Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth
Abstract
1. Introduction
2. Materials and Methods
2.1. The Primary Culture of SHED
2.2. The Optimization of FBS and hPL Concentration
2.3. Cell Morphology
2.4. Proliferation and Self-Renewing Capacity
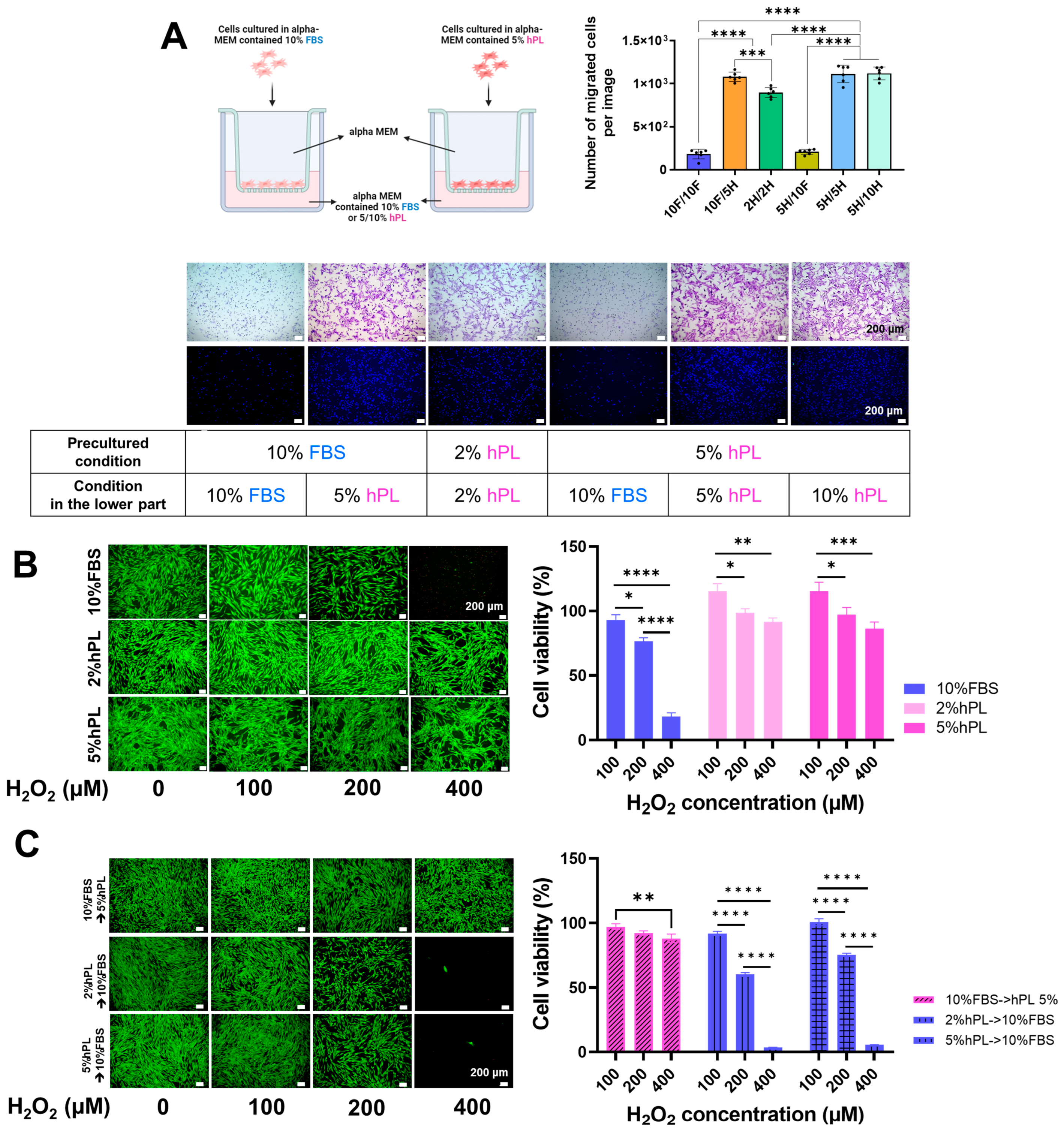
2.5. Cell Migration Assay
2.6. Cell Protective Effect of hPL under ROS Condition Induced by Hydrogen Peroxide (H2O2)
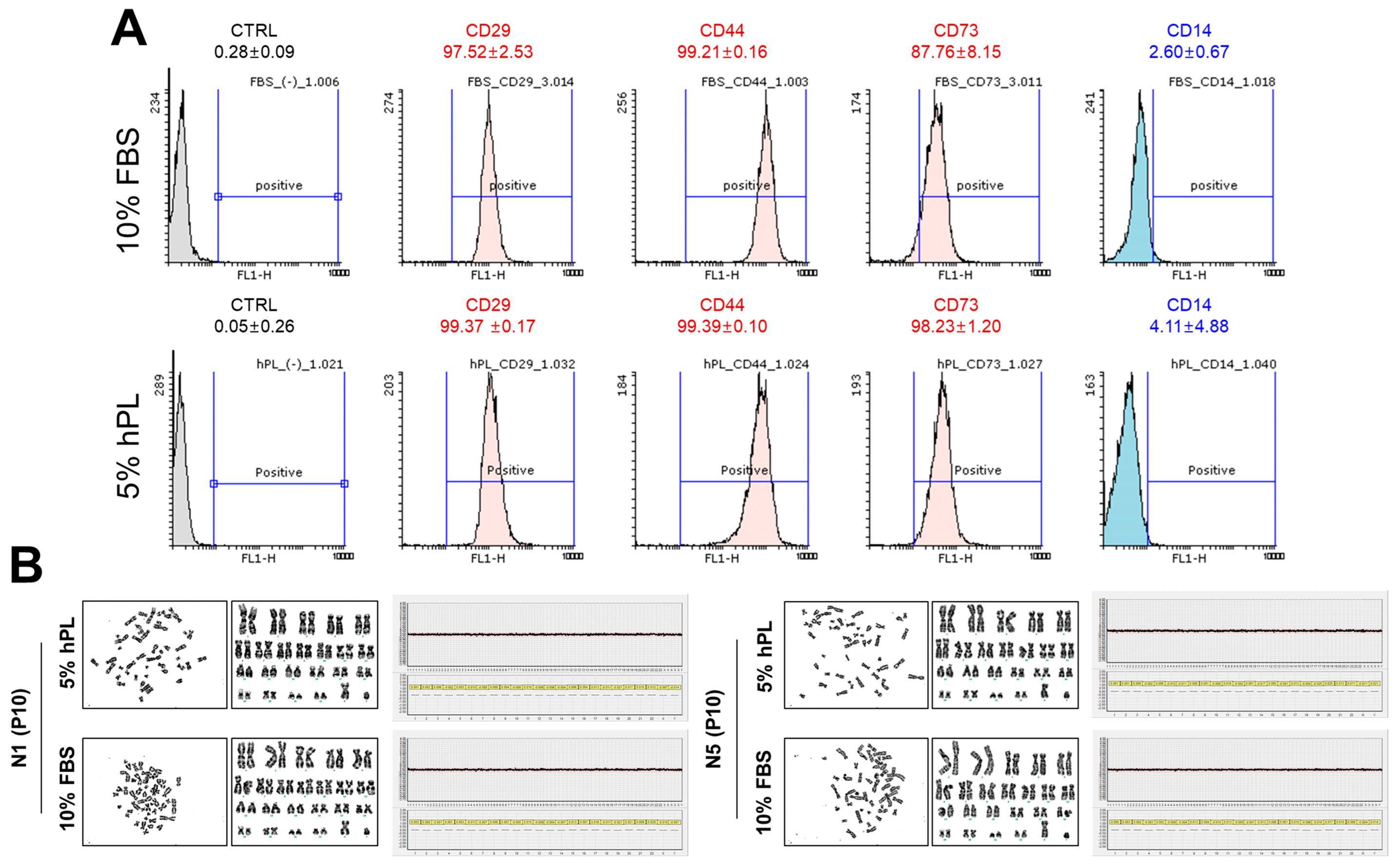
2.7. Analysis of Chromosomal Microarray-Cytogenetic Microarray (CMA)
2.8. Flow Cytometric Analysis
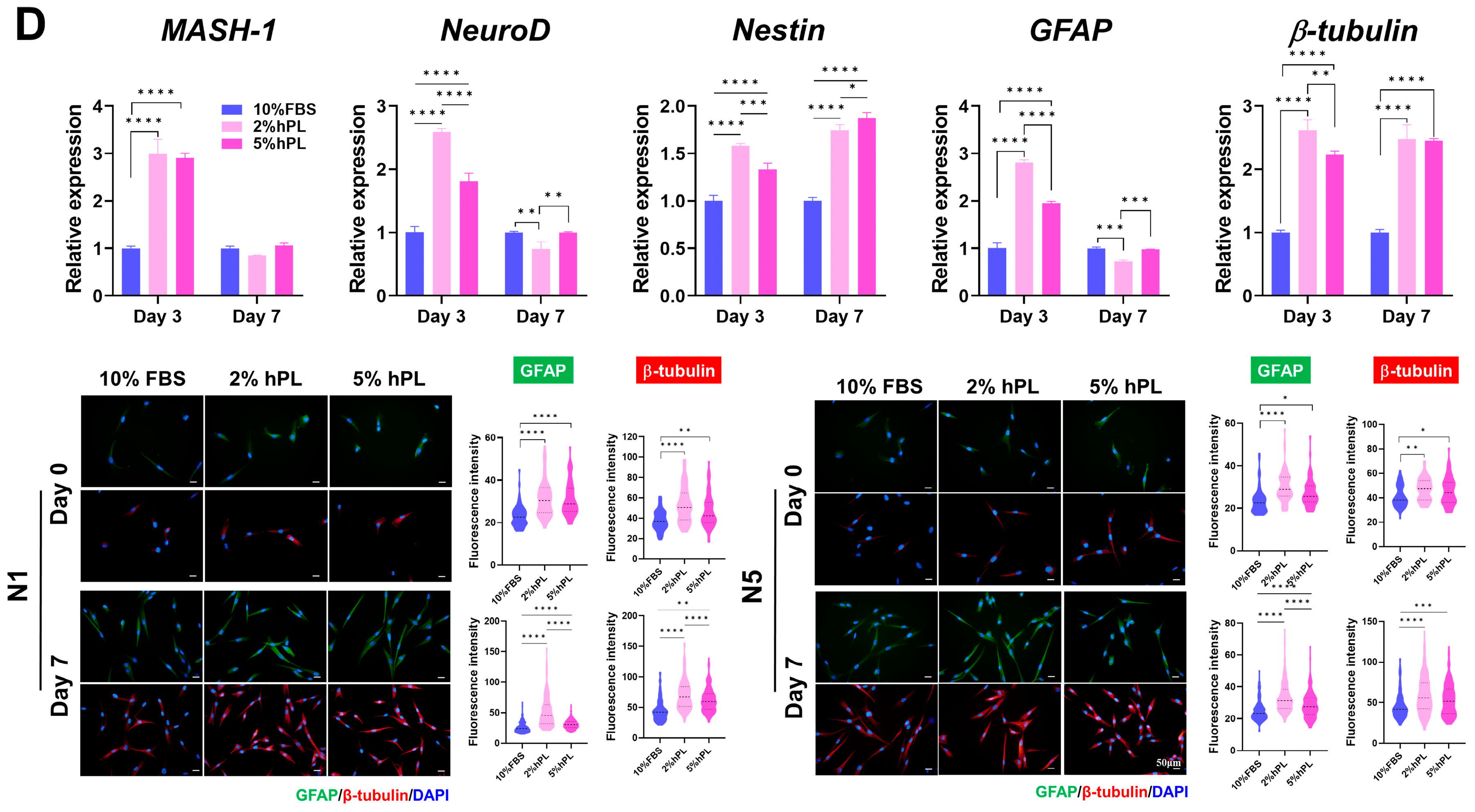
2.9. SHED Differentiation Assays
2.10. Immunocytochemistry (ICC)
2.11. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
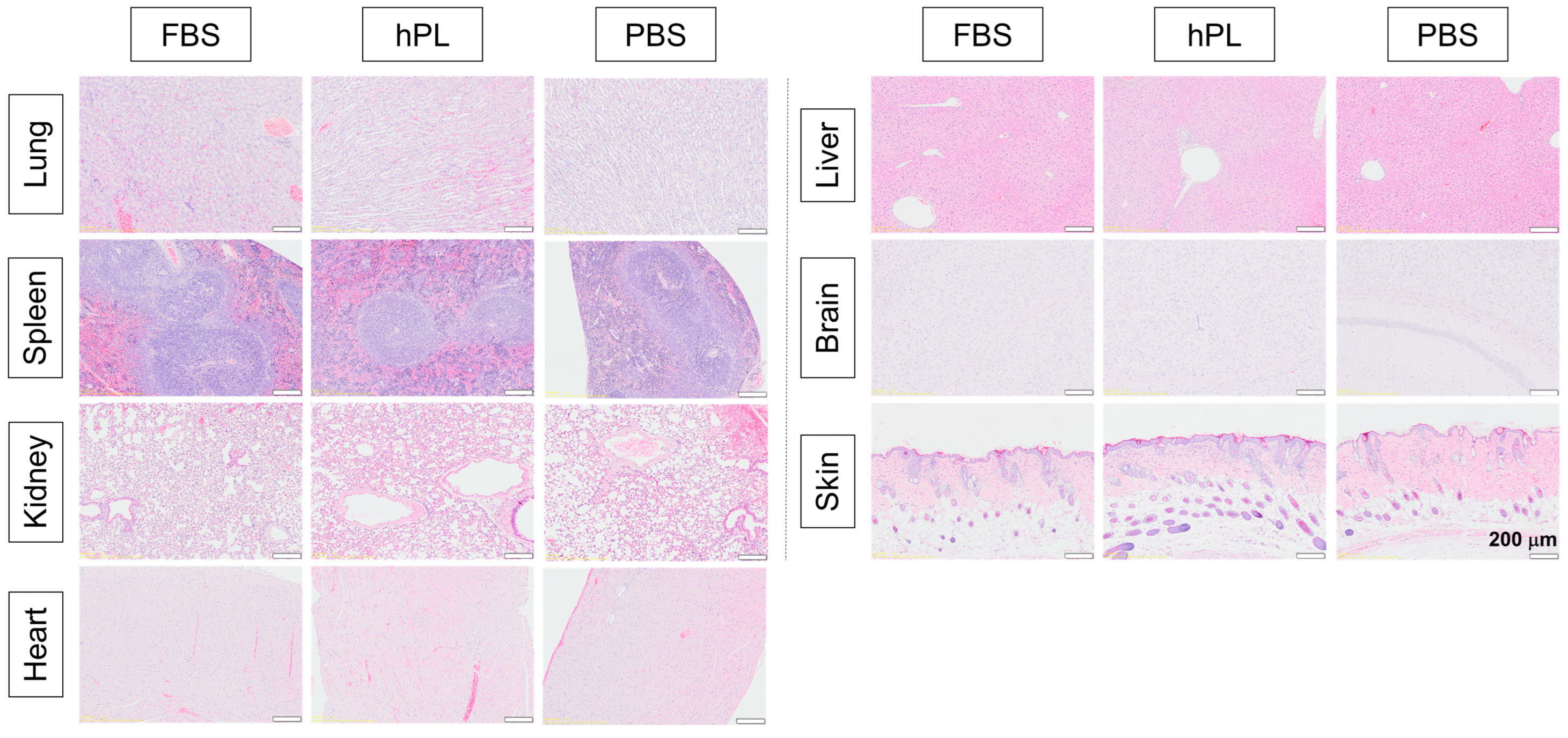
2.12. Organ Toxicity and Tumor Formation Test In Vivo
2.13. Statistical Analyses
3. Results
3.1. The Evaluation hPL as an Alternative to FBS in SHED Culture
3.1.1. Optimization of hPL and FBS Concentration for SHED Culture
3.1.2. The Effect of Cell Proliferation of hPL and FBS
3.1.3. The Effect of Cell Migration of hPL and FBS
3.1.4. The Cell Protection Effect of hPL and FBS against Oxidative Stress on Cells
3.2. Characterization of hPL-SHEDs and FBS-SHEDs
3.2.1. Surface Markers and Chromosomal Stability
3.2.2. Differentiation Capacity of hPL-SHEDs and FBS-SHEDs
3.2.3. In Vivo Studies of Tumorigenesis and Organ Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.L.W.; Janes, M.E.; Kumbhojkar, N.; Kapate, N.; Clegg, J.R.; Prakash, S.; Heavey, M.K.; Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Cell therapies in the clinic. Bioeng. Transl. Med. 2021, 6, e10214. [Google Scholar] [CrossRef] [PubMed]
- El-Kadiry, A.E.-H.; Rafei, M.; Shammaa, R. Cell therapy: Types, regulation, and clinical benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef]
- Ajmal, L.; Ajmal, S.; Ajmal, M.; Nawaz, G. Organ Regeneration Through Stem Cells and Tissue Engineering. Cureus 2023, 15, e34336. [Google Scholar] [CrossRef]
- Gazarian, K.G.; Ramírez-García, L.R. Human deciduous teeth stem cells (SHED) display neural crest signature characters. PLoS ONE 2017, 12, e0170321. [Google Scholar] [CrossRef]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P., Jr.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [CrossRef]
- Karaöz, E.; Demircan, P.C.; Sağlam, Ö.; Aksoy, A.; Kaymaz, F.; Duruksu, G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol. 2011, 136, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Sang, Y.; Zhang, F.; Liu, Z.; Qi, N.; Chen, Y. Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cells Int. 2016, 2016, 3516574. [Google Scholar] [CrossRef]
- Anoop, M.; Datta, I. Stem cells derived from human exfoliated deciduous teeth (SHED) in neuronal disorders: A review. Curr. Stem Cell Res. Ther. 2021, 16, 535–550. [Google Scholar]
- Wang, H.; Zhong, Q.; Yang, T.; Qi, Y.; Fu, M.; Yang, X.; Qiao, L.; Ling, Q.; Liu, S.; Zhao, Y. Comparative characterization of SHED and DPSCs during extended cultivation in vitro. Mol. Med. Rep. 2018, 17, 6551–6559. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Kuang, G.; Fengying, S.; Shoumei, X.; Duohong, Z.; Jiacai, H.; Xuyan, T. Comparison of the angiogenic ability between SHED and DPSC in a mice model with critical limb ischemic. Tissue Eng. Regen. Med. 2022, 19, 861–870. [Google Scholar] [CrossRef]
- Cobo, F.; Talavera, P.; Concha, A. Diagnostic approaches for viruses and prions in stem cell banks. Virology 2006, 347, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Diaz, R.; Behfar, A.; Butler, G.W.; Padley, D.J.; Sarr, M.G.; Bartunek, J.; Dietz, A.B.; Terzic, A. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011, 20, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Coates, D.E.; Alansary, M.; Friedlander, L.; Zanicotti, D.G.; Duncan, W.J. Dental pulp stem cells in serum-free medium for regenerative medicine. J. R. Soc. N. Z. 2020, 50, 80–90. [Google Scholar] [CrossRef]
- Duarte, A.C.; Costa, E.C.; Filipe, H.; Saraiva, S.; Jacinto, T.; Miguel, S.; Ribeiro, M.; Coutinho, P. Animal-derived products in science and current alternatives. Biomater. Adv. 2023, 151, 213428. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.R.; Begot, L.; Holy, X.; Lataillade, J.J. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Agostini, F.; Chieregato, K.; Amati, E.; Durante, C.; Rassu, M.; Ruggeri, M.; Sella, S.; Lombardi, E.; Mazzucato, M. The production method affects the efficacy of platelet derivatives to expand mesenchymal stromal cells in vitro. J. Transl. Med. 2017, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Dessels, C.; Potgieter, M.; Pepper, M.S. Making the switch: Alternatives to fetal bovine serum for adipose-derived stromal cell expansion. Front. Cell Dev. Biol. 2016, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Lischer, M.; di Summa, P.G.; Oranges, C.M.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Human platelet lysate stimulated adipose stem cells exhibit strong neurotrophic potency for nerve tissue engineering applications. Regen. Med. 2020, 15, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Guiotto, M.; Raffoul, W.; Hart, A.; Riehle, M.; Di Summa, P. Human platelet lysate to substitute fetal bovine serum in hMSC expansion for translational applications: A systematic review. J. Transl. Med. 2020, 18, 351. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Yoon, J.-Y.; Park, J.-H.; Lee, H.-H.; Dashnyam, K.; Kim, H.-W.; Lee, J.-H.; Shin, J.-S.; Kim, J.-B. The Potential Application of Human Gingival Fibroblast-Conditioned Media in Pulp Regeneration: An In Vitro Study. Cells 2022, 11, 3398. [Google Scholar] [CrossRef]
- Vu, H.T.; Han, M.-R.; Lee, J.-H.; Kim, J.-S.; Shin, J.-S.; Yoon, J.-Y.; Park, J.-H.; Dashnyam, K.; Knowles, J.C.; Lee, H.-H. Investigating the effects of conditioned media from stem cells of human exfoliated deciduous teeth on dental pulp stem cells. Biomedicines 2022, 10, 906. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, D.; Li, Q.; Tian, W.; Guo, W. The establishment of a chemically defined serum-free culture system for human dental pulp stem cells. Stem Cell Res. Ther. 2018, 9, 191. [Google Scholar] [CrossRef]
- Kawase-Koga, Y.; Fujii, Y.; Yamakawa, D.; Sato, M.; Chikazu, D. Identification of neurospheres generated from human dental pulp stem cells in xeno-/serum-free conditions. Regen. Ther. 2020, 14, 128–135. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Mankani, M.H.; Robey, P.G. Effect of serum on human bone marrow stromal cells: Ex vivo expansion and in vivo bone formation. Transplantation 2000, 70, 1780–1787. [Google Scholar] [CrossRef]
- Morimoto, N.; Ito, T.; Takemoto, S.; Katakami, M.; Kanda, N.; Tada, H.; Tanaka, S.; Teramukai, S.; Kawai, K.; Nakamura, Y. An exploratory clinical study on the safety and efficacy of an autologous fibroblast-seeded artificial skin cultured with animal product-free medium in patients with diabetic foot ulcers. Int. Wound J. 2014, 11, 183–189. [Google Scholar] [CrossRef]
- Schallmoser, K.; Strunk, D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. JoVE J. Vis. Exp. 2009, 32, e1523. [Google Scholar]
- Lohmann, M.; Walenda, G.; Hemeda, H.; Joussen, S.; Drescher, W.; Jockenhoevel, S.; Hutschenreuter, G.; Zenke, M.; Wagner, W. Donor age of human platelet lysate affects proliferation and differentiation of mesenchymal stem cells. PLoS ONE 2012, 7, e37839. [Google Scholar] [CrossRef]
- Kilian, O.; Flesch, I.; Wenisch, S.; Taborski, B.; Jork, A.; Schnettler, R.; Jonuleit, T. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur. J. Med. Res. 2004, 9, 337–344. [Google Scholar]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef]
- Flemming, A.; Schallmoser, K.; Strunk, D.; Stolk, M.; Volk, H.-D.; Seifert, M. Immunomodulative efficacy of bone marrow-derived mesenchymal stem cells cultured in human platelet lysate. J. Clin. Immunol. 2011, 31, 1143–1156. [Google Scholar] [CrossRef]
- Stultz, B.G.; McGinnis, K.; Thompson, E.E.; Surdo, J.L.L.; Bauer, S.R.; Hursh, D.A. Chromosomal stability of mesenchymal stromal cells during in vitro culture. Cytotherapy 2016, 18, 336–343. [Google Scholar] [CrossRef]
- Binato, R.; de Souza Fernandez, T.; Lazzarotto-Silva, C.; Du Rocher, B.; Mencalha, A.; Pizzatti, L.; Bouzas, L.; Abdelhay, E. Stability of human mesenchymal stem cells during in vitro culture: Considerations for cell therapy. Cell Prolif. 2013, 46, 10–22. [Google Scholar] [CrossRef]
- Irani, K. Oxidant signaling in vascular cell growth, death, and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef]
- PARk, W.H. The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. Int. J. Mol. Med. 2013, 31, 471–476. [Google Scholar] [CrossRef]
- Tan, J.-q.; Li, P.-c.; Li, Q.; Tang, J.-t.; Xue, H.-k. Protective effect of procyanidin B2 on hydrogen peroxide (H2O2)-induced oxidative damage in MCF-7 cells. Appl. Biol. Chem. 2020, 63, 58. [Google Scholar] [CrossRef]
- Pang, Q.Q.; Kim, J.H.; Kim, H.Y.; Kim, J.-H.; Cho, E.J. Protective Effects and Mechanisms of Pectolinarin against H2O2-Induced Oxidative Stress in SH-SY5Y Neuronal Cells. Molecules 2023, 28, 5826. [Google Scholar] [CrossRef]
- Wei, H.; Li, Z.; Hu, S.; Chen, X.; Cong, X. Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J. Cell. Biochem. 2010, 111, 967–978. [Google Scholar] [CrossRef]
- Widyaningrum, R.; Burnouf, T.; Nebie, O.; Delila, L.; Wang, T.-J. A purified human platelet pellet lysate rich in neurotrophic factors and antioxidants repairs and protects corneal endothelial cells from oxidative stress. Biomed. Pharmacother. 2021, 142, 112046. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Pisciotta, A.; Bertoni, L.; Vallarola, A.; Bertani, G.; Mecugni, D.; Carnevale, G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen. Res. 2020, 15, 373–381. [Google Scholar]
- Abe, S.; Hamada, K.; Miura, M.; Yamaguchi, S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol. Int. 2012, 36, 927–936. [Google Scholar] [CrossRef]
- Thesleff, I.; Åberg, T. Molecular regulation of tooth development. Bone 1999, 25, 123–125. [Google Scholar] [CrossRef]
- Peters, H.; Balling, R. Teeth: Where and how to make them. Trends Genet. 1999, 15, 59–65. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef]
- Rosmarwati, E.; Ellistasari, E.Y.; Kusumawardani, A.; Julianto, I.; Widhiati, S.; Setyawan, N.A.; Yanuar, F. Human platelet lysate-derived exosomes are superior to the lysate at increasing collagen deposition in a rat model of intrinsic aging. J. Appl. Pharm. Sci. 2023, 13, 211–216. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M. Risk of tumorigenicity in mesenchymal stromal cell–based therapies—Bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.-Y.; Vu, H.T.; Lee, J.H.; Shin, J.-S.; Kim, H.-W.; Lee, H.-H.; Kim, J.-B.; Lee, J.-H. Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth. Cells 2024, 13, 847. https://doi.org/10.3390/cells13100847
Yoon J-Y, Vu HT, Lee JH, Shin J-S, Kim H-W, Lee H-H, Kim J-B, Lee J-H. Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth. Cells. 2024; 13(10):847. https://doi.org/10.3390/cells13100847
Chicago/Turabian StyleYoon, Ji-Young, Huong Thu Vu, Jun Hee Lee, Ji-Sun Shin, Hae-Won Kim, Hae-Hyoung Lee, Jong-Bin Kim, and Jung-Hwan Lee. 2024. "Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth" Cells 13, no. 10: 847. https://doi.org/10.3390/cells13100847
APA StyleYoon, J.-Y., Vu, H. T., Lee, J. H., Shin, J.-S., Kim, H.-W., Lee, H.-H., Kim, J.-B., & Lee, J.-H. (2024). Evaluation of Human Platelet Lysate as an Alternative to Fetal Bovine Serum for Potential Clinical Applications of Stem Cells from Human Exfoliated Deciduous Teeth. Cells, 13(10), 847. https://doi.org/10.3390/cells13100847





