Hyperspectral and Chlorophyll Fluorescence Analyses of Comparative Leaf Surfaces Reveal Cellular Influences on Leaf Optical Properties in Tradescantia Plants
Abstract
1. Introduction
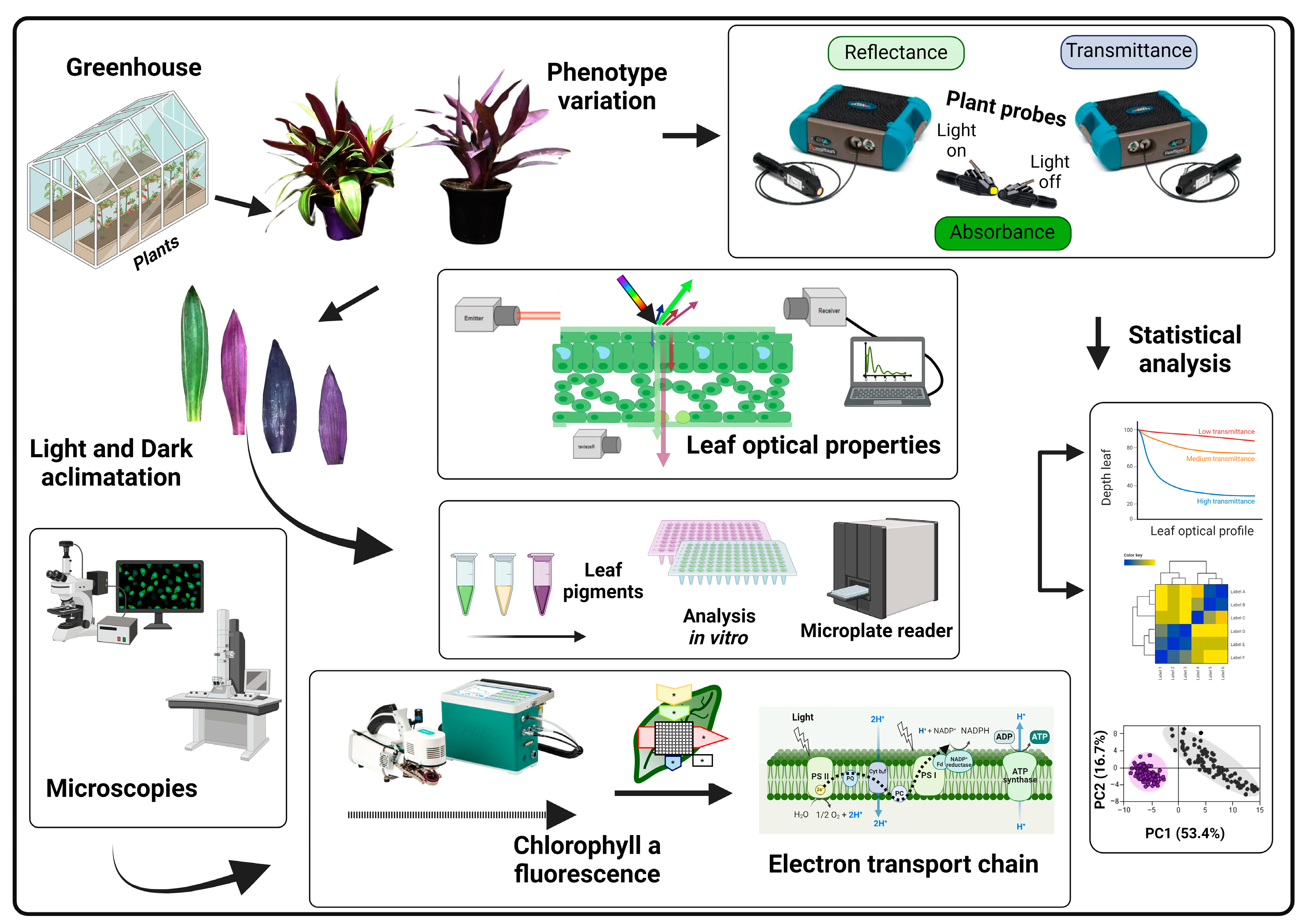
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Spectral Characterisation of Leaf Optical Properties through Hyperspectral Analysis
2.3. Assessment of Leaf Tissue Composition
2.3.1. Measurement of Chlorophyll and Carotenoids
2.3.2. Measurement of Anthocyanins and Flavonoids
2.3.3. Evaluation of Antioxidant Capacity
2.3.4. Measurement of Phenolic Compounds in Leaves
2.4. OJIP Chlorophyll a Fluorescence Transient
2.5. Preparation and Microscopic Analyses
2.5.1. Sample Preparation for Microscopy
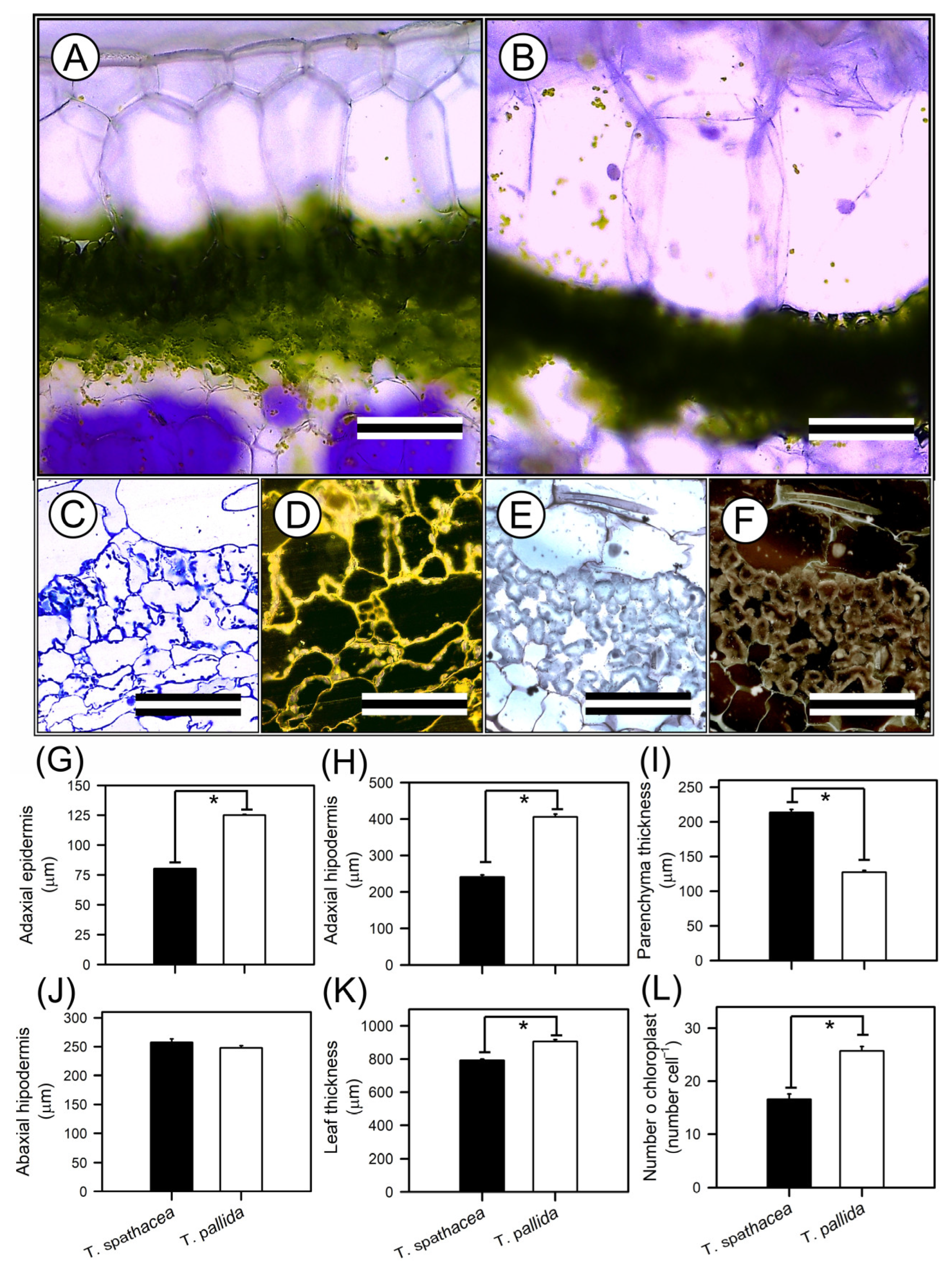
2.5.2. Optical Microscopy
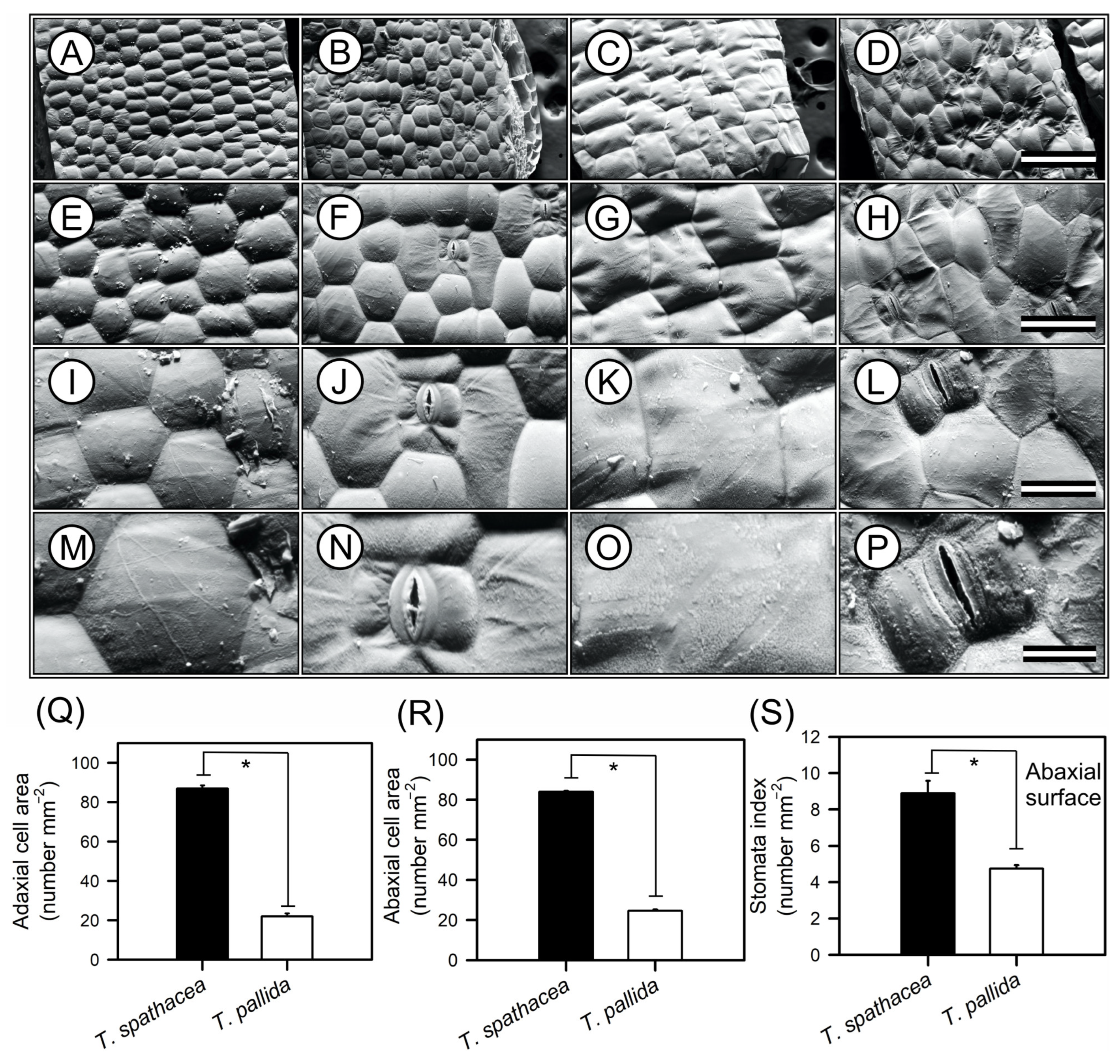
2.5.3. Scanning Electron Microscopy
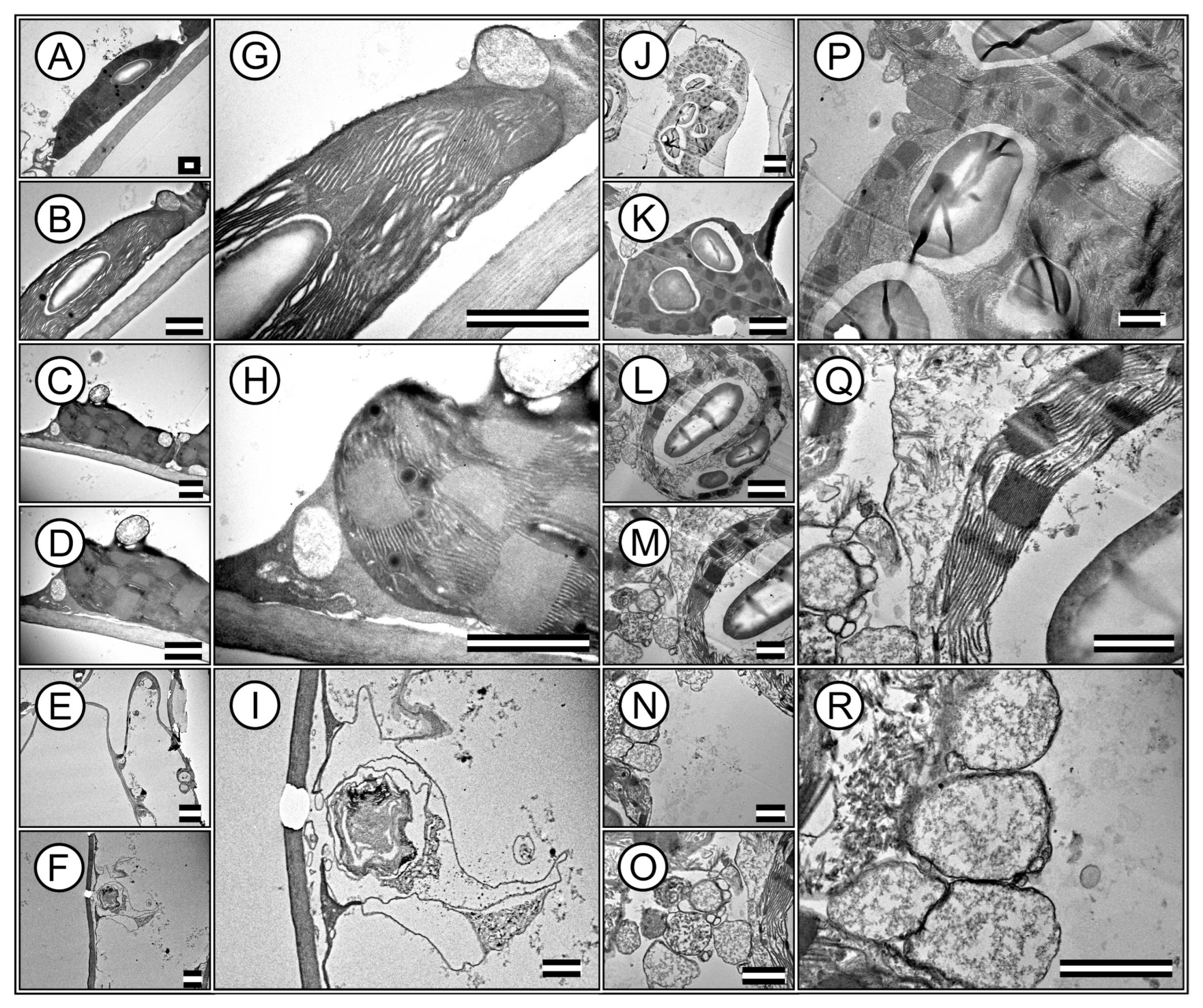
2.5.4. Transmission Electron Microscopy
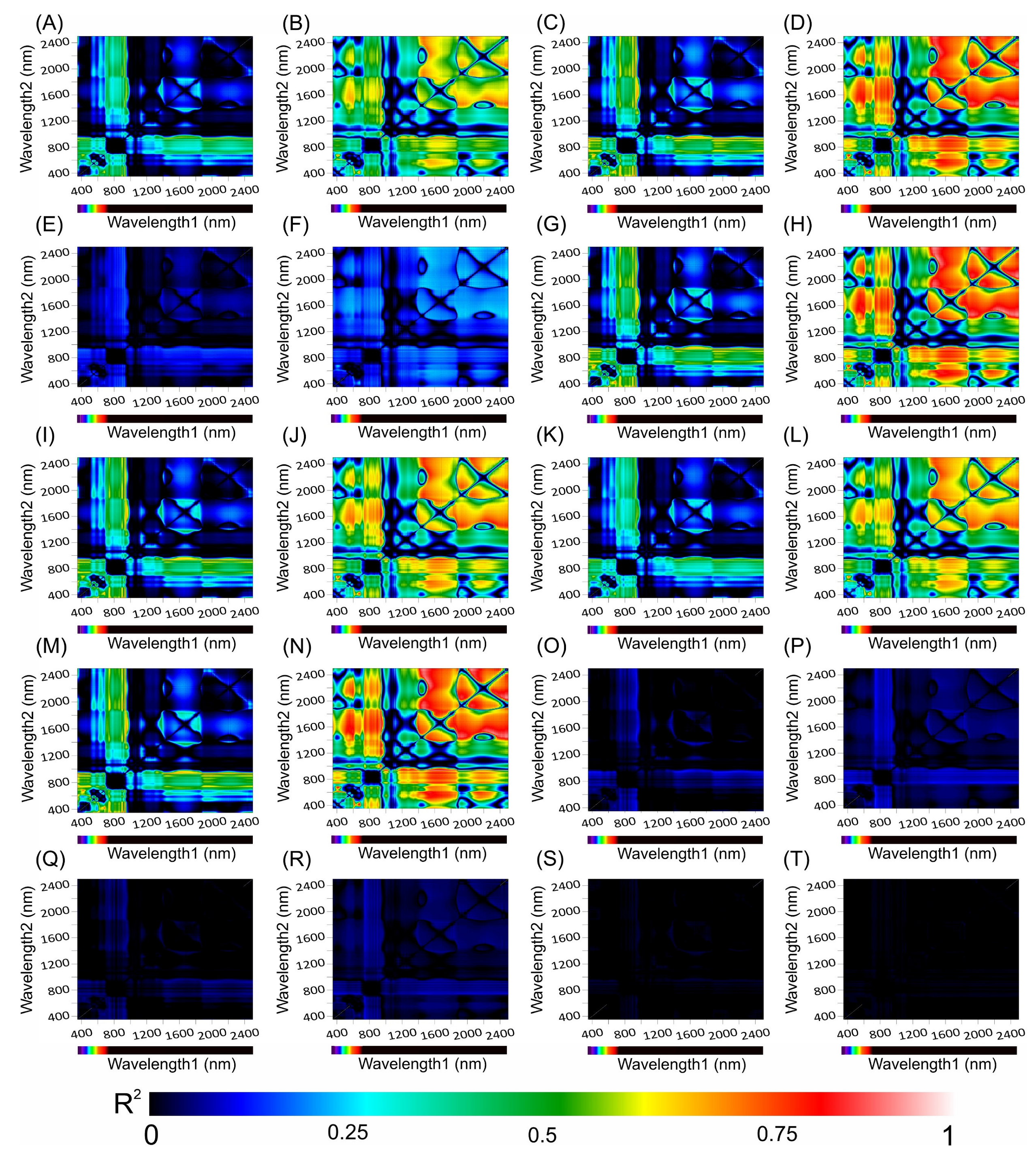
2.6. Hyperspectral Vegetation Indices Using Optimal Wavelengths for Biophysical Cell Parameters
2.7. Univariate and Multivariate Analyses
3. Results
3.1. Morphological Characteristics
3.2. Microscopic Epidermal Architecture of Tradescantia Species
3.3. Leaf Anatomy Structures
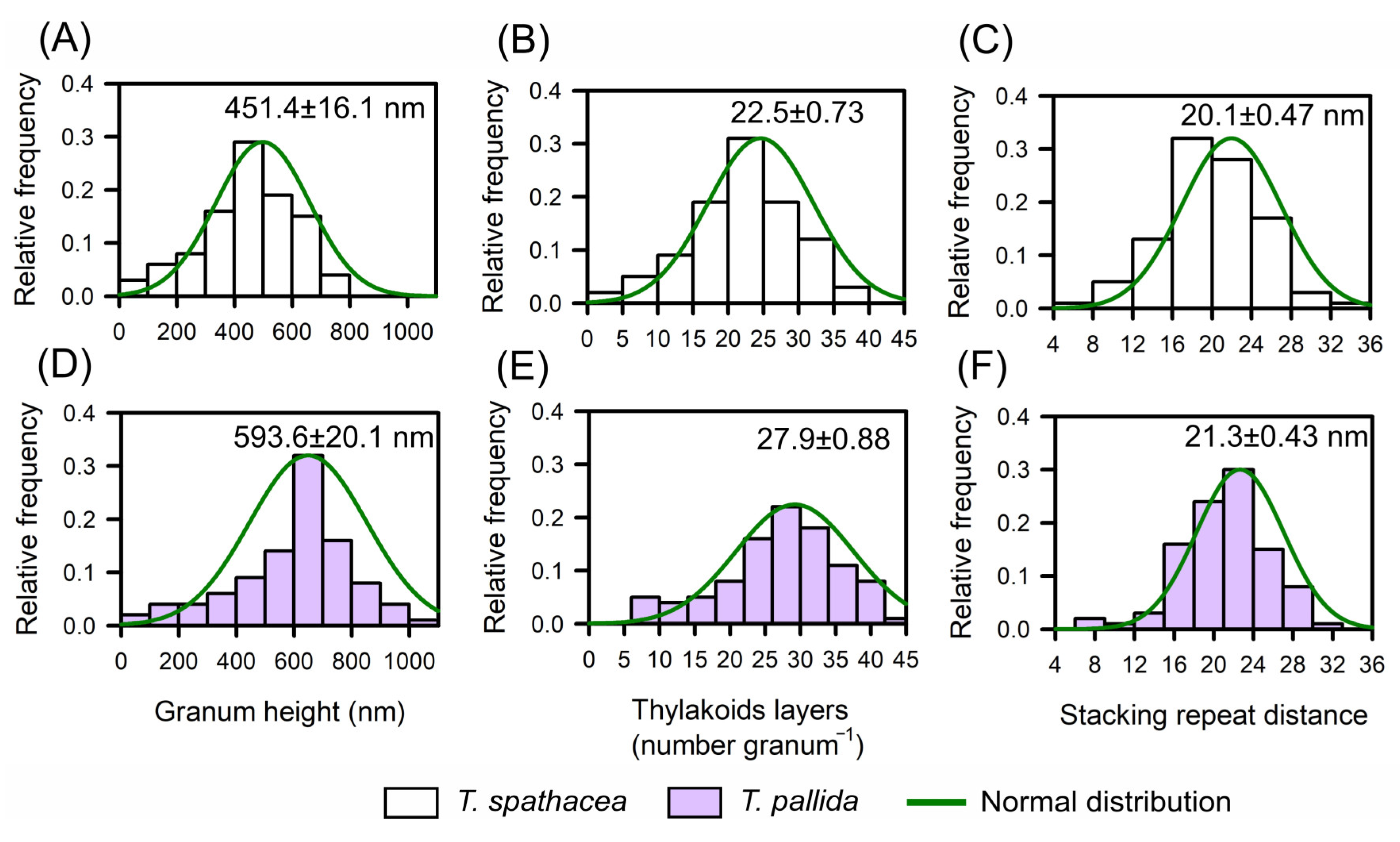
3.4. Leaf Anatomy Ultrastructures by Transmission Electron Microscopy
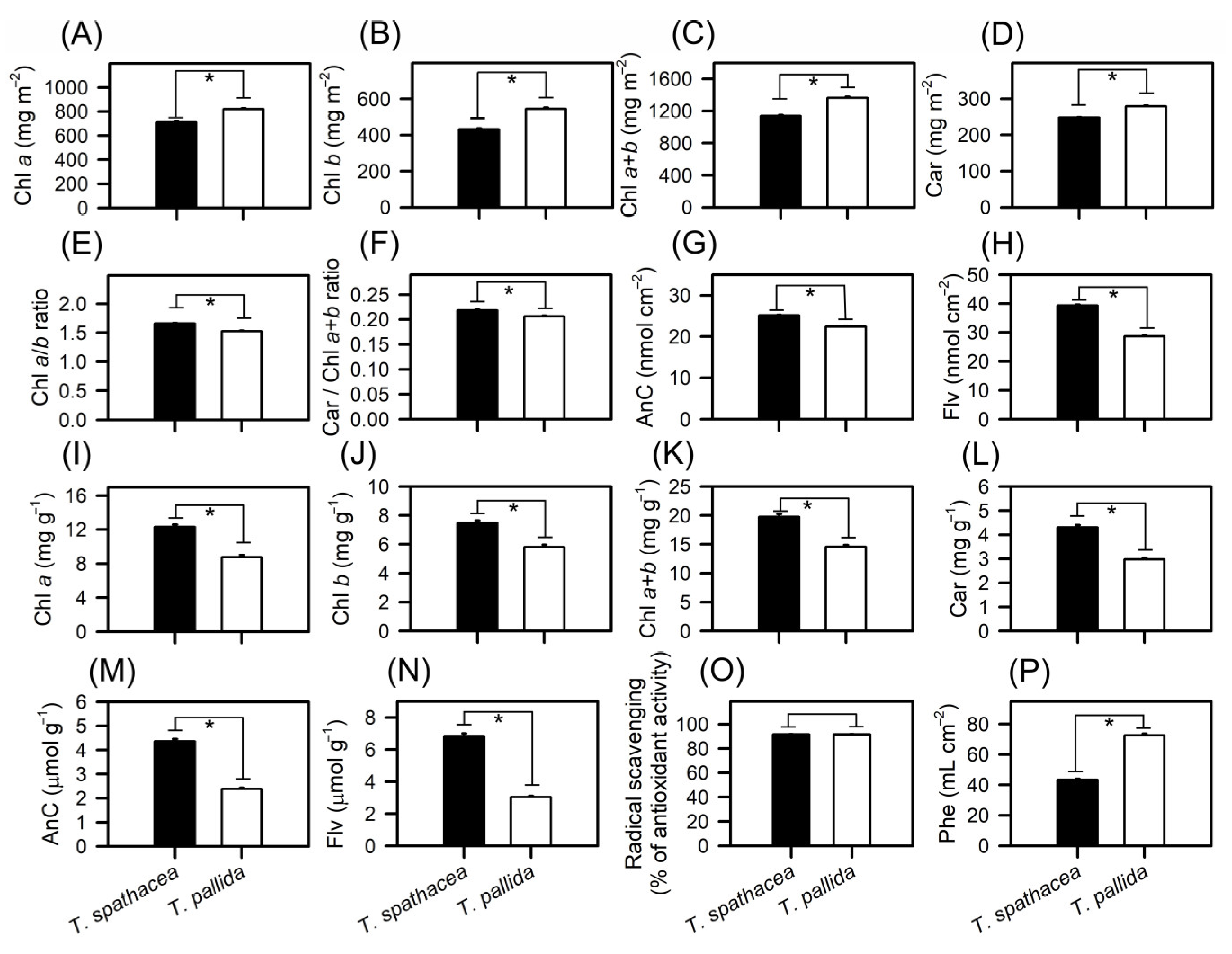
3.5. Pigment Components in Cell Structure and Ultrastructure
3.6. Leaf Optical Profile and Absorbance Spectrum of Pigments
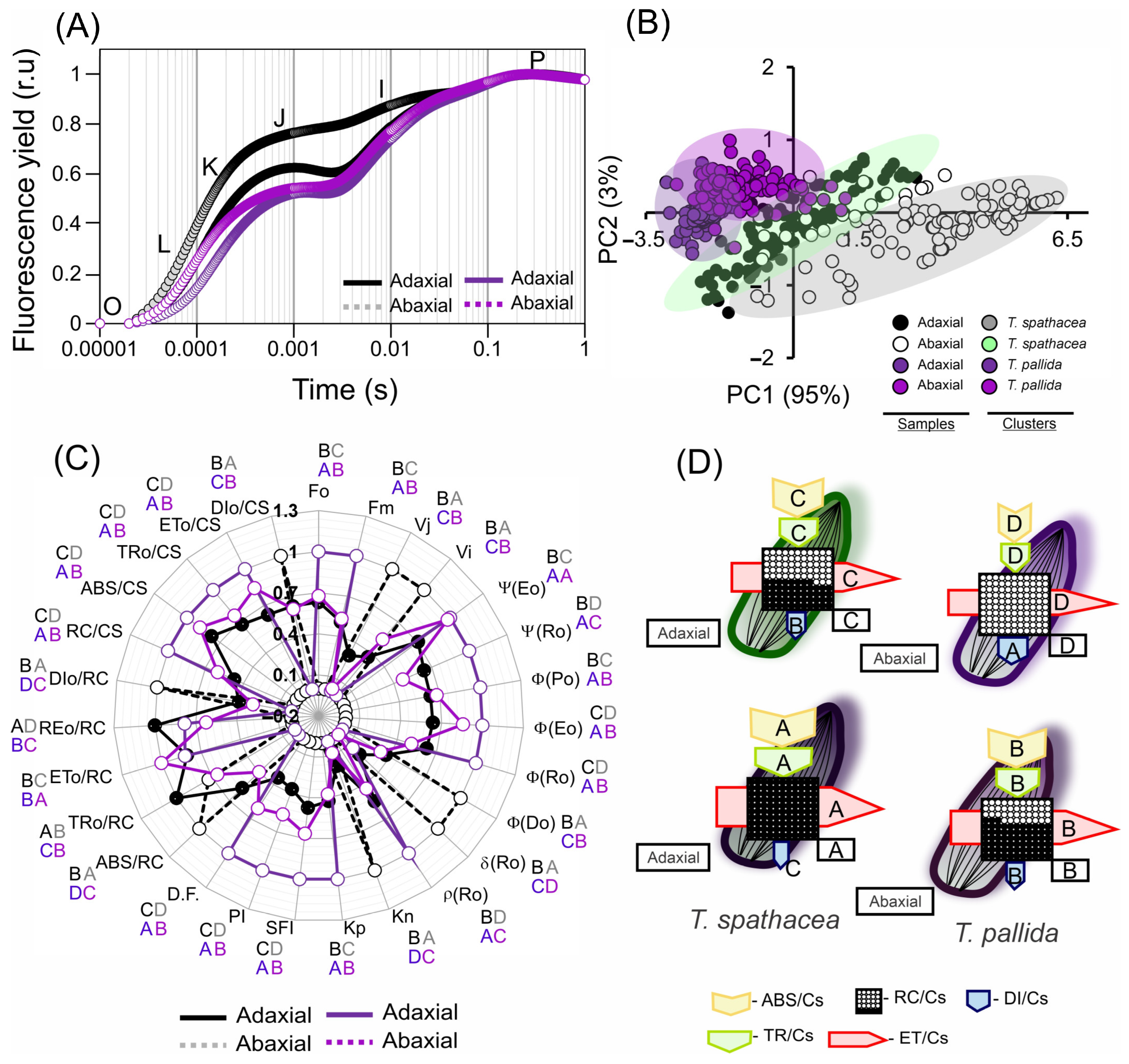
3.7. Chlorophyll a Fluorescence Kinetics-Based Pigments and the Structure and Ultrastructure of Cells
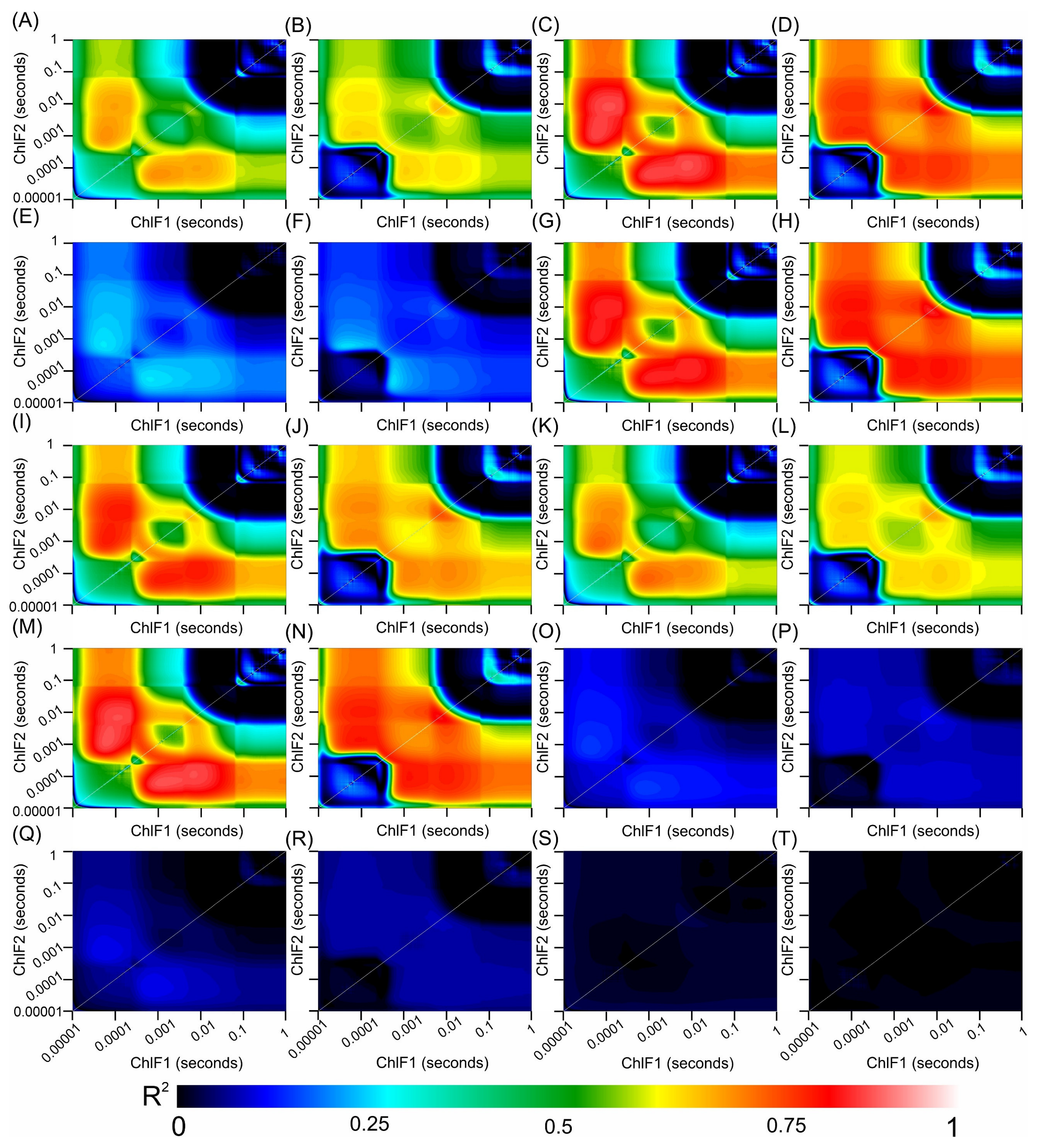
3.8. Correlation Coefficients between the Hyperspectral Vegetation Indices and Biophysical Parameters of the Leaves
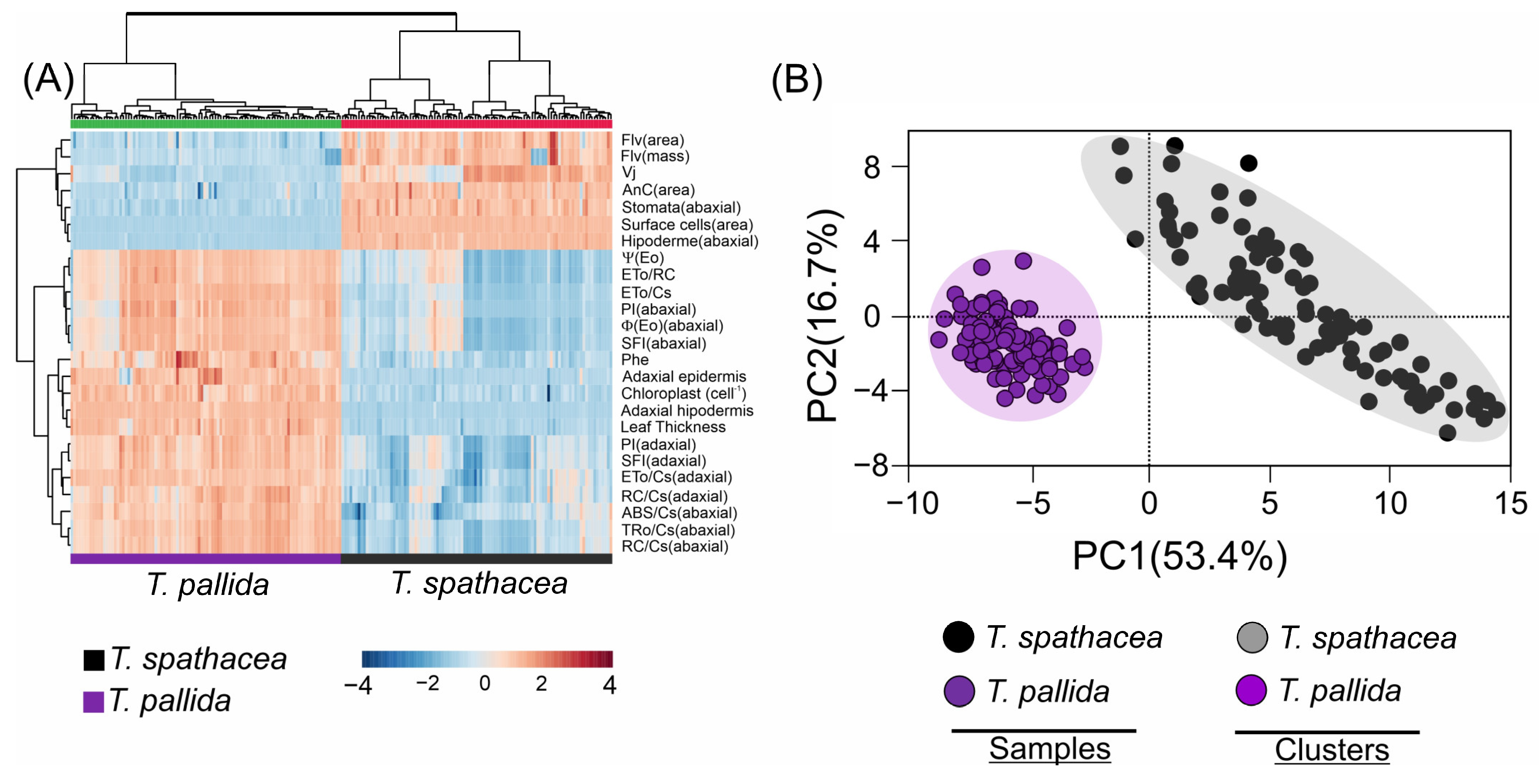
3.9. Multivariate Analyses
4. Discussion
4.1. Comparative Analysis of Morphological and Biochemical Characteristics
4.2. Adaptations to Cell and Tissue Structures from Spectral Data
4.3. Photosynthetic Strategies and Cellular Adaptations
4.4. Interaction Implications of Adaxial and Abaxial Cell Surfaces in Leaves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, R.J.; Furtado, A.; Rangan, P. Pathways of Photosynthesis in Non-Leaf Tissues. Biology 2020, 9, 438. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. Biophysical, Biochemical, and Photochemical Analyses Using Reflectance Hyperspectroscopy and Chlorophyll a Fluorescence Kinetics in Variegated Leaves. Biology 2023, 12, 704. [Google Scholar] [CrossRef]
- Mélida, H.; Largo-Gosens, A.; Novo-Uzal, E.; Santiago, R.; Pomar, F.; García, P.; García-Angulo, P.; Acebes, J.L.; Álvarez, J.; Encina, A. Ectopic Lignification in Primary Cellulose-Deficient Cell Walls of Maize Cell Suspension Cultures. J. Integr. Plant Biol. 2015, 57, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Glińska, S.; Gabara, B. The Effects of the Anthocyanin-Rich Extract from Red Cabbage Leaves on Allium cepa L. Root Tip Cell Ultrastructure. Ecotoxicol. Environ. Saf. 2011, 74, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wei, C.; Zhang, Y.; Blackburn, G.A.; Wang, X.; Wei, C.; Wang, J. Meta-Analysis of the Detection of Plant Pigment Concentrations Using Hyperspectral Remotely Sensed Data. PLoS ONE 2015, 10, e0137029. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral Remote Sensing of Plant Pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A.; Viña, A. Foliar Absorption Coefficient Derived from Reflectance Spectra: A Gauge of the Efficiency of in Situ Light-Capture by Different Pigment Groups. J. Plant Physiol. 2020, 254, 153277. [Google Scholar] [CrossRef]
- Antonio, É.; Paiva, S.; Mary, R.; Henrique, F.; Vale, A. The Influence of Light Intensity on Anatomical Structure and Pigment Contents of Tradescantia pallida (Rose) Hunt. Cv. Purpurea Boom (Commelinaceae) Leaves. Braz. Arch. Biol. Technol. 2003, 46, 617–624. [Google Scholar]
- Bercu, R. Histoanatomical Study on the Vegetative Organs of Tradescantia spathacea (Commelinaceae). Bot. Serbica 2013, 37, 121–126. [Google Scholar]
- Chimpan, C.; Sipos, M. Anatomy of the Vegetative Organs of Tradescantia Pallida Purpurea. Biharean Biol. 2009, 3, 1–4. [Google Scholar]
- Cuba, N.I.; Torres, R.; San Román, E.; Lagorio, M.G. Influence of Surface Structure, Pigmentation and Particulate Matter on Plant Reflectance and Fluorescence. Photochem. Photobiol. 2021, 97, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.; Maffi, D.; Faoro, F.; Cerabolini, B.E.L.; Spada, A. The Leaf Anatomical Trade-Offs Associated with Plant Ecological Strategy Variation. Plant Ecol. 2022, 223, 1233–1246. [Google Scholar] [CrossRef]
- Shabala, S.N.; Lew, R.R. Turgor Regulation in Osmotically Stressed Arabidopsis Epidermal Root Cells. Direct Support for the Role of Cell Turgor Measurement. Plant Physiol. 2002, 129, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Ptushenko, V.V.; Ptushenko, O.S.; Samoilova, O.P.; Solovchenko, A.E. Analysis of Photoprotection and Apparent Non-Photochemical Quenching of Chlorophyll Fluorescence in Tradescantia Leaves Based on the Rate of Irradiance-Induced Changes in Optical Transparence. Biochemistry 2017, 82, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Yuan, S.; Xu, F.; Zhu, F.; Yuan, M.; Ye, H.X.; Guo, H.Q.; Lv, X.; Yin, Y.; Lin, H.H. Light Intensity Affects Chlorophyll Synthesis during Greening Process by Metabolite Signal from Mitochondrial Alternative Oxidase in Arabidopsis. Plant Cell Environ. 2016, 39, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, M.N.; Chivkunova, O.B.; Melø, T.B.; Naqvi, K.R. Does a Leaf Absorb Radiation in the near Infrared (780-900 Nm) Region? A New Approach to Quantifying Optical Reflection, Absorption and Transmission of Leaves. Photosynth. Res. 2002, 72, 263–270. [Google Scholar] [CrossRef]
- Gould, K.S.; Jay-Allemand, C.; Logan, B.A.; Baissac, Y.; Bidel, L.P.R. When Are Foliar Anthocyanins Useful to Plants? Re-Evaluation of the Photoprotection Hypothesis Using Arabidopsis Thaliana Mutants That Differ in Anthocyanin Accumulation. Environ. Exp. Bot. 2018, 154, 11–22. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; de Oliveira, R.B.; Chicati, M.L.; Demattê, J.A.M.; Nanni, M.R. Assessment of Combined Reflectance, Transmittance, and Absorbance Hyperspectral Sensors for Prediction of Chlorophyll a Fluorescence Parameters. Remote Sens. 2023, 15, 5067. [Google Scholar] [CrossRef]
- Colpo, A.; Molinari, A.; Boldrini, P.; Živčak, M.; Brestič, M.; Demaria, S.; Baldisserotto, C.; Pancaldi, S.; Ferroni, L. Thylakoid Membrane Appression in the Giant Chloroplast of Selaginella martensii Spring: A Lycophyte Challenges Grana Paradigms in Shade-Adapted Species. Plant Sci. 2023, 336, 111833. [Google Scholar] [CrossRef]
- Rodrigues, M.; Berti de Oliveira, R.; dos Santos, G.L.A.A.; Mayara de Oliveira, K.; Silveira Reis, A.; Herrig Furlanetto, R.; Antônio Yanes Bernardo Júnior, L.; Silva Coelho, F.; Rafael Nanni, M. Rapid Quantification of Alkaloids, Sugar and Yield of Tobacco (Nicotiana tabacum L.) Varieties by Using Vis–NIR–SWIR Spectroradiometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121082. [Google Scholar] [CrossRef] [PubMed]
- Féret, J.-B.; le Maire, G.; Jay, S.; Berveiller, D.; Bendoula, R.; Hmimina, G.; Cheraiet, A.; Oliveira, J.C.; Ponzoni, F.J.; Solanki, T.; et al. Estimating Leaf Mass per Area and Equivalent Water Thickness Based on Leaf Optical Properties: Potential and Limitations of Physical Modeling and Machine Learning. Remote Sens. Environ. 2019, 231, 110959. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Murphy, S.P.; Pethybridge, S.J.; van Aardt, J. Growth Stage Classification and Harvest Scheduling of Snap Bean Using Hyperspectral Sensing: A Greenhouse Study. Remote Sens. 2020, 12, 3809. [Google Scholar] [CrossRef]
- Crusiol, L.G.T.; Sun, L.; Sun, Z.; Chen, R.; Wu, Y.; Ma, J.; Song, C. In-Season Monitoring of Maize Leaf Water Content Using Ground-Based and UAV-Based Hyperspectral Data. Sustainability 2022, 14, 9039. [Google Scholar] [CrossRef]
- Braga, P.; Crusiol, L.G.T.; Nanni, M.R.; Caranhato, A.L.H.; Fuhrmann, M.B.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Koltun, A.; Gonçalves, L.S.A.; et al. Vegetation Indices and NIR-SWIR Spectral Bands as a Phenotyping Tool for Water Status Determination in Soybean. Precis. Agric. 2021, 22, 249–266. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Perez-Llorca, M.; Munné-Bosch, S.; Gibin, M.S.; Sato, F.; Pelozo, A.; Pattaro, M.C.; Giacomelli, M.E.; Rüggeberg, M.; et al. Cell Wall Structure and Composition Is Affected by Light Quality in Tomato Seedlings. J. Photochem. Photobiol. B Biol. 2020, 203, 111745. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; Furlanetto, R.H.; Nanni, M.R.; Antunes, W.C. Simple, Fast and Efficient Methods for Analysing the Structural, Ultrastructural and Cellular Components of the Cell Wall. Plants 2022, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Radotić, K.; Melø, T.B.; Lindgren, M. A Fluorescence Spectroscopic Study of Light Transmission and Adaxial-Abaxial Distribution of Emitting Compounds in Leaves of Christmas Star (Euphorbia pulcherrima). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123269. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Sidhu, G.P.S. Climate Change Regulated Abiotic Stress Mechanisms in Plants: A Comprehensive Review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Cordón, G.B.; Lagorio, M.G. Optical Properties of the Adaxial and Abaxial Faces of Leaves. Chlorophyll Fluorescence, Absorption and Scattering Coefficients. Photochem. Photobiol. Sci. 2007, 6, 873–882. [Google Scholar] [CrossRef]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. Reflectance Spectroscopy for the Classification and Prediction of Pigments in Agronomic Crops. Plants 2023, 12, 2347. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; Pattaro, M.; Herrig Furlanetto, R.; Nanni, M.R.; Camargos Antunes, W. High Resolution Leaf Spectral Signature as a Tool for Foliar Pigment Estimation Displaying Potential for Species Differentiation. J. Plant Physiol. 2020, 249, 153161. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. A Novel Method for Estimating Chlorophyll and Carotenoid Concentrations in Leaves: A Two Hyperspectral Sensor Approach. Sensors 2023, 23, 3843. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.; Solovchenko, A. Non-Invasive Quantification of Foliar Pigments: Possibilities and Limitations of Reflectance- and Absorbance-Based Approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of Polyphenols and Antioxidant Properties of Five Lettuce Varieties and Escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Ragaee, S. Antioxidant Activity and Nutrient Composition of Selected Cereals for Food Use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Hossain, Z. Chlorophyll a Fluorescence—A Useful Tool for the Early Detection of Temperature Stress in Spring Barley (Hordeum vulgare L.). Omi. A J. Integr. Biol. 2011, 15, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee Chlorophyll a Fluorescence Induction: Can Just a One-Second Measurement Be Used to Quantify Abiotic Stress Responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopiński, M.; Małkowski, E. Photosynthetic Efficiency as Bioindicator of Environmental Pressure in A. Halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Kraus, J.E.; de Sousa, H.C.; Rezende, M.H.; Castro, N.M.; Vecchi, C.; Luque, R. Astra Blue and Basic Fuchsin Double Staining of Plant Materials. Biotech. Histochem. 1998, 73, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.S. The Use of Lead Citrate at High PH as an Electron-Opaque Stain in Electron Microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2010; ISBN 0-13-100846-3. [Google Scholar]
- Jolliffe, I.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin Improved Anthocyanin Accumulation by Regulating Gene Expressions and Resulted in High Reactive Oxygen Species Scavenging Capacity in Cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Stecha, S.; Ueda, T.; Bohorquez-Restrepo, A.; Chanoca, A.; Eliceiri, K.W.; Burkel, B.; Kovinich, N.; Otegui, M.S.; Grotewold, E. Anthocyanin Vacuolar Inclusions Form by a Microautophagy Mechanism. Plant Cell 2015, 27, 2545–2559. [Google Scholar]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman Spectroscopic Features of Plant Cuticles: A Review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Kycko, M.; Zagajewski, B.; Lavender, S.; Dabija, A. In Situ Hyperspectral Remote Sensing for Monitoring of Alpine Trampled and Recultivated Species. Remote Sens. 2019, 11, 1296. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple Flavonols during Fruit Adaptation to Solar Radiation: Spectral Features and Technique for Non-Destructive Assessment. J. Plant Physiol. 2005, 162, 151–160. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Rooney, A.; Miguel Hernández-Hierro, J.; José Heredia, F.; Byrne, H.J. Linking ATR-FTIR and Raman Features to Phenolic Extractability and Other Attributes in Grape Skin. Talanta 2017, 167, 44–50. [Google Scholar] [CrossRef]
- Osman, S.O.M.; Saad, A.S.I.; Tadano, S.; Takeda, Y.; Konaka, T.; Yamasaki, Y.; Tahir, I.S.A.; Tsujimoto, H.; Akashi, K. Chemical Fingerprinting of Heat Stress Responses in the Leaves of Common Wheat by Fourier Transform Infrared Spectroscopy. Int. J. Mol. Sci. 2022, 23, 2842. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Fortini, E.A.; Müller, L.A.d.C.; Batista, D.S.; Vieira, L.M.; Silva, P.O.; Amaral, C.H.; Poethig, R.S.; Otoni, W.C. Leaf Development Stages and Ontogenetic Changes in Passionfruit (Passiflora edulis Sims.) Are Detected by Narrowband Spectral Signal. J. Photochem. Photobiol. B Biol. 2020, 209, 111931. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E.; Saponaro, V.; Hoshika, Y.; Arab, L.; Rennenberg, H.; Paoletti, E. Hyperspectral Reflectance of Light-Adapted Leaves Can Predict Both Dark- and Light-Adapted Chl Fluorescence Parameters, and the Effects of Chronic Ozone Exposure on Date Palm (Phoenix dactylifera). Int. J. Mol. Sci. 2020, 21, 6441. [Google Scholar] [CrossRef]
- Xiao, Y.; Tholen, D.; Zhu, X.G. The Influence of Leaf Anatomy on the Internal Light Environment and Photosynthetic Electron Transport Rate: Exploration with a New Leaf Ray Tracing Model. J. Exp. Bot. 2016, 67, 6021–6035. [Google Scholar] [CrossRef]
- Fu, P.; Meacham-Hensold, K.; Guan, K.; Bernacchi, C.J. Hyperspectral Leaf Reflectance as Proxy for Photosynthetic Capacities: An Ensemble Approach Based on Multiple Machine Learning Algorithms. Front. Plant Sci. 2019, 10, 730. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Mubushar, M.; Tahir, M.U.; Marey, S.; Refay, Y.; Tola, E. Combining Hyperspectral Reflectance and Multivariate Regression Models to Estimate Plant Biomass of Advanced Spring Wheat Lines in Diverse Phenological Stages under Salinity Conditions. Appl. Sci. 2022, 12, 1983. [Google Scholar] [CrossRef]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The Transcription Factor MYB115 Contributes to the Regulation of Proanthocyanidin Biosynthesis and Enhances Fungal Resistance in Poplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Aluru, M.R.; Zola, J.; Foudree, A.; Rodermel, S.R. Chloroplast Photooxidation-Induced Transcriptome Reprogramming in Arabidopsis Immutans White Leaf Sectors. Plant Physiol. 2009, 150, 904–923. [Google Scholar] [CrossRef] [PubMed]
- Ďúranová, H.; Šimora, V.; Ďurišová, Ľ.; Olexiková, L.; Kovár, M.; Požgajová, M. Modifications in Ultrastructural Characteristics and Redox Status of Plants under Environmental Stress: A Review. Plants 2023, 12, 1666. [Google Scholar] [CrossRef]
- Iqbal, I.M.; Balzter, H.; Firdaus-e-Bareen; Shabbir, A. Identifying the Spectral Signatures of Invasive and Native Plant Species in Two Protected Areas of Pakistan through Field Spectroscopy. Remote Sens. 2021, 13, 4009. [Google Scholar] [CrossRef]
- Olmos, V.; Marro, M.; Loza-Alvarez, P.; Raldúa, D.; Prats, E.; Padrós, F.; Piña, B.; Tauler, R.; de Juan, A. Combining Hyperspectral Imaging and Chemometrics to Assess and Interpret the Effects of Environmental Stressors on Zebrafish Eye Images at Tissue Level. J. Biophotonics 2018, 11, e201700089. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Wilson, S. The Mechanism of Non-Photochemical Quenching in Plants: Localization and Driving Forces. Plant Cell Physiol. 2021, 62, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Tissue Specific Disruption of Photosynthetic Electron Transport Rate in Pigeonpea (Cajanus cajan L.) under Elevated Temperature. Plant Signal. Behav. 2019, 14, 1601952. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Almeida Rodrigues, A.; Carvalho Vasconcelos Filho, S.; Müller, C.; Almeida Rodrigues, D.; de Fátima Sales, J.; Zuchi, J.; Carlos Costa, A.; Lino Rodrigues, C.; Alves da Silva, A.; Pereira Barbosa, D. Tolerance of Eugenia dysenterica to Aluminum: Germination and Plant Growth. Plants 2019, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.-Q.; Liu, D.-H.; Sang, M.; Jiang, C.-D. Sunflower Leaf Structure Affects Chlorophyll a Fluorescence Induction Kinetics In Vivo. Int. J. Mol. Sci. 2022, 23, 14996. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic Action of Blue and Red Light on Shoot Elongation in Petunia Depends on Gibberellin, but the Effects on Flowering Are Not Generally Linked to Gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Weng, J.-K.; Chapple, C. The Origin and Evolution of Lignin Biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef]
- Kimura, H.; Hashimoto-Sugimoto, M.; Iba, K.; Terashima, I.; Yamori, W. Improved Stomatal Opening Enhances Photosynthetic Rate and Biomass Production in Fluctuating Light. J. Exp. Bot. 2020, 71, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D. Limitation to Photosynthesis in Water-Stressed Leaves: Stomata vs. Metabolism and the Role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Enriquez, S.; Sand-Jensen, K. Variation in Light Absorption Properties of Mentha aquatica L. as a Function of Leaf Form: Implications for Plant Growth. Int. J. Plant Sci. 2003, 164, 125–136. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.R.; Matthews, J.S.; Simkin, A.; Raines, C.A.; Lawson, T. Importance of Fluctuations in Light on Plant Photosynthetic Acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef]
- Kume, A. Importance of the Green Color, Absorption Gradient, and Spectral Absorption of Chloroplasts for the Radiative Energy Balance of Leaves. J. Plant Res. 2018, 131, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Bykowski, M.; Mazur, R.; Wójtowicz, J.; Suski, S.; Garstka, M.; Mostowska, A.; Kowalewska, Ł. Too Rigid to Fold: Carotenoid-Dependent Decrease in Thylakoid Fluidity Hampers the Formation of Chloroplast Grana. Plant Physiol. 2021, 185, 210–227. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple Functional Roles of Anthocyanins in Plant-Environment Interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Quina, F.H.; Moreira, P.F.; Vautier-Giongo, C.; Rettori, D.; Rodrigues, R.F.; Freitas, A.A.; Silva, P.F.; Maçanita, A.L. Photochemistry of Anthocyanins and Their Biological Role in Plant Tissues. Pure Appl. Chem. 2009, 81, 1687–1694. [Google Scholar] [CrossRef]
- Neil, S.O.; Gould, K.S. Anthocyanins in Leaves: Light Attenuators or Antioxidants? Funct. Plant Biol. 2003, 30, 865–873. [Google Scholar] [CrossRef]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin Positively Influences the Photosynthetic Machinery and Antioxidant System of Avena Sativa during Salinity Stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Photosynthetic Properties and Potentials for Improvement of Photosynthesis in Pale Green Leaf Rice under High Light Conditions. Front. Plant Sci. 2017, 8, 1082. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Surfus, J.S. Assessing Leaf Pigment Content and Activity with a Reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Subhash, N.; Mohanan, C.N. Curve-Fit Analysis of Chlorophyll Fluorescence Spectra: Application to Nutrient Stress Detection in Sunflower. Remote Sens. Environ. 1997, 60, 347–356. [Google Scholar] [CrossRef]
- Lang, M.; Stober, F.; Lichtenthaler, H.K. Fluorescence Emission Spectra of Plant Leaves and Plant Constituents. Radiat. Environ. Biophys. 1991, 30, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Mohiddin, G.; Ortiz, J.; Selvanayagam, M. International Journal of Pharmacology and Pharmaceutical Sciences. Int. J. Pharmacol. 2014, 2, 7–15. [Google Scholar]
- Ndlovu, E.; van Staden, J.; Maphosa, M. Morpho-Physiological Effects of Moisture, Heat and Combined Stresses on Sorghum bicolor [Moench (L.)] and Its Acclimation Mechanisms. Plant Stress 2021, 2, 100018. [Google Scholar] [CrossRef]
- Terashima, I.; Hiroki, O.; Takashi, F.; Riichi, O. Light Environment within a Leaf. II. Progress in the Past One-Third Century. J. Plant Res. 2016, 129, 353–363. [Google Scholar]
- Hatier, J.H.B.; Gould, K.S. Black Coloration in Leaves of Ophiopogon planiscapus “Nigrescens”. Leaf Optics, Chromaticity, and Internal Light Gradients. Funct. Plant Biol. 2007, 34, 130–138. [Google Scholar] [CrossRef]
- Gitelson, A.; Chivkunova, O.; Zhigalova, T.; Solovchenko, A. In Situ Optical Properties of Foliar Flavonoids: Implication for Non-Destructive Estimation of Flavonoid Content. J. Plant Physiol. 2017, 218, 258–264. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y.U. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light Acts as a Stressor and Influences Abiotic and Biotic Stress Responses in Plants. Plant. Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.A.; P’yankov, V.I. Structural Adaptation of the Leaf Mesophyll to Shading. Russ. J. Plant Physiol. 2002, 49, 419–431. [Google Scholar] [CrossRef]
- Romero, J.M.; Cordon, G.B.; Lagorio, M.G. Re-Absorption and Scattering of Chlorophyll Fluorescence in Canopies: A Revised Approach. Remote Sens. Environ. 2020, 246, 111860. [Google Scholar] [CrossRef]
- Boshkovski, B.; Doupis, G.; Zapolska, A.; Kalaitzidis, C.; Koubouris, G. Hyperspectral Imagery Detects Water Deficit and Salinity Effects on Photosynthesis and Antioxidant Enzyme Activity of Three Greek Olive Varieties. Sustainability 2022, 14, 1432. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcioni, R.; Antunes, W.C.; Berti de Oliveira, R.; Chicati, M.L.; Demattê, J.A.M.; Nanni, M.R. Hyperspectral and Chlorophyll Fluorescence Analyses of Comparative Leaf Surfaces Reveal Cellular Influences on Leaf Optical Properties in Tradescantia Plants. Cells 2024, 13, 952. https://doi.org/10.3390/cells13110952
Falcioni R, Antunes WC, Berti de Oliveira R, Chicati ML, Demattê JAM, Nanni MR. Hyperspectral and Chlorophyll Fluorescence Analyses of Comparative Leaf Surfaces Reveal Cellular Influences on Leaf Optical Properties in Tradescantia Plants. Cells. 2024; 13(11):952. https://doi.org/10.3390/cells13110952
Chicago/Turabian StyleFalcioni, Renan, Werner Camargos Antunes, Roney Berti de Oliveira, Marcelo Luiz Chicati, José Alexandre M. Demattê, and Marcos Rafael Nanni. 2024. "Hyperspectral and Chlorophyll Fluorescence Analyses of Comparative Leaf Surfaces Reveal Cellular Influences on Leaf Optical Properties in Tradescantia Plants" Cells 13, no. 11: 952. https://doi.org/10.3390/cells13110952
APA StyleFalcioni, R., Antunes, W. C., Berti de Oliveira, R., Chicati, M. L., Demattê, J. A. M., & Nanni, M. R. (2024). Hyperspectral and Chlorophyll Fluorescence Analyses of Comparative Leaf Surfaces Reveal Cellular Influences on Leaf Optical Properties in Tradescantia Plants. Cells, 13(11), 952. https://doi.org/10.3390/cells13110952






