“All for One and One for All”: The Secreted Heat Shock Protein gp96-Ig Based Vaccines
Abstract
1. Introduction
2. Gp96 as Molecular Chaperon
3. Gp96 as an Immune Modulator
4. Recombinant Fusion Protein gp96-Ig
5. How Does gp96 Induce Mucosal Immune Response?
6. “All for One”
7. “One for All”
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zinkernagel, R.M.; Doherty, P.C. Restriction of in Vitro T Cell-Mediated Cytotoxicity in Lymphocytic Choriomeningitis within a Syngeneic or Semiallogeneic System. Nature 1974, 248, 701–702. [Google Scholar] [CrossRef]
- Deres, K.; Schild, H.; Wiesmuller, K.H.; Jung, G.; Rammensee, H.G. In vivo Priming of Virus-Specific Cytotoxic T Lymphocytes with Synthetic Lipopeptide Vaccine. Nature 1989, 342, 561–564. [Google Scholar] [CrossRef]
- Strbo, N.; Vaccari, M.; Pahwa, S.; Kolber, M.A.; Doster, M.N.; Fisher, E.; Gonzalez, L.; Stablein, D.; Franchini, G.; Podack, E.R. Cutting Edge: Novel Vaccination Modality Provides Significant Protection against Mucosal Infection by Highly Pathogenic Simian Immunodeficiency Virus. J. Immunol. 2013, 190, 2495–2499. [Google Scholar] [CrossRef]
- Strbo, N.; Vaccari, M.; Pahwa, S.; Kolber, M.A.; Fisher, E.; Gonzalez, L.; Doster, M.N.; Hryniewicz, A.; Felber, B.K.; Pavlakis, G.N.; et al. Gp96 Siv Ig Immunization Induces Potent Polyepitope Specific, Multifunctional Memory Responses in Rectal and Vaginal Mucosa. Vaccine 2011, 29, 2619–2625. [Google Scholar] [CrossRef]
- Strbo, N.; Romero, L.; Fisher, E.; Garcia, D. Induction of Zika Specific Cd8 T Cell Responses in the Placenta after Heat Shock Protein Gp96-Ig Vaccination. J. Immunol. 2018, 200, 180. [Google Scholar]
- Strbo, N.; Romero, L.; Garcia, D. Heat Shock Protein Gp96-Ig Vaccine Induces Antigen Specific Cd8 T Cell Response in the Placenta. J. Immunol. 2017, 198, 73. [Google Scholar]
- Fisher, E.; Padula, L.; Podack, K.; O’Neill, K.; Seavey, M.M.; Jayaraman, P.; Jasuja, R.; Strbo, N. Induction of SARS-CoV-2 Protein S-Specific CD8+ T Cells in the Lungs of Gp96-Ig-S Vaccinated Mice. Front. Immunol. 2020, 11, 602254. [Google Scholar] [CrossRef]
- Padula, L.; Fisher, E.; Rivas, K.; Podack, K.; Frasca, D.; Kupritz, J.; Seavey, M.M.; Jayaraman, P.; Dixon, E.; Jasuja, R.; et al. Secreted Heat Shock Protein Gp96-Ig and Ox40l-Fc Combination Vaccine Enhances SARS-CoV-2 Spike (S) Protein-Specific B and T Cell Immune Responses. Vaccine X 2022, 12, 100202. [Google Scholar] [CrossRef]
- Padula, L.; Fisher, E.; Wijayalath, W.; Patterson, N.B.; Huang, J.; Ganeshan, H.; Robinson, T.; Bates, F.A.; Hanson, M.A.; Martin, M.L.; et al. Induction of Antigen Specific Intrahepatic Cd8+ T Cell Responses by a Secreted Heat Shock Protein based Gp96-Ig-PfCA Malaria Vaccine. Front. Immunol. 2023, 14, 1130054. [Google Scholar] [CrossRef]
- Ritossa, F. Discovery of the Heat Shock Response. Cell Stress Chaperones 1996, 1, 97–98. [Google Scholar] [CrossRef]
- Ritossa, F.M. Experimental Activation of Specific Loci in Polytene Chromosomes of Drosophila. Exp. Cell Res. 1964, 35, 601–607. [Google Scholar] [CrossRef]
- Ritossa, F.M.; Vonborstel, R.C. Chromosome Puffs in Drosophila Induced by Ribonuclease. Science 1964, 145, 513–514. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the Nomenclature of the Human Heat Shock Proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Picard, D. Heat-Shock Protein 90, a Chaperone for Folding and Regulation. Cell Mol. Life Sci. 2002, 59, 1640–1648. [Google Scholar] [CrossRef]
- Srivastava, P. Interaction of Heat Shock Proteins with Peptides and Antigen Presenting Cells: Chaperoning of the Innate and Adaptive Immune Responses. Annu. Rev. Immunol. 2002, 20, 395–425. [Google Scholar] [CrossRef]
- Joslin, G.; Hafeez, W.; Perlmutter, D.H. Expression of Stress Proteins in Human Mononuclear Phagocytes. J. Immunol. 1991, 147, 1614–1620. [Google Scholar]
- Lee, A.S. Mammalian Stress Response: Induction of the Glucose-Regulated Protein Family. Curr. Opin. Cell Biol. 1992, 4, 267–273. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z. Roles of Heat Shock Protein Gp96 in the Er Quality Control: Redundant or Unique Function? Mol. Cells 2005, 20, 173–182. [Google Scholar]
- Yang, Y.; Liu, B.; Dai, J.; Srivastava, P.K.; Zammit, D.J.; Lefrancois, L.; Li, Z. Heat Shock Protein Gp96 is a Master Chaperone for Toll-like Receptors and is Important in the Innate Function of Macrophages. Immunity 2007, 26, 215–226. [Google Scholar] [CrossRef]
- Staron, M.; Wu, S.; Hong, F.; Stojanovic, A.; Du, X.; Bona, R.; Liu, B.; Li, Z. Heat-Shock Protein Gp96/Grp94 is an Essential Chaperone for the Platelet Glycoprotein Ib-IX-V Complex. Blood 2011, 117, 7136–7144. [Google Scholar] [CrossRef]
- Barton, E.R.; Park, S.; James, J.K.; Makarewich, C.A.; Philippou, A.; Eletto, D.; Lei, H.; Brisson, B.; Ostrovsky, O.; Li, Z.; et al. Deletion of Muscle Grp94 Impairs Both Muscle and Body Growth by Inhibiting Local Igf Production. FASEB J. 2012, 26, 3691–3702. [Google Scholar] [CrossRef]
- Ostrovsky, O.; Ahmed, N.T.; Argon, Y. The Chaperone Activity of Grp94 Toward Insulin-like Growth Factor II is Necessary for the Stress Response to Serum Deprivation. Mol. Biol. Cell 2009, 20, 1855–1864. [Google Scholar] [CrossRef]
- Ghiasi, S.M.; Dahlby, T.; Hede Andersen, C.; Haataja, L.; Petersen, S.; Omar-Hmeadi, M.; Yang, M.; Pihl, C.; Bresson, S.E.; Khilji, M.S.; et al. Endoplasmic Reticulum Chaperone Glucose-Regulated Protein 94 is Essential for Proinsulin Handling. Diabetes 2019, 68, 747–760. [Google Scholar] [CrossRef]
- Wu, S.; Hong, F.; Gewirth, D.; Guo, B.; Liu, B.; Li, Z. The Molecular Chaperone Gp96/Grp94 Interacts with Toll-like Receptors and Integrins via Its C-Terminal Hydrophobic Domain. J. Biol. Chem. 2012, 287, 6735–6742. [Google Scholar] [CrossRef]
- Kim, J.W.; Cho, Y.B.; Lee, S. Cell Surface Grp94 as a Novel Emerging Therapeutic Target for Monoclonal Antibody Cancer Therapy. Cells 2021, 10, 670. [Google Scholar] [CrossRef]
- Van, P.N.; Peter, F.; Soling, H.D. Four Intracisternal Calcium-Binding Glycoproteins from Rat Liver Microsomes with High Affinity for Calcium. No Indication for Calsequestrin-Like Proteins in Inositol 1,4,5-Trisphosphate-Sensitive Calcium Sequestering Rat Liver Vesicles. J. Biol. Chem. 1989, 264, 17494–17501. [Google Scholar]
- Dollins, D.E.; Warren, J.J.; Immormino, R.M.; Gewirth, D.T. Structures of Grp94-Nucleotide Complexes Reveal Mechanistic Differences between the Hsp90 Chaperones. Mol. Cell 2007, 28, 41–56. [Google Scholar] [CrossRef]
- Munro, S.; Pelham, H.R. A C-Terminal Signal Prevents Secretion of Luminal Er Proteins. Cell 1987, 48, 899–907. [Google Scholar] [CrossRef]
- Frey, S.; Leskovar, A.; Reinstein, J.; Buchner, J. The Atpase Cycle of the Endoplasmic Chaperone Grp94. J. Biol. Chem. 2007, 282, 35612–35620. [Google Scholar] [CrossRef]
- Vogen, S.; Gidalevitz, T.; Biswas, C.; Simen, B.B.; Stein, E.; Gulmen, F.; Argon, Y. Radicicol-Sensitive Peptide Binding to the N-Terminal Portion of GRP94. J. Biol. Chem. 2002, 277, 40742–40750. [Google Scholar] [CrossRef]
- Dutta, R.; Inouye, M. GHKL, an Emergent Atpase/Kinase Superfamily. Trends Biochem. Sci. 2000, 25, 24–28. [Google Scholar] [CrossRef]
- Yamada, S.; Ono, T.; Mizuno, A.; Nemoto, T.K. A Hydrophobic Segment within the C-Terminal Domain is Essential for Both Client-Binding and Dimer Formation of the Hsp90-Family Molecular Chaperone. Eur. J. Biochem. 2003, 270, 146–154. [Google Scholar] [CrossRef]
- Li, Z.; Srivastava, P.K. Tumor Rejection Antigen Gp96/Grp94 is an Atpase: Implications for Protein Folding and Antigen Presentation. EMBO J. 1993, 12, 3143–3151. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nunez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Wallin, R.P.; Lundqvist, A.; More, S.H.; von Bonin, A.; Kiessling, R.; Ljunggren, H.G. Heat-Shock Proteins as Activators of the Innate Immune System. Trends Immunol. 2002, 23, 130–135. [Google Scholar] [CrossRef]
- Strbo, N.; Garcia-Soto, A.; Schreiber, T.H.; Podack, E.R. Secreted Heat Shock Protein Gp96-Ig: Next-Generation Vaccines for Cancer and Infectious Diseases. Immunol. Res. 2013, 57, 311–325. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Udono, H.; Blachere, N.E.; Li, Z. Heat Shock Proteins Transfer Peptides during Antigen Processing and Ctl Priming. Immunogenetics 1994, 39, 93–98. [Google Scholar] [CrossRef]
- Schild, H.; Arnold-Schild, D.; Lammert, E.; Rammensee, H.G. Stress Proteins and Immunity Mediated by Cytotoxic T Lymphocytes. Curr. Opin. Immunol. 1999, 11, 109–113. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Menoret, A.; Basu, S.; Binder, R.J.; McQuade, K.L. Heat Shock Proteins Come of Age: Primitive Functions Acquire New Roles in an Adaptive World. Immunity 1998, 8, 657–665. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Braedel, S.; Hilf, N.; Singh-Jasuja, H.; Herter, S.; Ahmad-Nejad, P.; Kirschning, C.J.; Da Costa, C.; Rammensee, H.G.; Wagner, H.; et al. The Endoplasmic Reticulum-Resident Heat Shock Protein Gp96 Activates Dendritic Cells via the Toll-like Receptor 2/4 Pathway. J. Biol. Chem. 2002, 277, 20847–20853. [Google Scholar] [CrossRef]
- Berwin, B.; Delneste, Y.; Lovingood, R.V.; Post, S.R.; Pizzo, S.V. SREC-I, a Type F Scavenger Receptor, is an Endocytic Receptor for Calreticulin. J. Biol. Chem. 2004, 279, 51250–51257. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Mambula, S.S.; Gray, P.J., Jr. Extracellular Heat Shock Proteins in Cell Signaling and Immunity. Ann. N. Y. Acad. Sci. 2007, 1113, 28–39. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Ramalingam, T.; Srivastava, P.K. Cd91 is a Common Receptor for Heat Shock Proteins Gp96, Hsp90, Hsp70, and Calreticulin. Immunity 2001, 14, 303–313. [Google Scholar] [CrossRef]
- Binder, R.J.; Han, D.K.; Srivastava, P.K. Cd91: A Receptor for Heat Shock Protein Gp96. Nat. Immunol. 2000, 1, 151–155. [Google Scholar] [CrossRef]
- Binder, R.J.; Srivastava, P.K. Essential Role of Cd91 in Re-Presentation of gp96-Chaperoned Peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 6128–6133. [Google Scholar] [CrossRef]
- Oizumi, S.; Strbo, N.; Pahwa, S.; Deyev, V.; Podack, E.R. Molecular and Cellular Requirements for Enhanced Antigen Cross-Presentation to Cd8 Cytotoxic T lymphocytes. J. Immunol. 2007, 179, 2310–2317. [Google Scholar] [CrossRef]
- Singh-Jasuja, H.; Toes, R.E.; Spee, P.; Munz, C.; Hilf, N.; Schoenberger, S.P.; Ricciardi-Castagnoli, P.; Neefjes, J.; Rammensee, H.G.; Arnold-Schild, D.; et al. Cross-Presentation of Glycoprotein 96-Associated Antigens on Major Histocompatibility Complex Class I Molecules Requires Receptor-Mediated Endocytosis. J. Exp. Med. 2000, 191, 1965–1974. [Google Scholar] [CrossRef]
- Strbo, N.; Oizumi, S.; Sotosek-Tokmadzic, V.; Podack, E.R. Perforin is Required for Innate and Adaptive Immunity Induced by Heat Shock Protein Gp96. Immunity 2003, 18, 381–390. [Google Scholar] [CrossRef]
- Arnold, D.; Faath, S.; Rammensee, H.; Schild, H. Cross-Priming of Minor Histocompatibility Antigen-Specific Cytotoxic T Cells Upon Immunization with the Heat Shock Protein Gp96. J. Exp. Med. 1995, 182, 885–889. [Google Scholar] [CrossRef]
- Singh-Jasuja, H.; Scherer, H.U.; Hilf, N.; Arnold-Schild, D.; Rammensee, H.G.; Toes, R.E.; Schild, H. The Heat Shock Protein Gp96 Induces Maturation of Dendritic Cells and Down-Regulation of Its Receptor. Eur. J. Immunol. 2000, 30, 2211–2215. [Google Scholar] [CrossRef]
- Tobian, A.A.; Canaday, D.H.; Boom, W.H.; Harding, C.V. Bacterial Heat Shock Proteins Promote Cd91-Dependent Class I MHC Cross-Presentation of Chaperoned Peptide to Cd8+ T Cells by Cytosolic Mechanisms in Dendritic Cells Versus Vacuolar Mechanisms in Macrophages. J. Immunol. 2004, 172, 5277–5286. [Google Scholar] [CrossRef]
- Suto, R.; Srivastava, P.K. A Mechanism for the Specific Immunogenicity of Heat Shock Protein-Chaperoned Peptides. Science 1995, 269, 1585–1588. [Google Scholar] [CrossRef]
- Schirmbeck, R.; Bohm, W.; Reimann, J. Stress Protein (Hsp73)-Mediated, TAP-Independent Processing of Endogenous, Truncated Sv40 Large T Antigen for Db-Restricted Peptide Presentation. Eur. J. Immunol. 1997, 27, 2016–2023. [Google Scholar] [CrossRef]
- Schirmbeck, R.; Reimann, J. Peptide Transporter-Independent, Stress Protein-Mediated Endosomal Processing of Endogenous Protein Antigens for Major Histocompatibility Complex Class I Presentation. Eur. J. Immunol. 1994, 24, 1478–1486. [Google Scholar] [CrossRef]
- He, W.; Gea-Mallorqui, E.; Colin-York, H.; Fritzsche, M.; Gillespie, G.M.; Brackenridge, S.; Borrow, P.; McMichael, A.J. Intracellular Trafficking of Hla-E and ITS Regulation. J. Exp. Med. 2023, 220, e20221941. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current concepts of antigen cross-presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar]
- Kinner-Bibeau, L.B.; Sedlacek, A.L.; Messmer, M.N.; Watkins, S.C.; Binder, R.J. Hsps Drive Dichotomous T-Cell Immune Responses via DNA Methylome Remodelling in Antigen Presenting Cells. Nat. Commun. 2017, 8, 15648. [Google Scholar] [CrossRef]
- Broere, F.; van der Zee, R.; van Eden, W. Heat Shock Proteins are no Damps, Rather ‘DAMPERs’. Nat. Rev. Immunol. 2011, 11, 565, author reply 565. [Google Scholar] [CrossRef]
- Chandawarkar, R.Y.; Wagh, M.S.; Kovalchin, J.T.; Srivastava, P. Immune Modulation with High-Dose Heat-Shock Protein Gp96: Therapy of Murine Autoimmune Diabetes and Encephalomyelitis. Int. Immunol. 2004, 16, 615–624. [Google Scholar] [CrossRef]
- Chandawarkar, R.Y.; Wagh, M.S.; Srivastava, P.K. The Dual Nature of Specific Immunological Activity of Tumor-Derived Gp96 Preparations. J. Exp. Med. 1999, 189, 1437–1442. [Google Scholar] [CrossRef]
- Kovalchin, J.T.; Mendonca, C.; Wagh, M.S.; Wang, R.; Chandawarkar, R.Y. In Vivo Treatment of Mice with heat Shock Protein, Gp 96, Improves Survival of Skin Grafts with Minor and Major Antigenic Disparity. Transpl. Immunol. 2006, 15, 179–185. [Google Scholar] [CrossRef]
- Kovalchin, J.T.; Murthy, A.S.; Horattas, M.C.; Guyton, D.P.; Chandawarkar, R.Y. Determinants of Efficacy of Immunotherapy with Tumor-Derived Heat Shock Protein Gp96. Cancer Immun. 2001, 1, 7. [Google Scholar]
- Li, X.; Liu, Z.; Yan, X.; Zhang, X.; Li, Y.; Zhao, B.; Wang, S.; Zhou, X.; Gao, G.F.; Meng, S. Induction of Regulatory T Cells by High-Dose Gp96 Suppresses Murine Liver Immune Hyperactivation. PLoS ONE 2013, 8, e68997. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Qiu, L.; Zhang, X.; Chen, L.; Cao, S.; Wang, F.; Meng, S. Treg Suppress Ctl Responses upon Immunization with HSP Gp96. Eur. J. Immunol. 2009, 39, 3110–3120. [Google Scholar] [CrossRef]
- Srivastava, P.K.; DeLeo, A.B.; Old, L.J. Tumor Rejection Antigens of Chemically Induced Sarcomas of Inbred Mice. Proc. Natl. Acad. Sci. USA 1986, 83, 3407–3411. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nguyen, T.; Podack, E.R. Cutting Edge: Tumor Secreted Heat Shock-Fusion Protein Elicits Cd8 Cells for Rejection. J. Immunol. 1999, 163, 5178–5182. [Google Scholar]
- Aguilera, R.; Saffie, C.; Tittarelli, A.; Gonzalez, F.E.; Ramirez, M.; Reyes, D.; Pereda, C.; Hevia, D.; Garcia, T.; Salazar, L.; et al. Heat-Shock Induction of Tumor-Derived Danger Signals Mediates Rapid Monocyte Differentiation into Clinically Effective Dendritic Cells. Clin. Cancer Res. 2011, 17, 2474–2483. [Google Scholar] [CrossRef]
- Oizumi, S.; Deyev, V.; Yamazaki, K.; Schreiber, T.; Strbo, N.; Rosenblatt, J.; Podack, E.R. Surmounting Tumor-Induced Immune Suppression by Frequent Vaccination or Immunization in the Absence of B cells. J. Immunother. 2008, 31, 394–401. [Google Scholar] [CrossRef][Green Version]
- Strbo, N.; Pahwa, S.; Kolber, M.A.; Gonzalez, L.; Fisher, E.; Podack, E.R. Cell-Secreted Gp96-Ig-Peptide Complexes Induce Lamina Propria and Intraepithelial Cd8+ Cytotoxic T Lymphocytes in the Intestinal Mucosa. Mucosal. Immunol. 2010, 3, 182–192. [Google Scholar] [CrossRef]
- Morgensztern, D.; Bazhenova, L.; Waqar, S.N.; McDermott, L.; Hutchins, J.; Harb, W.; Pennell, N.; Cohen, R.B. Viagenpumatucel-L (HS-110) Plus Nivolumab in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer (NSCLC). Cancer Immunol. Res. 2020, 8, 101. [Google Scholar]
- Bedoui, S.; Whitney, P.G.; Waithman, J.; Eidsmo, L.; Wakim, L.; Caminschi, I.; Allan, R.S.; Wojtasiak, M.; Shortman, K.; Carbone, F.R.; et al. Cross-Presentation of Viral and Self Antigens by Skin-Derived Cd103+ Dendritic Cells. Nat. Immunol. 2009, 10, 488–495. [Google Scholar] [CrossRef]
- Beura, L.K.; Mitchell, J.S.; Thompson, E.A.; Schenkel, J.M.; Mohammed, J.; Wijeyesinghe, S.; Fonseca, R.; Burbach, B.J.; Hickman, H.D.; Vezys, V.; et al. Intravital Mucosal Imaging of Cd8(+) Resident Memory T Cells Shows Tissue-Autonomous Recall Responses That Amplify Secondary Memory. Nat. Immunol. 2018, 19, 173–182. [Google Scholar] [CrossRef]
- Park, S.L.; Zaid, A.; Hor, J.L.; Christo, S.N.; Prier, J.E.; Davies, B.; Alexandre, Y.O.; Gregory, J.L.; Russell, T.A.; Gebhardt, T.; et al. Local Proliferation Maintains a Stable Pool of Tissue-Resident Memory T Cells after Antiviral Recall Responses. Nat. Immunol. 2018, 19, 183–191. [Google Scholar] [CrossRef]
- Wakim, L.M.; Waithman, J.; van Rooijen, N.; Heath, W.R.; Carbone, F.R. Dendritic Cell-Induced Memory T Cell Activation in Nonlymphoid Tissues. Science 2008, 319, 198–202. [Google Scholar] [CrossRef]
- Wein, A.N.; McMaster, S.R.; Takamura, S.; Dunbar, P.R.; Cartwright, E.K.; Hayward, S.L.; McManus, D.T.; Shimaoka, T.; Ueha, S.; Tsukui, T.; et al. Cxcr6 Regulates Localization of Tissue-Resident Memory Cd8 T Cells to the Airways. J. Exp. Med. 2019, 216, 2748–2762. [Google Scholar] [CrossRef]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-Generated Lung Tissue-Resident Memory T Cells Provide Heterosubtypic Protection to Influenza Infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef]
- Cohen, R.B.; Peoples, G.E.; Kawashima, T.; Arana, B.; Cui, X.; Bazhenova, L.; Sanborn, R.E.; Harb, W.A.; Pennell, N.A.; Morgensztern, D. Interim Results of Viagenpumatucel-L (HS-110) Plus Nivolumab in Previously Treated Patients (pts) with Advanced Non-Small Cell Lung Cancer (NSCLC) in Two Treatment Settings. J. Clin. Oncol. 2021, 39, 9100. [Google Scholar] [CrossRef]
- Jacqueline, C.; Dracz, M.; Xue, J.; Binder, R.J.; Minden, J.; Finn, O. LCVM Infection Generates Tumor Antigen-Specific Immunity and Inhibits Growth of Nonviral Tumors. Oncoimmunology 2022, 11, 2029083. [Google Scholar] [CrossRef]
- Iheagwara, U.K.; Beatty, P.L.; Van, P.T.; Ross, T.M.; Minden, J.S.; Finn, O.J. Influenza Virus Infection Elicits Protective Antibodies and T Cells Specific for Host Cell Antigens Also Expressed as Tumor-Associated Antigens: A New View of Cancer Immunosurveillance. Cancer Immunol. Res. 2014, 2, 263–273. [Google Scholar] [CrossRef]
- Brewer, B.G.; Mitchell, R.A.; Harandi, A.; Eaton, J.W. Embryonic Vaccines against Cancer: An Early History. Exp. Mol. Pathol. 2009, 86, 192–197. [Google Scholar] [CrossRef]
- Kropp, L.E.; Garg, M.; Binder, R.J. Ovalbumin-Derived Precursor Peptides are Transferred Sequentially from Gp96 and Calreticulin to MHC Class I in the Endoplasmic Reticulum. J. Immunol. 2010, 184, 5619–5627. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Wu, B.; Liu, S.; Wu, E.; Fan, H.; Gui, M.; Chen, L.; Li, C.; Ju, Y.; et al. Placenta-Derived Gp96 as a Multivalent Prophylactic Cancer Vaccine. Sci. Rep. 2013, 3, 1947. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, L.; Zhang, H.; Kan, F.; Wang, S.; Li, Y.; Tian, H.; Meng, S. Dendritic Cells Pulsed with Placental Gp96 Promote Tumor-Reactive Immune Responses. PLoS ONE 2019, 14, e0211490. [Google Scholar] [CrossRef]
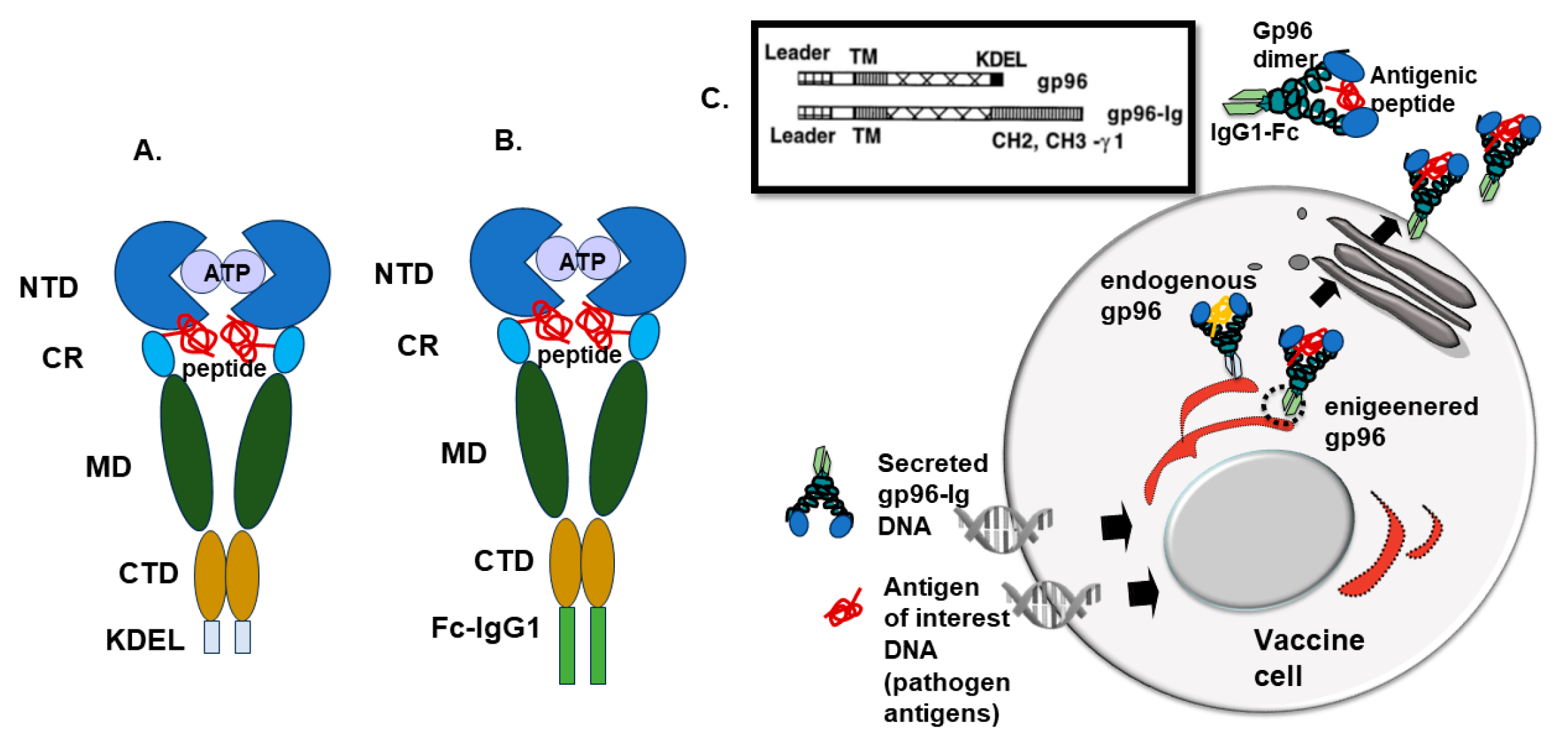
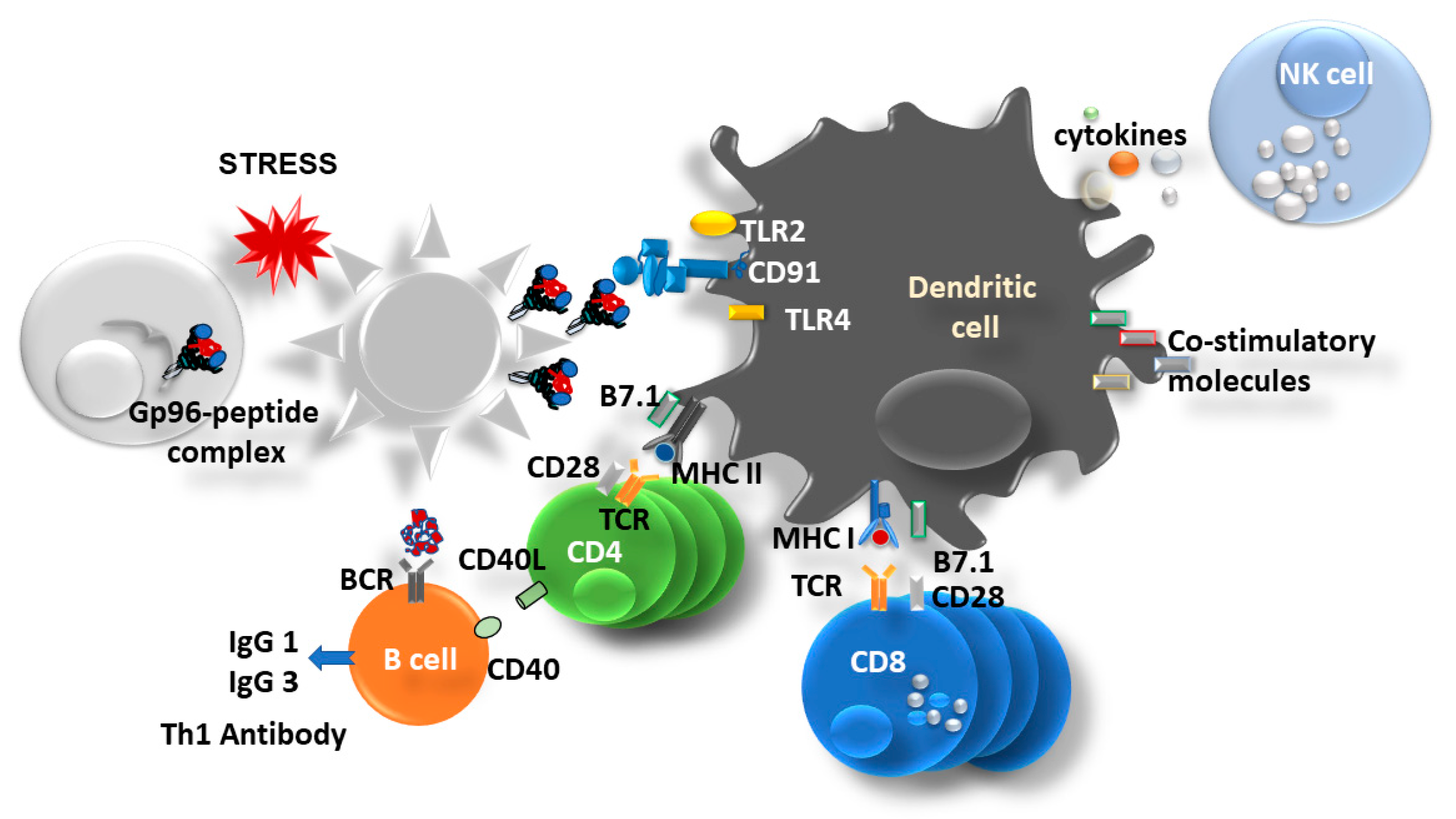
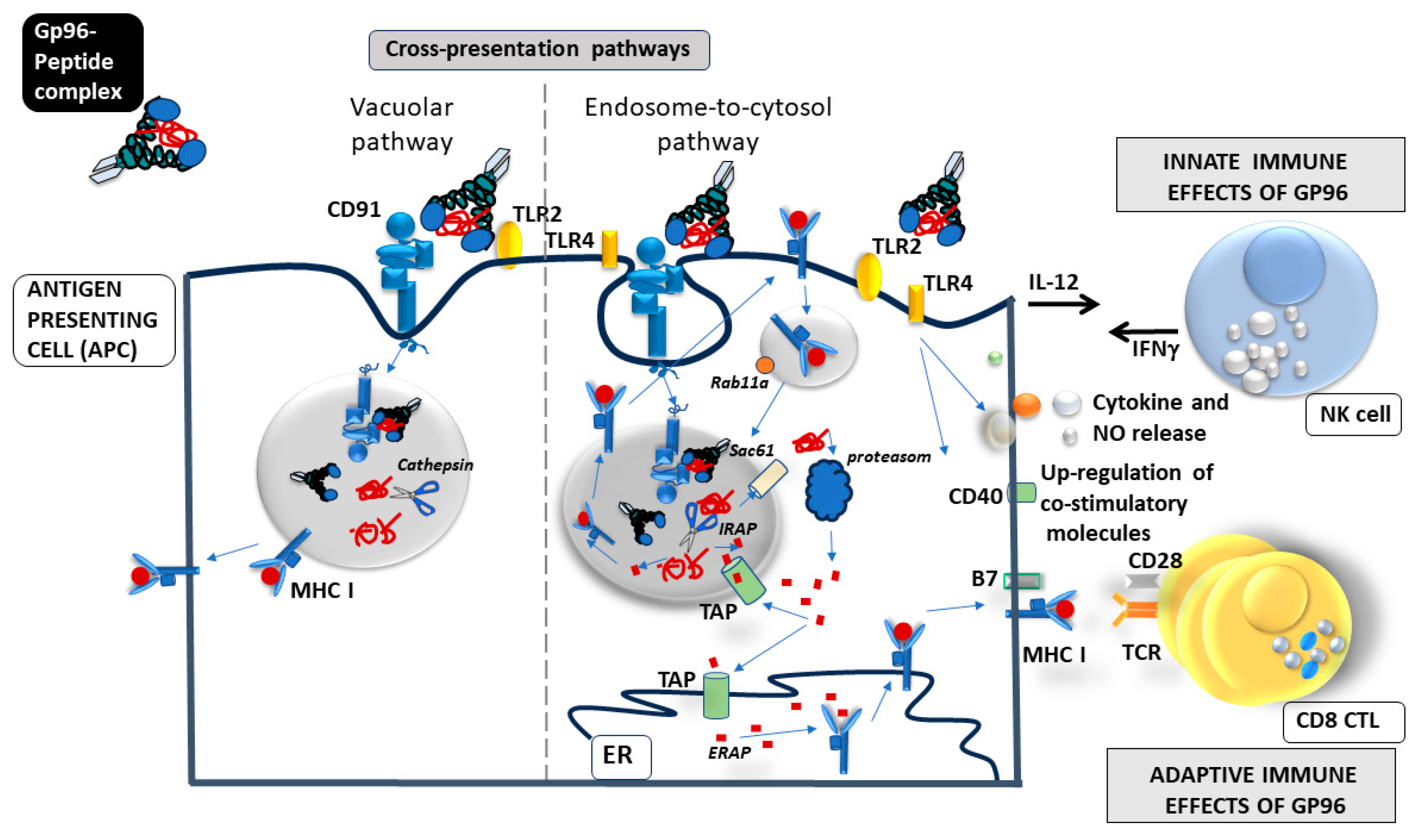
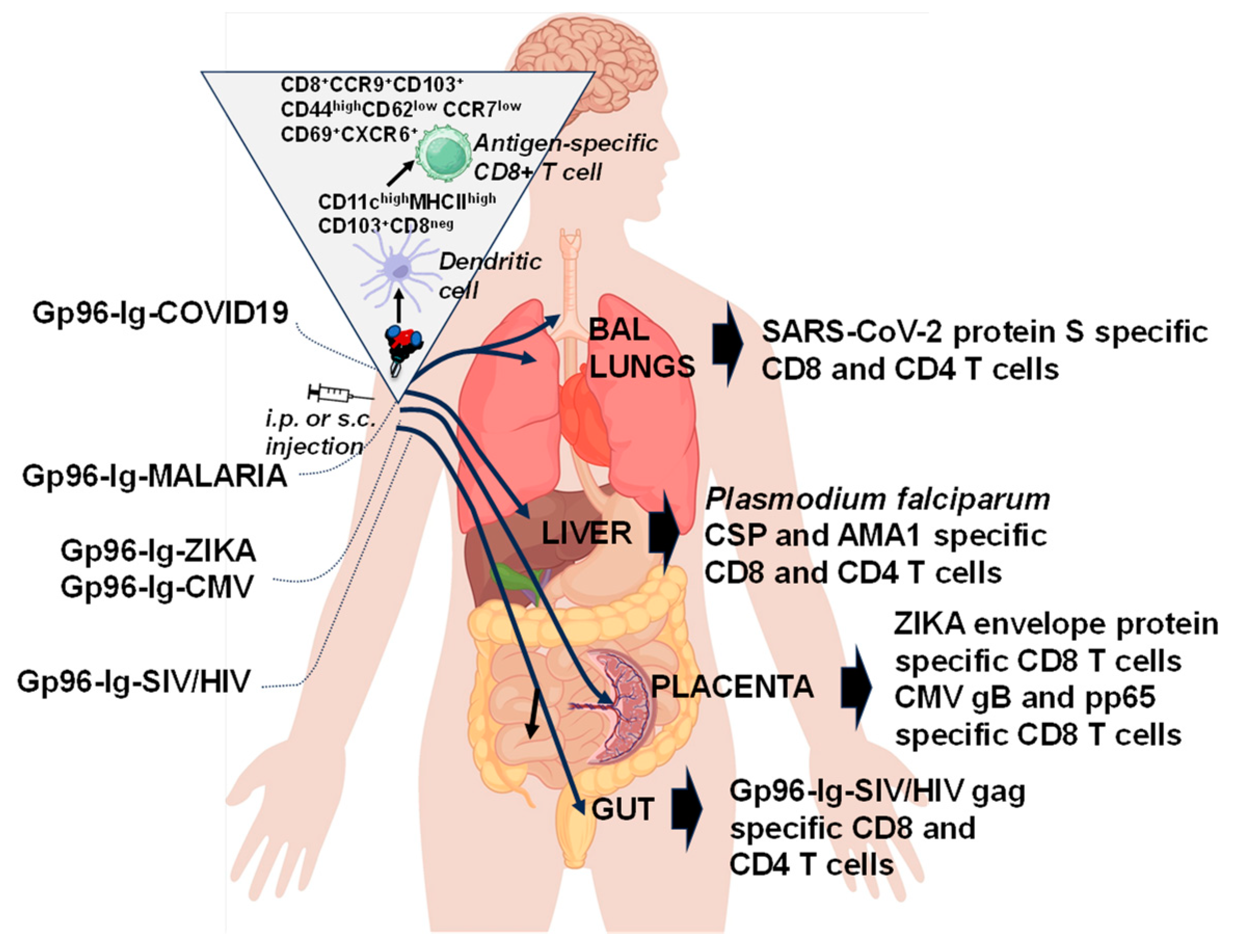
| Vaccine | Gp96-Ig Concentration * | Co-Expressed Antigens (Full-Length Proteins) | Vaccine Induced Immune Responses |
|---|---|---|---|
| SIV/HIV vaccine | 1 μg/mL | SIV/HIV gag | Blood |
| SIV/HIV ReTaNef | Rectal and vaginal lamina propria | ||
| SIV/HIV gp120 | Rectal and Vaginal Epithelial [3,4] | ||
| Malaria vaccine | 2 μg/mL | Plasmodium falciparum CSP | Blood Liver [9] |
| Plasmodium falciparum AMA1 | |||
| Zika vaccine | 0.5 μg/mL | Zika Pre-membrane Envelop | Blood Spleen Placenta |
| Decidua [5,6] | |||
| CMV vaccine | 0.5–1 μg/mL | Human cytomegalo virus (HCMV) gBpp65 | Blood Spleen Placenta Decidua [6] |
| 1 μg/mL | SARS-CoV-2- protein S | Blood Spleen Lungs Air ducts [7,8] | |
| COVID-19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padula, L.; Fisher, E.; Strbo, N. “All for One and One for All”: The Secreted Heat Shock Protein gp96-Ig Based Vaccines. Cells 2024, 13, 72. https://doi.org/10.3390/cells13010072
Padula L, Fisher E, Strbo N. “All for One and One for All”: The Secreted Heat Shock Protein gp96-Ig Based Vaccines. Cells. 2024; 13(1):72. https://doi.org/10.3390/cells13010072
Chicago/Turabian StylePadula, Laura, Eva Fisher, and Natasa Strbo. 2024. "“All for One and One for All”: The Secreted Heat Shock Protein gp96-Ig Based Vaccines" Cells 13, no. 1: 72. https://doi.org/10.3390/cells13010072
APA StylePadula, L., Fisher, E., & Strbo, N. (2024). “All for One and One for All”: The Secreted Heat Shock Protein gp96-Ig Based Vaccines. Cells, 13(1), 72. https://doi.org/10.3390/cells13010072






