Mol. Cancer 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Verdone, L.; Caserta, M.; Di Mauro, E. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 2005, 83, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed]
- D’Oto, A.; Tian, Q.-W.; Davidoff, A.M.; Yang, J. Histone Demethylases and Their Roles in Cancer Epigenetics. J. Med. Oncol. Ther. 2016, 1, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Chakravarti, D. A Peek into the Complex Realm of Histone Phosphorylation. Mol. Cell. Biol. 2011, 31, 4858–4873. [Google Scholar] [CrossRef]
- Gong, F.; Miller, K.M. Histone methylation and the DNA damage response. Mutat. Res. Mol. Mech. Mutagen. 2019, 780, 37–47. [Google Scholar] [CrossRef]
- Mlodawska, O.W.; Saini, P.; Parker, J.B.; Wei, J.-J.; E Bulun, S.; A Simon, M.; Chakravarti, D. Epigenomic and enhancer dysregulation in uterine leiomyomas. Hum. Reprod. Updat. 2022, 28, 518–547. [Google Scholar] [CrossRef]
- Islam, S.; Protic, O.; Stortoni, P.; Grechi, G.; Lamanna, P.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil. Steril. 2013, 100, 178–193. [Google Scholar] [CrossRef]
- Yang, Q.; Falahati, A.; Khosh, A.; Mohammed, H.; Kang, W.; Corachán, A.; Bariani, M.V.; Boyer, T.G.; Al-Hendy, A. Targeting Class I Histone Deacetylases in Human Uterine Leiomyosarcoma. Cells 2022, 11, 3801. [Google Scholar] [CrossRef]
- Baek, M.-H.; Park, J.-Y.; Park, Y.; Kim, K.-R.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. The combination of histone deacetylase and p53 expressions and histological subtype has prognostic implication in uterine leiomyosarcoma. Jpn. J. Clin. Oncol. 2019, 49, 719–726. [Google Scholar] [CrossRef]
- de Leval, L.; Waltregny, D.; Boniver, J.; Young, R.H.; Castronovo, V.; Oliva, E. Use of Histone Deacetylase 8 (HDAC8), a New Marker of Smooth Muscle Differentiation, in the Classification of Mesenchymal Tumors of the Uterus. Am. J. Surg. Pathol. 2006, 30, 319–327. [Google Scholar] [CrossRef]
- Lou, S.; Balluff, B.; de Graaff, M.A.; Cleven, A.H.; de Bruijn, I.B.; Bovée, J.V.; McDonnell, L.A. High-grade sarcoma diagnosis and prognosis: Biomarker discovery by mass spectrometry imaging. Proteomics 2016, 16, 1802–1813. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Ivan, C.; Huang, J.; Shen, D.-Y.; Kavanagh, J.J.; Bast, R.C.; Fu, S.; Hu, W.; Sood, A.K. Enhanced Cytotoxic Effects of Combined Valproic Acid and the Aurora Kinase Inhibitor VE465 on Gynecologic Cancer Cells. Front. Oncol. 2013, 3, 41997. [Google Scholar] [CrossRef]
- Hrzenjak, A.; Moinfar, F.; Kremser, M.-L.; Strohmeier, B.; Petru, E.; Zatloukal, K.; Denk, H. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol. Cancer 2010, 9, 49. [Google Scholar] [CrossRef]
- Sampson, E.R.; Amin, V.; Schwarz, E.M.; O’Keefe, R.J.; Rosier, R.N. The histone deacetylase inhibitor vorinostat selectively sensitizes fibrosarcoma cells to chemotherapy. J. Orthop. Res. 2011, 29, 623–632. [Google Scholar] [CrossRef]
- Choy, E.; Flamand, Y.; Balasubramanian, S.; Butrynski, J.E.; Harmon, D.C.; George, S.; Cote, G.M.; Wagner, A.J.; Morgan, J.A.; Sirisawad, M.; et al. Phase 1 study of oral abexinostat, a histone deacetylase inhibitor, in combination with doxorubicin in patients with metastatic sarcoma. Cancer 2014, 121, 1223–1230. [Google Scholar] [CrossRef]
- Tavallai, S.; A Hamed, H.; Grant, S.; Poklepovic, A.; Dent, P. Pazopanib and HDAC inhibitors interact to kill sarcoma cells. Cancer Biol. Ther. 2014, 15, 578–585. [Google Scholar] [CrossRef]
- Mastoraki, A.; Schizas, D.; Vlachou, P.; Melissaridou, N.M.; Charalampakis, N.; Fioretzaki, R.; Kole, C.; Savvidou, O.; Vassiliu, P.; Pikoulis, E. Assessment of Synergistic Contribution of Histone Deacetylases in Prognosis and Therapeutic Management of Sarcoma. Mol. Diagn. Ther. 2020, 24, 557–569. [Google Scholar] [CrossRef]
- Choy, E.; Ballman, K.; Chen, J.; Dickson, M.A.; Chugh, R.; George, S.; Okuno, S.; Pollock, R.; Patel, R.M.; Hoering, A.; et al. SARC018_SPORE02: Phase II Study of Mocetinostat Administered with Gemcitabine for Patients with Metastatic Leiomyosarcoma with Progression or Relapse following Prior Treatment with Gemcitabine-Containing Therapy. Sarcoma 2018, 2018, 2068517. [Google Scholar] [CrossRef]
- Monga, V.; Swami, U.; Tanas, M.; Bossler, A.; Mott, S.L.; Smith, B.J.; Milhem, M. A Phase I/II Study Targeting Angiogenesis Using Bevacizumab Combined with Chemotherapy and a Histone Deacetylase Inhibitor (Valproic Acid) in Advanced Sarcomas. Cancers 2018, 10, 53. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chao, A.; Wu, R.-C.; Lee, L.-Y.; Ueng, S.-H.; Tsai, C.-L.; Lee, Y.-S.; Peng, M.-T.; Yang, L.-Y.; Huang, H.-J.; et al. Synergistic effects of pazopanib and hyperthermia against uterine leiomyosarcoma growth mediated by downregulation of histone acetyltransferase 1. J. Mol. Med. 2020, 98, 1175–1188. [Google Scholar] [CrossRef]
- Yang, Q.; Vafaei, S.; Falahati, A.; Khosh, A.; Bariani, M.V.; Omran, M.M.; Bai, T.; Siblini, H.; Ali, M.; He, C.; et al. Bromodomain-Containing Protein 9 Regulates Signaling Pathways and Reprograms the Epigenome in Immortalized Human Uterine Fibroid Cells. Int. J. Mol. Sci. 2024, 25, 905. [Google Scholar] [CrossRef]
- Yang, Q.; Falahati, A.; Khosh, A.; Vafaei, S.; Al-Hendy, A. Targeting Bromodomain-Containing Protein 9 in Human Uterine Fibroid Cells. Reprod. Sci. 2024, 31. [Google Scholar] [CrossRef]
- Jain, A.K.; Barton, M.C. Bromodomain Histone Readers and Cancer. J. Mol. Biol. 2017, 429, 2003–2010. [Google Scholar] [CrossRef]
- Yang, Q.; Bariani, M.V.; Falahati, A.; Khosh, A.; Lastra, R.R.; Siblini, H.; Boyer, T.G.; Al-Hendy, A. The Functional Role and Regulatory Mechanism of Bromodomain-Containing Protein 9 in Human Uterine Leiomyosarcoma. Cells 2022, 11, 2160. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef]
- Psilopatis, I.; Vrettou, K.; Kokkali, S.; Theocharis, S. The Role of MicroRNAs in Uterine Leiomyosarcoma Diagnosis and Treatment. Cancers 2023, 15, 2420. [Google Scholar] [CrossRef]
- Yoshida, K.; Yokoi, A.; Kitagawa, M.; Sugiyama, M.; Yamamoto, T.; Nakayama, J.; Yoshida, H.; Kato, T.; Kajiyama, H.; Yamamoto, Y. Downregulation of miR-10b-5p facilitates the proliferation of uterine leiomyosarcoma cells: A microRNA sequencing-based approach. Oncol. Rep. 2023, 49, 86. [Google Scholar] [CrossRef]
- Danielson, L.S.; Menendez, S.; Attolini, C.S.-O.; Guijarro, M.V.; Bisogna, M.; Wei, J.; Socci, N.D.; Levine, D.A.; Michor, F.; Hernando, E. A Differentiation-Based MicroRNA Signature Identifies Leiomyosarcoma as a Mesenchymal Stem Cell-Related Malignancy. Am. J. Pathol. 2010, 177, 908–917. [Google Scholar] [CrossRef]
- de Almeida, B.C.; Garcia, N.; Maffazioli, G.; Gonzalez Dos Anjos, L.; Chada Baracat, E.; Candido Carvalho, K. Oncomirs expression profiling in uterine leiomyosarcoma cells. Int. J. Mol. Sci. 2017, 19, 52. [Google Scholar] [CrossRef]
- Conconi, D.; Chiappa, V.; Perego, P.; Redaelli, S.; Bovo, G.; Lavitrano, M.; Milani, R.; Dalprà, L.; Lissoni, A.A. Potential role of BCL2 in the recurrence of uterine smooth muscle tumors of uncertain malignant potential. Oncol. Rep. 2017, 37, 41–47. [Google Scholar] [CrossRef]
- Chuang, T.-D.; Ho, M.; Khorram, O. The Regulatory Function of miR-200c on Inflammatory and Cell-Cycle Associated Genes in SK-LMS-1, A Leiomyosarcoma Cell Line. Reprod. Sci. 2015, 22, 563–571. [Google Scholar] [CrossRef]
- Danielson, L.S.; Guijarro, M.V.; Menendez, S.; Higgins, B.; Sun, Q.; Mittal, K.; Popiolek, D.A.; Overholtzer, M.; Palmer, G.D.; Hernando, E. MiR-130b modulates the invasive, migratory, and metastatic behavior of leiomyosarcoma. PLoS ONE 2023, 18, e0278844. [Google Scholar] [CrossRef]
- de Almeida, B.C.; dos Anjos, L.G.; Uno, M.; da Cunha, I.W.; Soares, F.A.; Baiocchi, G.; Baracat, E.C.; Carvalho, K.C. Let-7 miRNA’s Expression Profile and Its Potential Prognostic Role in Uterine Leiomyosarcoma. Cells 2019, 8, 1452. [Google Scholar] [CrossRef]
- Kojima, S.; Chiyomaru, T.; Kawakami, K.; Yoshino, H.; Enokida, H.; Nohata, N.; Fuse, M.; Ichikawa, T.; Naya, Y.; Nakagawa, M.; et al. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br. J. Cancer 2012, 106, 405–413. [Google Scholar] [CrossRef]
- Weiss, M.; Brandenburg, L.-O.; Burchardt, M.; Stope, M.B. MicroRNA-1 properties in cancer regulatory networks and tumor biology. Crit. Rev. Oncol. Hematol. 2016, 104, 71–77. [Google Scholar] [CrossRef]
- Stope, M.B.; Cernat, V.; Kaul, A.; Diesing, K.; Koensgen, D.; Burchardt, M.; Mustea, A. Functionality of the Tumor Suppressor microRNA-1 in Malignant Tissue and Cell Line Cells of Uterine Leiomyosarcoma. Anticancer. Res. 2018, 38, 1547–1550. [Google Scholar] [CrossRef]
- Gkioka, E.; Msaouel, P.; Philippou, A.; I Vlaghogiannis, N.; Vogkou, C.T.; Margiolis, A.; Koutsilieris, M. Review: The Role of Insulin-like Growth Factor-1 Signaling Pathways in Uterine Leiomyoma. In Vivo 2015, 29, 637–649. [Google Scholar]
- Tsuruta, T.; Kozaki, K.I.; Uesugi, A.; Furuta, M.; Hirasawa, A.; Imoto, I.; Susumu, N.; Aoki, D.; Inazawa, J. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011, 71, 6450–6462. [Google Scholar] [CrossRef]
- Wang, Z.-N.; Zhou, X.; Zhao, F.; Song, Y.-X.; Chang, H.; Chiang, Y.; Xu, H.-M. Altered expression of miR-152 and miR-148a in ovarian cancer is related to cell proliferation. Oncol. Rep. 2012, 27, 447–454. [Google Scholar] [CrossRef][Green Version]
- Lahat, G.; Zhang, P.; Zhu, Q.-S.; Torres, K.; Ghadimi, M.; Smith, K.D.; Wang, W.-L.; Lazar, A.J.; Lev, D. The expression of c-Met pathway components in unclassified pleomorphic sarcoma/malignant fibrous histiocytoma (UPS/MFH): A tissue microarray study. Histopathology 2011, 59, 556–561. [Google Scholar] [CrossRef]
- Ravid, Y.; Formanski, M.; Smith, Y.; Reich, R.; Davidson, B. Uterine leiomyosarcoma and endometrial stromal sarcoma have unique miRNA signatures. Gynecol. Oncol. 2016, 140, 512–517. [Google Scholar] [CrossRef]
- Wiemer, E.A.; Wozniak, A.; Burger, H.; Smid, M.; Floris, G.; Nzokirantevye, A.; Sciot, R.; Sleijfer, S.; Schöffski, P. Identification of microRNA biomarkers for response of advanced soft tissue sarcomas to eribulin: Translational results of the EORTC 62052 trial. Eur. J. Cancer 2017, 75, 33–40. [Google Scholar] [CrossRef]
- Agostini, M.; Knight, R.A. miR-34: From bench to bedside. Oncotarget 2014, 5, 872–881. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.; He, C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015, 29, 1343–1355. [Google Scholar] [CrossRef]
- Lv, D.; Zhong, C.; Dixit, D.; Yang, K.; Wu, Q.; Godugu, B.; Prager, B.C.; Zhao, G.; Wang, X.; Xie, Q.; et al. EGFR promotes ALKBH5 nuclear retention to attenuate N6-methyladenosine and protect against ferroptosis in glioblastoma. Mol. Cell 2023, 83, 4334–4351.e7. [Google Scholar] [CrossRef]
- Choe, J.; Lin, S.; Zhang, W.; Liu, Q.; Wang, L.; Ramirez-Moya, J.; Du, P.; Kim, W.; Tang, S.; Sliz, P.; et al. mRNA circularization by METTL3–eIF3h enhances translation and promotes oncogenesis. Nature 2018, 561, 556–560. [Google Scholar] [CrossRef]
- Barbieri, I.; Tzelepis, K.; Pandolfini, L.; Shi, J.; Millán-Zambrano, G.; Robson, S.C.; Aspris, D.; Migliori, V.; Bannister, A.J.; Han, N.; et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature 2017, 552, 126–131. [Google Scholar] [CrossRef]
- Vu, L.P.; Pickering, B.F.; Cheng, Y.; Zaccara, S.; Nguyen, D.; Minuesa, G.; Chou, T.; Chow, A.; Saletore, Y.; Mackay, M.; et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 2017, 23, 1369–1376. [Google Scholar] [CrossRef]
- Knuckles, P.; Carl, S.H.; Musheev, M.; Niehrs, C.; Wenger, A.; Bühler, M. RNA fate determination through cotranscriptional adenosine methylation and microprocessor binding. Nat. Struct. Mol. Biol. 2017, 24, 561–569. [Google Scholar] [CrossRef]
- Wei, J.; Liu, F.; Lu, Z.; Fei, Q.; Ai, Y.; He, P.C.; Shi, H.; Cui, X.; Su, R.; Klungland, A.; et al. Differential m(6)A, m(6)A(m), and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol. Cell 2018, 71, 973–985.e5. [Google Scholar] [CrossRef]
- Wei, J.; Yu, X.; Yang, L.; Liu, X.; Gao, B.; Huang, B.; Dou, X.; Liu, J.; Zou, Z.; Cui, X.-L.; et al. FTO mediates LINE1 m 6 A demethylation and chromatin regulation in mESCs and mouse development. Science 2022, 376, 968–973. [Google Scholar] [CrossRef]
- Zou, Z.; Sepich-Poore, C.; Zhou, X.; Wei, J.; He, C. The mechanism underlying redundant functions of the YTHDF proteins. Genome Biol. 2023, 24, 17. [Google Scholar] [CrossRef]
- Panneerdoss, S.; Eedunuri, V.K.; Yadav, P.; Timilsina, S.; Rajamanickam, S.; Viswanadhapalli, S.; Abdelfattah, N.; Onyeagucha, B.C.; Cui, X.; Rao, M.K. Cross-talk among writers, readers, and erasers of m(6)A regulates cancer growth and progression. Sci. Adv. 2018, 4, eaar8263. [Google Scholar] [CrossRef]
- Berlivet, S.; Scutenaire, J.; Deragon, J.-M.; Bousquet-Antonelli, C. Readers of the m6A epitranscriptomic code. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2019, 1862, 329–342. [Google Scholar] [CrossRef]
- Schmiechen, A.C.; Batista, P.J. An epitranscriptomic vulnerability in myeloid malignancies. Nat. Med. 2017, 23, 1252–1254. [Google Scholar] [CrossRef]
- Shen, S.; Faouzi, S.; Bastide, A.; Martineau, S.; Malka-Mahieu, H.; Fu, Y.; Sun, X.; Mateus, C.; Routier, E.; Roy, S.; et al. An epitranscriptomic mechanism underlies selective mRNA translation remodelling in melanoma persister cells. Nat. Commun. 2019, 10, 5713. [Google Scholar] [CrossRef]
- Relier, S.; Rivals, E.; David, A. The multifaceted functions of the Fat mass and Obesity-associated protein (FTO) in normal and cancer cells. RNA Biol. 2022, 19, 132–142. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Deng, H.; Xu, B.; Zhou, Y.; Liu, J.; Liu, Y.; Shi, Y.; Zheng, X.; Jiang, J. N6-methyladenosine demethylase FTO promotes growth and metastasis of gastric cancer via m6A modification of caveolin-1 and metabolic regulation of mitochondrial dynamics. Cell Death Dis. 2022, 13, 72. [Google Scholar] [CrossRef]
- Yang, Q.; Al-Hendy, A. The Functional Role and Regulatory Mechanism of FTO M6A RNA Demethylase In Human Uterine Leiomyosarcoma. J. Endocr. Soc. 2023, 7, bvad114.2202. [Google Scholar] [CrossRef]
- Sparić, R.; Andjić, M.; Babović, I.; Nejković, L.; Mitrović, M.; Štulić, J.; Pupovac, M.; Tinelli, A. Molecular Insights in Uterine Leiomyosarcoma: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9728. [Google Scholar] [CrossRef]
- Asano, H.; Isoe, T.; Ito, Y.M.; Nishimoto, N.; Watanabe, Y.; Yokoshiki, S.; Watari, H. Status of the Current Treatment Options and Potential Future Targets in Uterine Leiomyosarcoma: A Review. Cancers 2022, 14, 1180. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef]
- Kapp, D.S.; Shin, J.Y.; Chan, J.K. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: Emphasis on impact of lymphadenectomy and oophorectomy. Cancer 2008, 112, 820–830. [Google Scholar] [CrossRef]
- Zivanovic, O.; Jacks, L.M.; Iasonos, A.; Leitao, M.M.; Soslow, R.A.; Veras, E.; Chi, D.S.; Abu-Rustum, N.R.; Barakat, R.R.; Brennan, M.F.; et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer 2012, 118, 660–669. [Google Scholar] [CrossRef]
- Leitao, M.M.; Sonoda, Y.; Brennan, M.F.; Barakat, R.R.; Chi, D.S. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol. Oncol. 2003, 91, 209–212. [Google Scholar] [CrossRef]
- Sagae, S.; Yamashita, K.; Ishioka, S.; Nishioka, Y.; Terasawa, K.; Mori, M.; Yamashiro, K.; Kanemoto, T.; Kudo, R. Preoperative Diagnosis and Treatment Results in 106 Patients with Uterine Sarcoma in Hokkaido, Japan. Oncology 2004, 67, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.R.; Thoresen, S.O. Uterine sarcomas in Norway 1956–1992: Incidence, survival and mortality. Eur. J. Cancer 1997, 33, 907–911. [Google Scholar] [CrossRef]
- Marchese, M.J.; Liskow, A.S.; Crum, C.P.; McCaffrey, R.M.; Frick, H.C., 2nd. Uterine sarcomas: A clinicopathologic study, 1965–1981. Gynecol. Oncol. 1984, 18, 299–312. [Google Scholar] [CrossRef]
- Abeler, V.M.; Røyne, O.; Thoresen, S.; E Danielsen, H.; Nesland, J.M.; Kristensen, G.B. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009, 54, 355–364. [Google Scholar] [CrossRef]
- Reed, N.S.; Mangioni, C.; Malmström, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.J.; Coens, C.; et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur. J. Cancer 2008, 44, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Giuntoli, R.L., 2nd; Eisenhauer, E.; Lopez, M.A.; Krill, L.; Tanner, E.J., 3rd; Gehrig, P.A.; Havrilesky, L.J.; Secord, A.A.; Levinson, K.; et al. Does adjuvant chemotherapy improve survival for women with early-stage uterine leiomyosarcoma? Gynecol. Oncol. 2013, 131, 629–633. [Google Scholar] [CrossRef]
- Omura, G.A.; Blessing, J.A.; Major, F.; Lifshitz, S.; E Ehrlich, C.; Mangan, C.; Beecham, J.; Park, R.; Silverberg, S. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: A Gynecologic Oncology Group Study. J. Clin. Oncol. 1985, 3, 1240–1245. [Google Scholar] [CrossRef]
- Hensley, M.L.; Enserro, D.; Hatcher, H.; Ottevanger, P.B.; Krarup-Hansen, A.; Blay, J.-Y.; Fisher, C.; Moxley, K.M.; Lele, S.B.; Lea, J.S.; et al. Adjuvant Gemcitabine Plus Docetaxel Followed by Doxorubicin Versus Observation for High-Grade Uterine Leiomyosarcoma: A Phase III NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2018, 36, JCO1800454. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, R.M.; Baker, L.H.; Gottlieb, J.E.; Rivkin, S.E.; Balcerzak, S.P.; Grumet, G.N.; Salmon, S.E.; Moon, T.E.; Hoogstraten, B. Dose response evaluation of adriamycin in human neoplasia. Cancer 1977, 39, 1940–1948. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Hensley, M.L.; Blessing, J.A.; Mannel, R.; Rose, P.G. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II trial. Gynecol. Oncol. 2008, 109, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Balleyguier, C.; Lebrun-Ly, V.; et al. Doxorubicin alone versus doxorubicin with trabectedin followed by trabectedin alone as first-line therapy for metastatic or unresectable leiomyosarcoma (LMS-04): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2022, 23, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Blessing, J.A.; DeGeest, K.; Abulafia, O.; Rose, P.G.; Homesley, H.D. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: A Gynecologic Oncology Group phase II study. Gynecol. Oncol. 2008, 109, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pautier, P.; Floquet, A.; Chevreau, C.; Penel, N.; Guillemet, C.; Delcambre, C.; Cupissol, D.; Selle, F.; Isambert, N.; Piperno-Neumann, S.; et al. Trabectedin in combination with doxorubicin for first-line treatment of advanced uterine or soft-tissue leiomyosarcoma (LMS-02): A non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Pousa, A.L.; Peñas, R.d.L.; del Muro, X.G.; Gutierrez, A.; Martinez-Trufero, J.; Cruz, J.; Alvarez, R.; Cubedo, R.; Redondo, A.; et al. Randomized Phase II Study of Trabectedin and Doxorubicin Compared with Doxorubicin Alone as First-Line Treatment in Patients with Advanced Soft Tissue Sarcomas: A Spanish Group for Research on Sarcoma Study. J. Clin. Oncol. 2016, 34, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf, W.T.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Schöffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase 3 trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- Shammas, N.; Yang, T.; Abidi, A.; Amneus, M.; Hodeib, M. Clinical use of PARP inhibitor in recurrent uterine leiomyosarcoma with presence of a somatic BRCA2 mutation. Gynecol. Oncol. Rep. 2022, 42, 101044. [Google Scholar] [CrossRef]
- Pan, M.; Ganjoo, K.; Karam, A. Rapid Response of a BRCA2/TP53/PTEN-Deleted Metastatic Uterine Leiomyosarcoma to Olaparib: A Case Report. Perm. J. 2021, 25, 251. [Google Scholar] [CrossRef]
- Ingham, M.; Allred, J.B.; Chen, L.; Das, B.; Kochupurakkal, B.; Gano, K.; George, S.; Attia, S.; Burgess, M.A.; Seetharam, M.; et al. Phase II Study of Olaparib and Temozolomide for Advanced Uterine Leiomyosarcoma (NCI Protocol 10250). J. Clin. Oncol. 2023, 41, 4154–4163. [Google Scholar] [CrossRef]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Mai, P.L.; Wentzensen, N.; Greene, M.H. Challenges related to developing serum-based biomarkers for early ovarian cancer detection. Cancer Prev. Res. 2011, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.; Ulin, M.; Yang, Q.; Ali, M.; Bosland, M.C.; Zeng, W.; Chen, L.; Al-Hendy, A. Survivin-Sodium Iodide Symporter Reporter as a Non-Invasive Diagnostic Marker to Differentiate Uterine Leiomyosarcoma from Leiomyoma. Cells 2023, 12, 2830. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, P.; Alibrahim, E.; Dobrotwir, A.; Paul, E.; Goergen, S. Multiparametric MR evaluation of uterine leiomyosarcoma and STUMP versus leiomyoma in symptomatic women planned for high frequency focussed ultrasound: Accuracy of imaging parameters and interobserver agreement for identification of malignancy. Br. J. Radiol. 2021, 94, 20200483. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Nagiri, C.; Shihoya, W.; Inoue, A.; Kawakami, K.; Hiratsuka, S.; Aoki, J.; Ito, Y.; Suzuki, T.; Suzuki, T.; et al. N6-methyladenosine (m6A) is an endogenous A3 adenosine receptor ligand. Mol. Cell 2021, 81, 659–674.e7. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Poudyal, B.; Yadollahi, S.; Wright, D.E.; Gregory, A.V.; Warner, J.D.; Korfiatis, P.; Green, I.C.; Rassier, S.L.; Mariani, A.; et al. A systematic review on the use of artificial intelligence in gynecologic imaging—Background, state of the art, and future directions. Gynecol. Oncol. 2022, 166, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Jayes, F.L.; Segars, J.H. The Extracellular Matrix Contributes to Mechanotransduction in Uterine Fibroids. Obstet. Gynecol. Int. 2014, 2014, 783289. [Google Scholar] [CrossRef]
- Rafique, S.; Segars, J.H.; Leppert, P.C. Mechanical SIgnaling and Extracellular Matrix in uterine fibroids. Semin. Reprod. Med. 2017, 35, 487–493. [Google Scholar] [CrossRef]
- Mitchell, C.N.C.; Islam, M.S.; Afrin, S.; Brennan, J.; Psoter, K.J.; Segars, J.H. Mechanical stiffness augments ligand-dependent progesterone receptor B activation via MEK 1/2 and Rho/rock–dependent signaling pathways in uterine fibroid cells. Fertil. Steril. 2021, 116, 255–265. [Google Scholar] [CrossRef]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.N. Validation of shear wave elastography in skeletal muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef]
- Chatzistergos, P.E.; Behforootan, S.; Allan, D.; Naemi, R.; Chockalingam, N. Shear wave elastography can assess the in-vivo nonlinear mechanical behavior of heel-pad. J. Biomech. 2018, 80, 144–150. [Google Scholar] [CrossRef]
- Cosgrove, D.O.; Berg, W.A.; Doré, C.J.; Skyba, D.M.; Henry, J.P.; Gay, J.; Cohen-Bacrie, C.; the BE1 Study Group. Shear wave elastography for breast masses is highly reproducible. Eur. Radiol. 2011, 22, 1023–1032. [Google Scholar] [CrossRef]
- Gennisson, J.-L.; Muller, M.; Ami, O.; Kohl, V.; Gabor, P.; Musset, D.; Tanter, M. Shear wave elastography in obstetrics: Quantification of cervix elasticity and uterine contraction. In Proceedings of the 2011 IEEE International Ultrasonics Symposium (IUS), Orlando, FL, USA, 18–21 October 2021; pp. 2094–2097. [Google Scholar]
- Furukawa, S.; Soeda, S.; Watanabe, T.; Nishiyama, H.; Fujimori, K. The measurement of stiffness of uterine smooth muscle tumor by elastography. SpringerPlus 2014, 3, 294. [Google Scholar] [CrossRef][Green Version]
- Frank, M.L.; Schäfer, S.D.; Möllers, M.; Falkenberg, M.K.; Braun, J.; Möllmann, U.; Strube, F.; Fruscalzo, A.; Amler, S.; Klockenbusch, W.; et al. Importance of Transvaginal Elastography in the Diagnosis of Uterine Fibroids and Adenomyosis. Ultraschall Med. Eur. J. Ultrasound 2015, 37, 373–378. [Google Scholar] [CrossRef]
- Wang, X.-L.; Lin, S.; Lyu, G.-R. Advances in the clinical application of ultrasound elastography in uterine imaging. Insights into Imaging 2022, 13, 141. [Google Scholar] [CrossRef]
- Reynolds, A.R.; Moschetta, M.; Yohannes, A.R.; Walcott, F.; Ashford, M.; Szucs, Z.; Sarbajna, T.; Hadfield, J.; Harrison, E.; Challis, B.G.; et al. A View on Drug Development for Cancer Prevention. Cancer Discov. 2023, 13, 1058–1083. [Google Scholar] [CrossRef]
- Sherman, J.D. Tamoxifen and Prevention of Breast Cancer. Toxicol. Ind. Health 1998, 14, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Maisonneuve, P.; Costa, A.; Sacchini, V.; Maltoni, C.; Robertson, C.; Rotmensz, N.; Boyle, P. Prevention of breast cancer with tamoxifen: Preliminary findings from the Italian randomised trial among hysterectomised women. Lancet 1998, 352, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C. Tamoxifen or Raloxifene in Postmenopausal Women for Prevention of Breast Cancer: A Tale of Two Choices—Counterpoint. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2210–2212. [Google Scholar] [CrossRef][Green Version]
- Young, D. Raloxifene examined for breast cancer prevention. Am. J. Health Pharm. 2007, 64, 1774. [Google Scholar] [CrossRef]
- Guan, M.; Wu, X.; Chu, P.; Chow, W.A. Fatty acid synthase reprograms the epigenome in uterine leiomyosarcomas. PLoS ONE 2017, 12, e0179692. [Google Scholar] [CrossRef]
- Shi, G.; Perle, M.A.; Mittal, K.; Chen, H.; Zou, X.; Narita, M.; Hernando, E.; Lee, P.; Wei, J. Let-7 repression leads to HMGA2 overexpression in uterine leiomyosarcoma. J. Cell. Mol. Med. 2009, 13, 3898–3905. [Google Scholar] [CrossRef]
- Pazzaglia, L.; Novello, C.; Conti, A.; Pollino, S.; Picci, P.; Benassi, M.S. miR-152 down-regulation is associated with MET up-regulation in leiomyosarcoma and undifferentiated pleomorphic sarcoma. Cell. Oncol. 2016, 40, 77–88. [Google Scholar] [CrossRef]
- Yokoi, A.; Matsuzaki, J.; Yamamoto, Y.; Tate, K.; Yoneoka, Y.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Takizawa, S.; Aoki, Y.; et al. Serum microRNA profile enables preoperative diagnosis of uterine leiomyosarcoma. Cancer Sci. 2019, 110, 3718–3726. [Google Scholar] [CrossRef]

| Features | Uterine Leiomyoma | Uterine Leiomyosarcoma |
|---|---|---|
| Clinicopathologic | Similarities
| Similarities
|
| Tissue Origin | Similarities
| Similarities
|
| Genetic Abnormality |
|
|
| Morphology | Similarities
| Similarities
|
| Behavior, Onset, Incidence | Similarities
| Similarities
|
| Histologic Features | Conventional LMS | Epithelioid LMS | Myxoid LMS |
|---|---|---|---|
| Essential diagnostic criteria | |||
| (2 of 3 histologic features): | (≥1 feature): | (≥1 feature): | |
| Cytologic Atypia | Severe | Moderate to severe | Moderate to severe |
| Necrosis | Present | Present | Coagulative necrosis |
| Mitosis | ≥10 mitoses/10 high power fields | often about 3 mitoses/10 high power fields | often about 3 mitosis/10 high power fields |
| Margins | infiltrative border | infiltrative borders | infiltrative borders |
| Cytologic features | |||
| Cell type | Spindle/elongated | >50% round or polygonal | |
| Cytoplasm | Eosinophilic | Eosinophilic, extensive hyalinization | scant |
| Nucleus | Hyperchromatic/pleomorphic Multinucleated Atypical mitoses | mild nuclear atypia | nuclear pleomorphism |
| Growth pattern | |||
| Cellular tumor comprised of long intersecting or haphazard fascicles | Arranged in nests, cords, or sheets May show pseudo-glandular spaces | Hypocellular tumor with abundant myxoid stroma Myxoid stroma may be difficult to differentiate from hydropic change in small/limited samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Madueke-Laveaux, O.S.; Cun, H.; Wlodarczyk, M.; Garcia, N.; Carvalho, K.C.; Al-Hendy, A. Comprehensive Review of Uterine Leiomyosarcoma: Pathogenesis, Diagnosis, Prognosis, and Targeted Therapy. Cells 2024, 13, 1106. https://doi.org/10.3390/cells13131106
Yang Q, Madueke-Laveaux OS, Cun H, Wlodarczyk M, Garcia N, Carvalho KC, Al-Hendy A. Comprehensive Review of Uterine Leiomyosarcoma: Pathogenesis, Diagnosis, Prognosis, and Targeted Therapy. Cells. 2024; 13(13):1106. https://doi.org/10.3390/cells13131106
Chicago/Turabian StyleYang, Qiwei, Obianuju Sandra Madueke-Laveaux, Han Cun, Marta Wlodarczyk, Natalia Garcia, Katia Candido Carvalho, and Ayman Al-Hendy. 2024. "Comprehensive Review of Uterine Leiomyosarcoma: Pathogenesis, Diagnosis, Prognosis, and Targeted Therapy" Cells 13, no. 13: 1106. https://doi.org/10.3390/cells13131106
APA StyleYang, Q., Madueke-Laveaux, O. S., Cun, H., Wlodarczyk, M., Garcia, N., Carvalho, K. C., & Al-Hendy, A. (2024). Comprehensive Review of Uterine Leiomyosarcoma: Pathogenesis, Diagnosis, Prognosis, and Targeted Therapy. Cells, 13(13), 1106. https://doi.org/10.3390/cells13131106









