NADH Intraperitoneal Injection Prevents Lung Inflammation in a BALB/C Mice Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
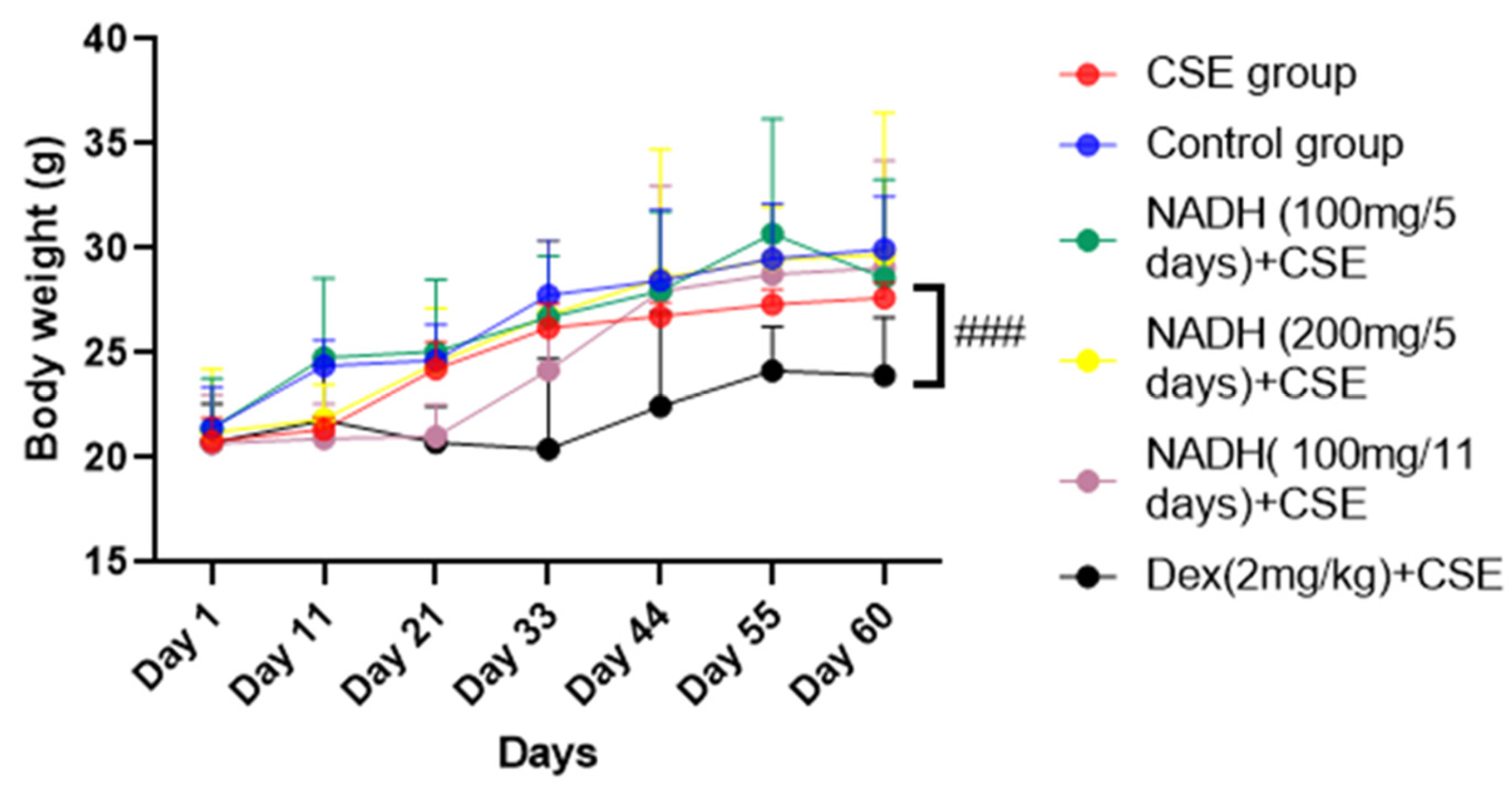
3.1. Effect of NADH Treatment on Body Weights of Mice
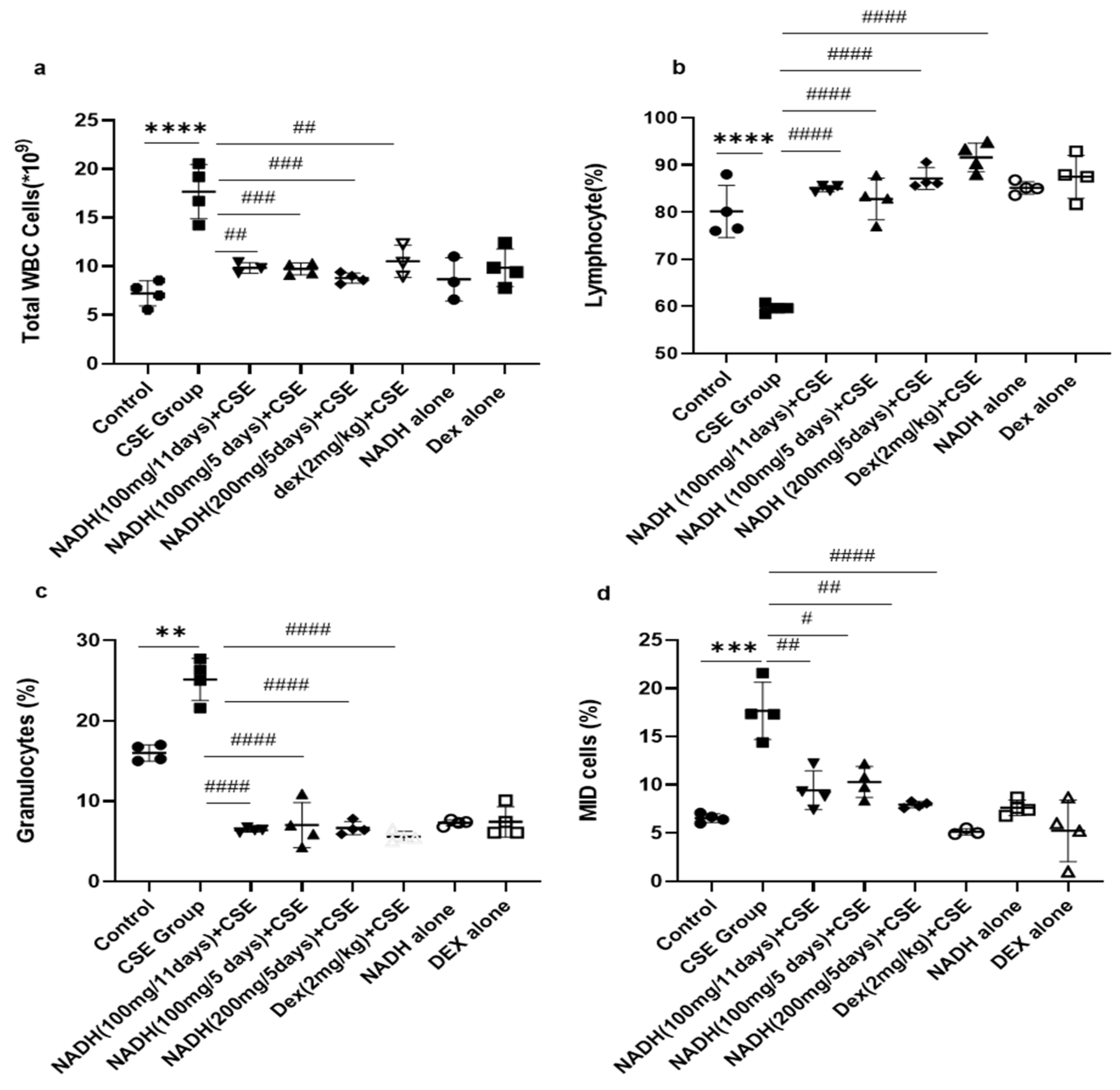
3.2. Effect of NADH on CSE-Induced Changes in Total White Blood Cell Count
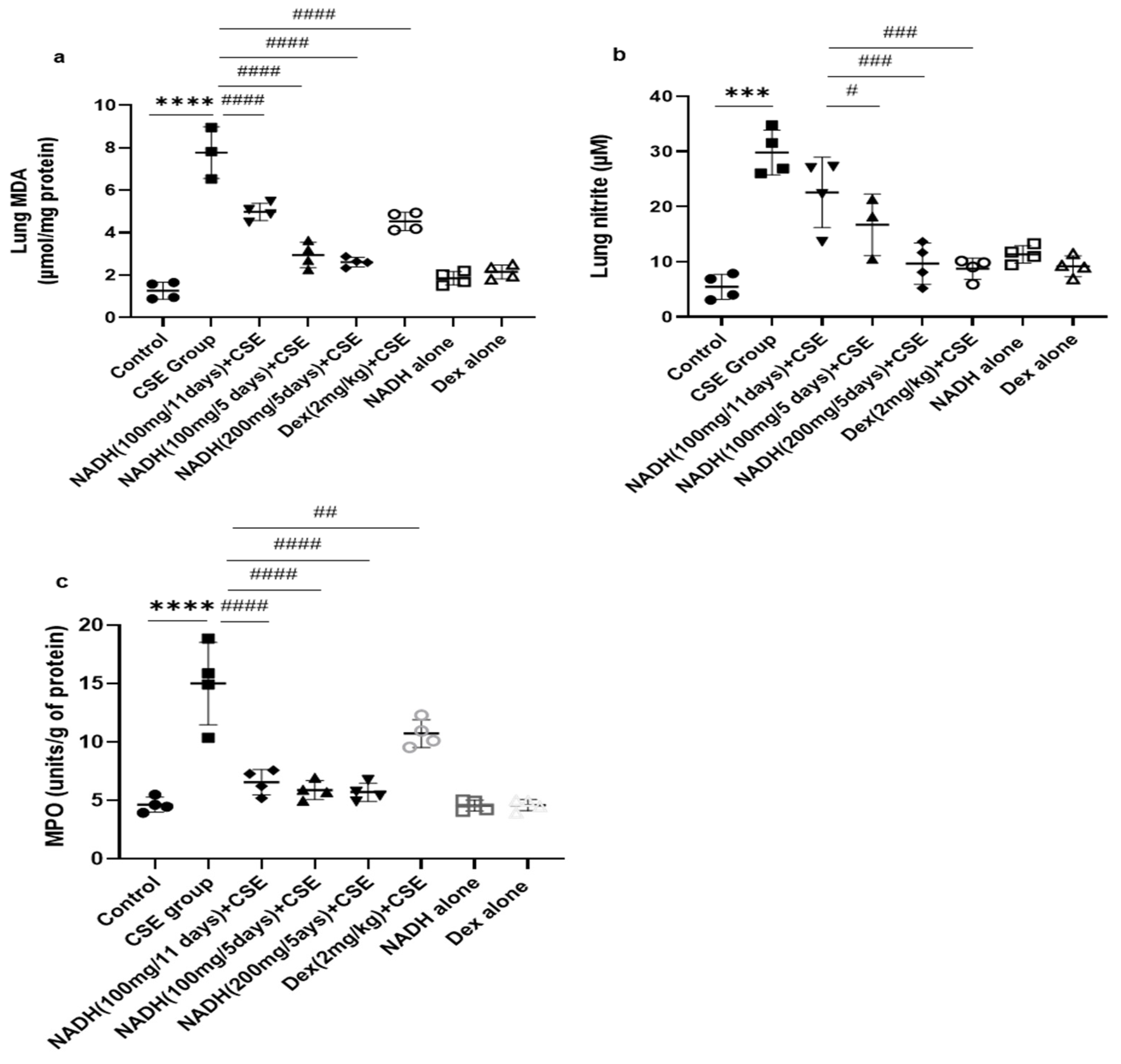
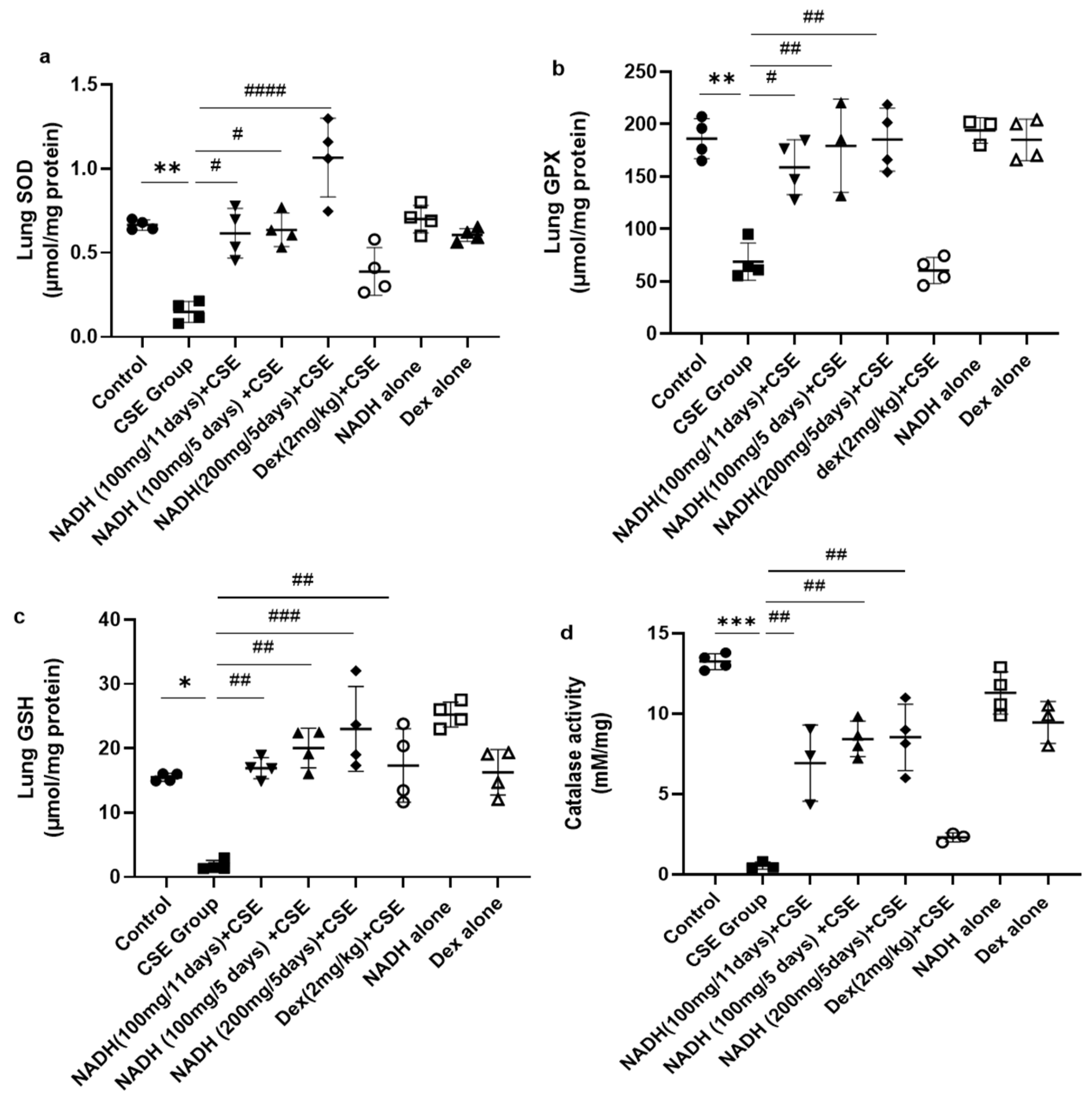
3.3. Effect of NADH on CSE-Induced Lung Changes in Oxidative Stress and Antioxidant Defense Biomarkers
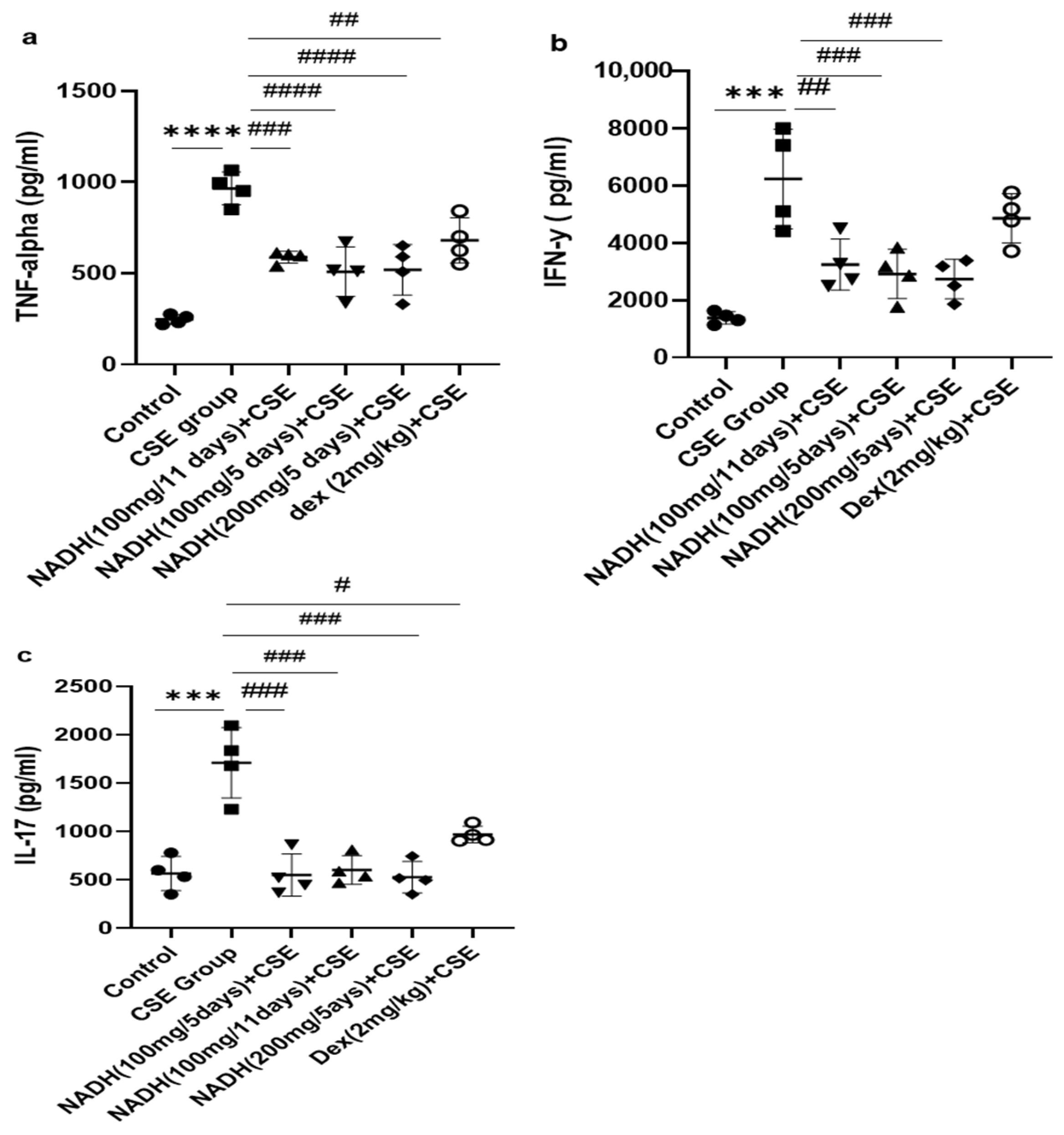
3.4. Effect of NADH on CSE-Induced Changes in Inflammatory Cytokines
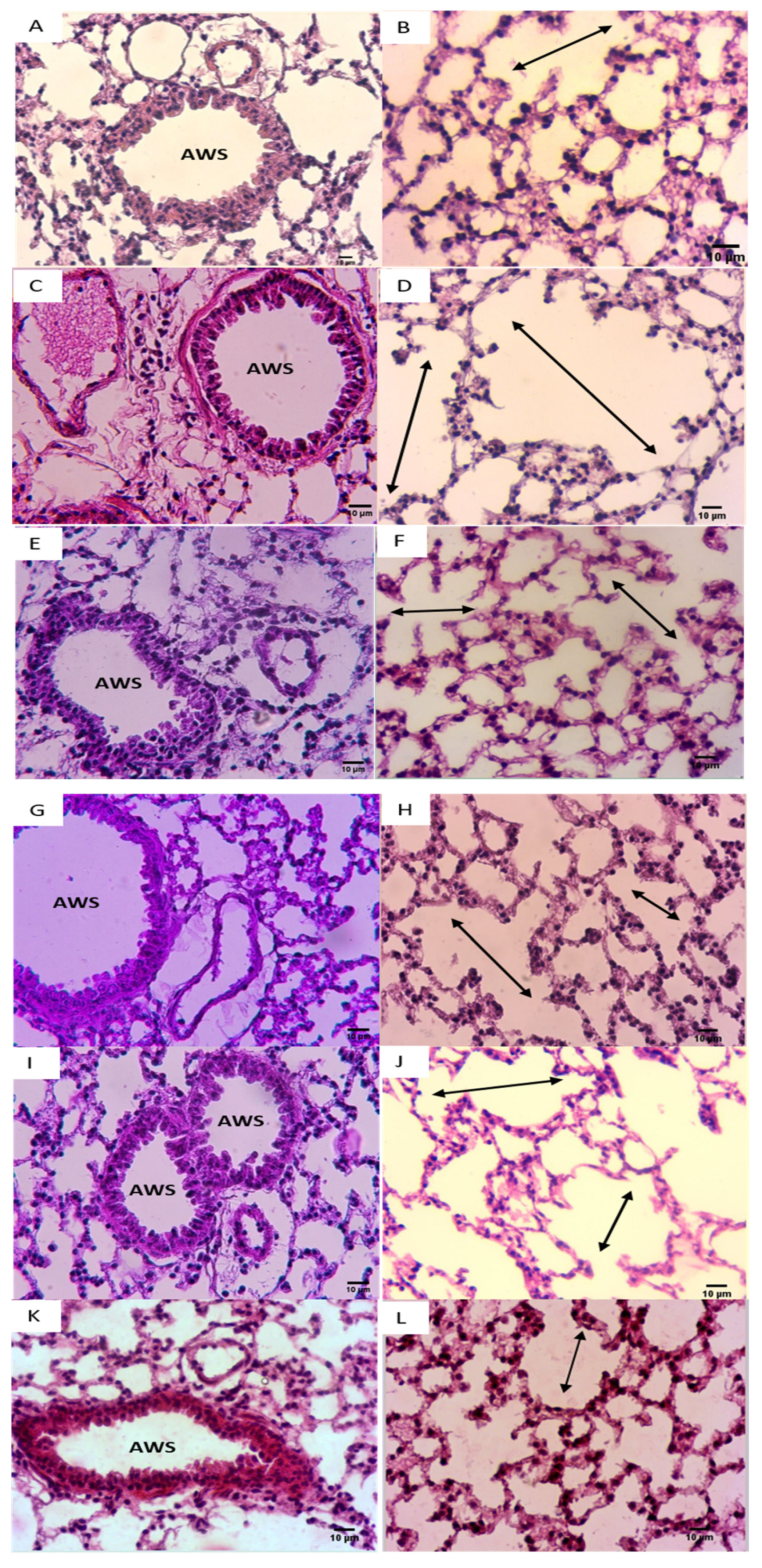
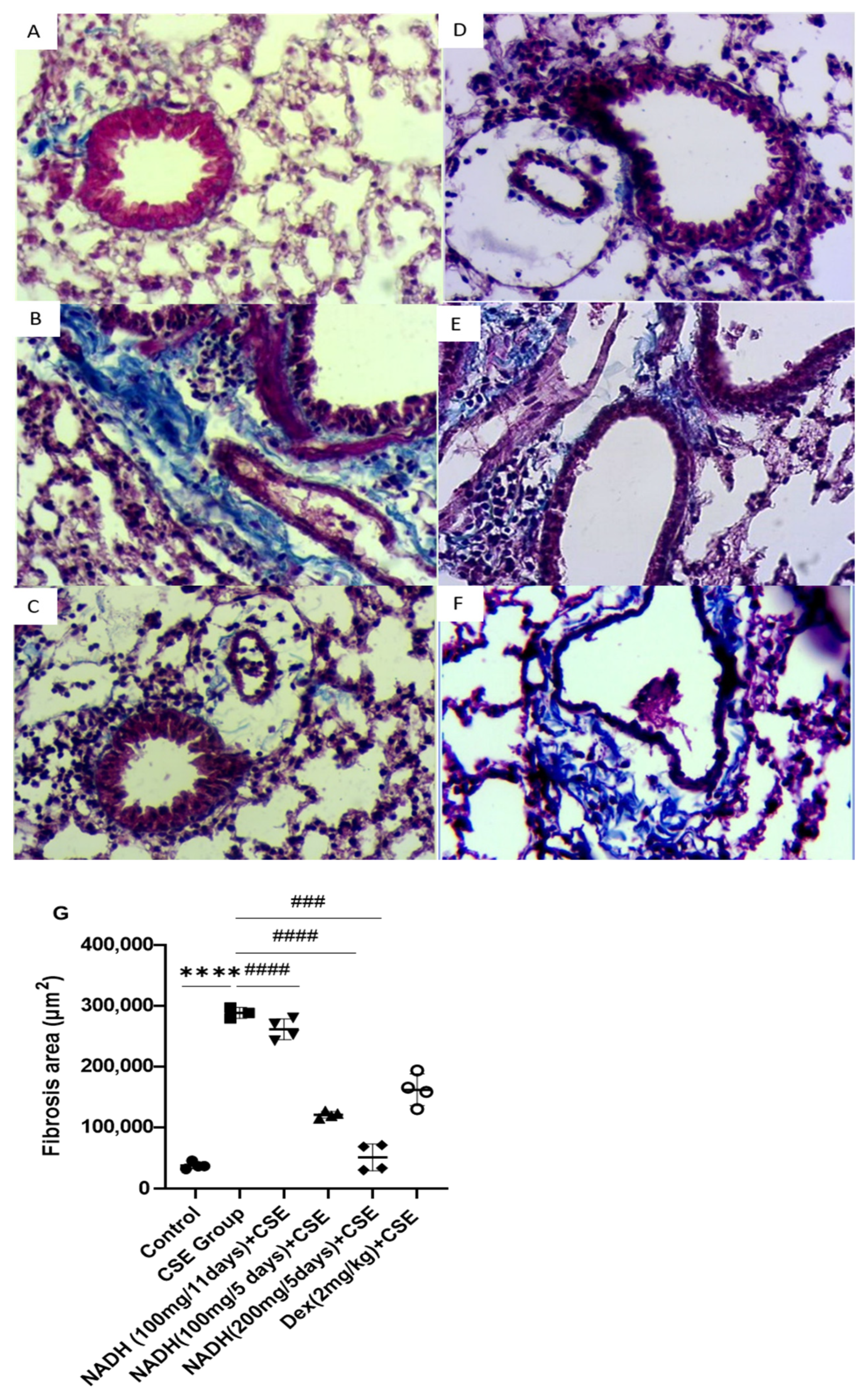
3.5. Effect of NADH on CSE-Induced Inflammatory Changes in Lungs
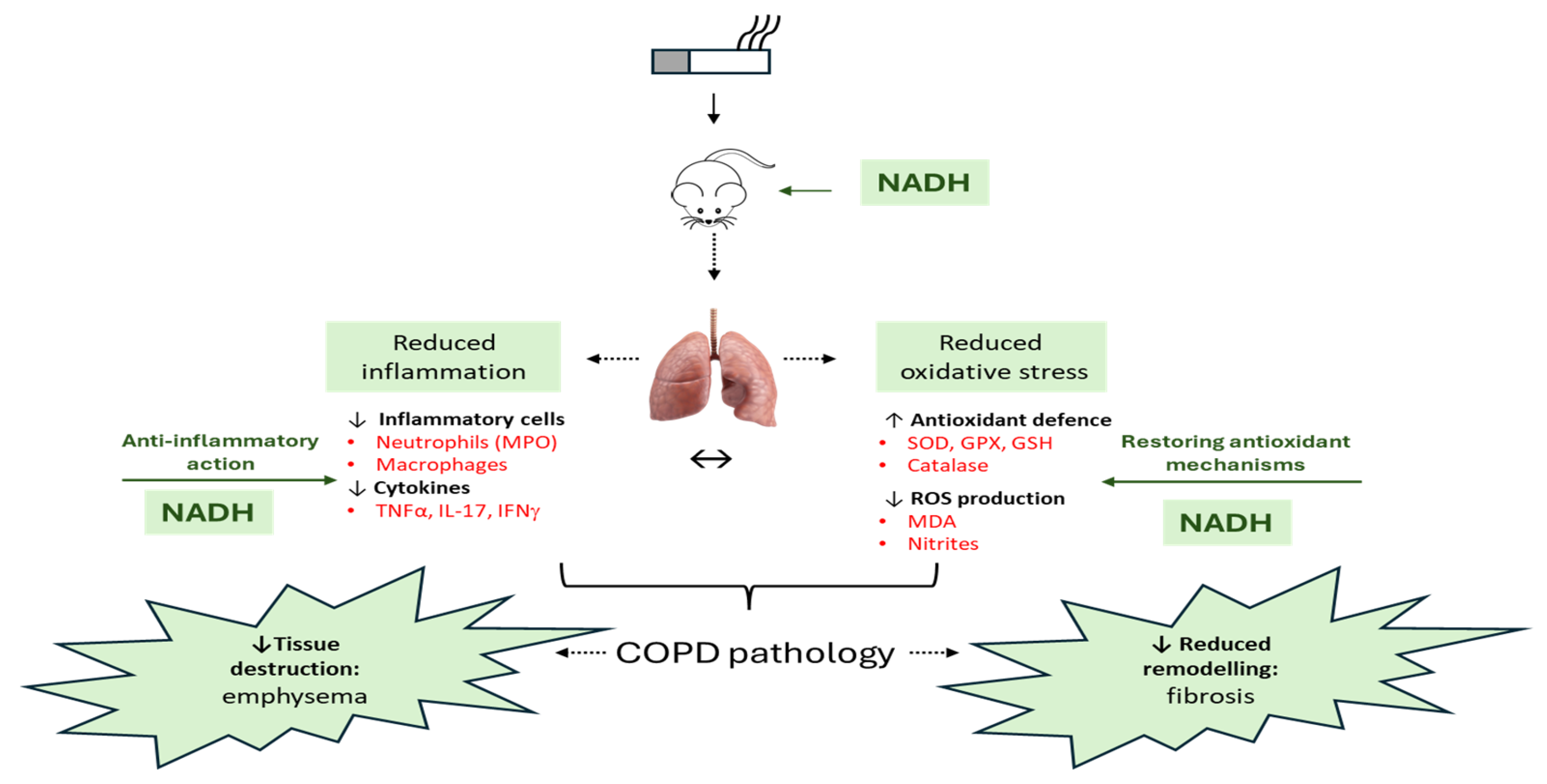
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorababu, A.; Maraswami, M. Recent Advances (2015–2020) in Drug Discovery for Attenuation of Pulmonary Fibrosis and COPD. Molecules 2023, 28, 3674. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Cheng, S.-L. Pathophysiology, Therapeutic Targets, and Future Therapeutic Alternatives in COPD: Focus on the Importance of the Cholinergic System. Biomolecules 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory Mechanisms in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, V.K.; Koval, M. Lung Epithelial Biology in the Pathogenesis of Pulmonary Disease; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-S.; Izumchenko, E.; Dasgupta, S.; Hoque, M.O. Mitochondria in Chronic Obstructive Pulmonary Disease and Lung Cancer: Where Are We Now? Biomark. Med. 2017, 11, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.D.A.F.; Weiss, D.J.; Rocco, P.R.M.; Silva, P.L.; Cruz, F.F. Mitochondria in Focus: From Function to Therapeutic Strategies in Chronic Lung Diseases. Front. Immunol. 2021, 12, 782074. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Kapoor, H.; Chaudhary, N.; Roy, S.S. Cellular and Mitochondrial Calcium Communication in Obstructive Lung Disorders. Mitochondrion 2021, 58, 184–199. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wu, D.; Walker, M.; Wang, P.; Tian, R.; Wang, W. Genetically Encoded Biosensors for Evaluating NAD+/NADH Ratio in Cytosolic and Mitochondrial Compartments. Cell Rep. Methods 2021, 1, 100116. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, B.; Ashok, D.; Liu, T. Mitochondrial Ca2+ in Heart Failure: Not Enough or Too Much? J. Mol. Cell. Cardiol. 2021, 151, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.; Bahria, K.; Slama, N.; Oukrif, D.; Shalaby, A.; Birkmayer, G.; Oumouna, M.; Benachour, K. NADH Intraperitoneal Injection Prevents Massive Pancreatic Beta Cell Destruction in a Streptozotocin-Induced Diabetes in Rats. Histochem. Cell Biol. 2024, 161, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Salech, F.; Ponce, D.P.; Paula-Lima, A.C.; SanMartin, C.D.; Behrens, M.I. Nicotinamide, a Poly [ADP-Ribose] Polymerase 1 (PARP-1) Inhibitor, as an Adjunctive Therapy for the Treatment of Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, W.; Zheng, Z.; Wang, W.; Yuan, Y.; Hong, Q.; Lin, J.; Li, X.; Meng, Y. Cigarette Smoke-Inactivated SIRT1 Promotes Autophagy-Dependent Senescence of Alveolar Epithelial Type 2 Cells to Induce Pulmonary Fibrosis. Free Radic. Biol. Med. 2021, 166, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Huang, Q.; Wang, Y.; Yao, Y.; Li, J.; Chen, J.; Wu, M.; Zhang, Z.; E, M.; Qi, H.; et al. Therapeutic Application of Natural Products: NAD+ Metabolism as Potential Target. Phytomedicine 2023, 114, 154768. [Google Scholar] [CrossRef] [PubMed]
- Alegre, J.; Rosés, J.M.; Javierre, C.; Ruiz-Baqués, A.; Segundo, M.J.; de Sevilla, T.F. Nicotinamide adenine dinucleotide (NADH) in patients with chronic fatigue syndrome. Rev. Clínica Española 2010, 210, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Nicotinamide Adenine Dinucleotide (NADH)—A New Therapeutic Approach to Parkinson’s Disease. Comparison of Oral and Parenteral Application. Available online: https://pubmed.ncbi.nlm.nih.gov/8101414/ (accessed on 9 March 2024).
- Birkmayer, J. Coenzyme Nicotinamide Adenine Dinucleotide: New Therapeutic Approach for Improving Dementia of the Alzheimer Type. Ann. Clin. Lab. Sci. 1996, 26, 1–9. [Google Scholar]
- He, Z.H.; Chen, P.; Chen, Y.; He, S.D.; Ye, J.R.; Zhang, H.L.; Cao, J. Comparison between Cigarette Smoke-Induced Emphysema and Cigarette Smoke Extract-Induced Emphysema. Tob. Induc. Dis. 2015, 13, 6. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Arias-Dìaz, J.; Vara, E.; Torres-Melero, J.; García, C.; Baki, W.; Ramσrez-Armengol, J.A.; Balibrea, J.L. Nitrite/nitrate and cytokine levels in bronchoalvelar lavage fluid of lung cancer patients. Cancer 2014, 74, 1546–1551. [Google Scholar] [CrossRef]
- Ruyssers, N.E.; De Winter, B.Y.; De Man, J.G.; Loukas, A.; Pearson, M.S.; Weinstock, J.V.; Van den Bossche, R.M.; Martinet, W.; Pelckmans, P.A.; Moreels, T.G. Therapeutic Potential of Helminth Soluble Proteins in TNBS-Induced Colitis in Mice. Inflamm. Bowel Dis. 2009, 15, 491–500. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Friedberg, T.; Milbert, U.; Bentley, P.; Guenther, T.M.; Oesch, F. Purification and Characterization of a New Cytosolic Glutathione S-Transferase (Glutathione S-Transferase X) from Rat Liver. Biochem. J. 1983, 215, 617–625. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Salaets, T.; Tack, B.; Gie, A.; Pavie, B.; Sindhwani, N.; Jimenez, J.; Regin, Y.; Allegaert, K.; Deprest, J.; Toelen, J. A Semi-Automated Method for Unbiased Alveolar Morphometry: Validation in a Bronchopulmonary Dysplasia Model. PLoS ONE 2020, 15, e0239562. [Google Scholar] [CrossRef]
- Lin, W.; Jianbo, S.; Wanzhong, L.; Yanna, L.; Weiwei, S.; Gang, W.; Chunzhen, Z. Protective Effect of Eucalyptus Oil on Pulmonary Destruction and Inflammation in Chronic Obstructive Pulmonary Disease (COPD) in Rats. J. Med. Plants Res. 2017, 11, 129–136. [Google Scholar] [CrossRef]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of Oxidant Generation by Catalase. Ann. N. Y. Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef]
- Adcock, I.M.; Marwick, J.; Casolari, P.; Contoli, M.; Fan Chung, K.; Kirkham, P.; Papi, A.; Caramori, G. Mechanisms of corticosteroid resistance in severe asthma and chronic obstructive pulmonary disease (COPD). Curr. Pharm. Des. 2010, 16, 3554–3573. [Google Scholar] [CrossRef]
- Shu, J.; Li, D.; Ouyang, H.; Huang, J.; Long, Z.; Liang, Z.; Chen, Y.; Chen, Y.; Zheng, Q.; Kuang, M.; et al. Comparison and Evaluation of Two Different Methods to Establish the Cigarette Smoke Exposure Mouse Model of COPD. Sci. Rep. 2017, 7, 15454. [Google Scholar] [CrossRef]
- Vlahos, R.; Bozinovski, S. Recent Advances in Pre-Clinical Mouse Models of COPD. Clin. Sci. 2014, 126, 253–265. [Google Scholar] [CrossRef]
- Yang, Y.; Di, T.; Zhang, Z.; Liu, J.; Fu, C.; Wu, Y.; Bian, T. Dynamic Evolution of Emphysema and Airway Remodeling in Two Mouse Models of COPD. BMC Pulm. Med. 2021, 21, 134. [Google Scholar] [CrossRef]
- Ghorani, V.; Boskabady, M.H.; Khazdair, M.R.; Kianmeher, M. Experimental Animal Models for COPD: A Methodological Review. Tob. Induc. Dis. 2017, 15, 25. [Google Scholar] [CrossRef]
- Serré, J.; Tanjeko, A.T.; Mathyssen, C.; Vanherwegen, A.-S.; Heigl, T.; Janssen, R.; Verbeken, E.; Maes, K.; Vanaudenaerde, B.; Janssens, W.; et al. Enhanced Lung Inflammatory Response in Whole-Body Compared to Nose-Only Cigarette Smoke-Exposed Mice. Respir. Res. 2021, 22, 86. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Chen, Y.; Chen, P.; Peng, H.; Cai, S.; Luo, H.; Wu, S.-J. Intraperitoneal Injection of Cigarette Smoke Extract Induced Emphysema, and Injury of Cardiac and Skeletal Muscles in BALB/C Mice. Exp. Lung Res. 2013, 39, 18–31. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, P.; Zeng, H.; Zhang, Y.; Peng, H.; Chen, Y.; He, Z. Protective Effect of Demethylation Treatment on Cigarette Smoke Extract-Induced Mouse Emphysema Model. J. Pharmacol. Sci. 2013, 123, 159–166. [Google Scholar] [CrossRef]
- Li, A.; Liu, Y.; Zhu, X.; Sun, X.; Feng, X.; Li, D.; Zhang, J.; Zhu, M.; Zhao, Z. Methylallyl Sulfone Attenuates Inflammation, Oxidative Stress and Lung Injury Induced by Cigarette Smoke Extract in Mice and RAW264.7 Cells. Int. Immunopharmacol. 2018, 59, 369–374. [Google Scholar] [CrossRef]
- Kettawan, A.; Ruangklai, S.; Rungruang, T.; Thongam, J.; Kettawan, A.K.; Nirmal, N.; Srisuma, S. Rice Bran Oil Improves Emphysema in Cigarette Smoke Extract-Induced Mice through Anti-Inflammatory and Antioxidative Effects. Nutrients 2024, 16, 433. [Google Scholar] [CrossRef]
- Campos, K.K.D.; Araújo, G.R.; Martins, T.L.; Bandeira, A.C.B.; de Paula Costa, G.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Costa, D.C.; Bezerra, F.S. The Antioxidant and Anti-Inflammatory Properties of Lycopene in Mice Lungs Exposed to Cigarette Smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef]
- Wang, S.; He, N.; Xing, H.; Sun, Y.; Ding, J.; Liu, L. Function of Hesperidin Alleviating Inflammation and Oxidative Stress Responses in COPD Mice Might Be Related to SIRT1/PGC-1α/NF-κB Signaling Axis. J. Recept. Signal Transduct. 2020, 40, 388–394. [Google Scholar] [CrossRef]
- Sørensen, A.K.; Holmgaard, D.B.; Mygind, L.H.; Johansen, J.; Pedersen, C. Neutrophil-to-lymphocyte ratio, calprotectin and YKL-40 in patients with chronic obstructive pulmonary disease: Correlations and 5-year mortality—A cohort study. J. Inflamm. 2015, 12, 20. [Google Scholar] [CrossRef]
- Turato, G.; Zuin, R.; Saetta, M. Pathogenesis and pathology of COPD. Respiration 2001, 68, 117–128. [Google Scholar] [CrossRef]
- Wang, W.; Zha, G.; Zou, J.j.; Wang, X.; Li, C.n.; Wu, X.j. Berberine Attenuates Cigarette Smoke Extract-Induced Airway Inflammation in Mice: Involvement of TGF-Β1/Smads Signaling Pathway. Curr. Med. Sci. 2019, 39, 748–753. [Google Scholar] [CrossRef]
- Deng, M.; Tong, R.; Bian, Y.; Hou, G. Astaxanthin Attenuates Cigarette Smoking-Induced Oxidative Stress and Inflammation in a Sirtuin 1-Dependent Manner. Biomed. Pharmacother. 2023, 159, 114230. [Google Scholar] [CrossRef]
- Lee, K.H.; Woo, J.; Kim, J.; Lee, C.H.; Yoo, C.G. YPL-001 Shows Various Beneficial Effects against Cigarette Smoke Extract-Induced Emphysema Formation: Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Effects. Antioxidants 2023, 12, 15. [Google Scholar] [CrossRef]
- Rovina, N.; Koutsoukou, A.; Koulouris, N.G. Inflammation and immune response in COPD: Where do we stand? Mediat. Inflamm. 2013, 2013, 413735. [Google Scholar] [CrossRef]
- Guo, P.; Li, R.; Piao, T.H.; Wang, C.L.; Wu, X.L.; Cai, H.Y. Pathological mechanism and targeted drugs of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1565–1575. [Google Scholar] [CrossRef]
- Sharma, A.; Chabloz, S.; Lapides, R.A.; Roider, E.; Ewald, C.Y. Potential Synergistic Supplementation of NAD+ Promoting Compounds as a Strategy for Increasing Healthspan. Nutrients 2023, 15, 445. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Ma, R.; Su, H.; Jiao, K.; Liu, J. Association Between IL-17 and Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1681–1690. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its Role for Host Defense, Inflammation, and Neutrophil Function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Zhou, W.C.; Qu, J.; Xie, S.Y.; Sun, Y.; Yao, H.W. Mitochondrial dysfunction in chronic respiratory diseases: Implications for the pathogenesis and potential therapeutics. Oxid. Med. Cell. Longev. 2021, 2021, 10245–10288. [Google Scholar] [CrossRef]
- Mahalanobish, S.; Dutta, S.; Saha, S.; Sil, P.C. Melatonin Induced Suppression of ER Stress and Mitochondrial Dysfunction Inhibited NLRP3 Inflammasome Activation in COPD Mice. Food Chem. Toxicol. 2020, 144, 111588. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, Y.S.; Jun, W.; Yang, S.J. Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J. Med. Food 2015, 18, 1207–1213. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef]
- Eto, K.; Suga, S.; Wakui, M.; Tsubamoto, Y.; Terauchi, Y.; Taka, J.; Kadowaki, T. NADH Shuttle System Regulates KATPChannel-dependent Pathway and Steps Distal to Cytosolic Ca2+ Concentration Elevation in Glucose-induced Insulin Secretion. J. Biol. Chem. 1999, 274, 25386–25392. [Google Scholar] [CrossRef]
- Eto, K.; Tsubamoto, Y.; Terauchi, Y.; Sugiyama, T.; Kishimoto, T.; Takahashi, N.; Yamauchi, N.; Kubota, N.; Murayama, S.; Aizawa, T.; et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 1999, 283, 981–985. [Google Scholar] [CrossRef]
- Ning, W.; Li, C.J.; Kaminski, N.; Feghali-Bostwick, C.A.; Alber, S.M.; Di, Y.P.; Choi, A.M. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2004, 101, 14895–14900. [Google Scholar] [CrossRef]
- Kang, H.-R.; Lee, J.-Y.; Lee, C.G. TGF-Β1 as a Therapeutic Target for Pulmonary Fibrosis and COPD. Expert Rev. Clin. Pharmacol. 2008, 1, 547–558. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Sukkar, M.B.; Khorasani, N.M.; Bhavsar, P.K.; Chung, K.F. TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 300, L295–L304. [Google Scholar] [CrossRef]
- Repine, J.E.; Bast, A.A.L.T.; Lankhorst, I.D.A.; Oxidative Stress Study Group. Oxidative stress in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 156, 341–357. [Google Scholar] [CrossRef]
- Lu, H.; Burns, D.; Garnier, P.; Wei, G.; Zhu, K.; Ying, W. P2X7 Receptors Mediate NADH Transport across the Plasma Membranes of Astrocytes. Biochem. Biophys. Res. Commun. 2007, 362, 946–950. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The Potential of P2X7 Receptors as a Therapeutic Target, Including Inflammation and Tumour Progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef]
- Lucattelli, M.; Cicko, S.; Müller, T.; Lommatzsch, M.; De Cunto, G.; Cardini, S.; Sundas, W.; Grimm, M.; Zeiser, R.; Dürk, T.; et al. P2X7 Receptor Signaling in the Pathogenesis of Smoke-Induced Lung Inflammation and Emphysema. Am. J. Respir. Cell Mol. Biol. 2011, 44, 423–429. [Google Scholar] [CrossRef]
- Munk, S.H.N.; Merchut-Maya, J.M.; Adelantado Rubio, A.; Hall, A.; Pappas, G.; Milletti, G.; Lee, M.; Johnsen, L.G.; Guldberg, P.; Bartek, J.; et al. NAD+ Regulates Nucleotide Metabolism and Genomic DNA Replication. Nat. Cell Biol. 2023, 25, 1774–1786. [Google Scholar] [CrossRef]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 Hemi Channels Mediate Ca2+-Regulated Transmembrane NAD+ Fluxes in Intact Cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef]
- Billington, R.A.; Travelli, C.; Ercolano, E.; Galli, U.; Roman, C.B.; Grolla, A.A.; Canonico, P.L.; Condorelli, F.; Genazzani, A.A. Characterization of NAD Uptake in Mammalian Cells. J. Biol. Chem. 2008, 283, 6367–6374. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slama, N.; Abdellatif, A.; Bahria, K.; Gasmi, S.; Khames, M.; Hadji, A.; Birkmayer, G.; Oumouna, M.; Amrani, Y.; Benachour, K. NADH Intraperitoneal Injection Prevents Lung Inflammation in a BALB/C Mice Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease. Cells 2024, 13, 881. https://doi.org/10.3390/cells13100881
Slama N, Abdellatif A, Bahria K, Gasmi S, Khames M, Hadji A, Birkmayer G, Oumouna M, Amrani Y, Benachour K. NADH Intraperitoneal Injection Prevents Lung Inflammation in a BALB/C Mice Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease. Cells. 2024; 13(10):881. https://doi.org/10.3390/cells13100881
Chicago/Turabian StyleSlama, Nada, Amina Abdellatif, Karima Bahria, Sara Gasmi, Maamar Khames, Abderrahmene Hadji, George Birkmayer, Mustapha Oumouna, Yassine Amrani, and Karine Benachour. 2024. "NADH Intraperitoneal Injection Prevents Lung Inflammation in a BALB/C Mice Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease" Cells 13, no. 10: 881. https://doi.org/10.3390/cells13100881
APA StyleSlama, N., Abdellatif, A., Bahria, K., Gasmi, S., Khames, M., Hadji, A., Birkmayer, G., Oumouna, M., Amrani, Y., & Benachour, K. (2024). NADH Intraperitoneal Injection Prevents Lung Inflammation in a BALB/C Mice Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease. Cells, 13(10), 881. https://doi.org/10.3390/cells13100881







