Abstract
Regulatory T cells (Tregs) are essential to maintain immune homeostasis by promoting self-tolerance. Reduced Treg numbers or functionality can lead to a loss of tolerance, increasing the risk of developing autoimmune diseases. An overwhelming variety of human Tregs has been described, based on either specific phenotype, tissue compartment, or pathological condition, yet the bulk of the literature only addresses CD25-positive and CD127-negative cells, coined by naturally occurring Tregs (nTregs), most of which express the transcription factor Forkhead box protein 3 (FOXP3). While the discovery of FOXP3 was seminal to understanding the origin and biology of nTregs, there is evidence in humans that not all T cells expressing FOXP3 are regulatory, and that not all Tregs express FOXP3. Namely, the activation of human T cells induces the transient expression of FOXP3, irrespective of whether they are regulatory or inflammatory effectors, while some induced T cells that may be broadly defined as Tregs (e.g., Tr1 cells) typically lack demethylation and do not express FOXP3. Furthermore, it is unknown whether and how many nTregs exist without FOXP3 expression. Several other candidate regulatory molecules, such as GITR, Lag-3, GARP, GPA33, Helios, and Neuropilin, have been identified but subsequently discarded as Treg-specific markers. Multiparametric analyses have uncovered a plethora of Treg phenotypes, and neither single markers nor combinations thereof can define all and only Tregs. To date, only the functional capacity to inhibit immune responses defines a Treg and distinguishes Tregs from inflammatory T cells (Teffs) in humans. This review revisits current knowledge of the Treg universe with respect to their heterogeneity in phenotype and function. We propose that it is unavoidable to characterize human Tregs by their phenotype in combination with their function, since phenotype alone does not unambiguously define Tregs. There is an unmet need to align the expression of specific markers or combinations thereof with a particular suppressive function to coin functional Treg entities and categorize Treg diversity.
1. Introduction
It is important for the immune system to have the ability to control inflammatory responses and prevent chronic reactions against self-antigens. In addition, foreign antigens introduced by pregnancy, organ transplants, or commensal bacteria in the intestine also need to be tolerated by the immune system. One of the mechanisms to control immune responses involves regulatory T cells (Tregs). Tregs reside in lymphoid and non-lymphoid organs and adapt to their environment in order to maintain tissue homeostasis. An imbalance between inflammatory T cells (i.e., effector T cells or Teff) and Tregs can cause a loss of tolerance and disease. For example, decreased Treg counts or low suppression activity are associated with autoimmunity [1] and chronic inflammation [2]. Persistent Treg activity, on the other hand, can favor tumor progression [3], chronic infections [4], and low responsiveness to vaccinations [5,6].
An overwhelming variety of Tregs has been described, either generated in vitro or isolated from human tissues in various pathological situations, using novel phenotypic markers and different functional assays [7,8,9,10,11,12,13]. This complicates the assessment of whether a certain Treg is related to a previously described Treg type and reaching a conclusion as to whether a disbalance between the same Treg and Teff subsets supports different pathologies. One of the efforts to characterize Tregs was made by establishing a minimum level of information on in vitro cultured Tregs [14], but a consensus on how to characterize human Tregs ex vivo is lacking at the moment. With different, partially overlapping phenotypic marker combinations, and with a universal marker for Tregs lacking, only the functional capacity to inhibit immune responses defines all Tregs and distinguishes these from Teff cells. An additional limitation of characterizing immune cells by the available surface markers is that these markers often do not correlate with the functional diversity of Tregs. In this review, we revisit general Treg knowledge, beliefs, and simplifications since some of these are still used as a standard to identify human Tregs. We propose to combine the phenotype with suppressive functional features to characterize the population of interest and define phenotype markers that align with particular suppressive functionalities to classify Treg populations and understand their relations.
2. Revisiting Human Treg Phenotypes
A ubiquitously accepted definition of Tregs was of any cell expressing FOXP3, but this proved problematic for human Tregs. In search of an alternative, improved techniques such as multidimensional flow cytometry and single-cell sequencing revealed many different Treg subtypes, introduced new markers that were claimed to be Treg-specific, described multiple Treg origins and antigen specificities, and challenged us to revisit the omnipresent view of the identity of human Treg cells, Treg phenotypes, and functions, as well as better delineation from Teff cells.
2.1. FOXP3 Is Neither Specific nor Exclusive to Human Tregs
The transcription factor Forkhead box protein 3 (FOXP3) was discovered in scurfy mice [15]. These mice have a missense mutation in the Foxp3 gene and, therefore, lack Foxp3 expression in T cells, resulting in a lymphoproliferative disease with multiorgan inflammation [16,17,18]. While, in humans, autoimmunity can occur independently of dysfunctional Tregs [19,20,21,22], patients with IPEX (immune dysfunction/polyendocrinopathy/enteropathy/X-linked) syndrome often show mutations in the FOXP3 gene [23,24,25,26,27,28] and present with symptoms like severe enteropathy, polyendocrinopathy, and immune dysregulation, similar to scurfy mice [29,30,31]. However, not all IPEX patients have a mutation in FOXP3, implying that immune (dys)regulation can occur either downstream or independently of FOXP3 [32,33,34,35,36]. Indeed, a study involving 15 IPEX patients showed that Treg signature genes were expressed despite a loss-of-function mutation in FOXP3, indicating that Tregs can exist without functional FOXP3 [37,38]. In vitro-induced or expanded antigen-specific Tregs can be negative or can transiently express FOXP3 [7,39,40,41,42,43,44]; therefore, FOXP3 has been primarily associated with the so-called naturally occurring Tregs (nTregs) rather than acting as a universal Treg marker.
Furthermore, in human T cells, FOXP3 is expressed in non-suppressive Teff cells shortly after T cell receptor (TCR) stimulation (e.g., with anti-CD3) [45,46,47] (Table 1). Hence, detecting FOXP3 in T cells alone is not sufficient to specify Tregs in cross-sectional analyses of human tissue samples, since staining for FOXP3 in inflammatory lesions may include recently activated Teff cells while leaving some induced Tregs with unstable FOXP3 that are undetected and ignored. On the other hand, analyzing the Treg-specific demethylated region (TSDR) can discriminate between Tregs with sustained FOXP3 expression and Teff transiently expressing FOXP3 [48]. This method, however, is not unambiguous since the TSDR can be partially demethylated in bonafide Tregs [49], as well as in some Teff subpopulations [50].
Notwithstanding the importance of FOXP3 as a checkpoint in human Treg differentiation and the suppression of Teff, and considering the available literature, we conclude that FOXP3 in humans does not exclusively identify Tregs, or all Tregs, and should not be used as a stand-alone proxy for immune regulation in vivo [51].
2.2. The Complexity of the Treg Universe, as Revealed by Their Phenotypes
The appreciation of the limitations of FOXP3 in humans as an unambiguous Treg marker has stimulated an extensive search for other molecules that differentiate suppressive from non-suppressive T cells. This approach, however, made defining Tregs in human samples even more difficult since it unraveled a whole universe of Treg subsets, based on either specific phenotypes, tissue compartments, or functions.
For classification, human Tregs have been divided into three groups: tTregs, generated in the thymus and also commonly known as nTregs, pTregs, generated in the peripheral tissues, and iTregs, induced in vitro. The tTregs/nTregs express CD25 and FOXP3 and lack CD127. They can sometimes express molecules like cytotoxic T-lymphocyte-associated protein 4 (CTLA4), glucocorticoid-induced TNFR-related protein (GITR), latency-associated peptide (LAP), and/or lymphocyte-activation gene 3 (Lag-3), and can produce inhibitory cytokines such as interleukine-10 (IL-10), transforming growth factor β (TGF-β), and/or IL-35. However, not all the proposed molecules are expressed on all nTregs, and over twenty subsets have been described within the nTregs population [52]. It remains a subject of debate how to distinguish pTregs from tTregs/nTregs (markers like Helios and Neuropilin (Nrp-1) have been proposed but discarded since the publication of [53,54,55,56]) and whether some of the pTregs found in patients compare to those induced in vitro, such as Type 1 regulatory T cells (Tr1), which are characterized by CD25lo, FOXP3neg, Lag-3pos, and CD49bpos expression, and the production of high levels of IL-10 [7,39,40,41,42], along with Type 2 regulatory T cells (Tr2 or Th3), which produce high levels of TGF-β and express CD25lo, FOXP3neg, LAPpos, CD69pos, CTLA4lo, and the GITRlo phenotype [43,44].
The continuous splitting of Treg populations into subsets on the basis of new candidate markers adds to the confusion when classifying Treg sub-entities, which can share phenotypic and functional features irrespective of their origin that sometimes overlap with activated Teff cells (Figure 1). Furthermore, supposed Treg markers such as GITR, Lag-3, and glycoprotein A33 (GPA33) and others (Table 1) can be expressed by activated Teff cells to regulate/modulate T cell activation and may be linked to the contraction of T cells after activation to regain immune homeostasis [57,58,59,60,61]. For example, Lag-3 is upregulated on all T cells upon TCR activation or by certain cytokines [57]. By binding to HLA class II, Lag-3 inhibits the early steps of the TCR pathway, suppressing any subsequent stimulation [62]. Activated T cells (Tregs and Teff cells) thus express Lag-3, enabling them to blunt any overt immune reactivity and contract Teff expansion. Nevertheless, when Tregs express Lag-3, they do so with greater magnitude compared to CD4+ Teff cells [63], giving them an advantage when interacting with and inhibiting antigen-presenting cells (APCs). Thus, some of the described markers may not be Treg-specific but are, nevertheless, employed by these cells for their regulatory function. These markers should be monitored even though their changing expression rates, upon activation, hamper the qualification of Treg subtypes or distinction between Tregs and Teff cells.
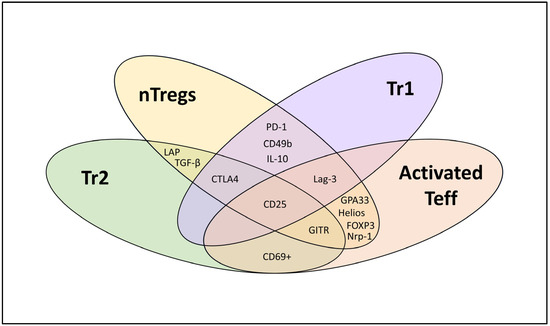
Figure 1.
Phenotypic characterization of different T cell subsets (nTregs, Tr1, Tr2, and activated effector T cells (Teff)).
When cytokines, chemokines, and other transcription factors are included, further subsets of Tregs have been suggested, such as IL-35-producing induced Tregs (iTr35, FOXP3−IL-35+) [64] or TGFb and IL-10-producing Tregs induced by B cells (Tregs-of-B cells, FOXP3− IL-10+ TGF-β+) [65,66,67,68] and the regulatory subtype of Tfh cells (Tfr, FOXP3+ CXCR5+ CD25+/− Bcl-6+ PD-1+ ICOS+) [69,70]. Adding to the confusion, the newly discovered Treg subtypes have been given new names without testing their similarity to previously described subsets (indeed, we, too, are guilty as charged; see [49]), resulting in many seemingly different but potentially related Treg subtypes, making the comparison of Tregs between studies difficult.

Table 1.
Markers claimed to be specific for regulatory T cells (Tregs) but shown to also be expressed by activated effector T cells (Teff).
Table 1.
Markers claimed to be specific for regulatory T cells (Tregs) but shown to also be expressed by activated effector T cells (Teff).
| Markers | ‘Treg-Specific’ [Reference] | Also Expressed in Teff Cells [Reference] |
|---|---|---|
| 4-1BB/CD137 | [71] | [72] |
| CD25 | [8] | [46,73] |
| FOXP3 | [74,75,76] | [45,46,47] |
| GARP | [77] | [78,79] |
| GITR | [9,80] | [81] |
| GPA33 | [82] | [61] |
| Helios | [54,83,84,85] | [53] |
| Lag-3 | [86,87] | [88] |
| LAP | [10] | [79] |
| Neuropilin | [11,56] | [55] |
| OX40 | [71] | [89] |
| TNFR2/CD120b | [90] | [91] |
The challenge to measure all described Treg markers simultaneously, due to their intracellular expression or release and limited technical capacities, was partially addressed by multidimensional analyses (e.g., CyTOF analysis and spectral flow cytometry), revealing many distinct subpopulations of nTregs alone [52], and confirming the complexity of the Treg universe. These subsets may also represent nTregs in different development/activation stages, reflect their changes in time, their different tissues of origin, or variations in the functions they exert. Moreover, multidimensional phenotyping does not yet resolve the problem of discriminating between Tregs and Teff cells effectively [92]. In conclusion, the search for a universal Treg-specific marker, or one at least shared between most Treg subtypes but not with activated Teff cells, has not been successful as yet, and functional assays remain indispensable to characterize Tregs in concert with their phenotypes.
2.3. Does the Priming Site Determine the Treg Type?
In our view, the attempt to classify human Tregs based on their origin (i.e., where they acquired their regulatory function) into tTregs, pTregs, and iTregs does not reduce confusion since all T cells derive from the thymus by definition. While the priming and differentiation site of tTregs or pTregs/iTregs may differ, similarities in specificity, phenotype, or function cannot be excluded. The assumed differentiation of Tregs from thymocytes follows a similar route to that of conventional T cells, both requiring TCR-dependent signaling after engagement with human leukocyte antigen (HLA) class II molecules on specialized APCs presenting a variety of peptides derived mainly from available autoantigens [93,94,95,96,97]. The differentiation of nTregs is presumed to occur during negative selection, where high-avidity TCR/HLA-interactive T cells undergo apoptosis [98,99], low-avidity TCR/HLA interactive T cells favor egressing into conventional naïve T cells, and intermediate-avidity TCR/HLA interactive T cells would favor sustained FOXP3 expression and differentiate into nTregs/tTregs [100,101,102,103]. The low-avidity TCR/HLA-binding naïve T cells may subsequently develop into pTregs/iTregs upon encountering the antigens presented by tolerance-inducing APCs in specialized tolerogenic niches in the periphery or in vitro [49,92,104,105,106,107,108]. As a result, tTregs are believed to be biased toward the immune recognition of self-antigens presented in the thymus, whereas pTregs/iTreg cells will recognize non-self- and neoantigens not presented in the thymus, such as allergens, food, tumor- or stress-induced antigens, and microbiota [109,110]. This strict division, however, knows exceptions; we showed that Tregs reacting against the self-derived proinsulin peptide can be induced from naïve CD4 T cells by tolerogenic dendritic cells (tolDCs) [49,92], and these Tregs would be classified as iTregs. Also, IL-10-producing islet-specific CD4 T cells that qualify as pTregs were isolated from nondiabetic individuals ex vivo [111]. Thus, pTregs and iTregs can respond to self-antigens and are not exclusively biased toward recognizing non-self-antigens.
3. Lessons from Treg Clones
Most studies of Tregs and Treg subtypes have been performed on polyclonal populations. Tregs in polyclonal populations can now be analyzed on a single-cell level [112,113,114]. However, to accurately investigate Treg diversity and relate phenotypes with functions, Tregs must be studied at the clonal level. Isolating and expanding such antigen-specific Treg clones, however, is extremely challenging since Tregs are endorsed with (self-) regulatory properties that impair their expansion. Nonetheless, attempts to propagate and characterize a range of a-specific expanded (e.g., with anti-CD3) or melanoma antigen family A3 (MAGE-A3)-specific Treg clones [115,116], or induced/expanded proinsulin-specific Treg clones did succeed [49,111]. Some MAGE-specific Tregs that were isolated from melanoma patients vaccinated with MAGE were CD4+CD25+ and demethylated FOXP3, whereas other CD4+ clones lacking CD25 could also suppress Teff cells, despite FOXP3 being methylated [116]. Autoreactive Tregs that were induced by tolDC against proinsulin were functionally and phenotypically indistinguishable from islet autoantigen-specific Tregs sorted directly from blood by IL-10 capture assays [49,111]. Unsupervised clustering of these proinsulin-specific Treg clones based on their regulatory features (including their function and phenotype, such as the intracellular expression of IL-10, IFN-γ, granzyme B, the surface expression of CTLA-4 and IL-10, IFN-γ, TNF, and IL-13 production in combination with functional characterization, including monocyte killing, the prevention of the activation of naïve T cells (the ‘classic’ suppression assay), and the inhibition of pre-primed, activated effector Th1 cells) revealed three discrete clusters, in spite of an identical induction protocol and the antigen-specificity of these Treg clones (Table 2) [49]. Cluster 1 involved Treg clones producing high amounts of cytokines (IL-10, TNF, IFN-γ, and IL-13), which were able to kill monocytes and inhibited both naïve T cells and, to a lesser degree, activated Th1 responses. Cluster 2 exhibited strong monocyte killing performance, often accompanied by granzyme B secretion, whereas cluster 3 defined efficient monocyte killers that also strongly inhibited both naïve T cell and Th1 responses.

Table 2.
Summary data of Treg clusters induced by tolerogenic dendritic cells (tolDCs) against proinsulin peptide.
Clones from cluster 3 can induce infectious tolerance [117]. These iTregs, primed by tolDCs, were able to functionally modify proinflammatory mDCs to become anti-inflammatory dendritic cells (DCs) through mechanisms including ICOS-L and B7-H3 ligation. These converted DCs in turn lost their capacity to stimulate Teff cells and, instead, induced IL-10-producing Tregs from the naïve T cell repertoire.
Surprisingly, the Treg clones from cluster 1 exhibited a completely inversed polarity of their TCR, leading to reversed docking onto the proinsulin peptide/HLA complex [49]; the α-chain and the β-chain of these TCRs interacted with the α-chain and β-chain of the HLA class II molecule, respectively. Nevertheless, this unorthodox TCR–peptide–HLA interaction resulted in HLA-restricted and antigen-specific (proinsulin) Treg activation. Thus, we propose that some Tregs may employ uniquely reversed TCR docking, contributing to their regulatory nature, a feature that would distinguish Tregs from Teffs. Indeed, similar inversed TCR docking has since been observed in the T cells of mice unresponsive to flu vaccination [118]. It is, therefore, important to consider that such a reverse TCR docking feature might be characteristic of antigen-specific Tregs when using TCRs in CAR or ‘avatar’ Treg models. Hence, endorsing Tregs with TCR derived from Teff may not necessarily result in immune regulation being exerted through the adopted TCR.
In conclusion, Treg clones have taught us that even when Tregs share antigen-specificity, their phenotypic and functional characteristics divide them into clusters, supporting the finding that not all Tregs are equal and that functional features assigned to polyclonal populations may, in fact, represent combinations of separate mechanistic features asserted by different Treg clones.
4. Functional Diversity of Tregs
Similar to our findings using the proinsulin-specific iTreg clones [49], nTregs may exert a range of regulatory mechanisms (reviewed in [119]), such as their interaction with and modulation of APCs, the release of inhibitory cytokines, interference with metabolic signals, direct interaction with Teff cells, and the induction of apoptosis (Figure 2). However, it remains unresolved whether all these mechanisms are employed simultaneously, or whether individual nTregs show different patterns of functionality. Since our clonal iTregs showed that clones within different phenotype clusters can share regulatory mechanisms (e.g., the induction of apoptosis), we propose that the detailed characterization of diverse regulatory mechanisms is indispensable for distinguishing different types of Tregs and should be included in Treg phenotyping. In the following section, we will briefly review various mechanisms of T cell regulation that should be considered when assessing Treg functionality (Figure 2).
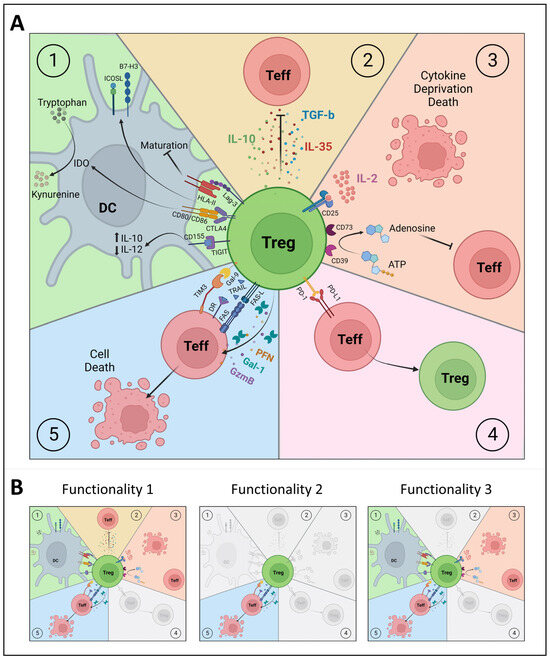
Figure 2.
Functional characterization of polyclonal and clonal Treg subsets. (A) Different mechanisms of immunosuppression by regulatory T cells (Tregs): 1. Interaction with antigen-presenting cells (dendritic cell, DC). 2. Secretion of inhibitory or immunosuppressive cytokines. 3. Metabolic disruption. 4. Direct contact with effector T cells (Teff). 5. Induction of apoptosis. (B) The revisited Treg universe, based on functional properties at the clonal level rather than on phenotype at the polyclonal level. There are Tregs with a single inhibitory function (e.g., killing, Functionality 2), while other Tregs may exert multiple immunoregulatory functionalities (some functions might be shared, as with Functionalities 1 and 3). CTLA4: cytotoxic T-lymphocyte associated protein 4, DR: death receptor, Gal-1: galectin 1, Gal-9: galectin 9, GzmB: granzyme B, HLA-II: human leukocyte antigen class II, ICOSL: inducible co-stimulator ligand, IDO: indoleamine 2,3 dioxygenase, Lag-3: lymphocyte-activation gene 3, PD-1: programmed cell death protein 1, PD-L1: programmed death-ligand 1, PFN: perforin, TIGIT: T cell immunoreceptor with immunoglobulin and ITIM domain, TIM3: T cell immunoglobulin and mucin domain 3, TRAIL: tumor necrosis factor-related apoptosis-inducing ligand. Figures were created with Biorender.com.
4.1. Interaction with Antigen-Presenting Cells
There are several mechanisms by which Tregs interact with APCs; these can alter the maturation and/or function of DCs, affecting antigen presentation and T cell activation (Figure 2). DCs provide the required costimulation for Teff activation. One of the mechanisms that Tregs employ to interfere with costimulation is via CTLA4 (or CD152) expression. CTLA4 competes against CD28 to bind to CD80/CD86 on DCs [120,121] and, in this way, prevents the proliferation, activation, and differentiation of Teff cells via CD28. The binding of CTLA4 to CD80/CD86 will also lead to the production of the enzyme indoleamine 2,3 dioxygenase (IDO) by DCs, which initiates the breaking down of the essential amino acid tryptophan into kynurenine. The scarcity of tryptophan suppresses the protein synthesis of Teff cells, resulting in cell cycle arrest and, thus, the inactivity or anergy of Teff cells [122,123,124,125].
Tregs also use the Lag-3 (or CD223) molecule to interact with APCs by binding with the HLA class II molecules with a high affinity, like CD4. Although this does not interfere with the interaction between HLA class II and CD4 on T cells [86], it does have functional consequences for T cell activation, since the binding of Lag-3 to HLA class II suppresses DC maturation and the capacity of DCs to present antigens. Namely, Lag-3 binding induces the cross-linking of HLA class II molecules, and the activation of immunoreceptor tyrosine-based activation motif (ITAM)-mediated inhibitory signaling in DCs via extracellular-signal-regulated kinase (ERK) and protein tyrosine phosphatase 1 (SHP1) [126,127].
Additionally, Tregs can express a molecule named TIGIT (T cell immunoreceptor with immunoglobulin and ITIM domain). TIGIT competes with CD226 for its ligand CD155 on DCs. CD226 is a costimulatory receptor that promotes cell contact and TCR signaling [128] and induces the production of proinflammatory cytokines by Teff cells [129]. TIGIT binds to CD155 with greater affinity than CD226, thereby limiting CD226-mediated activation and Teff function [130,131,132]. Additionally, the binding of TIGIT to CD155 on DCs induces CD155 phosphorylation and triggers a signaling cascade, resulting in the increased production of IL-10 and the reduced production of IL-12 by DCs, further inhibiting the activation of Teff cells [131,133].
Another important feature of some Tregs is their capacity to modulate proinflammatory DCs to induce new Tregs (Figure 2) [117] and, through this ‘infectious tolerance’, create a legacy of antigen-specific immune regulation that lasts beyond their lifetime. This process requires Treg-mDC contact and the activation of the Tregs by their cognate (auto)antigen, and critically involves immune regulatory molecules such as ICOS-L and B7-H3. Indeed, blocking these molecules prevented the transfer of tolerogenic properties to mature DCs by Tregs [117].
4.2. Inhibitory Cytokines
Tregs can suppress immune cells via the release of soluble mediators or inhibitory cytokines such as IL-10, TGF-β, and IL-35 (Figure 2) [134,135]. These cytokines restrict the stimulation and/or survival of Teff cells and induce survival signals in Tregs, supporting peripheral homeostasis [136]. IL-10 inhibits the CD28 pathway, which T cells need as co-stimulation to become activated [137,138]. The active form of TGF-β can function in either a surface-bound or secreted form, after which it binds its receptor on Teff cells [139,140]. This eventually affects the expression of genes such as GATA3, T-bet, STAT4, IFN-γ, and granzyme B, which are important for T cell differentiation and function [141,142,143,144,145]. IL-35 is a heterodimeric cytokine that belongs to the IL-12 family [135] and plays a role in immunosuppression by inhibiting T cell proliferation and promoting the induction of Tregs cells from naïve T cells without the requirement of IL-10, TGF-β, and FOXP3 [146].
4.3. Metabolic Disruption
Tregs influence Teff metabolism by competing for IL-2 and by energy deprivation using CD39 and CD73 (Figure 2). Tregs express high levels of CD25, the high-affinity subunit of the IL-2 receptor, thereby possessing a stronger capacity to bind IL-2 than other T cells [122,147]. By limiting IL-2 access for proliferating cells, Tregs trigger metabolic disruption and apoptosis [148,149]. The ATP apyrase (CD39) and ecto-5′-AMP-nucleotidase (CD73) suppress Teff cell function by converting ATP into adenosine [150]. The CD39 expressed by Tregs breaks down ATP into AMP, while CD73 converts AMP into adenosine. When adenosine binds the adenosine receptor (A2A) on Teff cells, this increases c-AMP via the G-protein-coupled receptor and results in an inhibitory signal in T cells [151]. CD39 also functions to sustain Treg stability since CD39high Tregs remain unchanged when cultured in the presence of the proinflammatory cytokines IL-1β and IL-6, whereas CD39low Tregs can differentiate into Th1 and Th17 cells [152]. Also, CD39high Tregs showed stronger suppressive capacity to inhibit PBMCs compared to CD39low Tregs in vitro and protected against xenograft-versus-host disease in a mouse model [152]. Tregs can also disrupt metabolism indirectly by CTLA-4-induced IDO expression in DCs (as described in Section 4.1). IDO breaks down tryptophan and the resulting scarcity of tryptophan suppresses Teff cells [122,123,124,125].
4.4. Direct Interaction with Effector T Cells
Tregs can directly interact with Teff cells through the programmed cell death protein (PD-1) and programmed death-ligand 1 (PD-L1) (Figure 2) [153,154]. PD-L1 ligation on CD4 T cells can prevent their activation and polarization [155]. PD-1high Tregs have a much stronger suppressive capacity than PD-1low Tregs during chronic LCMV infection [156]. A PD-1 blockade on PD-1high Tregs prevented the suppression of Teff cells in vitro (stimulated with anti-CD3/CD28 without the presence of APCs), pointing to the direct interaction of PD-1 on Tregs with PD-L1 on Teff. In addition, PD-L1 engagement on memory T cells (CD4posCD25negCD45RAnegCD45ROpos) promoted their conversion into iTregs when using an antibody crosslinking PD-L1 [157]. It is, therefore, possible that PD-1 on Tregs binds to PD-L1 on Teff, leading to the crosslinking of PD-L1 and the conversion of Teff into iTregs. Furthermore, PD-1 signaling is important for FOXP3 expression and for maintaining Treg homeostasis [158,159].
4.5. Induction of Apoptosis
Finally, Tregs can induce apoptosis in Teff cells (Figure 2) through the release of granzyme B (GzmB) and perforin (PFN) [160,161] or by the expression of FAS-L [162,163,164,165]. Tregs can also use β-galactoside binding proteins (galectin) to suppress immune cells. The blockade of galectin-1 (Gal-1) reduced the suppressive capacity of human nTregs in vitro [166]. Galectin 9 (Gal-9) is expressed on the surface of Tregs and binds T cell immunoglobulin and mucin domain 3 (TIM3) on Teff cells, which results in anergy or apoptosis, mediated by intracellular calcium release [167,168]. Furthermore, activated Tregs upregulate the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which binds death receptor (DR) 4 and 5 on Teff cells and thereby triggers the intracellular signaling components forming the death-inducing signaling complex (DISC), subsequent caspase cascade activation, and cell death [169].
4.6. Evolving Understanding of a Regulatory Role in HLA-DR Expression and Extracellular Vesicles
There are a few additional but so far poorly investigated mechanisms that Tregs may use to suppress immune responses.
Activated human Teffs and Tregs express HLA class II [170,171,172,173,174], the role of which remains unclear, given that these cells do not have antigen-processing machinery. The antibody blockade of HLA class II resulted in the loss of the suppressive activity of Treg in vitro [170,175]. Tregs could express HLA class II, loaded with self-peptides, to connect with autoreactive Teff cells and use this interaction to inhibit Teff cells via one or several of the regulatory mechanisms described in this review. Alternatively, the expression of HLA class II could allow a self-regulating mechanism through an interaction with Lag-3.
Another interesting option involves extracellular vesicles (EVs) being released by Tregs. EVs are membranous structures that can be produced by any cell, the content of which reflects the cell of origin. Human Treg EVs can contain immunomodulatory molecules such as CD25, CD73, Nrp1, and CTLA-4 [176], cytokines such as IL-35 [177], and microRNAs such as Let-7d, Let-7b, miR-155, and miR-146a-5p [178,179]. These EVs have been demonstrated to influence processes like miRNA-induced gene silencing, surface protein activity, and enzyme activity in vitro [176,180,181]. Still, Treg EVs were not as effective as Tregs in suppressing Teff, indicating that cell-based processes are indispensable for optimal suppression [178]. Though less effective, EVs from Tregs could contribute to the intercellular exchange with Teff or between Tregs themselves, governing immunological responses and, therefore, establishing a tolerogenic milieu in a cell-free manner.
The current results raise new questions about the involvement of HLA-DR on Tregs and the release of EVs in the suppressive capacity of Tregs, and both need to be studied more closely to understand their role.
5. A New View on the Treg Universe
Recent advances in multidimensional analyses have enabled us to appreciate the complexity of the Treg universe and that a single marker or a functional property does not suffice to define this cell type. Our unique Treg clones illustrate that even when Tregs share their antigen-specificity and surface phenotypes, they can differ in the number and type of regulatory functions that they use for suppression (Figure 2). As an analogy, some Tregs may have a single inhibitory function in vivo (e.g., the killing of Teffs or APCs), while others may exert multiple immunoregulatory functionalities, irrespective of their origin or antigen specificity. Classifying Tregs based on their different functional properties will help to define, measure, and understand Tregs more accurately, particularly in combination with analyses of the phenotypes that accompany them, to identify or confirm markers that are tightly linked with a certain function, for example, the expression of FAS-L and the production of granzymes associated with killing capacity, and the expression of CTLA4 with the conditioning of APCs. Such markers can be used to assign the functional property of a certain Treg and allow us to dissect the regulatory T cell population in general, instead of focusing on ‘specific’ Treg subtype definitions such as Tr1 and Tr2 cells (defined on the basis of different markers in different studies).
In conclusion, we propose to view the Treg universe with fresh eyes and consider the functional properties of Tregs and the related markers, rather than merely the expression of FOXP3 or any other supposedly Treg-specific markers. In this way, we hope that a consensus can be reached on characterizing human Tregs and studying Tregs categorically. This will also help to relate Tregs induced in vitro with Tregs ex vivo, which is difficult at this moment.
However, there are reassuring examples that islet-antigen-specific Tregs that are induced in vitro by tolDC are very similar to Tregs that are isolated and expanded ex vivo [49,111]. Furthermore, identifying Tregs by function may help in moving forward strategies that aim to employ Tregs in immunotherapy. It is conceivable that certain Treg entities are superior to others, which may explain and overcome the disappointing results from various Treg-based immune intervention trials (as reviewed in [182]).
Author Contributions
All the authors listed have made a substantial, direct, and intellectual contribution to the work and. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are supported by grants from the Helmsley Charitable Trust and Stichting DON.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| APCs | Antigen-presenting cells |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DR | Death receptor |
| EVs | Extracellular vesicles |
| Gal | Galectin |
| GITR | Glucocorticoid-induced TNFR-related protein |
| GPA33 | Glycoprotein A33 |
| GzmB | Granzyme B |
| IDO | Indoleamine 2,3 dioxygenase |
| IPEX | Immune dysfunction/polyendocrinopathy/enteropathy/X-linked |
| iTregs | Tregs induced in vitro |
| Lag-3 | Lymphocyte-activation gene 3 |
| LAP | Latency-associated peptide |
| MAGE-A3 | Melanoma antigen family A3 |
| mDCs | Inflammatory monocyte-derived dendritic cell |
| Nrp-1 | Neuropilin |
| nTregs | Naturally occurring Tregs |
| PD-1 | Programmed cell death protein |
| PD-L1 | Programmed death-ligand 1 |
| PFN | Perforin |
| pTregs | Tregs generated in the peripheral tissues |
| TCR | T cell receptor |
| Teff | Effector T cells |
| TIGIT | T cell immunoreceptor with immunoglobulin and ITIM domain |
| TIM3 | T cell immunoglobulin and mucin domain 3 |
| tolDCs | Tolerogenic dendritic cells |
| Tr1 | Type 1 regulatory T cells |
| Tr2 | Type 2 regulatory T cells |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| Tregs | Regulatory T cells |
| TSDR | Treg-specific demethylated region |
| tTregs | Tregs generated in the thymus |
References
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Reichel, A.; Bonhagen, K.; Morawietz, L.; Rauchhaus, U.; Kamradt, T. Regulatory T cells control the transition from acute into chronic inflammation in glucose-6-phosphate isomerase-induced arthritis. Ann. Rheum. Dis. 2010, 69, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Zelinskyy, G.; Dietze, K.K.; Husecken, Y.P.; Schimmer, S.; Nair, S.; Werner, T.; Gibbert, K.; Kershaw, O.; Gruber, A.D.; Sparwasser, T.; et al. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood 2009, 114, 3199–3207. [Google Scholar] [CrossRef]
- Goldeck, D.; Theeten, H.; Hassouneh, F.; Oettinger, L.; Wistuba-Hamprecht, K.; Cools, N.; Tsitsilonis, O.E.; Pawelec, G. Frequencies of peripheral immune cells in older adults following seasonal influenza vaccination with an adjuvanted vaccine. Vaccine 2017, 35, 4330–4338. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Fernandez, I.; Rosado-Sanchez, I.; Alvarez-Rios, A.I.; Galva, M.I.; De Luna-Romero, M.; Sanbonmatsu-Gamez, S.; Perez-Ruiz, M.; Navarro-Mari, J.M.; Carrillo-Vico, A.; Sanchez, B.; et al. Effect of homeostatic T-cell proliferation in the vaccine responsiveness against influenza in elderly people. Immun. Ageing 2019, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Gagliani, N.; Magnani, C.F.; Huber, S.; Gianolini, M.E.; Pala, M.; Licona-Limon, P.; Guo, B.; Herbert, D.R.; Bulfone, A.; Trentini, F.; et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 2013, 19, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.M.; Shevach, E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998, 188, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, R.; Bistoni, O.; Alunno, A.; Petrillo, M.G.; Ronchetti, S.; Sportoletti, P.; Bocci, E.B.; Nocentini, G.; Gerli, R.; Riccardi, C. CD4+ CD25(low) GITR+ cells: A novel human CD4+ T-cell population with regulatory activity. Eur. J. Immunol. 2011, 41, 2269–2278. [Google Scholar] [CrossRef]
- Tran, D.Q.; Andersson, J.; Hardwick, D.; Bebris, L.; Illei, G.G.; Shevach, E.M. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood 2009, 113, 5125–5133. [Google Scholar] [CrossRef]
- Bruder, D.; Probst-Kepper, M.; Westendorf, A.M.; Geffers, R.; Beissert, S.; Loser, K.; von Boehmer, H.; Buer, J.; Hansen, W. Neuropilin-1: A surface marker of regulatory T cells. Eur. J. Immunol. 2004, 34, 623–630. [Google Scholar] [CrossRef]
- Burzyn, D.; Benoist, C.; Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 2013, 14, 1007–1013. [Google Scholar] [CrossRef]
- Edozie, F.C.; Nova-Lamperti, E.A.; Povoleri, G.A.; Scotta, C.; John, S.; Lombardi, G.; Afzali, B. Regulatory T-cell therapy in the induction of transplant tolerance: The issue of subpopulations. Transplantation 2014, 98, 370–379. [Google Scholar] [CrossRef]
- Fuchs, A.; Gliwinski, M.; Grageda, N.; Spiering, R.; Abbas, A.K.; Appel, S.; Bacchetta, R.; Battaglia, M.; Berglund, D.; Blazar, B.; et al. Minimum Information about T Regulatory Cells: A Step toward Reproducibility and Standardization. Front. Immunol. 2017, 8, 1844. [Google Scholar] [CrossRef]
- Brunkow, M.E.; Jeffery, E.W.; Hjerrild, K.A.; Paeper, B.; Clark, L.B.; Yasayko, S.A.; Wilkinson, J.E.; Galas, D.; Ziegler, S.F.; Ramsdell, F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001, 27, 68–73. [Google Scholar] [CrossRef]
- Godfrey, V.L.; Wilkinson, J.E.; Rinchik, E.M.; Russell, L.B. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: Potential model for thymic education. Proc. Natl. Acad. Sci. USA 1991, 88, 5528–5532. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, V.L.; Wilkinson, J.E.; Russell, L.B. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am. J. Pathol. 1991, 138, 1379–1387. [Google Scholar] [PubMed]
- Sharma, R.; Sung, S.S.; Fu, S.M.; Ju, S.T. Regulation of multi-organ inflammation in the regulatory T cell-deficient scurfy mice. J. Biomed. Sci. 2009, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.M.; Tremble, J.; Dayan, C.; Beyan, H.; Leslie, R.D.; Peakman, M.; Tree, T.I. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin. Exp. Immunol. 2008, 154, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Rieck, M.; Sanda, S.; Pihoker, C.; Greenbaum, C.; Buckner, J.H. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J. Immunol. 2008, 181, 7350–7355. [Google Scholar] [CrossRef]
- Venigalla, R.K.; Tretter, T.; Krienke, S.; Max, R.; Eckstein, V.; Blank, N.; Fiehn, C.; Ho, A.D.; Lorenz, H.M. Reduced CD4+,CD25− T cell sensitivity to the suppressive function of CD4+,CD25high, CD127 -/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008, 58, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Yamana, J.; Yamamura, M.; Okamoto, A.; Aita, T.; Iwahashi, M.; Sunahori, K.; Makino, H. Resistance to IL-10 inhibition of interferon gamma production and expression of suppressor of cytokine signaling 1 in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, R567–R577. [Google Scholar] [CrossRef] [PubMed]
- Gambineri, E.; Torgerson, T.R.; Ochs, H.D. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 2003, 15, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Ochs, H.D.; Ziegler, S.F.; Torgerson, T.R. FOXP3 acts as a rheostat of the immune response. Immunol. Rev. 2005, 203, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.J.; Jennings, C.E.; Imrie, H.; Lachaux, A.; Bridges, N.A.; Cheetham, T.D.; Pearce, S.H. Mutational analysis of the FOXP3 gene and evidence for genetic heterogeneity in the immunodysregulation, polyendocrinopathy, enteropathy syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Shiari, R.; Yamada, M.; Kawamura, N.; Okano, M.; Yara, A.; Iguchi, A.; Ishikawa, N.; Ariga, T.; Sakiyama, Y.; et al. Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX). J. Med. Genet. 2001, 38, 874–876. [Google Scholar] [CrossRef]
- Wildin, R.S.; Ramsdell, F.; Peake, J.; Faravelli, F.; Casanova, J.L.; Buist, N.; Levy-Lahad, E.; Mazzella, M.; Goulet, O.; Perroni, L.; et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001, 27, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001, 27, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Barzaghi, F.; Passerini, L.; Bacchetta, R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: A paradigm of immunodeficiency with autoimmunity. Front. Immunol. 2012, 3, 211. [Google Scholar] [CrossRef]
- d’Hennezel, E.; Bin Dhuban, K.; Torgerson, T.; Piccirillo, C.A. The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 2012, 49, 291–302. [Google Scholar] [CrossRef]
- Gambineri, E.; Ciullini Mannurita, S.; Hagin, D.; Vignoli, M.; Anover-Sombke, S.; DeBoer, S.; Segundo, G.R.S.; Allenspach, E.J.; Favre, C.; Ochs, H.D.; et al. Clinical, Immunological, and Molecular Heterogeneity of 173 Patients With the Phenotype of Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked (IPEX) Syndrome. Front. Immunol. 2018, 9, 2411. [Google Scholar] [CrossRef] [PubMed]
- Caudy, A.A.; Reddy, S.T.; Chatila, T.; Atkinson, J.P.; Verbsky, J.W. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J. Allergy Clin. Immunol. 2007, 119, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Moes, N.; Rieux-Laucat, F.; Begue, B.; Verdier, J.; Neven, B.; Patey, N.; Torgerson, T.T.; Picard, C.; Stolzenberg, M.C.; Ruemmele, C.; et al. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology 2010, 139, 770–778. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Moes, N.; de Serre, N.P.; Rieux-Laucat, F.; Goulet, O. Clinical and molecular aspects of autoimmune enteropathy and immune dysregulation, polyendocrinopathy autoimmune enteropathy X-linked syndrome. Curr. Opin. Gastroenterol. 2008, 24, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.L.; Russo, P.; Sullivan, K.E. Use of sirolimus in IPEX and IPEX-like children. J. Clin. Immunol. 2008, 28, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Zuber, J.; Viguier, M.; Lemaitre, F.; Senee, V.; Patey, N.; Elain, G.; Geissmann, F.; Fakhouri, F.; Ferradini, L.; Julier, C.; et al. Severe FOXP3+ and naive T lymphopenia in a non-IPEX form of autoimmune enteropathy combined with an immunodeficiency. Gastroenterology 2007, 132, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Zemmour, D.; Charbonnier, L.M.; Leon, J.; Six, E.; Keles, S.; Delville, M.; Benamar, M.; Baris, S.; Zuber, J.; Chen, K.; et al. Single-cell analysis of FOXP3 deficiencies in humans and mice unmasks intrinsic and extrinsic CD4+ T cell perturbations. Nat. Immunol. 2021, 22, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Gavin, M.A.; Rasmussen, J.P.; Fontenot, J.D.; Vasta, V.; Manganiello, V.C.; Beavo, J.A.; Rudensky, A.Y. Foxp3-dependent programme of regulatory T-cell differentiation. Nature 2007, 445, 771–775. [Google Scholar] [CrossRef]
- Levings, M.K.; Gregori, S.; Tresoldi, E.; Cazzaniga, S.; Bonini, C.; Roncarolo, M.G. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood 2005, 105, 1162–1169. [Google Scholar] [CrossRef]
- Brun, V.; Neveu, V.; Pers, Y.M.; Fabre, S.; Quatannens, B.; Bastian, H.; Clerget-Chossat, N.; Jorgensen, C.; Foussat, A. Isolation of functional autologous collagen-II specific IL-10 producing Tr1 cell clones from rheumatoid arthritis blood. Int. Immunopharmacol. 2011, 11, 1074–1078. [Google Scholar] [CrossRef]
- Brun, V.; Bastian, H.; Neveu, V.; Foussat, A. Clinical grade production of IL-10 producing regulatory Tr1 lymphocytes for cell therapy of chronic inflammatory diseases. Int. Immunopharmacol. 2009, 9, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Passerini, L.; Di Nunzio, S.; Gregori, S.; Gambineri, E.; Cecconi, M.; Seidel, M.G.; Cazzola, G.; Perroni, L.; Tommasini, A.; Vignola, S.; et al. Functional type 1 regulatory T cells develop regardless of FOXP3 mutations in patients with IPEX syndrome. Eur. J. Immunol. 2011, 41, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Scurr, M.; Ladell, K.; Besneux, M.; Christian, A.; Hockey, T.; Smart, K.; Bridgeman, H.; Hargest, R.; Phillips, S.; Davies, M.; et al. Highly prevalent colorectal cancer-infiltrating LAP+ Foxp3− T cells exhibit more potent immunosuppressive activity than Foxp3+ regulatory T cells. Mucosal Immunol. 2014, 7, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Farez, M.F.; Wang, Y.; Kozoriz, D.; Quintana, F.J.; Weiner, H.L. Cutting edge: Human latency-associated peptide+ T cells: A novel regulatory T cell subset. J. Immunol. 2010, 184, 4620–4624. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ioan-Facsinay, A.; van der Voort, E.I.; Huizinga, T.W.; Toes, R.E. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007, 37, 129–138. [Google Scholar] [CrossRef]
- Kmieciak, M.; Gowda, M.; Graham, L.; Godder, K.; Bear, H.D.; Marincola, F.M.; Manjili, M.H. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J. Transl. Med. 2009, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.E.; Crome, S.Q.; Crellin, N.K.; Passerini, L.; Steiner, T.S.; Bacchetta, R.; Roncarolo, M.G.; Levings, M.K. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007, 19, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Baron, U.; Floess, S.; Wieczorek, G.; Baumann, K.; Grutzkau, A.; Dong, J.; Thiel, A.; Boeld, T.J.; Hoffmann, P.; Edinger, M.; et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur. J. Immunol. 2007, 37, 2378–2389. [Google Scholar] [CrossRef]
- Beringer, D.X.; Kleijwegt, F.S.; Wiede, F.; van der Slik, A.R.; Loh, K.L.; Petersen, J.; Dudek, N.L.; Duinkerken, G.; Laban, S.; Joosten, A.; et al. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat. Immunol. 2015, 16, 1153–1161. [Google Scholar] [CrossRef]
- Schmidl, C.; Klug, M.; Boeld, T.J.; Andreesen, R.; Hoffmann, P.; Edinger, M.; Rehli, M. Lineage-specific DNA methylation in T cells correlates with histone methylation and enhancer activity. Genome Res. 2009, 19, 1165–1174. [Google Scholar] [CrossRef]
- Voss, K.; Lake, C.; Luthers, C.R.; Lott, N.M.; Dorjbal, B.; Arjunaraja, S.; Bauman, B.M.; Soltis, A.R.; Sukumar, G.; Dalgard, C.L.; et al. FOXP3 protects conventional human T cells from premature restimulation-induced cell death. Cell. Mol. Immunol. 2021, 18, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.M.; Lowe, K.; Melchiotti, R.; Ellis, R.; de Rinaldis, E.; Peakman, M.; Heck, S.; Lombardi, G.; Tree, T.I. Phenotypic Complexity of the Human Regulatory T Cell Compartment Revealed by Mass Cytometry. J. Immunol. 2015, 195, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Akimova, T.; Beier, U.H.; Wang, L.; Levine, M.H.; Hancock, W.W. Helios expression is a marker of T cell activation and proliferation. PLoS ONE 2011, 6, e24226. [Google Scholar] [CrossRef] [PubMed]
- Thornton, A.M.; Korty, P.E.; Tran, D.Q.; Wohlfert, E.A.; Murray, P.E.; Belkaid, Y.; Shevach, E.M. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010, 184, 3433–3441. [Google Scholar] [CrossRef] [PubMed]
- Milpied, P.; Renand, A.; Bruneau, J.; Mendes-da-Cruz, D.A.; Jacquelin, S.; Asnafi, V.; Rubio, M.T.; MacIntyre, E.; Lepelletier, Y.; Hermine, O. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur. J. Immunol. 2009, 39, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Louvet, C.; Davini, D.; Gardner, J.M.; Martinez-Llordella, M.; Bailey-Bucktrout, S.; Anthony, B.A.; Sverdrup, F.M.; Head, R.; Kuster, D.J.; et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012, 209, 1713–1722, S1711–S1719. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Tournier, M.; Triebel, F. LAG-3 does not define a specific mode of natural killing in human. Immunol. Lett. 1998, 61, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, G.; Giunchi, L.; Ronchetti, S.; Krausz, L.T.; Bartoli, A.; Moraca, R.; Migliorati, G.; Riccardi, C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 6216–6221. [Google Scholar] [CrossRef]
- Gurney, A.L.; Marsters, S.A.; Huang, R.M.; Pitti, R.M.; Mark, D.T.; Baldwin, D.T.; Gray, A.M.; Dowd, A.D.; Brush, A.D.; Heldens, A.D.; et al. Identification of a new member of the tumor necrosis factor family and its receptor, a human ortholog of mouse GITR. Curr. Biol. 1999, 9, 215–218. [Google Scholar] [CrossRef]
- Kanamaru, F.; Youngnak, P.; Hashiguchi, M.; Nishioka, T.; Takahashi, T.; Sakaguchi, S.; Ishikawa, I.; Azuma, M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J. Immunol. 2004, 172, 7306–7314. [Google Scholar] [CrossRef]
- Opstelten, R.; Suwandi, J.S.; Slot, M.C.; Morgana, F.; Scott, A.M.; Laban, S.; Nikolic, T.; Turksma, A.W.; Kroeze, A.; Voermans, C.; et al. GPA33 is expressed on multiple human blood cell types and distinguishes CD4+ central memory T cells with and without effector function. Eur. J. Immunol. 2021, 51, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Cauley, L.S.; Kim, I.J.; Blackman, M.A.; Woodland, D.L.; Vignali, D.A. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 2004, 172, 5450–5455. [Google Scholar] [CrossRef]
- Keane, C.; Law, S.C.; Gould, C.; Birch, S.; Sabdia, M.B.; Merida de Long, L.; Thillaiyampalam, G.; Abro, E.; Tobin, J.W.; Tan, X.; et al. LAG3: A novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv. 2020, 4, 1367–1377. [Google Scholar] [CrossRef]
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A.; et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010, 11, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, P.; Dornbach, B.; Rong, S.; Beissert, S.; Gueler, F.; Loser, K.; Gunzer, M. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood 2007, 110, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.H.; Chiang, B.L. Regulatory T cells induced by mucosal B cells alleviate allergic airway hypersensitivity. Am. J. Respir. Cell Mol. Biol. 2012, 46, 651–659. [Google Scholar] [CrossRef]
- Chu, K.H.; Chiang, B.L. Characterization and functional studies of forkhead box protein 3− lymphocyte activation gene 3+ CD4+ regulatory T cells induced by mucosal B cells. Clin. Exp. Immunol. 2015, 180, 316–328. [Google Scholar] [CrossRef]
- Hsu, L.H.; Li, K.P.; Chu, K.H.; Chiang, B.L. A B-1a cell subset induces Foxp3− T cells with regulatory activity through an IL-10-independent pathway. Cell. Mol. Immunol. 2015, 12, 354–365. [Google Scholar] [CrossRef]
- Hao, H.; Nakayamada, S.; Tanaka, Y. Differentiation, functions, and roles of T follicular regulatory cells in autoimmune diseases. Inflamm. Regen. 2021, 41, 14. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.M.; Dent, A.L. Unexpected Help: Follicular Regulatory T Cells in the Germinal Center. Front. Immunol. 2018, 9, 1536. [Google Scholar] [CrossRef]
- Schoenbrunn, A.; Frentsch, M.; Kohler, S.; Keye, J.; Dooms, H.; Moewes, B.; Dong, J.; Loddenkemper, C.; Sieper, J.; Wu, P.; et al. A converse 4-1BB and CD40 ligand expression pattern delineates activated regulatory T cells (Treg) and conventional T cells enabling direct isolation of alloantigen-reactive natural Foxp3+ Treg. J. Immunol. 2012, 189, 5985–5994. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Valbracht, J.; Tuckwell, J.; von Kempis, J.; Lotz, M. ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood 1995, 85, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Shatrova, A.N.; Mityushova, E.V.; Vassilieva, I.O.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; Marakhova, I.I. Time-Dependent Regulation of IL-2R alpha-Chain (CD25) Expression by TCR Signal Strength and IL-2-Induced STAT5 Signaling in Activated Human Blood T Lymphocytes. PLoS ONE 2016, 11, e0167215. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Schubert, L.A.; Jeffery, E.; Zhang, Y.; Ramsdell, F.; Ziegler, S.F. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem. 2001, 276, 37672–37679. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wan, Q.; Kozhaya, L.; Fujii, H.; Unutmaz, D. Identification of a regulatory T cell specific cell surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS ONE 2008, 3, e2705. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.A.; Neuhoff, A.; Landsberg, J.; Schupp, J.; Eberts, D.; Leukel, P.; Bros, M.; Weilbaecher, M.; Schuppan, D.; Grabbe, S.; et al. A key role of GARP in the immune suppressive tumor microenvironment. Oncotarget 2016, 7, 42996–43009. [Google Scholar] [CrossRef] [PubMed]
- Elkord, E.; Abd Al Samid, M.; Chaudhary, B. Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget 2015, 6, 20026–20036. [Google Scholar] [CrossRef]
- Nocentini, G.; Alunno, A.; Petrillo, M.G.; Bistoni, O.; Bartoloni, E.; Caterbi, S.; Ronchetti, S.; Migliorati, G.; Riccardi, C.; Gerli, R. Expansion of regulatory GITR+CD25 low/-CD4+ T cells in systemic lupus erythematosus patients. Arthritis Res. Ther. 2014, 16, 444. [Google Scholar] [CrossRef]
- Li, Z.; Mahesh, S.P.; Kim, B.J.; Buggage, R.R.; Nussenblatt, R.B. Expression of glucocorticoid induced TNF receptor family related protein (GITR) on peripheral T cells from normal human donors and patients with non-infectious uveitis. J. Autoimmun. 2003, 21, 83–92. [Google Scholar] [CrossRef]
- Opstelten, R.; de Kivit, S.; Slot, M.C.; van den Biggelaar, M.; Iwaszkiewicz-Grzes, D.; Gliwinski, M.; Scott, A.M.; Blom, B.; Trzonkowski, P.; Borst, J.; et al. GPA33: A Marker to Identify Stable Human Regulatory T Cells. J. Immunol. 2020, 204, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Getnet, D.; Grosso, J.F.; Goldberg, M.V.; Harris, T.J.; Yen, H.R.; Bruno, T.C.; Durham, N.M.; Hipkiss, E.L.; Pyle, K.J.; Wada, S.; et al. A role for the transcription factor Helios in human CD4+CD25+ regulatory T cells. Mol. Immunol. 2010, 47, 1595–1600. [Google Scholar] [CrossRef]
- Morina, L.; Jones, M.E.; Oguz, C.; Kaplan, M.J.; Gangaplara, A.; Fitzhugh, C.D.; Kanakry, C.G.; Shevach, E.M.; Buszko, M. Co-expression of Foxp3 and Helios facilitates the identification of human T regulatory cells in health and disease. Front. Immunol. 2023, 14, 1114780. [Google Scholar] [CrossRef]
- Sugimoto, N.; Oida, T.; Hirota, K.; Nakamura, K.; Nomura, T.; Uchiyama, T.; Sakaguchi, S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int. Immunol. 2006, 18, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef]
- Camisaschi, C.; Casati, C.; Rini, F.; Perego, M.; De Filippo, A.; Triebel, F.; Parmiani, G.; Belli, F.; Rivoltini, L.; Castelli, C. LAG-3 expression defines a subset of CD4+CD25(high)Foxp3+ regulatory T cells that are expanded at tumor sites. J. Immunol. 2010, 184, 6545–6551. [Google Scholar] [CrossRef] [PubMed]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Godfrey, W.R.; Fagnoni, F.F.; Harara, M.A.; Buck, D.; Engleman, E.G. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J. Exp. Med. 1994, 180, 757–762. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Lazzeri, E.; Manetti, R.; Vanini, V.; Romagnani, P.; Maggi, E.; Romagnani, S. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J. Exp. Med. 2002, 196, 379–387. [Google Scholar] [CrossRef]
- Mercer, F.; Kozhaya, L.; Unutmaz, D. Expression and function of TNF and IL-1 receptors on human regulatory T cells. PLoS ONE 2010, 5, e8639. [Google Scholar] [CrossRef]
- Suwandi, J.S.; Laban, S.; Vass, K.; Joosten, A.; van Unen, V.; Lelieveldt, B.P.F.; Hollt, T.; Zwaginga, J.J.; Nikolic, T.; Roep, B.O. Multidimensional analyses of proinsulin peptide-specific regulatory T cells induced by tolerogenic dendritic cells. J. Autoimmun. 2020, 107, 102361. [Google Scholar] [CrossRef]
- Brennecke, P.; Reyes, A.; Pinto, S.; Rattay, K.; Nguyen, M.; Kuchler, R.; Huber, W.; Kyewski, B.; Steinmetz, L.M. Single-cell transcriptome analysis reveals coordinated ectopic gene-expression patterns in medullary thymic epithelial cells. Nat. Immunol. 2015, 16, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.N.; Thyagarajan, H.M.; Srinivasan, J.; Li, Y.; Hu, Z.; Ehrlich, L.I.R. Live-cell imaging reveals the relative contributions of antigen-presenting cell subsets to thymic central tolerance. Nat. Commun. 2019, 10, 2220. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.; Zemmour, D.; Mathis, D.; Benoist, C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat. Immunol. 2015, 16, 942–949. [Google Scholar] [CrossRef]
- Perry, J.S.A.; Lio, C.J.; Kau, A.L.; Nutsch, K.; Yang, Z.; Gordon, J.I.; Murphy, K.M.; Hsieh, C.S. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 2014, 41, 414–426. [Google Scholar] [CrossRef]
- Sansom, S.N.; Shikama-Dorn, N.; Zhanybekova, S.; Nusspaumer, G.; Macaulay, I.C.; Deadman, M.E.; Heger, A.; Ponting, C.P.; Hollander, G.A. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014, 24, 1918–1931. [Google Scholar] [CrossRef]
- Starr, T.K.; Jameson, S.C.; Hogquist, K.A. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003, 21, 139–176. [Google Scholar] [CrossRef] [PubMed]
- Anderton, S.M.; Wraith, D.C. Selection and fine-tuning of the autoimmune T-cell repertoire. Nat. Rev. Immunol. 2002, 2, 487–498. [Google Scholar] [CrossRef]
- Hsieh, C.S.; Zheng, Y.; Liang, Y.; Fontenot, J.D.; Rudensky, A.Y. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 2006, 7, 401–410. [Google Scholar] [CrossRef]
- Lee, H.M.; Bautista, J.L.; Scott-Browne, J.; Mohan, J.F.; Hsieh, C.S. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity 2012, 37, 475–486. [Google Scholar] [CrossRef]
- Moran, A.E.; Holzapfel, K.L.; Xing, Y.; Cunningham, N.R.; Maltzman, J.S.; Punt, J.; Hogquist, K.A. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011, 208, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Sakaguchi, S. Genetic and epigenetic basis of Treg cell development and function: From a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol. Rev. 2014, 259, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, P.; Doherty, T.A.; Duan, W.; Mehta, A.K.; Choi, H.; Adams, Y.F.; Mikulski, Z.; Khorram, N.; Rosenthal, P.; Broide, D.H.; et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 2013, 210, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.M.; Ruane, D.; Moran, B.; Dunne, P.J.; Keane, J.; Mills, K.H. Alveolar macrophages contribute to respiratory tolerance by inducing FoxP3 expression in naive T cells. Am. J. Respir. Cell Mol. Biol. 2013, 48, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.J.; Pino-Lagos, K.; Rosemblatt, M.; Noelle, R.J. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med. 2007, 204, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Esterhazy, D.; Loschko, J.; London, M.; Jove, V.; Oliveira, T.Y.; Mucida, D. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat. Immunol. 2016, 17, 545–555. [Google Scholar] [CrossRef]
- Bilate, A.M.; Lafaille, J.J. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu. Rev. Immunol. 2012, 30, 733–758. [Google Scholar] [CrossRef]
- Hsieh, C.S.; Lee, H.M.; Lio, C.W. Selection of regulatory T cells in the thymus. Nat. Rev. Immunol. 2012, 12, 157–167. [Google Scholar] [CrossRef]
- Tree, T.I.; Lawson, J.; Edwards, H.; Skowera, A.; Arif, S.; Roep, B.O.; Peakman, M. Naturally arising human CD4 T-cells that recognize islet autoantigens and secrete interleukin-10 regulate proinflammatory T-cell responses via linked suppression. Diabetes 2010, 59, 1451–1460. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, C.; Wang, B.; Niu, Q.; Su, X.; Bai, Y.; Zhu, S.; Zhao, C.; Sun, Y.; Wang, J.; et al. Single-cell transcriptomic analysis reveals disparate effector differentiation pathways in human T(reg) compartment. Nat. Commun. 2021, 12, 3913. [Google Scholar] [CrossRef]
- Miragaia, R.J.; Gomes, T.; Chomka, A.; Jardine, L.; Riedel, A.; Hegazy, A.N.; Whibley, N.; Tucci, A.; Chen, X.; Lindeman, I.; et al. Single-Cell Transcriptomics of Regulatory T Cells Reveals Trajectories of Tissue Adaptation. Immunity 2019, 50, 493–504.e497. [Google Scholar] [CrossRef] [PubMed]
- Xydia, M.; Rahbari, R.; Ruggiero, E.; Macaulay, I.; Tarabichi, M.; Lohmayer, R.; Wilkening, S.; Michels, T.; Brown, D.; Vanuytven, S.; et al. Common clonal origin of conventional T cells and induced regulatory T cells in breast cancer patients. Nat. Commun. 2021, 12, 1119. [Google Scholar] [CrossRef] [PubMed]
- Stockis, J.; Fink, W.; Francois, V.; Connerotte, T.; de Smet, C.; Knoops, L.; van der Bruggen, P.; Boon, T.; Coulie, P.G.; Lucas, S. Comparison of stable human Treg and Th clones by transcriptional profiling. Eur. J. Immunol. 2009, 39, 869–882. [Google Scholar] [CrossRef]
- Francois, V.; Ottaviani, S.; Renkvist, N.; Stockis, J.; Schuler, G.; Thielemans, K.; Colau, D.; Marchand, M.; Boon, T.; Lucas, S.; et al. The CD4+ T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res. 2009, 69, 4335–4345. [Google Scholar] [CrossRef]
- Kleijwegt, F.S.; Laban, S.; Duinkerken, G.; Joosten, A.M.; Koeleman, B.P.; Nikolic, T.; Roep, B.O. Transfer of regulatory properties from tolerogenic to proinflammatory dendritic cells via induced autoreactive regulatory T cells. J. Immunol. 2011, 187, 6357–6364. [Google Scholar] [CrossRef] [PubMed]
- Gras, S.; Chadderton, J.; Del Campo, C.M.; Farenc, C.; Wiede, F.; Josephs, T.M.; Sng, X.Y.X.; Mirams, M.; Watson, K.A.; Tiganis, T.; et al. Reversed T Cell Receptor Docking on a Major Histocompatibility Class I Complex Limits Involvement in the Immune Response. Immunity 2016, 45, 749–760. [Google Scholar] [CrossRef]
- Grover, P.; Goel, P.N.; Greene, M.I. Regulatory T Cells: Regulation of Identity and Function. Front. Immunol. 2021, 12, 750542. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef]
- Onishi, Y.; Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 2008, 105, 10113–10118. [Google Scholar] [CrossRef]
- Arce-Sillas, A.; Alvarez-Luquin, D.D.; Tamaya-Dominguez, B.; Gomez-Fuentes, S.; Trejo-Garcia, A.; Melo-Salas, M.; Cardenas, G.; Rodriguez-Ramirez, J.; Adalid-Peralta, L. Regulatory T Cells: Molecular Actions on Effector Cells in Immune Regulation. J. Immunol. Res. 2016, 2016, 1720827. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, G.X.; Gran, B.; Fallarino, F.; Yu, S.; Li, H.; Cullimore, M.L.; Rostami, A.; Xu, H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 2010, 185, 5953–5961. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Wang, X. Indoleamine 2,3-dioxygenase in tumor induced tolerance. Chin. Med. J. 2009, 122, 3072–3077. [Google Scholar]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Liang, B.; Workman, C.; Lee, J.; Chew, C.; Dale, B.M.; Colonna, L.; Flores, M.; Li, N.; Schweighoffer, E.; Greenberg, S.; et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol. 2008, 180, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Vignali, D.A. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J. Immunol. 2005, 174, 688–695. [Google Scholar] [CrossRef]
- Shibuya, K.; Lanier, L.L.; Phillips, J.H.; Ochs, H.D.; Shimizu, K.; Nakayama, E.; Nakauchi, H.; Shibuya, A. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity 1999, 11, 615–623. [Google Scholar] [CrossRef]
- Lozano, E.; Joller, N.; Cao, Y.; Kuchroo, V.K.; Hafler, D.A. The CD226/CD155 interaction regulates the proinflammatory (Th1/Th17)/anti-inflammatory (Th2) balance in humans. J. Immunol. 2013, 191, 3673–3680. [Google Scholar] [CrossRef]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; Johnston, J.; Hammond, A.; Bontadelli, K.; Ardourel, D.; et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur. J. Immunol. 2011, 41, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Kucan Brlic, P.; Lenac Rovis, T.; Cinamon, G.; Tsukerman, P.; Mandelboim, O.; Jonjic, S. Targeting PVR (CD155) and its receptors in anti-tumor therapy. Cell. Mol. Immunol. 2019, 16, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Turnis, M.E.; Sawant, D.V.; Szymczak-Workman, A.L.; Andrews, L.P.; Delgoffe, G.M.; Yano, H.; Beres, A.J.; Vogel, P.; Workman, C.J.; Vignali, D.A. Interleukin-35 Limits Anti-Tumor Immunity. Immunity 2016, 44, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Joss, A.; Akdis, M.; Faith, A.; Blaser, K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000, 14, 1666–1668. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Akdis, M.; Faith, A.; Blaser, K.; Akdis, C.A. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur. J. Immunol. 2000, 30, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Green, E.A.; Gorelik, L.; McGregor, C.M.; Tran, E.H.; Flavell, R.A. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2003, 100, 10878–10883. [Google Scholar] [CrossRef]
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef]
- Chen, C.H.; Seguin-Devaux, C.; Burke, N.A.; Oriss, T.B.; Watkins, S.C.; Clipstone, N.; Ray, A. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J. Exp. Med. 2003, 197, 1689–1699. [Google Scholar] [CrossRef]
- Gorelik, L.; Constant, S.; Flavell, R.A. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 2002, 195, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, L.; Fields, P.E.; Flavell, R.A. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 2000, 165, 4773–4777. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Martin, S.L.; Xia, L.; Gorham, J.D. TGF-beta 1 uses distinct mechanisms to inhibit IFN-gamma expression in CD4+ T cells at priming and at recall: Differential involvement of Stat4 and T-bet. J. Immunol. 2005, 174, 5950–5958. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Massague, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef] [PubMed]
- Oberle, N.; Eberhardt, N.; Falk, C.S.; Krammer, P.H.; Suri-Payer, E. Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: Independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J. Immunol. 2007, 179, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Burchill, M.A.; Yang, J.; Vang, K.B.; Farrar, M.A. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 2007, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ni, X.; Pan, X.; Lu, H.; Lu, Y.; Zhao, J.; Guo Zheng, S.; Hippen, K.L.; Wang, X.; Lu, L. Human CD39(hi) regulatory T cells present stronger stability and function under inflammatory conditions. Cell. Mol. Immunol. 2017, 14, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Knoechel, B.; Abbas, A.K. Regulatory T cells in the periphery. Immunol. Rev. 2006, 212, 149–162. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Fierabracci, A. Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front. Immunol. 2018, 9, 2374. [Google Scholar] [CrossRef]
- Diskin, B.; Adam, S.; Cassini, M.F.; Sanchez, G.; Liria, M.; Aykut, B.; Buttar, C.; Li, E.; Sundberg, B.; Salas, R.D.; et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 2020, 21, 442–454. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.S.; Jeong, Y.H.; Son, J.; Ban, Y.H.; Lee, B.H.; Chen, L.; Chang, J.; Chung, D.H.; Choi, I.; et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J. Immunol. 2015, 194, 5801–5811. [Google Scholar] [CrossRef]
- Fanelli, G.; Romano, M.; Nova-Lamperti, E.; Werner Sunderland, M.; Nerviani, A.; Scotta, C.; Bombardieri, M.; Quezada, S.A.; Sacks, S.H.; Noelle, R.J.; et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PLoS Biol. 2021, 19, e3001199. [Google Scholar] [CrossRef]
- Duraiswamy, J.; Freeman, G.J.; Coukos, G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013, 73, 6900–6912. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Idema, A.J.; Bol, K.F.; Nierkens, S.; Grauer, O.M.; Wesseling, P.; Grotenhuis, J.A.; Hoogerbrugge, P.M.; de Vries, I.J.; Adema, G.J. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009, 11, 394–402. [Google Scholar] [CrossRef]
- Long, M.; Adler, A.J. Cutting edge: Paracrine, but not autocrine, IL-2 signaling is sustained during early antiviral CD4 T cell response. J. Immunol. 2006, 177, 4257–4261. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Gratz, I.K.; Paw, J.S.; Lee, K.; Marshak-Rothstein, A.; Abbas, A.K. Response to self antigen imprints regulatory memory in tissues. Nature 2011, 480, 538–542. [Google Scholar] [CrossRef]
- Baatar, D.; Olkhanud, P.; Sumitomo, K.; Taub, D.; Gress, R.; Biragyn, A. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4− Tregs, regulate effector T cells using FasL. J. Immunol. 2007, 178, 4891–4900. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Liu, S.; Park, D.; Kang, Y.; Zheng, G. Depleting intratumoral CD4+CD25+ regulatory T cells via FasL protein transfer enhances the therapeutic efficacy of adoptive T cell transfer. Cancer Res. 2007, 67, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Gritzapis, A.D.; Voutsas, I.F.; Lekka, E.; Papamichail, M.; Baxevanis, C.N. Peptide vaccination breaks tolerance to HER-2/neu by generating vaccine-specific FasL+ CD4+ T cells: First evidence for intratumor apoptotic regulatory T cells. Cancer Res. 2010, 70, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.; Wang, A.; McKay, D.M. Transient local depletion of Foxp3+ regulatory T cells during recovery from colitis via Fas/Fas ligand-induced death. J. Immunol. 2008, 180, 8316–8326. [Google Scholar] [CrossRef] [PubMed]
- Garin, M.I.; Chu, C.C.; Golshayan, D.; Cernuda-Morollon, E.; Wait, R.; Lechler, R.I. Galectin-1: A key effector of regulation mediated by CD4+CD25+ T cells. Blood 2007, 109, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Fujita, K.; Iwama, H.; Sakamoto, T.; Okura, R.; Kobayashi, K.; Takano, J.; Katsura, A.; Tatsuta, M.; Maeda, E.; Mimura, S.; et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int. J. Oncol. 2015, 46, 2419–2430. [Google Scholar] [CrossRef]
- Ren, X.; Ye, F.; Jiang, Z.; Chu, Y.; Xiong, S.; Wang, Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4+CD25+ regulatory T cells. Cell Death Differ. 2007, 14, 2076–2084. [Google Scholar] [CrossRef]
- Peiser, M.; Becht, A.; Wanner, R. Antibody blocking of MHC II on human activated regulatory T cells abrogates their suppressive potential. Allergy 2007, 62, 773–780. [Google Scholar] [CrossRef]
- Ahmed, A.; Adiga, V.; Nayak, S.; Uday Kumar, J.A.J.; Dhar, C.; Sahoo, P.N.; Sundararaj, B.K.; Souza, G.D.; Vyakarnam, A. Circulating HLA-DR+CD4+ effector memory T cells resistant to CCR5 and PD-L1 mediated suppression compromise regulatory T cell function in tuberculosis. PLoS Pathog. 2018, 14, e1007289. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cao, L.; Raneri, M.; Wang, H.; Cao, Q.; Zhao, Y.; Bediaga, N.G.; Naselli, G.; Harrison, L.C.; Hawthorne, W.J.; et al. Human HLA-DR+CD27+ regulatory T cells show enhanced antigen-specific suppressive function. JCI Insight 2023, 8, e162978. [Google Scholar] [CrossRef] [PubMed]
- Schaier, M.; Seissler, N.; Becker, L.E.; Schaefer, S.M.; Schmitt, E.; Meuer, S.; Hug, F.; Sommerer, C.; Waldherr, R.; Zeier, M.; et al. The extent of HLA-DR expression on HLA-DR+ Tregs allows the identification of patients with clinically relevant borderline rejection. Transpl. Int. 2013, 26, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ye, S.; Goswami, S.; Li, T.; Wu, J.; Cao, C.; Ma, J.; Lu, B.; Pei, X.; Chen, Y.; et al. Highly immunosuppressive HLADR(hi) regulatory T cells are associated with unfavorable outcomes in cervical squamous cell carcinoma. Int. J. Cancer 2020, 146, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Tree, T.I.; Duinkerken, G.; Willemen, S.; de Vries, R.R.; Roep, B.O. HLA-DQ-regulated T-cell responses to islet cell autoantigens insulin and GAD65. Diabetes 2004, 53, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.A.; Ratnasothy, K.; Tsang, J.Y.; Boardman, D.; Warley, A.; Lechler, R.; Lombardi, G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 2013, 43, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020, 30, 1039–1051.e1035. [Google Scholar] [CrossRef] [PubMed]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Torri, A.; Carpi, D.; Bulgheroni, E.; Crosti, M.C.; Moro, M.; Gruarin, P.; Rossi, R.L.; Rossetti, G.; Di Vizio, D.; Hoxha, M.; et al. Extracellular MicroRNA Signature of Human Helper T Cell Subsets in Health and Autoimmunity. J. Biol. Chem. 2017, 292, 2903–2915. [Google Scholar] [CrossRef]
- Tung, S.L.; Fanelli, G.; Matthews, R.I.; Bazoer, J.; Letizia, M.; Vizcay-Barrena, G.; Faruqu, F.N.; Philippeos, C.; Hannen, R.; Al-Jamal, K.T.; et al. Regulatory T Cell Extracellular Vesicles Modify T-Effector Cell Cytokine Production and Protect Against Human Skin Allograft Damage. Front. Cell Dev. Biol. 2020, 8, 317. [Google Scholar] [CrossRef]
- Rojas, C.; Campos-Mora, M.; Carcamo, I.; Villalon, N.; Elhusseiny, A.; Contreras-Kallens, P.; Refisch, A.; Galvez-Jiron, F.; Emparan, I.; Montoya-Riveros, A.; et al. T regulatory cells-derived extracellular vesicles and their contribution to the generation of immune tolerance. J. Leukoc. Biol. 2020, 108, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders—Advances and challenges. Hum. Vaccin. Immunother. 2022, 18, 2035117. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).