The Role of Apoptosis and Oxidative Stress in a Cell Spheroid Model of Calcific Aortic Valve Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Spheroid Formation and Media Supplementation
2.3. Calcium Staining
2.4. Cell Viability
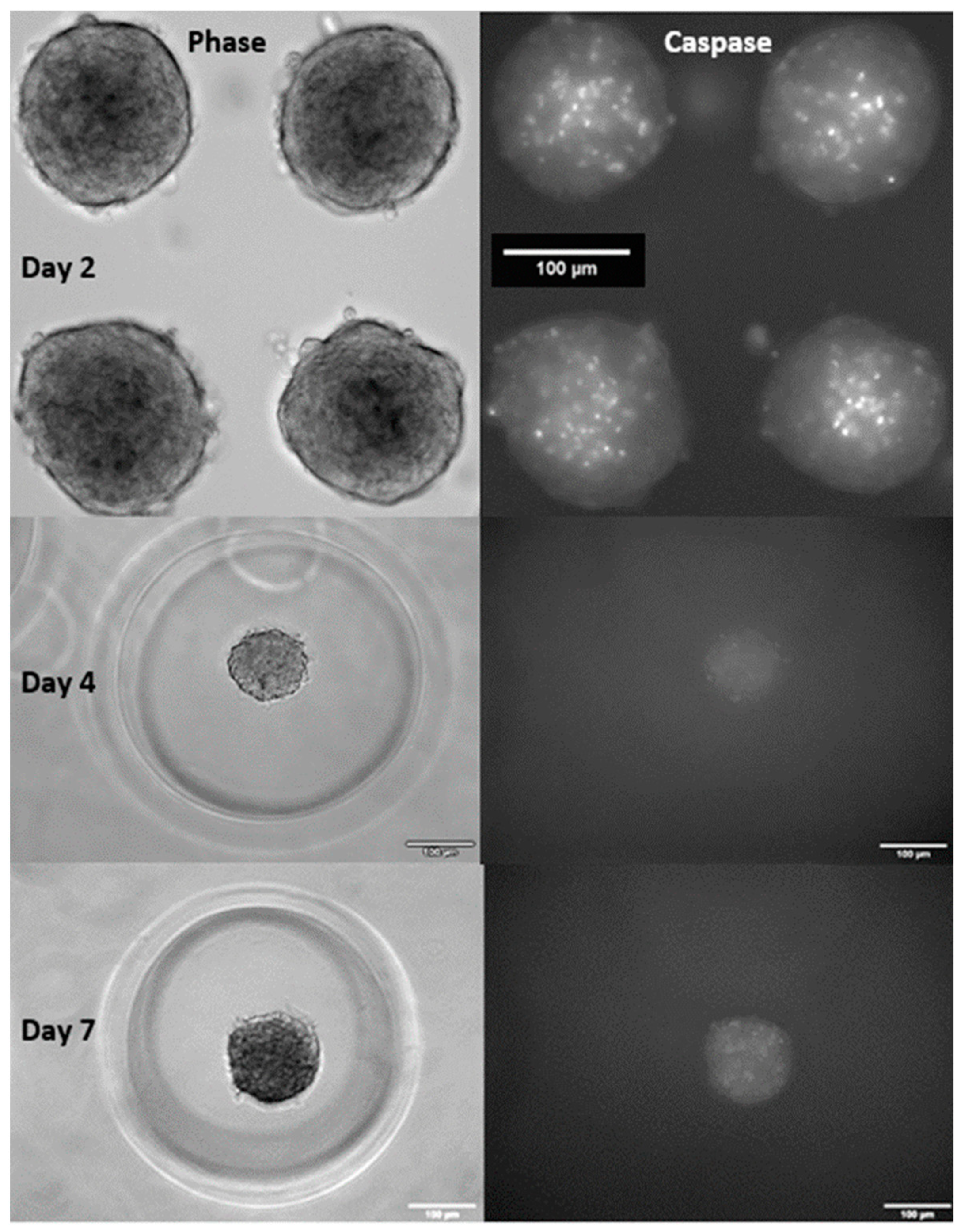
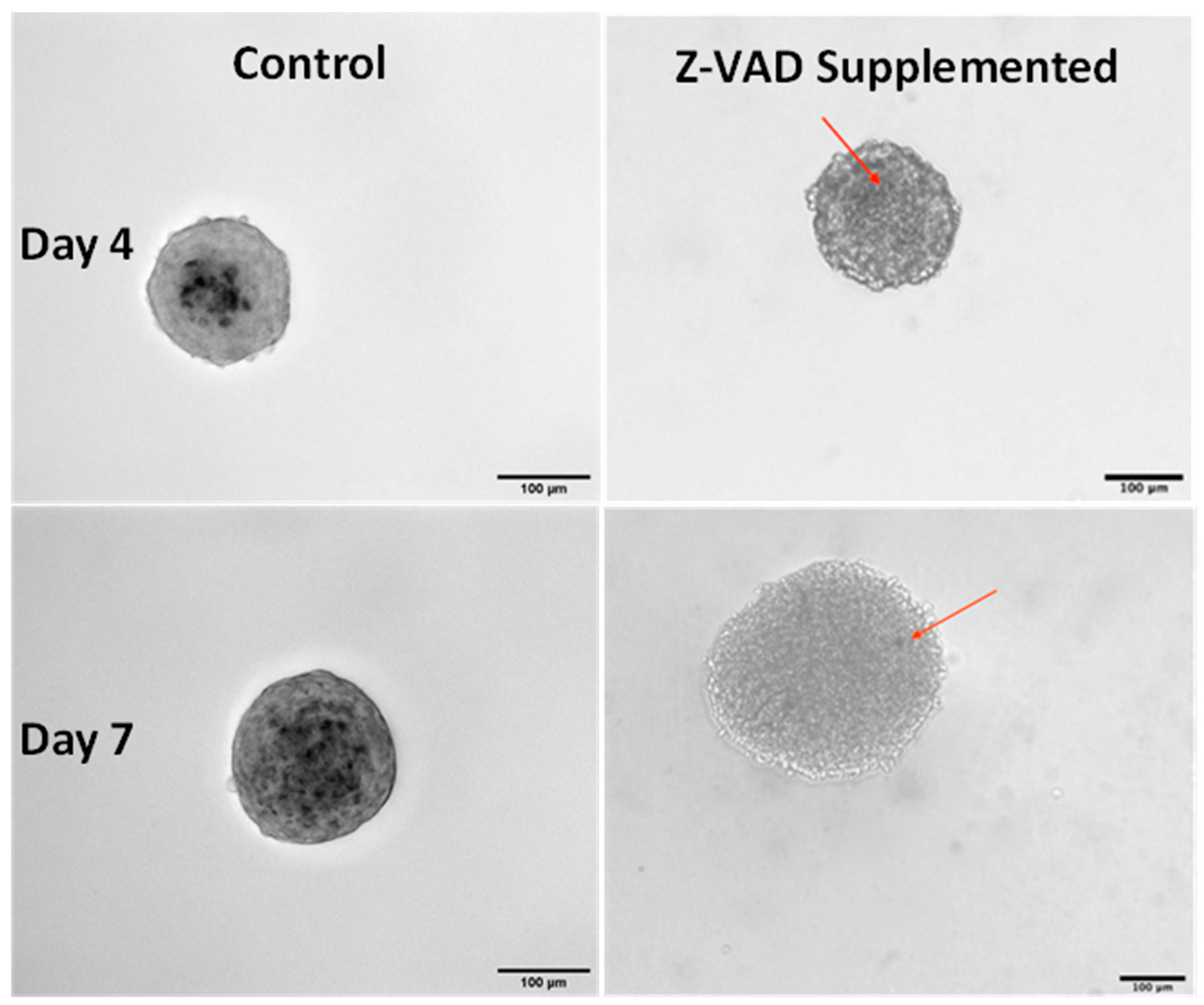
2.5. Measuring and Blocking Apoptosis
2.6. Inducing Oxidative Stress
2.7. Statistical Analysis
3. Results
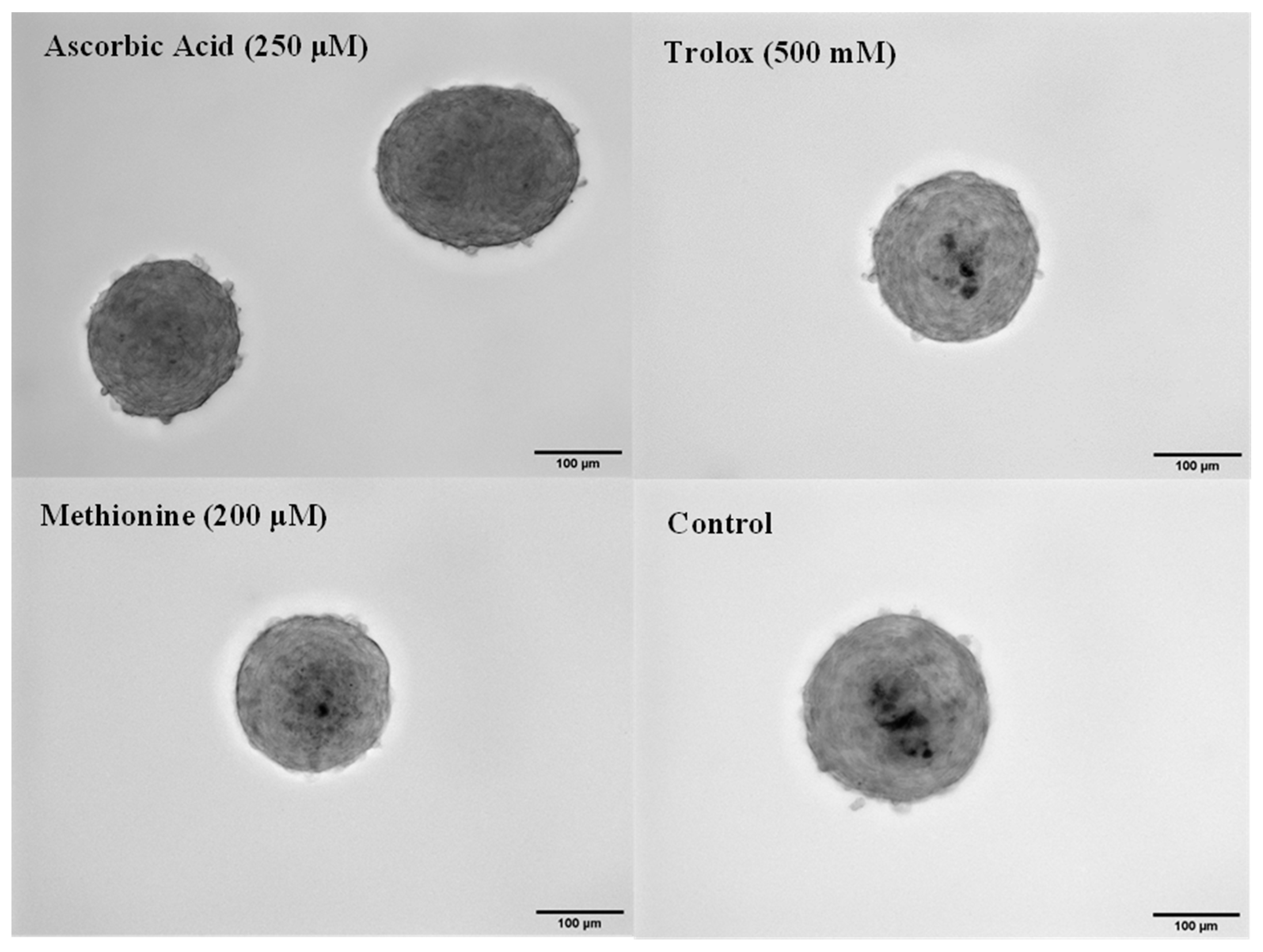
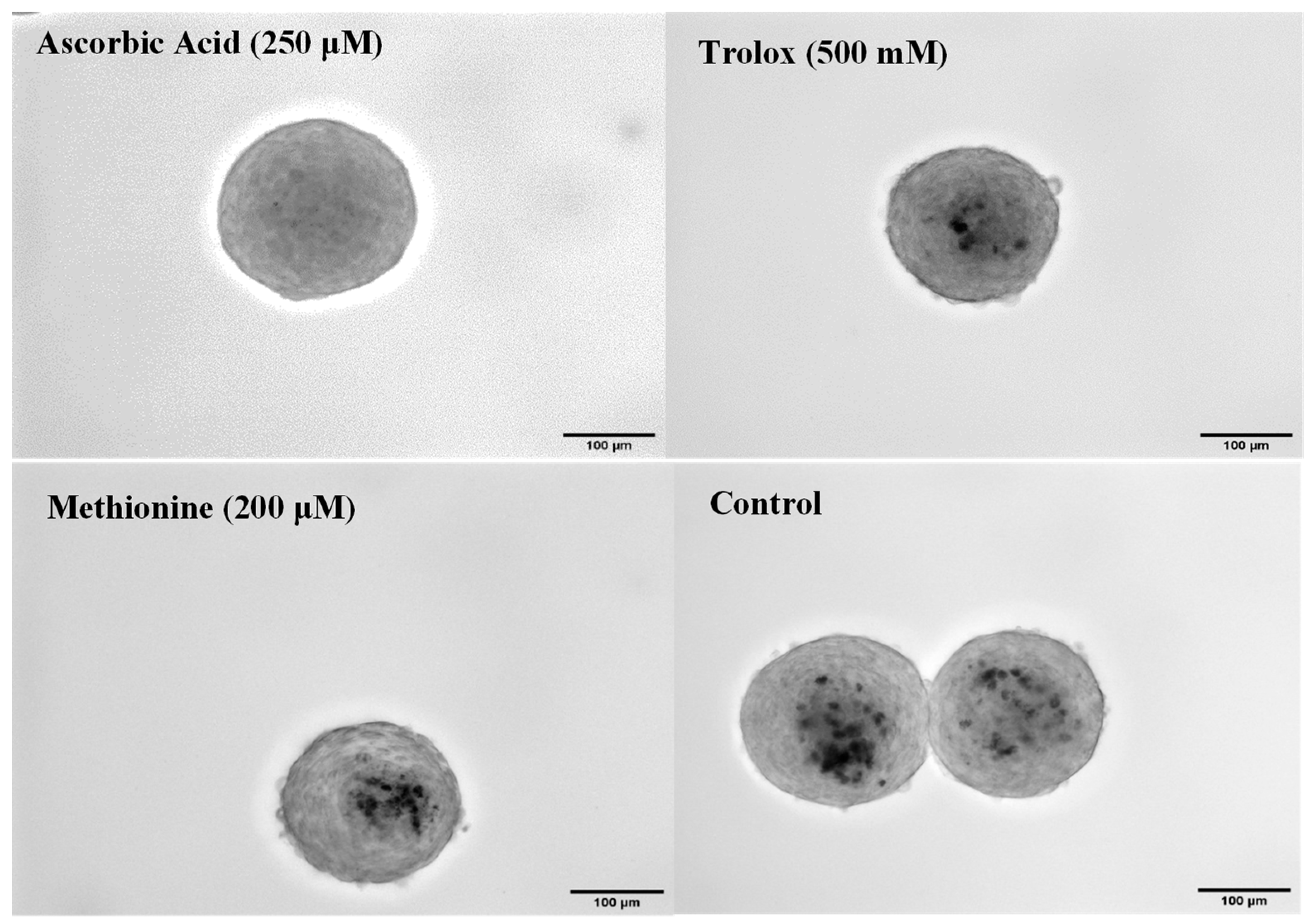
3.1. Effect of Ascorbic Acid Supplementation on Spheroids
3.2. Effect of Blocking Apoptosis with Z-VAD
3.3. Determination of Antioxidant Concentrations in 2D Culture
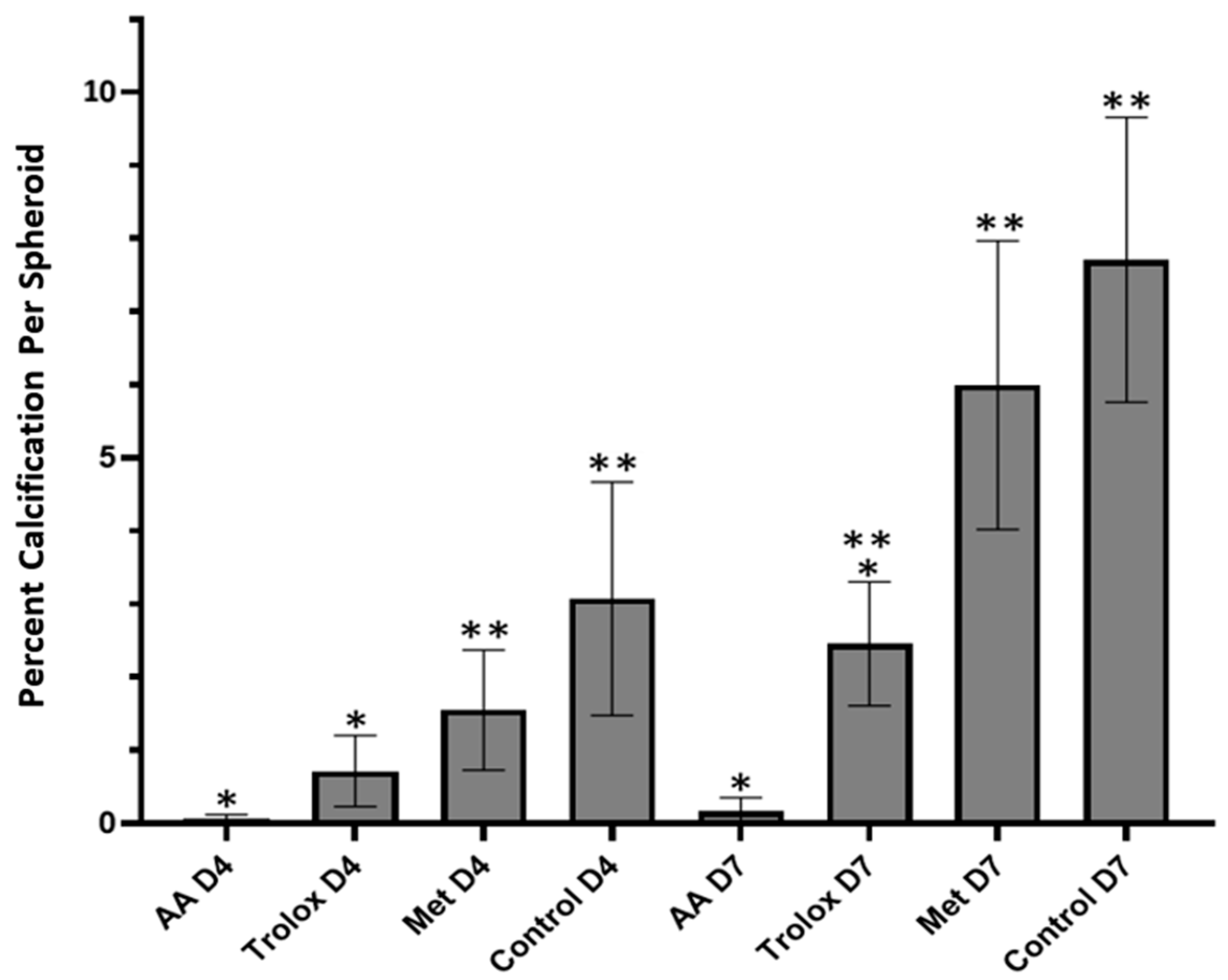
3.4. Antioxidant Effects in 3D Spheroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerman, D.A.; Prasad, S.; Alotti, N. Calcific Aortic Valve Disease: Molecular Mechanisms and Therapeutic Approaches. Eur. Cardiol. 2015, 10, 108–112. [Google Scholar] [CrossRef]
- Yutzey, K.E.; Demer, L.L.; Body, S.C.; Huggins, G.S.; Towler, D.A.; Giachelli, C.M.; Hofmann-Bowman, M.A.; Mortlock, D.P.; Rogers, M.B.; Sadeghi, M.M.; et al. Calcific aortic valve disease: A consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Desai, C.S.; Roselli, E.E.; Svensson, L.G.; Bonow, R.O. Transcatheter aortic valve replacement: Current status and future directions. Semin. Thorac. Cardiovasc. Surg. 2013, 25, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.R., 3rd; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.Y.; Chen, J.H.; Zhao, R.; Simmons, C.A. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Benton, J.A.; Kern, H.B.; Anseth, K.S. Substrate properties influence calcification in valvular interstitial cell culture. J. Heart Valve Dis. 2008, 17, 689–699. [Google Scholar] [PubMed]
- Jian, B.; Narula, N.; Li, Q.Y.; Mohler, E.R., 3rd; Levy, R.J. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 2003, 75, 457–465; discussion 465–466. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; Masters, K.S.; Shah, D.N.; Anseth, K.S.; Leinwand, L.A. Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ. Res. 2004, 95, 253–260. [Google Scholar] [CrossRef]
- Fisher, C.I.; Chen, J.; Merryman, W.D. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech. Model Mechanobiol. 2013, 12, 5–17. [Google Scholar] [CrossRef]
- Cirka, H.A.; Uribe, J.; Liang, V.; Schoen, F.J.; Billiar, K.L. Reproducible in vitro model for dystrophic calcification of cardiac valvular interstitial cells: Insights into the mechanisms of calcific aortic valvular disease. Lab Chip 2017, 17, 814–829. [Google Scholar] [CrossRef]
- Duan, B.; Yin, Z.; Kang, L.H.; Magin, R.L.; Butcher, J.T. Active tissue stiffness modulation controls valve interstitial cell phenotype and osteogenic potential in 3D culture. Acta Biomater. 2016, 36, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Mabry, K.M.; Lawrence, R.L.; Anseth, K.S. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 2015, 49, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hjorntaes, J.; Goettsch, C.; Hutcheson, J.; Camci-Unal, G.; Lax, L.; Sherer, K.; Body, S.; Schoen, F.; Kluin, J.; Khademhosseini, A.; et al. Simulation of Early Calcific Aortic Valve Disease in a 3D Platform: A Role for Myofibroblast Differentiation. J. Or Mol. Cell. Cardiol. 2016, 94, 13–20. [Google Scholar]
- Monroe, M.N.; Nikonowicz, R.C.; Grande-Allen, K.J. Heterogeneous multi-laminar tissue constructs as a platform to evaluate aortic valve matrix-dependent pathogenicity. Acta Biomater. 2019, 97, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Roosens, A.; Puype, I.; Cornelissen, R. Scaffold-free high throughput generation of quiescent valvular microtissues. J. Mol. Cell. Cardiol. 2017, 106, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Antioxidants suppress apoptosis. J. Nutr. 2004, 134, 3179S–3180S. [Google Scholar] [CrossRef]
- Quinlan, A.M.; Billiar, K.L. Investigating the role of substrate stiffness in the persistence of valvular interstitial cell activation. J. Biomed. Mater. Res. A 2012, 100, 2474–2482. [Google Scholar] [CrossRef]
- Koziel, R.; Ruckenstuhl, C.; Albertini, E.; Neuhaus, M.; Netzberger, C.; Bust, M.; Madeo, F.; Wiesner, R.J.; Jansen-Durr, P. Methionine restriction slows down senescence in human diploid fibroblasts. Aging Cell 2014, 13, 1038–1048. [Google Scholar] [CrossRef]
- Gibson, G.E.; Zhang, H.; Sheu, K.R.; Park, L.C. Differential alterations in antioxidant capacity in cells from Alzheimer patients. Biochim. Biophys. Acta 2000, 1502, 319–329. [Google Scholar] [CrossRef]
- Deegan, A.J.; Aydin, H.M.; Hu, B.; Konduru, S.; Kuiper, J.H.; Yang, Y. A facile in vitro model to study rapid mineralization in bone tissues. Biomed. Eng. Online 2014, 13, 136. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Cullen, S.P.; Sheridan, C.; Luthi, A.U.; Gerner, C.; Martin, S.J. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc. Natl. Acad. Sci. USA 2008, 105, 12815–12819. [Google Scholar] [CrossRef] [PubMed]
- Pienkowska, N.; Bartosz, G.; Pichla, M.; Grzesik-Pietrasiewicz, M.; Gruchala, M.; Sadowska-Bartosz, I. Effect of antioxidants on the H2O2-induced premature senescence of human fibroblasts. Aging 2020, 12, 1910–1927. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Farzaneh-Far, A.; Shanahan, C.M.; Weissberg, P.L. The role of apoptosis in the initiation of vascular calcification. Z. Kardiol. 2001, 90 (Suppl. S3), 43–46. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Skepper, J.N.; Hegyi, L.; Bennett, M.R.; Shanahan, C.M.; Weissberg, P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000, 87, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Masters, K.S. Role of the MAPK/ERK pathway in valvular interstitial cell calcification. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1748–H1757. [Google Scholar] [CrossRef]
- Fujita, H.; Yamamoto, M.; Ogino, T.; Kobuchi, H.; Ohmoto, N.; Aoyama, E.; Oka, T.; Nakanishi, T.; Inoue, K.; Sasaki, J. Necrotic and apoptotic cells serve as nuclei for calcification on osteoblastic differentiation of human mesenchymal stem cells in vitro. Cell Biochem. Funct. 2014, 32, 77–86. [Google Scholar] [CrossRef]
- Mathieu, P.; Bouchareb, R.; Boulanger, M.C. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. J. Immunol. Res. 2015, 2015, 851945. [Google Scholar] [CrossRef]
- Galeone, A.; Brunetti, G.; Oranger, A.; Greco, G.; Di Benedetto, A.; Mori, G.; Colucci, S.; Zallone, A.; Paparella, D.; Grano, M. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int. J. Cardiol. 2013, 169, 296–304. [Google Scholar] [CrossRef]
- Hutson, H.N.; Marohl, T.; Anderson, M.; Eliceiri, K.; Campagnola, P.; Masters, K.S. Calcific Aortic Valve Disease Is Associated with Layer-Specific Alterations in Collagen Architecture. PLoS ONE 2016, 11, e0163858. [Google Scholar] [CrossRef]
- Fondard, O.; Detaint, D.; Iung, B.; Choqueux, C.; Adle-Biassette, H.; Jarraya, M.; Hvass, U.; Couetil, J.P.; Henin, D.; Michel, J.B.; et al. Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 2005, 26, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, H.A.; Satta, J.; Risteli, J.; Veijola, M.; Vare, P.; Soini, Y. Type I and type III collagen synthesis and composition in the valve matrix in aortic valve stenosis. Atherosclerosis 2006, 189, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.J.; Piechura, L.M.; Porras, A.M.; Masters, K.S. Manipulation of valve composition to elucidate the role of collagen in aortic valve calcification. BMC Cardiovasc. Disord. 2014, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar] [PubMed]
- Rodriguez, K.J.; Masters, K.S. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J. Biomed. Mater. Res. A 2009, 90, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Saiz, A.M., Jr.; Leach, J.K. Engineered Cell-Secreted Extracellular Matrix Modulates Cell Spheroid Mechanosensing and Amplifies Their Response to Inductive Cues for the Formation of Mineralized Tissues. Adv. Healthcare Mater. 2022, 11, e2102337. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Greenberg, H.Z.E.; Zhao, G.; Shah, A.M.; Zhang, M. Role of oxidative stress in calcific aortic valve disease and its therapeutic implications. Cardiovasc. Res. 2021, 118, 1433–1451. [Google Scholar] [CrossRef]
- Branchetti, E.; Sainger, R.; Poggio, P.; Grau, J.B.; Patterson-Fortin, J.; Bavaria, J.E.; Chorny, M.; Lai, E.; Gorman, R.C.; Levy, R.J.; et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, e66–e74. [Google Scholar] [CrossRef]
- Miller, J.D.; Chu, Y.; Brooks, R.M.; Richenbacher, W.E.; Pena-Silva, R.; Heistad, D.D. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J. Am. Coll. Cardiol. 2008, 52, 843–850. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, K.C. Antioxidants as an Epidermal Stem Cell Activator. Antioxidants 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.A.; Butcher, J.T. Isolation of valvular endothelial cells. J. Vis. Exp. 2010, 46, e2158. [Google Scholar]
- Levonen, A.L.; Vahakangas, E.; Koponen, J.K.; Yla-Herttuala, S. Antioxidant gene therapy for cardiovascular disease: Current status and future perspectives. Circulation 2008, 117, 2142–2150. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutts, C.W.; Baldwin, A.M.; Jebeli, M.; Jolin, G.E.; Mungai, R.W.; Billiar, K.L. The Role of Apoptosis and Oxidative Stress in a Cell Spheroid Model of Calcific Aortic Valve Disease. Cells 2024, 13, 45. https://doi.org/10.3390/cells13010045
Coutts CW, Baldwin AM, Jebeli M, Jolin GE, Mungai RW, Billiar KL. The Role of Apoptosis and Oxidative Stress in a Cell Spheroid Model of Calcific Aortic Valve Disease. Cells. 2024; 13(1):45. https://doi.org/10.3390/cells13010045
Chicago/Turabian StyleCoutts, Colin W., Ashley M. Baldwin, Mahvash Jebeli, Grace E. Jolin, Rozanne W. Mungai, and Kristen L. Billiar. 2024. "The Role of Apoptosis and Oxidative Stress in a Cell Spheroid Model of Calcific Aortic Valve Disease" Cells 13, no. 1: 45. https://doi.org/10.3390/cells13010045
APA StyleCoutts, C. W., Baldwin, A. M., Jebeli, M., Jolin, G. E., Mungai, R. W., & Billiar, K. L. (2024). The Role of Apoptosis and Oxidative Stress in a Cell Spheroid Model of Calcific Aortic Valve Disease. Cells, 13(1), 45. https://doi.org/10.3390/cells13010045





