A microRNA Profile Regulates Inflammation-Related Signaling Pathways in Young Women with Locally Advanced Cervical Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. RNA Purification
2.3. Microarray Hybridization
2.4. Determination of miRNAs with Differential Expression
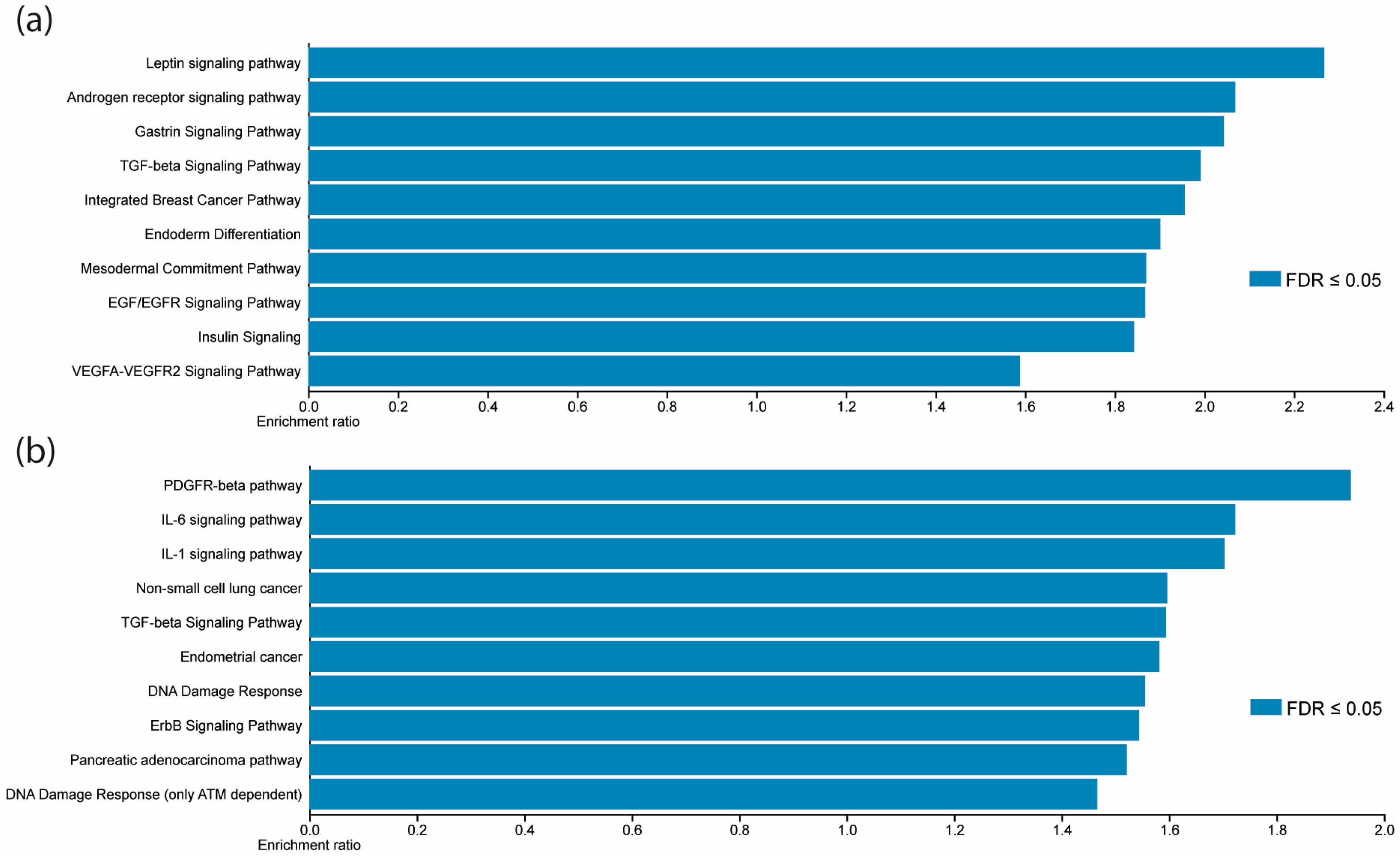
2.5. MiRNA Targets Prediction and KEGG Enrichment Analysis
2.6. Cell Culture and Transfection
2.7. RNA Expression Analysis
2.8. Luciferase Reporter Assays
2.9. Cell Viability Assays
2.10. Colony Formation Assay
2.11. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of Patients with LACC
3.2. A miRNA Expression Profile Differentiated between LACC and Healthy Tissues
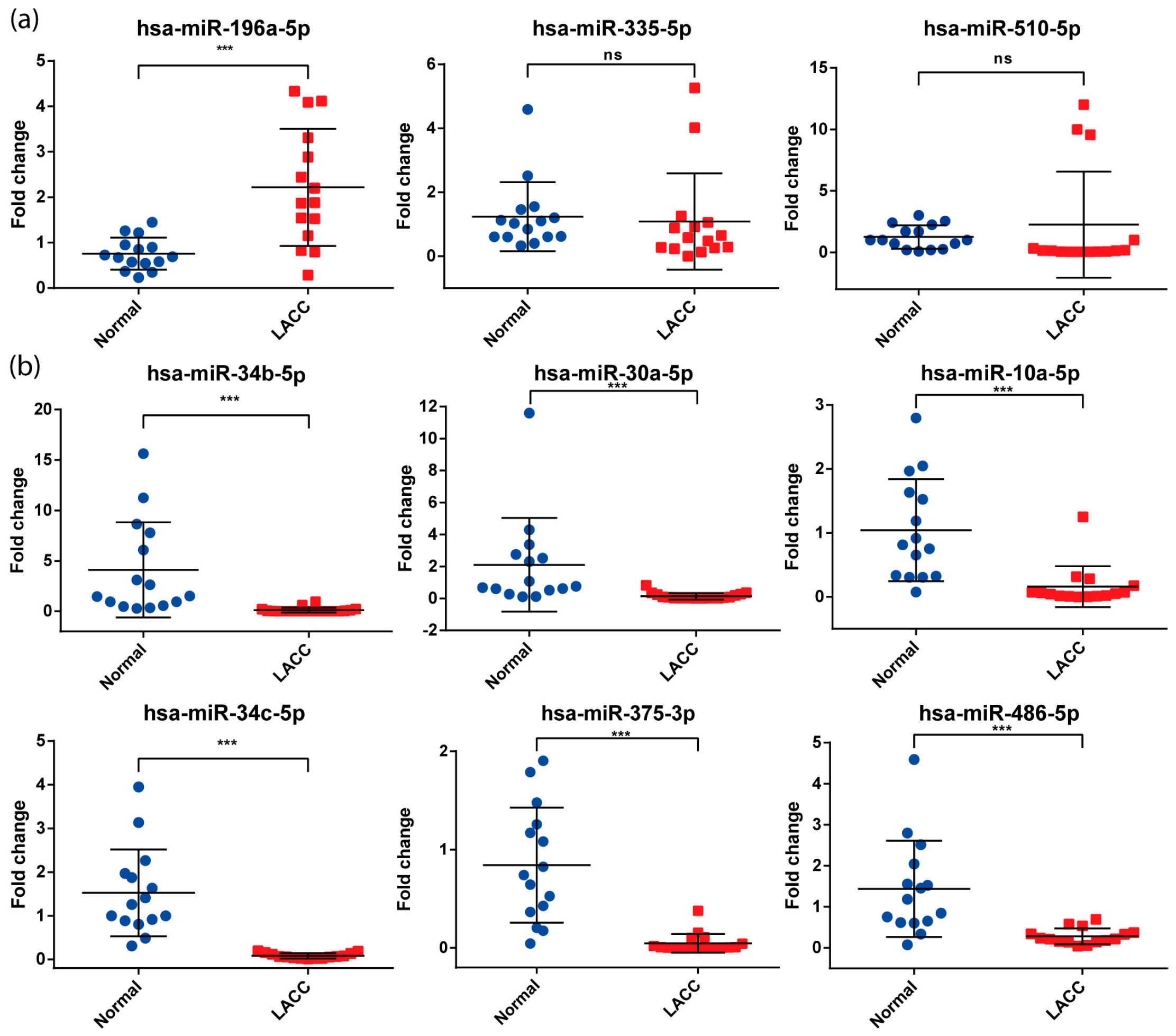
3.3. Validation of miRNAs Expression by qPCR
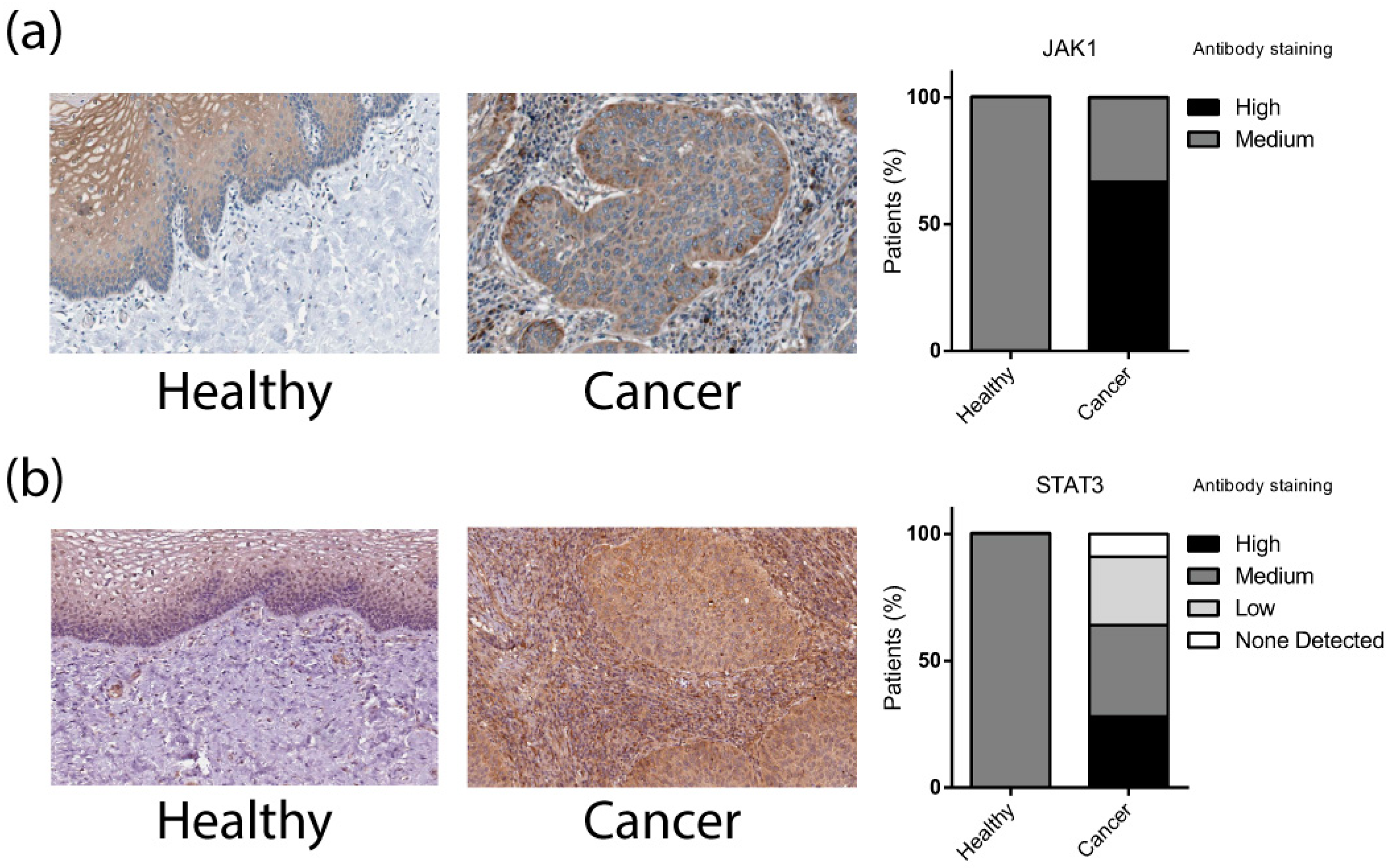
3.4. Differentially Expressed Validated miRNAs Regulate JAK/STAT Signaling Pathways Components
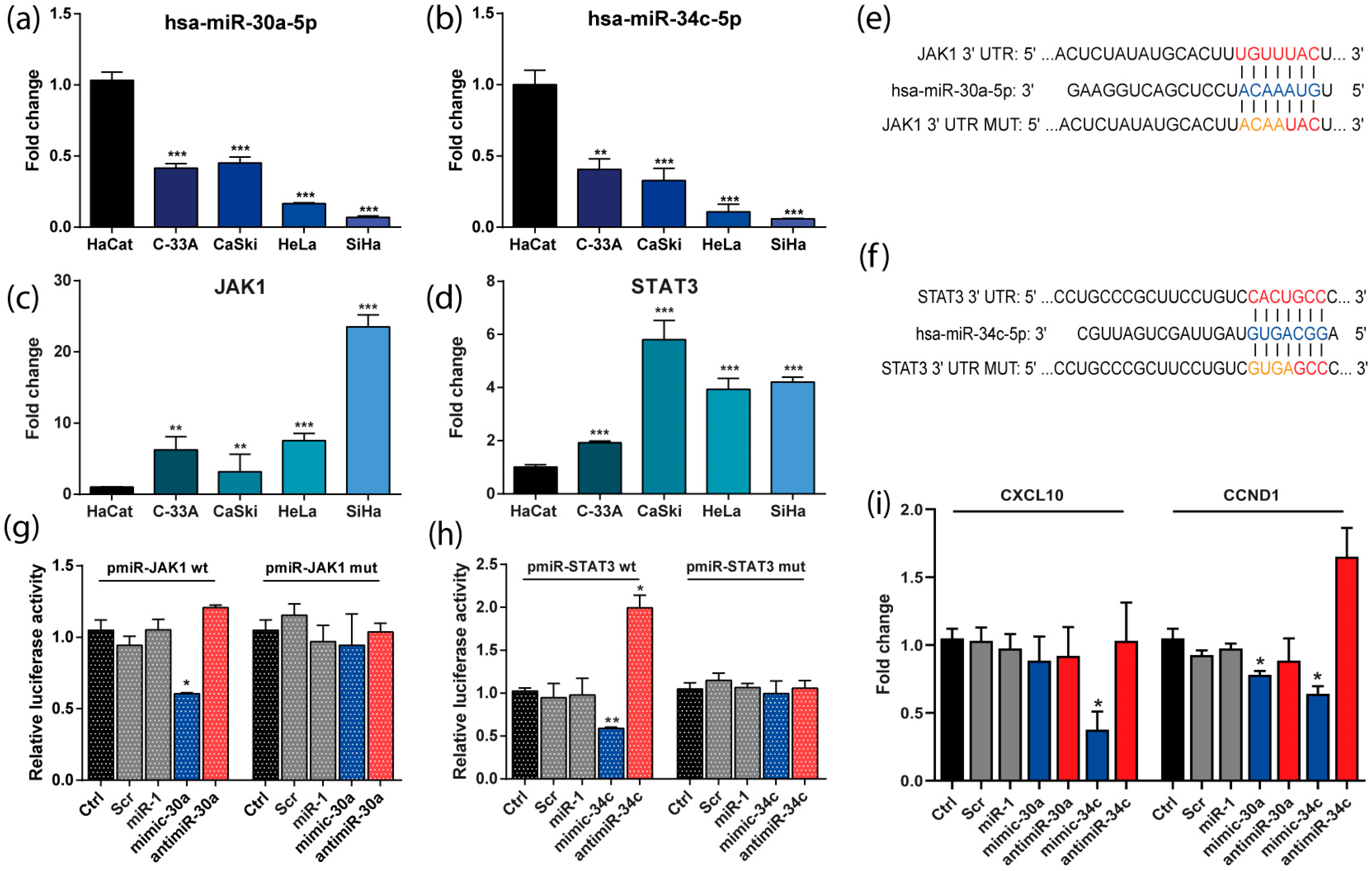
3.5. The Expression of miR-30a and miR-34c Was Negatively Correlated with the Expression of JAK/STAT Genes in CC-Derived Cell Lines
3.6. JAK1 and STAT3 Are Directly Regulated by miR-30a and miR-34c Respectively and Its Transcriptional Targets
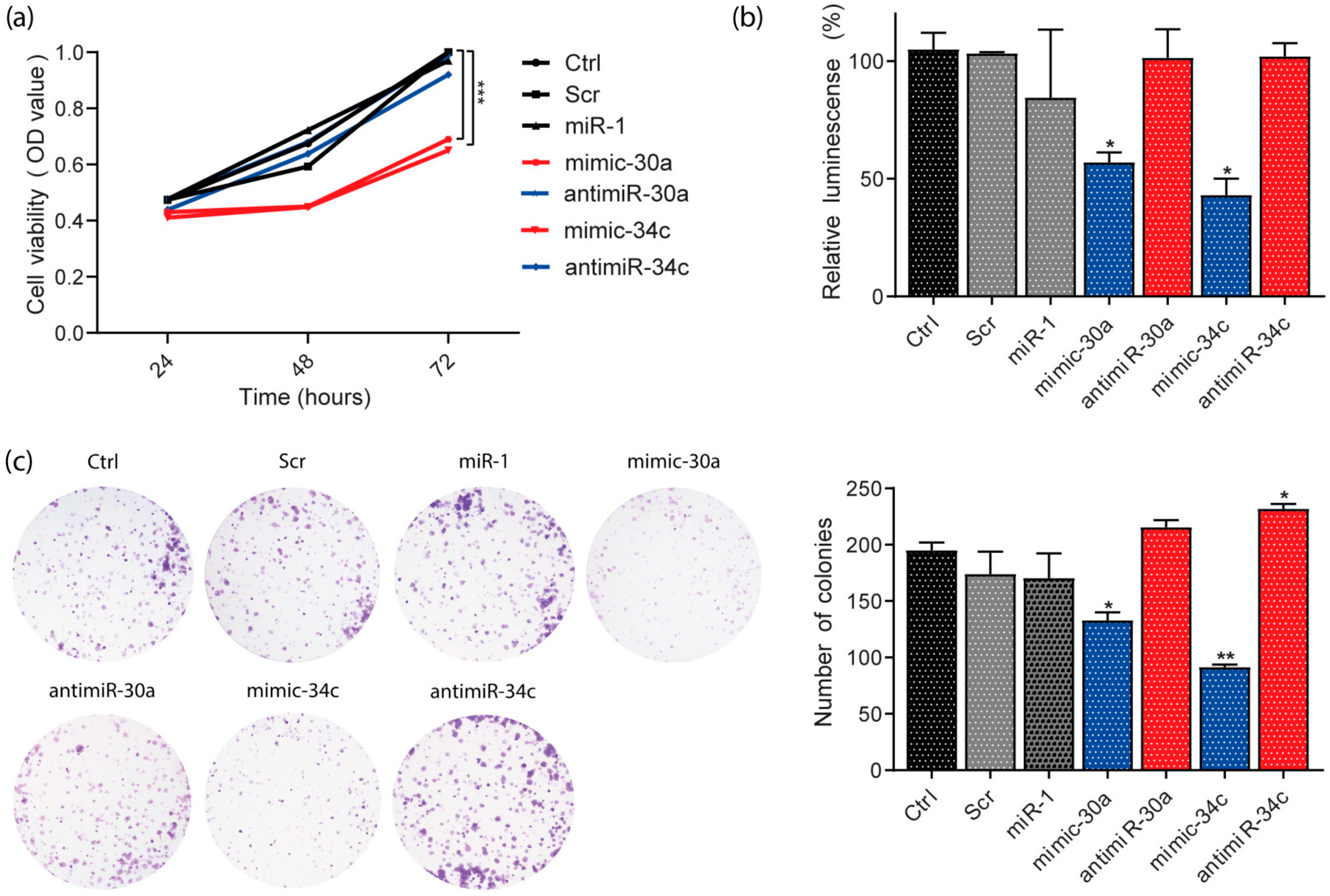
3.7. miR-30a and miR-34c Overexpression Decreased Viability and Proliferation in HeLa Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arango-Bravo, E.A.; Cetina-Pérez, L.D.C.; Galicia-Carmona, T.; Castro-Eguiluz, D.; Gallardo-Rincón, D.; Cruz-Bautista, I.; Duenas-Gonzalez, A. The Health System and Access to Treatment in Patients with Cervical Cancer in Mexico. Front. Oncol. 2022, 12, 1028291. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Tan, D.S.P.; Hernández Chagüi, J.D.; Takyar, J.; Paskow, M.J.; Nunes, A.T.; Pujade-Lauraine, E. Proportions and Incidence of Locally Advanced Cervical Cancer: A Global Systematic Literature Review. Int. J. Gynecol. Cancer 2022, 32, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Alemany, L.; De Sanjosé, S.; Tous, S.; Quint, W.; Vallejos, C.; Shin, H.R.; Bravo, L.E.; Alonso, P.; Lima, M.A.; Guimerà, N.; et al. Time Trends of Human Papillomavirus Types in Invasive Cervical Cancer, from 1940 to 2007. Int. J. Cancer 2014, 135, 88–95. [Google Scholar] [CrossRef]
- Gallardo-Alvarado, L.; Cantú-de León, D.; Ramirez-Morales, R.; Santiago-Concha, G.; Barquet-Muñoz, S.; Salcedo-Hernandez, R.; Reyes, C.; Perez-Alvarez, S.; Perez-Montiel, D.; Perez-Plasencia, C.; et al. Tumor Histology Is an Independent Prognostic Factor in Locally Advanced Cervical Carcinoma: A Retrospective Study. BMC Cancer 2022, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Isla-Ortiz, D.; Palomares-Castillo, E.; Mille-Loera, J.E.; Ramírez-Calderón, N.; Mohar-Betancourt, A.; Meneses-García, A.A.; Reynoso-Noverón, N. Cervical Cancer in Young Women: Do They Have a Worse Prognosis? A Retrospective Cohort Analysis in a Population of Mexico. Oncologist 2020, 25, e1363–e1371. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.Y.; Juang, C.M.; Chen, Y.J.; Twu, N.F.; Yen, M.S.; Chao, K.C. Aggressive Characteristics of Cervical Cancer in Young Women in Taiwan. Int. J. Gynecol. Obstet. 2009, 107, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Poka, R.; Juhász, B.; Lampé, L. Cervical Cancer in Younger Women: A Poorer Prognosis? Int. J. Gynecol. Obstet. 1994, 46, 33–37. [Google Scholar] [CrossRef]
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo-Ramírez, O.R.; Treviño, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV Integration and Its Progression to Cervical Cancer. Infect. Genet. Evol. 2018, 61, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hemmat, N.; Bannazadeh Baghi, H. Association of Human Papillomavirus Infection and Inflammation in Cervical Cancer. Pathog. Dis. 2019, 77, ftz048. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; De Sanjose, S.; Ascunce, N.; Gonzalez, L.C.; Kaldor, J.M.; Guerrero, E.; Lorinczi, A.; Santamariai, M.; Alonso De Ruiz, P.; et al. The Causal Link between Human Papillomavirus and Study in Colombia and Spain Invasive Cervical Cancer: A Population-Based Case-Control. Int. J. Cancer 1992, 52, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.H.; Bauer, H.M.; Hoover, R.N.; Glass, A.G.; Cadell, D.M.; Rush, B.B.; Scott, D.R.; Sherman, M.E.; Kurman, R.J.; Wacholder, S.; et al. ARTICLES Epidemiologic Evidence Showing That Human Papillomavirus Infection Causes Most Cervical Intraepithelial Neoplasia. J. Natl. Cancer Inst. 1993, 85, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical Cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Qiu, J.; Wei, H.; Li, J. A Bibliometric Analysis of Global Research Trends of Inflammation in Cervical Cancer: A Review. Medicine 2023, 102, e36598. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, S.D.; Balakrishnan, V.; Oon, C.E.; Kaur, G. Gene Expression Profiling of HPV-Associated Cervical Carcinogenesis in Formalin-Fixed Paraffin-Embedded (FFPE) Tissues Using the NanoString NCounterTM Platform. Gene 2022, 825, 146385. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Li, W.X. Role of the JAK-STAT Signalling Pathway in Cancer. Encycl. Life Sci. 2013, 6, 1–9. [Google Scholar] [CrossRef]
- Campos-Parra, A.D.; Padua-Bracho, A.; Pedroza-Torres, A.; Figueroa-González, G.; Fernández-Retana, J.; Millan-Catalan, O.; Peralta-Zaragoza, O.; Cantú de León, D.; Herrera, L.A.; Pérez-Plasencia, C. Comprehensive Transcriptome Analysis Identifies Pathways with Therapeutic Potential in Locally Advanced Cervical Cancer. Gynecol. Oncol. 2016, 143, 406–413. [Google Scholar] [CrossRef]
- Satapathy, S.; Batra, J.; Jeet, V.; Thompson, E.W.; Punyadeera, C. MicroRNAs in HPV Associated Cancers: Small Players with Big Consequences. Expert Rev. Mol. Diagn. 2017, 17, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Yang, Y.; Huang, X. MiR-199a-5p Promotes Proliferation and Metastasis and Epithelial-Mesenchymal Transition through Targeting PIAS3 in Cervical Carcinoma. J. Cell. Biochem. 2019, 120, 13562–13572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, J.; Zhao, L.; Li, X.; Xie, Q.; Chen, X.; Wang, J.; Lu, F. Down-Regulation of MicroRNA-9 Leads to Activation of IL-6/Jak/STAT3 Pathway through Directly Targeting IL-6 in HeLa Cell. Mol. Carcinog. 2016, 55, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. StarBase v2.0: Decoding MiRNA-CeRNA, MiRNA-NcRNA and Protein-RNA Interaction Networks from Large-Scale CLIP-Seq Data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.; Marques, J.P.; Soares, A.R.; Carreto, L.; Santos, M.A.S. Microrna Expression Variability in Human Cervical Tissues. PLoS ONE 2010, 5, e11780. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Ruiz, V.; Juárez-Méndez, S.; Pérez-González, O.A.; Arreola, H.; Paniagua-García, L.; Parra-Melquiadez, M.; Peralta-Rodríguez, R.; López-Romero, R.; Monroy-García, A.; Mantilla-Morales, A.; et al. Heterogeneity of MicroRNAs Expression in Cervical Cancer Cells: Over-Expression of MiR-196a. Int. J. Clin. Exp. Pathol. 2014, 7, 1389. [Google Scholar] [PubMed]
- Wang, C.; Jiang, T. MicroRNA-335 Represents an Independent Prognostic Marker in Cervical Cancer. Tumor Biol. 2015, 36, 5825–5830. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yokoi, A.; Yamamoto, Y.; Kajiyama, H. ChrXq27.3 MiRNA Cluster Functions in Cancer Development. J. Exp. Clin. Cancer Res. 2021, 40, 112. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Wences, H.; Martínez-Carrillo, D.N.; Peralta-Zaragoza, O.; Campos-Viguri, G.E.; Hernández-Sotelo, D.; Jiménez-López, M.A.; Muñoz-Camacho, J.G.; Garzón-Barrientos, V.H.; Illades-Aguiar, B.; Fernández-Tilapa, G. Methylation and Expression of MiRNAs in Precancerous Lesions and Cervical Cancer with HPV16 Infection. Oncol. Rep. 2016, 35, 2297–2305. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, G.; Xie, C.; Zhou, Y. Mir-34b Regulates Cervical Cancer Cell Proliferation and Apoptosis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu Tepe, N.; Bozgeyik, E.; Bozdag, Z.; Balat, O.; Ozcan, H.C.; Ugur, M.G. Identification of Autophagy-Associated MiRNA Signature for the Cervical Squamous Cell Cancer and High-Grade Cervical Intraepithelial Lesions. Reprod. Biol. 2021, 21, 100536. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Li, Y.; Lan, X.; Ai, L. MicroRNA-10a-5p Suppresses Cancer Proliferation and Division in Human Cervical Cancer by Targeting BDNF. Exp. Ther. Med. 2017, 14, 6147–6151. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, X.; Niu, X.; Jiao, R.; Li, X.; Wang, S. MiR-34c-5p Targets Notch1 and Suppresses the Metastasis and Invasion of Cervical Cancer. Mol. Med. Rep. 2021, 23, 120. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Xu, J.; Ye, F.; Shen, Y.; Zhou, J.; Lu, W.; Wan, X.; Ma, D.; Xie, X. Progressive MiRNA Expression Profiles in Cervical Carcinogenesis and Identification of HPV-Related Target Genes for MiR-29. J. Pathol. 2011, 224, 484–495. [Google Scholar] [CrossRef]
- Wilting, S.M.; Snijders, P.J.F.; Verlaat, W.; Jaspers, A.; Van De Wiel, M.A.; Van Wieringen, W.N.; Meijer, G.A.; Kenter, G.G.; Yi, Y.; Le Sage, C.; et al. Altered MicroRNA Expression Associated with Chromosomal Changes Contributes to Cervical Carcinogenesis. Oncogene 2013, 32, 106–116. [Google Scholar] [CrossRef]
- Tian, Q.; Li, Y.; Wang, F.; Li, Y.; Xu, J.; Shen, Y.; Ye, F.; Wang, X.; Cheng, X.; Chen, Y.; et al. MicroRNA Detection in Cervical Exfoliated Cells as a Triage for Human Papillomavirus-Positive Women. J. Natl. Cancer Inst. 2014, 106, dju241. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, X.; Li, W.; Bai, F.; Lyu, J.; Meng, Q.H. Serum MiR-486-5p as a Diagnostic Marker in Cervical Cancer: With Investigation of Potential Mechanisms. BMC Cancer 2018, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Plasencia, C.; Vázquez-Ortiz, G.; López-Romero, R.; Piña-Sanchez, P.; Moreno, J.; Salcedo, M. Genome Wide Expression Analysis in HPV16 Cervical Cancer: Identification of Altered Metabolic Pathways. Infect. Agent. Cancer 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Romero-Lorca, A.; Novillo, A.; Gaibar, M.; Gilsanz, M.F.; Galán, M.; Beltrán, L.; Antón, B.; Malón, D.; Moreno, A.; Fernández-Santander, A. Mir-7, Mir-10a and Mir-143 Expression May Predict Response to Bevacizumab plus Chemotherapy in Patients with Metastatic Colorectal Cancer. Pharmgenom. Pers. Med. 2021, 14, 1263–1273. [Google Scholar] [CrossRef]
- Wang, L.-S.; Li, L.; Li, L.; Chu, S.; Shiang, K.-D.; Li, M.; Sun, H.-Y.; Xu, J.; Xiao, F.-J.; Sun, G.; et al. MicroRNA-486 Regulates Normal Erythropoiesis and Enhances Growth and Modulates Drug Response in CML Progenitors Key Points. J. Am. Soc. Hematol. 2015, 125, 1302–1313. [Google Scholar] [CrossRef]
- Sobti, R.C.; Singh, N.; Hussain, S.; Suri, V.; Bharti, A.C.; Das, B.C. Overexpression of STAT3 in HPV-Mediated Cervical Cancer in a North Indian Population. Mol. Cell Biochem. 2009, 330, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sobti, R.C.; Singh, N.; Hussain, S.; Suri, V.; Bharadwaj, M.; Das, B.C. Deregulation of STAT-5 Isoforms in the Development of HPV-Mediated Cervical Carcinogenesis. J. Recept. Signal Transduct. 2010, 30, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Sobti, R.C.; Singh, N.; Hussain, S.; Suri, V.; Nijhawan, R.; Bharti, A.C.; Bharadwaj, M.; Das, B.C. Aberrant Promoter Methylation and Loss of Suppressor of Cytokine Signalling-1 Gene Expression in the Development of Uterine Cervical Carcinogenesis. Cell. Oncol. 2011, 34, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. Role of the JAK/STAT Pathway in Cervical Cancer: Its Relationship with HPV E6/E7 Oncoproteins. Cells 2020, 9, 2297. [Google Scholar] [CrossRef] [PubMed]
- Spiryda, L.B.; Whitaker, K.M.; Messersmith, A.; Banister, C.E.; Creek, K.E.; Pirisi-Creek, L.A. Clinical Utility of Molecular Biomarkers in Cervical Squamous Intraepithelial Lesions in a Young Adult Population. J. Low. Genit. Tract. Dis. 2016, 20, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, D.; Wei, Q.; Ren, L. CXCL10 Serves as a Potential Serum Biomarker Complementing SCC-Ag for Diagnosing Cervical Squamous Cell Carcinoma. BMC Cancer 2022, 22, 1052. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Diop, G.; Derbois, C.; Spadoni, J.-L.; Noirel, J.; Medina-Santos, R.; Coulonges, C.; Torres, M.; Dieye, A.; Sembene, M.; et al. Gene Expression Profiling of Peripheral Blood Mononuclear Cells from Women with Cervical Lesions Reveals New Markers of Cancer. Oncol. Rep. 2023, 49, 118. [Google Scholar] [CrossRef]
- Kemp, T.J.; Hildesheim, A.; García-Piñeres, A.; Williams, M.C.; Shearer, G.M.; Rodriguez, A.C.; Schiffman, M.; Burk, R.; Freer, E.; Bonilla, J.; et al. Elevated Systemic Levels of Inflammatory Cytokines in Older Women with Persistent Cervical Human Papillomavirus Infection. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Domenici, L.; Tonacci, A.; Aretini, P.; Garibaldi, S.; Perutelli, A.; Bottone, P.; Muzii, L.; Benedetti Panici, P. Inflammatory Biomarkers as Promising Predictors of Prognosis in Cervical Cancer Patients. Oncology 2021, 99, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shen, L.; Wang, Y.; Chen, Y.; Pan, X.; Liang, H.; Yu, H. Signatures of Immune Cell Infiltration for Predicting Immune Escape and Immunotherapy in Cervical Cancer. Aging 2023, 15, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.-R.; Huang, M.; Jin, C.; Wang, H.-C.; Yu, J.-T.; Zeng, L.-C.; Zheng, F.-Y.; Lin, F. Cervical Cancer Systemic Inflammation Score: A Novel Predictor of Prognosis. Oncotarget 2016, 7, 15230. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, M.V.; Mangino, G.; Iuliano, M.; Zangrillo, M.S.; De Lillis, I.; Vaccari, G.; Accardi, R.; Tommasino, M.; Columba Cabezas, S.; Federico, M.; et al. Human Papillomavirus E6 and E7 Oncoproteins Affect the Expression of Cancer-Related MicroRNAs: Additional Evidence in HPV-Induced Tumorigenesis. J. Cancer Res. Clin. Oncol. 2016, 142, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Torres, A.; López-Urrutia, E.; García-Castillo, V.; Jacobo-Herrera, N.; Herrera, L.A.; Peralta-Zaragoza, O.; López-Camarillo, C.; De Leon, D.C.; Fernández-Retana, J.; Cerna-Cortés, J.F.; et al. MicroRNAs in Cervical Cancer: Evidences for a MiRNA Profile Deregulated by HPV and Its Impact on Radio-Resistance. Molecules 2014, 19, 6263–6281. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; De Maria, D.; Francavilla, A.; Di Gaetano, C.; Ronco, G.; Naccarati, A. MicroRNAs as Markers of Progression in Cervical Cancer: A Systematic Review. BMC Cancer 2018, 18, 696. [Google Scholar] [CrossRef] [PubMed]
- Causin, R.L.; de Freitas, A.J.A.; Filho, C.M.T.H.; Dos Reis, R.; Reis, R.M.; Marques, M.M.C. A Systematic Review of Micrornas Involved in Cervical Cancer Progression. Cells 2021, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Castanon, A.; Tataru, D.; Sasieni, P. Survival from Cervical Cancer Diagnosed Aged 20–29 Years by Age at First Invitation to Screening in England: Population-Based Study. Cancers 2020, 12, 2079. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Pan, S.; Zou, S.; Zhu, H.; Zhu, X. Characteristics and Treatments of Patients Aged 65 Years or over with Cervical Cancer. Clin. Interv. Aging 2020, 15, 841–851. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Liu, J.B.; Zhang, Y.; Liu, J.; Li, Y. hua LncRNA AL592284.1 Facilitates Proliferation and Metastasis of Cervical Cancer Cells via MiR-30a-5p/Vimentin/EMT Axis. Biochem. Biophys. Res. Commun. 2021, 577, 95–102. [Google Scholar] [CrossRef]
- Córdova-Rivas, S.; Fraire-Soto, I.; Torres, A.M.C.; Servín-González, L.S.; Granados-López, A.J.; López-Hernández, Y.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; Castañeda-Delgado, J.E.; Ramírez-Hernández, L.; et al. 5p and 3p Strands of MiR-34 Family Members Have Differential Effects in Cell Proliferation, Migration, and Invasion in Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 545. [Google Scholar] [CrossRef] [PubMed]
- Roszik, J.; Ring, K.L.; Wani, K.M.; Lazar, A.J.; Yemelyanova, A.V.; Soliman, P.T.; Frumovitz, M.; Jazaeri, A.A. Gene Expression Analysis Identifies Novel Targets for Cervical Cancer Therapy. Front. Immunol. 2018, 9, 2102. [Google Scholar] [CrossRef]

| Characteristics | N | % |
|---|---|---|
| Age (mean, standard deviation) | 40 (±5) | |
| Clinical stage | ||
| IB2 | 2 | 4.34% |
| IIB | 32 | 69.56% |
| IIIB | 12 | 26.08% |
| Histological type | ||
| Adenocarcinoma | 6 | 13.04% |
| Squamous cell carcinoma | 40 | 86.95% |
| Tumor size | ||
| <4 cm | 6 | 13.04% |
| >4 cm | 40 | 86.95% |
| HPV Genotyping | ||
| Type 16 | 22 | 47.8% |
| Type 18 | 12 | 26.1% |
| Type 45 | 8 | 17.4% |
| Other | 4 | 8.7% |
| Treatment outcome | ||
| Complete (RC) | 22 | 47.82% |
| No response (PR, PD, SD) | 24 | 52.17% |
| miRNA | Fold Change | Adj p-Value | Previous Reports |
|---|---|---|---|
| hsa-miR-196a-5p | 3.07 | 2.84 × 10−5 | Pereira, et al., 2010 [25]; Villegas-Ruiz, et al., 2014 [26] |
| hsa-miR-335-5p | 2.63 | 7.17 × 10−7 | Wang and Jiang, 2015 [27] |
| hsa-miR-510-5p | 2.12 | 3.15 × 10−8 | Yoshida, et al., 2021 [28] |
| hsa-miR-34b-5p | −2.39 | 1.01 × 10−5 | Jiménez-Wences, et al., 2016 [29]; Cao, et al., 2019 [30] |
| hsa-miR-30a-5p | −2.75 | 4.52 × 10−5 | Bayramoglu et al., 2021 [31] |
| hsa-miR-10a-5p | −3.00 | 6.97 × 10−6 | Pereira, et al., 2010 [25]; Zhai et al., 2017 [32] |
| hsa-miR-34c-5p | −3.52 | 6.90 × 10−6 | Jiménez-Wences, et al., 2016 [29]; Wei, et al., 2021 [33] |
| hsa-miR-375-3p | −5.12 | 1.13 × 10−7 | Li, et al., 2011 [34]; Wilting, et al., 2013 [35]; Tiang, et al., 2014 [36] |
| hsa-miR-486-5p | −5.69 | 4.12 × 10−12 | Li et al., 2018 [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millan-Catalan, O.; Pérez-Yépez, E.A.; Martínez-Gutiérrez, A.D.; Rodríguez-Morales, M.; López-Urrutia, E.; Coronel-Martínez, J.; Cantú de León, D.; Jacobo-Herrera, N.; Peralta-Zaragoza, O.; López-Camarillo, C.; et al. A microRNA Profile Regulates Inflammation-Related Signaling Pathways in Young Women with Locally Advanced Cervical Cancer. Cells 2024, 13, 896. https://doi.org/10.3390/cells13110896
Millan-Catalan O, Pérez-Yépez EA, Martínez-Gutiérrez AD, Rodríguez-Morales M, López-Urrutia E, Coronel-Martínez J, Cantú de León D, Jacobo-Herrera N, Peralta-Zaragoza O, López-Camarillo C, et al. A microRNA Profile Regulates Inflammation-Related Signaling Pathways in Young Women with Locally Advanced Cervical Cancer. Cells. 2024; 13(11):896. https://doi.org/10.3390/cells13110896
Chicago/Turabian StyleMillan-Catalan, Oliver, Eloy Andrés Pérez-Yépez, Antonio Daniel Martínez-Gutiérrez, Miguel Rodríguez-Morales, Eduardo López-Urrutia, Jaime Coronel-Martínez, David Cantú de León, Nadia Jacobo-Herrera, Oscar Peralta-Zaragoza, César López-Camarillo, and et al. 2024. "A microRNA Profile Regulates Inflammation-Related Signaling Pathways in Young Women with Locally Advanced Cervical Cancer" Cells 13, no. 11: 896. https://doi.org/10.3390/cells13110896
APA StyleMillan-Catalan, O., Pérez-Yépez, E. A., Martínez-Gutiérrez, A. D., Rodríguez-Morales, M., López-Urrutia, E., Coronel-Martínez, J., Cantú de León, D., Jacobo-Herrera, N., Peralta-Zaragoza, O., López-Camarillo, C., Rodríguez-Dorantes, M., & Pérez-Plasencia, C. (2024). A microRNA Profile Regulates Inflammation-Related Signaling Pathways in Young Women with Locally Advanced Cervical Cancer. Cells, 13(11), 896. https://doi.org/10.3390/cells13110896









