NLRX1 Mediates the Disruption of Intestinal Mucosal Function Caused by Porcine Astrovirus Infection via the Extracellular Regulated Protein Kinases/Myosin Light–Chain Kinase (ERK/MLCK) Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Antibodies
2.2. Transfection and Silencing of the NLRX1 Gene Using siRNA
2.3. TCID50 for PAstV
2.4. Quantitative Real-Time PCR (qPCR)
2.5. Western Blotting Analysis
2.6. Statistical Analysis
3. Results
3.1. Down-Regulation of Tight-Junction Proteins in Caco–2 Cells Following PAstV–4 Infection
3.2. Up-Regulation of NLRX1 Expression Induced by PAstV Infection
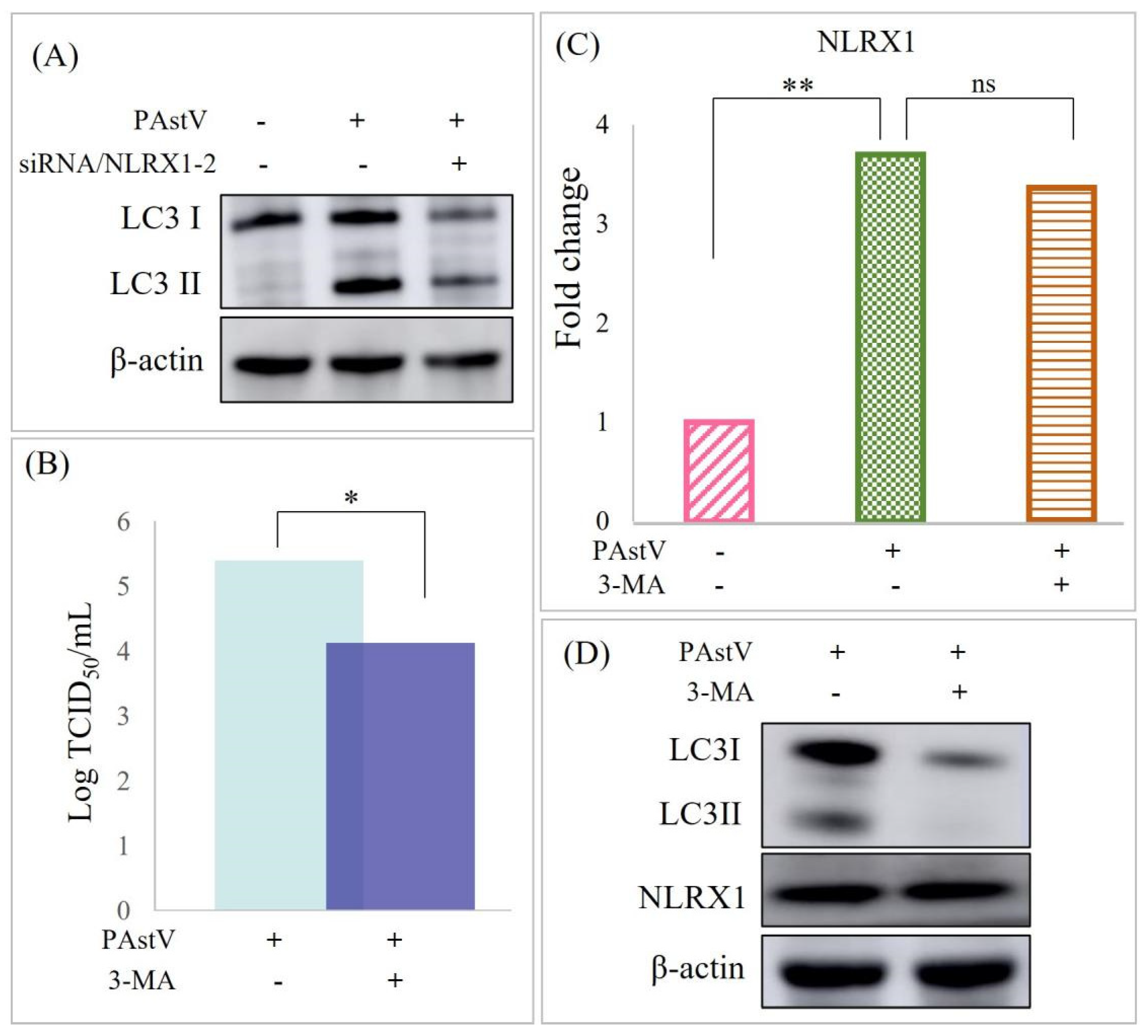
3.3. PAstV–4 Replication Augmented by NLRX1 Knockdown in Caco−2 Cells
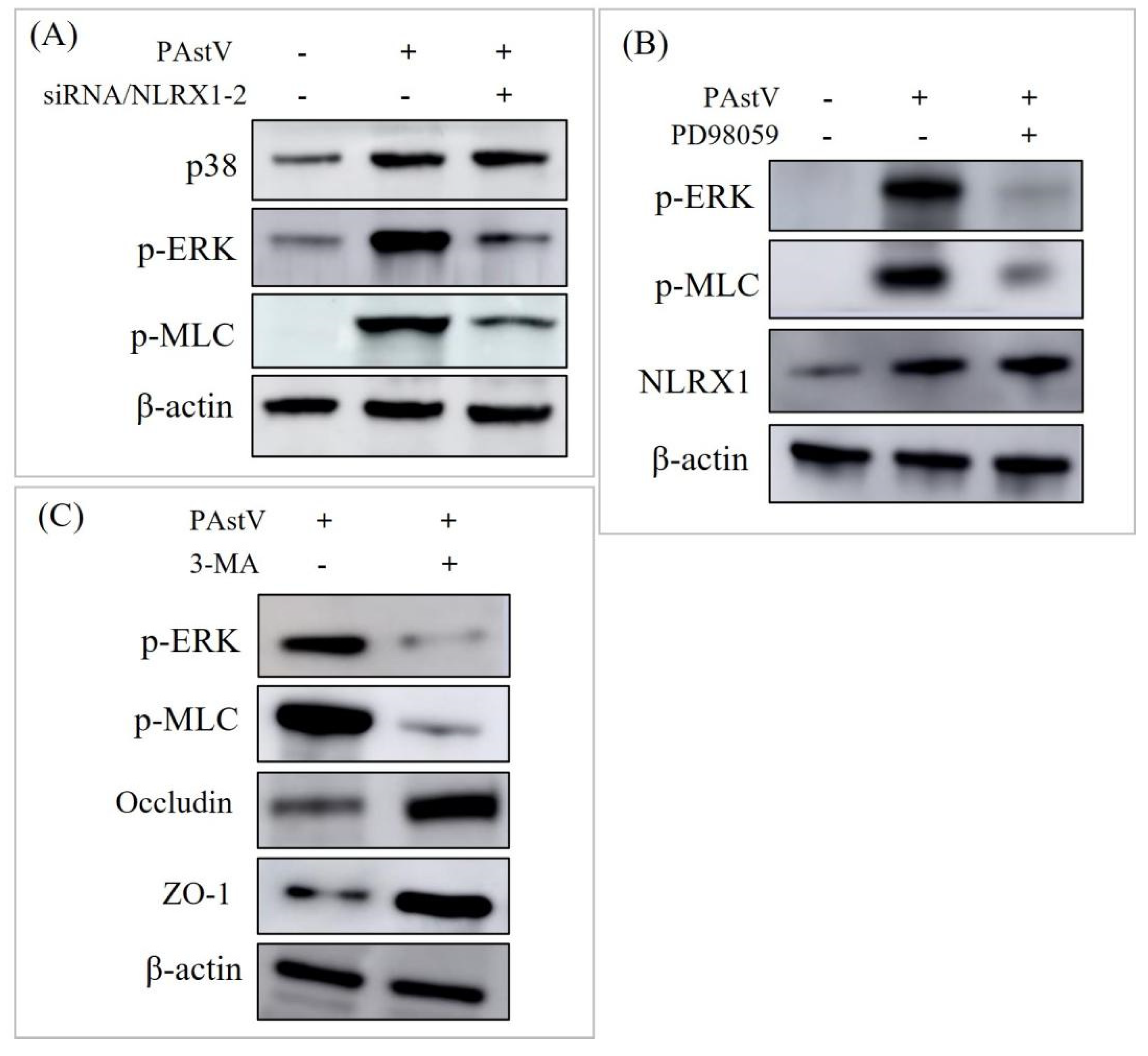
3.4. Disruption of Caco−2 Mucosal Barrier through ERK/MLC Pathway Activation by NLRX1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawal, G.; Linhares, D.C.L. Scoping review on the epidemiology, diagnostics and clinical significance of porcine astroviruses. Transbound. Emerg. Dis. 2022, 69, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Piewbang, C.; Techangamsuwan, S. Genetic characterization of canine astrovirus in non-diarrhea dogs and diarrhea dogs in Vietnam and Thailand reveals the presence of a unique lineage. Front. Vet. Sci. 2023, 10, 1278417. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wan, C.M.; Gong, S.T.; Fang, F.; Sun, M.; Qian, Y.; Huang, Y.; Wang, B.X.; Xu, C.D.; Ye, L.Y.; et al. Chinese clinical practice guidelines for acute infectious diarrhea in children. World J. Pediatr. 2018, 14, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.L.; Bosch, A.; Pinto, R.M.; Guix, S. Epidemiology of Classic and Novel Human Astrovirus: Gastroenteritis and Beyond. Viruses 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Bridger, J.C. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus–like particles in the faeces of piglets with diarrhoea. Vet. Rec. 1980, 107, 532–533. [Google Scholar]
- Jonassen, C.M.; Jonassen, T.O.; Saif, Y.M.; Snodgrass, D.R.; Ushijima, H.; Shimizu, M.; Grinde, B. Comparison of capsid sequences from human and animal astroviruses. J. Gen. Virol. 2001, 82 Pt 5, 1061–1067. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Sun, J.; Su, M.; Song, H. Research progress of porcine astrovirus (PAstV) epidemic in China. Chinese J. Pre. Vet. Med. 2024, 46, 1–6. [Google Scholar]
- Zhao, C.; Chen, C.; Li, Y.; Dong, S.; Tan, K.; Tian, Y.; Zhang, L.; Huang, J.; Zhang, L. Genomic characterization of a novel recombinant porcine astrovirus isolated in northeastern China. Arch. Virol. 2019, 164, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Arruda, B.; Arruda, P.; Hensch, M.; Chen, Q.; Zheng, Y.; Yang, C.; Gatto, I.R.H.; Ferreyra, F.M.; Gauger, P.; Schwartz, K.; et al. Porcine Astrovirus Type 3 in Central Nervous System of Swine with Polioencephalomyelitis. Emerg. Infect. Dis. 2017, 23, 2097–2100. [Google Scholar] [CrossRef]
- Shan, T.; Li, L.; Simmonds, P.; Wang, C.; Moeser, A.; Delwart, E. The fecal virome of pigs on a high–density farm. J. Virol. 2011, 85, 11697–11708. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Opriessnig, T.; Wen, D.; Gu, K.; Jiang, Y. Multiplex gel–based PCR assay for the simultaneous detection of 5 genotypes of porcine astroviruses. J. Vet. Diagn. Investig. 2023, 35, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Qi, S.; Yang, D.; Guo, D.; Yin, B.; Sun, D. Coinfection and Genetic Characterization of Porcine Astrovirus in Diarrheic Piglets in China From 2015 to 2018. Front. Vet. Sci. 2020, 7, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ullman, K.; Chowdry, V.; Reining, M.; Benyeda, Z.; Baule, C.; Juremalm, M.; Wallgren, P.; Schwarz, L.; Zhou, E.; et al. Molecular investigations on the prevalence and viral load of enteric viruses in pigs from five European countries. Vet. Microbiol. 2016, 182, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, J.J.; Malik, Y.S.; Saurabh, S.; Sircar, S.; Vinodhkumar, O.R.; Bora, D.P.; Dhama, K.; Ghosh, S.; Banyai, K.; Touil, N.; et al. First report and genetic characterization of porcine astroviruses of lineage 4 and 2 in diarrhoeic pigs in India. Transbound. Emerg. Dis. 2019, 66, 47–53. [Google Scholar] [CrossRef]
- Lee, S.; Jang, G.; Lee, C. Complete genome sequence of a porcine astrovirus from South Korea. Arch. Virol. 2015, 160, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Vaishali; Gupta, R.; Kumar, M.; Bansal, N.; Vivek; Kumar, P.; Kumar, P.; Jindal, N. Coinfection of porcine astrovirus and other porcine viruses in diarrheic pigs in Haryana, India. Arch. Virol. 2023, 168, 246. [Google Scholar] [CrossRef] [PubMed]
- Werid, G.M.; Ibrahim, Y.M.; Chen, H.; Fu, L.; Wang, Y. Molecular Detection and Genetic Characterization of Potential Zoonotic Swine Enteric Viruses in Northern China. Pathogens 2022, 11, 417. [Google Scholar] [CrossRef]
- Mor, S.K.; Chander, Y.; Marthaler, D.; Patnayak, D.P.; Goyal, S.M. Detection and molecular characterization of Porcine astrovirus strains associated with swine diarrhea. J. Vet. Diagn. Investig. 2012, 24, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Salvo Romero, E.; Alonso Cotoner, C.; Pardo Camacho, C.; Casado Bedmar, M.; Vicario, M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015, 107, 686–696. [Google Scholar] [CrossRef]
- Ho, G.T.; Theiss, A.L. Mitochondria and Inflammatory Bowel Diseases: Toward a Stratified Therapeutic Intervention. Annu. Rev. Physiol. 2022, 84, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Hargest, V.; Cortez, V.; Meliopoulos, V.A.; Schultz–Cherry, S. Astrovirus Pathogenesis. Viruses 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Li, B.; Cheng, J.; Shi, Y.; Qiao, C.; Lin, Z.; Liu, H. Genomic Divergence Characterization and Quantitative Proteomics Exploration of Type 4 Porcine Astrovirus. Viruses 2022, 14, 1383. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tao, J.; Li, B.; Cheng, J.; Shi, Y.; Xiaohui, S.; Liu, H. Molecular characterization of a porcine sapelovirus strain isolated in China. Arch. Virol. 2021, 166, 2683–2692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Tao, J.; Li, B.; Shi, Y.; Liu, H. Coinfection with PEDV and BVDV induces inflammatory bowel disease pathway highly enriched in PK–15 cells. Virol. J. 2022, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Khamrin, P.; Pham, N.T.K.; Shimizu-Onda, Y.; Trinh, Q.D.; Hoque, S.A.; Kumthip, K.; Nomura, A.; Okitsu, S.; Maneekarn, N.; Muller, W.E.G.; et al. Evaluation of an Immunochromatographic Test for Rapid Detection of Astrovirus in Acute Gastroenteritis Pediatric Patients. Clin. Lab. 2023, 69. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Guo, S.; Xing, C.; Xiao, C.; He, B.; Wu, B.; Xia, X.; Tu, C.; Gong, W. Isolation and Characterization of Porcine Astrovirus 5 from a Classical Swine Fever Virus–Infected Specimen. J. Virol. 2020, 95, e01513-20. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.B.; Bergstralh, D.T.; Duncan, J.A.; Lei, Y.; Morrison, T.E.; Zimmermann, A.G.; Accavitti–Loper, M.A.; Madden, V.J.; Sun, L.; Ye, Z.; et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 2008, 451, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Gu, X.; Zhang, X.; Jia, Z. NLRX1/FUNDC1/NIPSNAP1–2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury. Cell Prolif. 2021, 54, e12986. [Google Scholar] [CrossRef]
- Jing, H.; Song, T.; Cao, S.; Sun, Y.; Wang, J.; Dong, W.; Zhang, Y.; Ding, Z.; Wang, T.; Xing, Z.; et al. Nucleotide–binding oligomerization domain–like receptor X1 restricts porcine reproductive and respiratory syndrome virus–2 replication by interacting with viral Nsp9. Virus Res. 2019, 268, 18–26. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK–TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, J.; Wang, S.; Xiong, J.; Chen, Y.; Liu, Y.; Xiao, T.; Li, Y.; He, T.; Li, Y.; et al. Indoxyl sulfate induces intestinal barrier injury through IRF1–DRP1 axis–mediated mitophagy impairment. Theranostics 2020, 10, 7384–7400. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Malone, M.; Shah, N.; Anderson, G.; Gaspar, H.B.; Cubitt, W.D. Pathology of astrovirus associated diarrhoea in a paediatric bone marrow transplant recipient. J. Clin. Pathol. 2004, 57, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Moser, L.A.; Carter, M.; Schultz–Cherry, S. Astrovirus increases epithelial barrier permeability independently of viral replication. J. Virol. 2007, 81, 11937–11945. [Google Scholar] [CrossRef] [PubMed]
- Alfajaro, M.M.; Cho, E.H.; Kim, D.S.; Kim, J.Y.; Park, J.G.; Soliman, M.; Baek, Y.B.; Park, C.H.; Kang, M.I.; Park, S.I.; et al. Early Porcine Sapovirus Infection Disrupts Tight Junctions and Uses Occludin as a Coreceptor. J. Virol. 2019, 93, e01773-18. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Guo, L.; Zhang, J.; Xu, Y.; Gu, W.; Feng, L.; Wang, Y. Tight Junction Protein Occludin Is a Porcine Epidemic Diarrhea Virus Entry Factor. J. Virol. 2017, 91, 00202-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, W.; Roy, S.; Liu, H.; Roberts, R.M.; Wang, L.; Shi, L.; Ma, W. Tight junction protein occludin is an internalization factor for SARS–CoV–2 infection and mediates virus cell–to–cell transmission. Proc. Natl. Acad. Sci. USA 2023, 120, e2218623120. [Google Scholar] [CrossRef] [PubMed]
- Meliopoulos, V.A.; Marvin, S.A.; Freiden, P.; Moser, L.A.; Nighot, P.; Ali, R.; Blikslager, A.; Reddivari, M.; Heath, R.J.; Koci, M.D.; et al. Oral Administration of Astrovirus Capsid Protein Is Sufficient to Induce Acute Diarrhea In Vivo. mBio 2016, 7, e01494-16. [Google Scholar] [CrossRef]
- Hargest, V.; Bub, T.; Neale, G.; Schultz–Cherry, S. Astrovirus-induced epithelial–mesenchymal transition via activated TGF-beta increases viral replication. PLoS Pathog. 2022, 18, e1009716. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, C.; Liu, H.; Wu, Q.; Liang, S.; Cen, M.; Dong, Q.; Wei, Y.; Chen, Y.; Ouyang, K.; et al. Pathogenic Characteristics of a Porcine Astrovirus Strain Isolated in China. Viruses 2019, 11, 1156. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Chen, X.; Bian, Y.; Wang, Y.; Xie, K.; Yu, Y. Dexmedetomidine protects against burn-induced intestinal barrier injury via the MLCK/p-MLC signalling pathway. Burns 2021, 47, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, D.; Wang, J.; Cheng, Z.; Wang, C.; Zhang, X.; Xu, X.; Gao, J. An Inulin-Type Fructan CP-A from Codonopsis pilosula Alleviated 5-Fluorouracil-Induced Intestinal Mucositis via the ERK/MLCK/MLC2 Pathway and Regulation of Gut Microbiota. Pharmaceuticals 2024, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Coulombe, F.; Downey, J.; Tzelepis, F.; Shalaby, K.; Tattoli, I.; Berube, J.; Rousseau, S.; Martin, J.G.; Girardin, S.E.; et al. NLRX1 prevents mitochondrial induced apoptosis and enhances macrophage antiviral immunity by interacting with influenza virus PB1-F2 protein. Proc. Natl. Acad. Sci. USA 2014, 111, E2110–E2119. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Cheng, J.; Shi, Y.; Li, B.; Tang, P.; Jiao, J.; Liu, H. NLRX1 Mediates the Disruption of Intestinal Mucosal Function Caused by Porcine Astrovirus Infection via the Extracellular Regulated Protein Kinases/Myosin Light–Chain Kinase (ERK/MLCK) Pathway. Cells 2024, 13, 913. https://doi.org/10.3390/cells13110913
Tao J, Cheng J, Shi Y, Li B, Tang P, Jiao J, Liu H. NLRX1 Mediates the Disruption of Intestinal Mucosal Function Caused by Porcine Astrovirus Infection via the Extracellular Regulated Protein Kinases/Myosin Light–Chain Kinase (ERK/MLCK) Pathway. Cells. 2024; 13(11):913. https://doi.org/10.3390/cells13110913
Chicago/Turabian StyleTao, Jie, Jinghua Cheng, Ying Shi, Benqiang Li, Pan Tang, Jiajie Jiao, and Huili Liu. 2024. "NLRX1 Mediates the Disruption of Intestinal Mucosal Function Caused by Porcine Astrovirus Infection via the Extracellular Regulated Protein Kinases/Myosin Light–Chain Kinase (ERK/MLCK) Pathway" Cells 13, no. 11: 913. https://doi.org/10.3390/cells13110913
APA StyleTao, J., Cheng, J., Shi, Y., Li, B., Tang, P., Jiao, J., & Liu, H. (2024). NLRX1 Mediates the Disruption of Intestinal Mucosal Function Caused by Porcine Astrovirus Infection via the Extracellular Regulated Protein Kinases/Myosin Light–Chain Kinase (ERK/MLCK) Pathway. Cells, 13(11), 913. https://doi.org/10.3390/cells13110913





