Thrombin, a Key Driver of Pathological Inflammation in the Brain
Abstract
1. Neuroinflammation: A Common Mechanism in Neurodegenerative Diseases
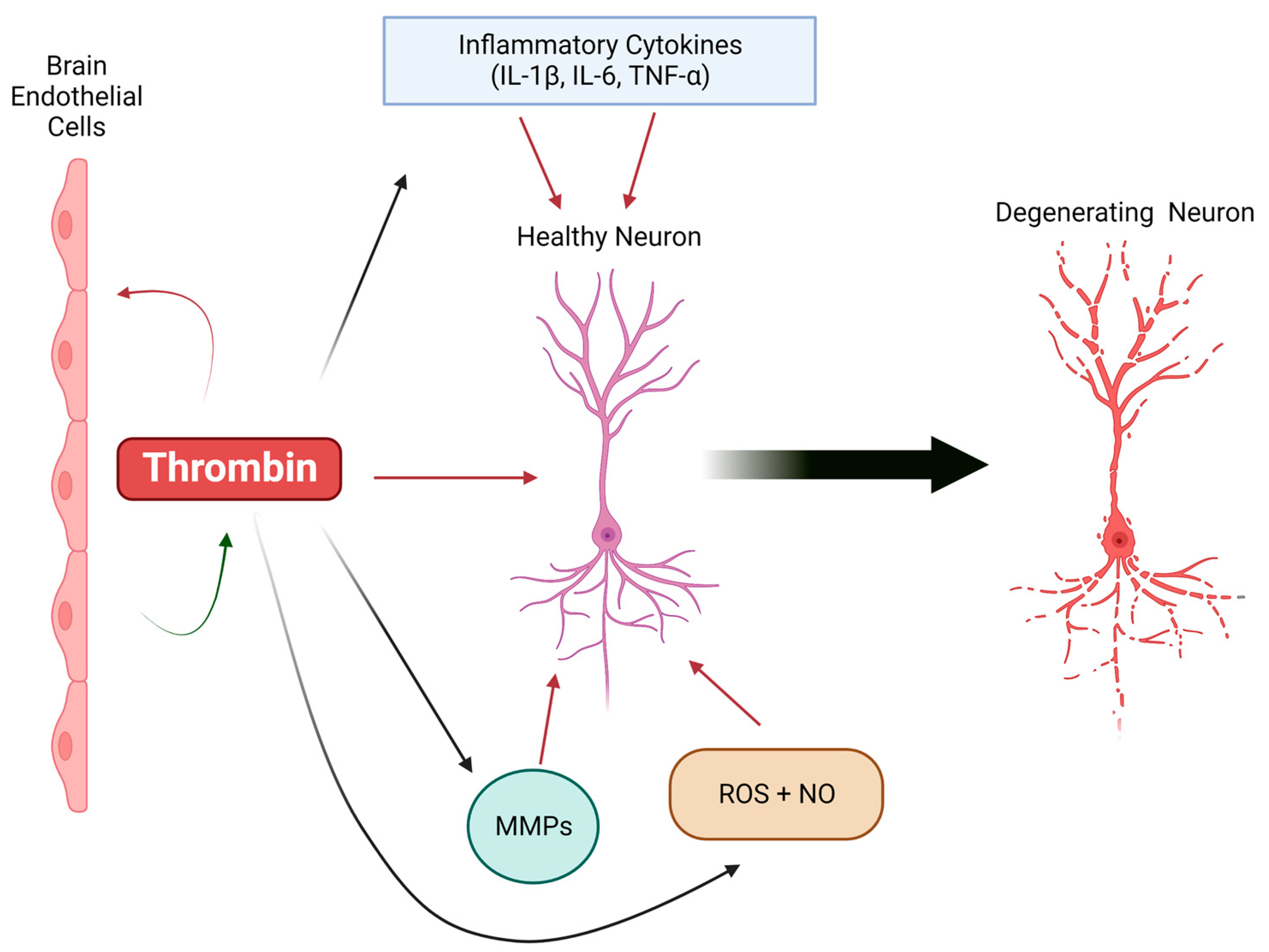
2. Thrombin, a Key Driver of Neuroinflammation
3. Thrombin-Mediated Inflammation: A Shared Mechanism among AD Risk Factors
3.1. Atherosclerosis
3.2. Diabetes
3.3. Traumatic Brain Injury (TBI)
4. Cellular Effects of Thrombin in the Brain: Implications for AD
4.1. Microglia
4.2. Astrocytes
4.3. Endothelial Cells
5. Thrombin: A Novel Inflammatory Therapeutic Target in AD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.L.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; Desai, R.; et al. Burden of Neurological Disorders across the US from 1990–2017: A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2017, 145, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Akiyama, H. Inflammatory response in Alzheimer’s disease. Tohoku J. Exp. Med. 1994, 174, 295–303. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.H. Chemokine receptors in the central nervous system: Role in brain inflammation and neurodegenerative diseases. Brain Res. Rev. 2005, 48, 16–42. [Google Scholar] [CrossRef]
- Hauss-Wegrzyniak, B.; Dobrzanski, P.; Stoehr, J.D.; Wenk, G.L. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998, 780, 294–303. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Picciano, M.; Malester, B.; LaFrancois, J.; Zehr, C.; Daeschner, J.M.; Olschowka, J.A.; Fonseca, M.I.; O’Banion, M.K.; Tenner, A.J.; et al. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. Am. J. Pathol. 2001, 158, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Lutshumba, J.; Nikolajczyk, B.S.; Bachstetter, A.D. Dysregulation of Systemic Immunity in Aging and Dementia. Front. Cell. Neurosci. 2021, 15, 652111. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 276. [Google Scholar] [CrossRef]
- Jones, L.; Holmans, P.A.; Hamshere, M.L.; Harold, D.; Moskvina, V.; Ivanov, D.; Pocklington, A.; Abraham, R.; Hollingworth, P.; Sims, R.; et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS ONE 2010, 5, e13950. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef]
- Evola, M.; Hall, A.; Wall, T.; Young, A.; Grammas, P. Oxidative stress impairs learning and memory in apoE knockout mice. Pharmacol. Biochem. Behav. 2010, 96, 181–186. [Google Scholar] [CrossRef]
- Tripathy, D.; Sanchez, A.; Yin, X.; Luo, J.; Martinez, J.; Grammas, P. Thrombin, a mediator of cerebrovascular inflammation in AD and hypoxia. Front. Aging Neurosci. 2013, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, N.; Soleimani, A.; Pashirzad, M.; Abdeahad, H.; Mohammadi, F.; Khoshakhlagh, M.; Khazaei, M.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Role of thrombin in the pathogenesis of atherosclerosis. J. Cell. Biochem. 2019, 120, 4757–4765. [Google Scholar] [CrossRef] [PubMed]
- Kalz, J.; ten Cate, H.; Spronk, H.M. Thrombin generation and atherosclerosis. J. Thromb. Thrombolysis 2014, 37, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ay, L.; Hoellerl, F.; Ay, C.; Brix, J.M.; Koder, S.; Schernthaner, G.H.; Pabinger, I.; Schernthaner, G. Thrombin generation in type 2 diabetes with albuminuria and macrovascular disease. Eur. J. Clin. Investig. 2012, 42, 470–477. [Google Scholar] [CrossRef]
- Itsekson-Hayosh, Z.; Shavit-Stein, E.; Katzav, A.; Rubovitch, V.; Maggio, N.; Chapman, J.; Harnof, S.; Pick, C.G. Minimal Traumatic Brain Injury in Mice: Protease-Activated Receptor 1 and Thrombin-Related Changes. J. Neurotrauma 2016, 33, 1848–1854. [Google Scholar] [CrossRef]
- Akiyama, H.; Ikeda, K.; Kondo, H.; McGeer, P.L. Thrombin accumulation in brains of patients with Alzheimer’s disease. Neurosci. Lett. 1992, 146, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P.; Ovase, R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol. Aging 2001, 22, 837–842. [Google Scholar] [CrossRef]
- Frohman, E.M.; Frohman, T.C.; Gupta, S.; de Fougerolles, A.; van den Noort, S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J. Neurol. Sci. 1991, 106, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.A.; Kumar, P.; Grammas, P. Expression of CAP37, a novel inflammatory mediator, in Alzheimer’s disease. Neurobiol. Aging 1996, 17, 753–759. [Google Scholar] [CrossRef]
- Yin, X.; Wright, J.; Wall, T.; Grammas, P. Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer’s disease. Am. J. Pathol. 2010, 176, 1600–1606. [Google Scholar] [CrossRef]
- Grammas, P.; Tripathy, D.; Sanchez, A.; Yin, X.; Luo, J. Brain microvasculature and hypoxia-related proteins in Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2011, 4, 616–627. [Google Scholar] [PubMed]
- Grammas, P. Neurovascular dysfunction, inflammation and endothelial activation: Implications for the pathogenesis of Alzheimer’s disease. J. Neuroinflamm. 2011, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The Coagulation Factors Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders—A Systematic Review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef]
- Coughlin, S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005, 3, 1800–1814. [Google Scholar] [CrossRef]
- Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 2000, 407, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Shavit Stein, E.; Ben Shimon, M.; Artan Furman, A.; Golderman, V.; Chapman, J.; Maggio, N. Thrombin Inhibition Reduces the Expression of Brain Inflammation Markers upon Systemic LPS Treatment. Neural Plast. 2018, 2018, 7692182. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Reiser, G. Protease-activated receptors in the brain: Receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res. Rev. 2007, 56, 331–345. [Google Scholar] [CrossRef]
- De Luca, C.; Virtuoso, A.; Maggio, N.; Papa, M. Neuro-Coagulopathy: Blood Coagulation Factors in Central Nervous System Diseases. Int. J. Mol. Sci. 2017, 18, 2128. [Google Scholar] [CrossRef]
- Choi, S.H.; Joe, E.H.; Kim, S.U.; Jin, B.K. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J. Neurosci. 2003, 23, 5877–5886. [Google Scholar] [CrossRef]
- Davalos, D.; Baeten, K.M.; Whitney, M.A.; Mullins, E.S.; Friedman, B.; Olson, E.S.; Ryu, J.K.; Smirnoff, D.S.; Petersen, M.A.; Bedard, C.; et al. Early detection of thrombin activity in neuroinflammatory disease. Ann. Neurol. 2014, 75, 303–308. [Google Scholar] [CrossRef]
- Krenzlin, H.; Lorenz, V.; Danckwardt, S.; Kempski, O.; Alessandri, B. The Importance of Thrombin in Cerebral Injury and Disease. Int. J. Mol. Sci. 2016, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Reiser, G. Prothrombin/thrombin and the thrombin receptors PAR-1 and PAR-4 in the brain: Localization, expression and participation in neurodegenerative diseases. Thromb. Haemost. 2008, 100, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P.; Samany, P.G.; Thirumangalakudi, L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: Implications for disease pathogenesis. J. Alzheimer’s Dis. 2006, 9, 51–58. [Google Scholar] [CrossRef]
- Vaughan, P.J.; Su, J.; Cotman, C.W.; Cunningham, D.D. Protease nexin-1, a potent thrombin inhibitor, is reduced around cerebral blood vessels in Alzheimer’s disease. Brain Res. 1994, 668, 160–170. [Google Scholar] [CrossRef]
- Wagner, S.L.; Geddes, J.W.; Cotman, C.W.; Lau, A.L.; Gurwitz, D.; Isackson, P.J.; Cunningham, D.D. Protease nexin-1, an antithrombin with neurite outgrowth activity, is reduced in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1989, 86, 8284–8288. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef]
- Mann, K.G.; Butenas, S.; Brummel, K. The dynamics of thrombin formation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S. Multifunctional roles of thrombin. Ann. Clin. Lab. Sci. 1999, 29, 275–280. [Google Scholar]
- Esmon, C.T. Crosstalk between inflammation and thrombosis. Maturitas 2008, 61, 122–131. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc. Med. 2005, 15, 254–259. [Google Scholar] [CrossRef]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.A.; Ryu, J.K.; Akassoglou, K. Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nat. Rev. Neurosci. 2018, 19, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Zamolodchikov, D.; Ahn, H.J.; Strickland, S.; Norris, E.H. Fibrinogen and altered hemostasis in Alzheimer’s disease. J. Alzheimer’s Dis. 2012, 32, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Merlini, M.; Rafalski, V.A.; Rios Coronado, P.E.; Gill, T.M.; Ellisman, M.; Muthukumar, G.; Subramanian, K.S.; Ryu, J.K.; Syme, C.A.; Davalos, D.; et al. Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer’s Disease Model. Neuron 2019, 101, 1099–1108.e1096. [Google Scholar] [CrossRef] [PubMed]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Ryu, J.K.; Merlini, M.; Baeten, K.M.; Le Moan, N.; Petersen, M.A.; Deerinck, T.J.; Smirnoff, D.S.; Bedard, C.; Hakozaki, H.; et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 2012, 3, 1227. [Google Scholar] [CrossRef]
- Ziliotto, N.; Bernardi, F.; Piazza, F. Hemostasis components in cerebral amyloid angiopathy and Alzheimer’s disease. Neurol. Sci. 2021, 42, 3177–3188. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Lin, J.R.; Jabalameli, M.R.; Mitra, J.; Nguyen, N.; Zhang, Z.D. Deep post-GWAS analysis identifies potential risk genes and risk variants for Alzheimer’s disease, providing new insights into its disease mechanisms. Sci. Rep. 2021, 11, 20511. [Google Scholar] [CrossRef]
- Han, M.H.; Hwang, S.I.; Roy, D.B.; Lundgren, D.H.; Price, J.V.; Ousman, S.S.; Fernald, G.H.; Gerlitz, B.; Robinson, W.H.; Baranzini, S.E.; et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 2008, 451, 1076–1081. [Google Scholar] [CrossRef]
- Strickland, S. Blood will out: Vascular contributions to Alzheimer’s disease. J. Clin. Investig. 2018, 128, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Spronk, H.M.; ten Cate, H. The hemostatic system as a modulator of atherosclerosis. N. Engl. J. Med. 2011, 364, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Spronk, H.M.H.; Heeneman, S.; ten Cate, H. Is thrombin a key player in the ‘coagulation-atherogenesis’ maze? Cardiovasc. Res. 2009, 82, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Spronk, H.M.; ten Cate, H.; Hofstra, L. Accelerated in vivo thrombin formation independently predicts the presence and severity of CT angiographic coronary atherosclerosis. JACC Cardiovasc. Imaging 2012, 5, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Sciacca, F.L.; Sironi, M.; Luini, W.; Rabiet, M.J.; Mantovani, A. Expression of monocyte chemotactic protein-1 by monocytes and endothelial cells exposed to thrombin. Am. J. Pathol. 1994, 144, 975–985. [Google Scholar]
- Minami, T.; Sugiyama, A.; Wu, S.Q.; Abid, R.; Kodama, T.; Aird, W.C. Thrombin and phenotypic modulation of the endothelium. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 41–53. [Google Scholar] [CrossRef]
- Bowen-Pope, D.F.; Raines, E.W. History of discovery: Platelet-derived growth factor. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2397–2401. [Google Scholar] [CrossRef]
- ten Cate, H. Tissue factor-driven thrombin generation and inflammation in atherosclerosis. Thromb. Res. 2012, 129 (Suppl. S2), S38–S40. [Google Scholar] [CrossRef]
- Steffel, J.; Lüscher, T.F.; Tanner, F.C. Tissue factor in cardiovascular diseases: Molecular mechanisms and clinical implications. Circulation 2006, 113, 722–731. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Otten, J.J.; Heeneman, S.; Leenders, P.; van Oerle, R.; Soehnlein, O.; Loubele, S.T.; Hamulyak, K.; Hackeng, T.M.; Daemen, M.J.; et al. Genetic and pharmacological modifications of thrombin formation in apolipoprotein e-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner. PLoS ONE 2013, 8, e55784. [Google Scholar] [CrossRef]
- Palekar, R.U.; Jallouk, A.P.; Myerson, J.W.; Pan, H.; Wickline, S.A. Inhibition of Thrombin With PPACK-Nanoparticles Restores Disrupted Endothelial Barriers and Attenuates Thrombotic Risk in Experimental Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 446–455. [Google Scholar] [CrossRef]
- Pingel, S.; Tiyerili, V.; Mueller, J.; Werner, N.; Nickenig, G.; Mueller, C. Thrombin inhibition by dabigatran attenuates atherosclerosis in ApoE deficient mice. Arch. Med. Sci. 2014, 10, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Preusch, M.R.; Ieronimakis, N.; Wijelath, E.S.; Cabbage, S.; Ricks, J.; Bea, F.; Reyes, M.; van Ryn, J.; Rosenfeld, M.E. Dabigatran etexilate retards the initiation and progression of atherosclerotic lesions and inhibits the expression of oncostatin M in apolipoprotein E-deficient mice. Drug Des. Dev. Ther. 2015, 9, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.J.; Li, Y.H.; Shi, G.Y.; Liu, S.L.; Chang, P.C.; Kuo, C.H.; Wu, H.L. Thrombomodulin domains attenuate atherosclerosis by inhibiting thrombin-induced endothelial cell activation. Cardiovasc. Res. 2011, 92, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Aoki, I.; Shimoyama, K.; Aoki, N.; Homori, M.; Yanagisawa, A.; Nakahara, K.; Kawai, Y.; Kitamura, S.I.; Ishikawa, K. Platelet-dependent thrombin generation in patients with diabetes mellitus: Effects of glycemic control on coagulability in diabetes. J. Am. Coll. Cardiol. 1996, 27, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J. Coagulation in inflammatory diseases of the central nervous system. Semin. Thromb. Hemost. 2013, 39, 876–880. [Google Scholar] [CrossRef]
- Undas, A.; Wiek, I.; Stepien, E.; Zmudka, K.; Tracz, W. Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care 2008, 31, 1590–1595. [Google Scholar] [CrossRef]
- Beijers, H.J.; Ferreira, I.; Spronk, H.M.; Bravenboer, B.; Dekker, J.M.; Nijpels, G.; ten Cate, H.; Stehouwer, C.D. Body composition as determinant of thrombin generation in plasma: The Hoorn study. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, J.; Didelot, M.; Mohamadi, A.; Walton, L.A.; Bloemen, S.; de Laat, B.; Louis, H.; Thornton, S.N.; Derby, B.; Sherratt, M.J.; et al. Implication of Free Fatty Acids in Thrombin Generation and Fibrinolysis in Vascular Inflammation in Zucker Rats and Evolution with Aging. Front. Physiol. 2017, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Chen, Z.; Wang, M.; Li, Q.; Feng, W.; Wu, Y.; Wu, W.; Graziano, M.P.; Chintala, M. Zucker Diabetic Fatty rats exhibit hypercoagulability and accelerated thrombus formation in the Arterio-Venous shunt model of thrombosis. Thromb. Res. 2014, 134, 433–439. [Google Scholar] [CrossRef]
- Shavit-Stein, E.; Aronovich, R.; Sylantiev, C.; Gofrit, S.G.; Chapman, J.; Dori, A. The role of thrombin in the pathogenesis of diabetic neuropathy. PLoS ONE 2019, 14, e0219453. [Google Scholar] [CrossRef]
- Rahadian, A.; Fukuda, D.; Salim, H.M.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Shimabukuro, M.; Sata, M. Thrombin inhibition by dabigatran attenuates endothelial dysfunction in diabetic mice. Vasc. Pharmacol. 2020, 124, 106632. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.S.; Zhu, C.J.; Yan, B.Y.; Yan, C.Y.; Ling, R. Stimulation of KLF14/PLK1 pathway by thrombin signaling potentiates endothelial dysfunction in Type 2 diabetes mellitus. Biomed. Pharmacother. 2018, 99, 859–866. [Google Scholar] [CrossRef]
- Vittal Rao, H.; Bihaqi, S.W.; Iannucci, J.; Sen, A.; Grammas, P. Thrombin Signaling Contributes to High Glucose-Induced Injury of Human Brain Microvascular Endothelial Cells. J. Alzheimer’s Dis. 2021, 79, 211–224. [Google Scholar] [CrossRef]
- Deselms, H.; Maggio, N.; Rubovitch, V.; Chapman, J.; Schreiber, S.; Tweedie, D.; Kim, D.S.; Greig, N.H.; Pick, C.G. Novel pharmaceutical treatments for minimal traumatic brain injury and evaluation of animal models and methodologies supporting their development. J. Neurosci. Methods 2016, 272, 69–76. [Google Scholar] [CrossRef]
- Lindblad, C.; Thelin, E.P.; Nekludov, M.; Frostell, A.; Nelson, D.W.; Svensson, M.; Bellander, B.M. Assessment of Platelet Function in Traumatic Brain Injury-A Retrospective Observational Study in the Neuro-Critical Care Setting. Front. Neurol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Piao, C.; Ralay Ranaivo, H.; Rusie, A.; Wadhwani, N.; Koh, S.; Wainwright, M.S. Thrombin decreases expression of the glutamate transporter GLAST and inhibits glutamate uptake in primary cortical astrocytes via the Rho kinase pathway. Exp. Neurol. 2015, 273, 288–300. [Google Scholar] [CrossRef]
- Li, Y.J.; Chang, G.Q.; Liu, Y.; Gong, Y.; Yang, C.; Wood, K.; Shi, F.D.; Fu, Y.; Yan, Y. Fingolimod alters inflammatory mediators and vascular permeability in intracerebral hemorrhage. Neurosci. Bull. 2015, 31, 755–762. [Google Scholar] [CrossRef]
- Maegele, M. Coagulopathy and Progression of Intracranial Hemorrhage in Traumatic Brain Injury: Mechanisms, Impact, and Therapeutic Considerations. Neurosurgery 2021, 89, 954–966. [Google Scholar] [CrossRef]
- Mortimer, J.A.; van Duijn, C.M.; Chandra, V.; Fratiglioni, L.; Graves, A.B.; Heyman, A.; Jorm, A.F.; Kokmen, E.; Kondo, K.; Rocca, W.A.; et al. Head trauma as a risk factor for Alzheimer’s disease: A collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int. J. Epidemiol. 1991, 20 (Suppl. S2), S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Nemetz, P.N.; Leibson, C.; Naessens, J.M.; Beard, M.; Kokmen, E.; Annegers, J.F.; Kurland, L.T. Traumatic brain injury and time to onset of Alzheimer’s disease: A population-based study. Am. J. Epidemiol. 1999, 149, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Itzekson, Z.; Maggio, N.; Milman, A.; Shavit, E.; Pick, C.G.; Chapman, J. Reversal of trauma-induced amnesia in mice by a thrombin receptor antagonist. J. Mol. Neurosci. 2014, 53, 87–95. [Google Scholar] [CrossRef]
- Piao, C.S.; Holloway, A.L.; Hong-Routson, S.; Wainwright, M.S. Depression following traumatic brain injury in mice is associated with down-regulation of hippocampal astrocyte glutamate transporters by thrombin. J. Cereb. Blood Flow Metab. 2019, 39, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, X.; Liu, H.; Cui, W.; Guo, W.; Guo, K.; Guo, H.; Tao, K.; Li, F.; Shi, Y.; et al. Antagonism of Protease-Activated Receptor 4 Protects against Traumatic Brain Injury by Suppressing Neuroinflammation via Inhibition of Tab2/NF-κB Signaling. Neurosci. Bull. 2021, 37, 242–254. [Google Scholar] [CrossRef]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef]
- Alabanza, L.M.; Bynoe, M.S. Thrombin induces an inflammatory phenotype in a human brain endothelial cell line. J. Neuroimmunol. 2012, 245, 48–55. [Google Scholar] [CrossRef][Green Version]
- Brailoiu, E.; Shipsky, M.M.; Yan, G.; Abood, M.E.; Brailoiu, G.C. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 2017, 1657, 167–175. [Google Scholar] [CrossRef]
- Arai, T.; Guo, J.P.; McGeer, P.L. Proteolysis of non-phosphorylated and phosphorylated tau by thrombin. J. Biol. Chem. 2005, 280, 5145–5153. [Google Scholar] [CrossRef]
- Suo, Z.; Wu, M.; Citron, B.A.; Palazzo, R.E.; Festoff, B.W. Rapid tau aggregation and delayed hippocampal neuronal death induced by persistent thrombin signaling. J. Biol. Chem. 2003, 278, 37681–37689. [Google Scholar] [CrossRef]
- Zamolodchikov, D.; Chen, Z.L.; Conti, B.A.; Renne, T.; Strickland, S. Activation of the factor XII-driven contact system in Alzheimer’s disease patient and mouse model plasma. Proc. Natl. Acad. Sci. USA 2015, 112, 4068–4073. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, M.; Hensley, K.; Nguyen, A.; Grammas, P. Chronic thrombin exposure results in an increase in apolipoprotein-E levels. J. Neurosci. Res. 2006, 84, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, M.; Nguyen, A.; Kashani, S.; Pham, T.; Adesina, A.; Grammas, P. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol. Aging 2004, 25, 783–793. [Google Scholar] [CrossRef]
- Han, C.; Xia, X.; Jiao, S.; Li, G.; Ran, Q.; Yao, S. Tripartite motif containing protein 37 involves in thrombin stimulated BV-2 microglial cell apoptosis and interleukin 1beta release. Biochem. Biophys. Res. Commun. 2019, 516, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zuo, D.; Yu, L.; Zhang, L.; Tang, J.; Cui, C.; Bao, L.; Zan, K.; Zhang, Z.; Yang, X.; et al. ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem. Biophys. Res. Commun. 2017, 485, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ma, R.; Sun, S.; Wei, G.; Fang, Y.; Liu, R.; Li, G. JAK2-STAT3 signaling pathway mediates thrombin-induced proinflammatory actions of microglia in vitro. J. Neuroimmunol. 2008, 204, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, K.W.; Jin, B.K. Thrombin induces neurodegeneration and microglial activation in the cortex in vivo and in vitro: Proteolytic and non-proteolytic actions. Biochem. Biophys. Res. Commun. 2006, 346, 727–738. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Kang, X.; Jiang, C.; Zhang, H.; Wang, P.; Li, J. Thrombin-induced microglial activation impairs hippocampal neurogenesis and spatial memory ability in mice. Behav. Brain Funct. 2015, 11, 30. [Google Scholar] [CrossRef]
- Suo, Z.; Wu, M.; Ameenuddin, S.; Anderson, H.E.; Zoloty, J.E.; Citron, B.A.; Andrade-Gordon, P.; Festoff, B.W. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J. Neurochem. 2002, 80, 655–666. [Google Scholar] [CrossRef]
- Yin, M.; Chen, Z.; Ouyang, Y.; Zhang, H.; Wan, Z.; Wang, H.; Wu, W.; Yin, X. Thrombin-induced, TNFR-dependent miR-181c downregulation promotes MLL1 and NF-κB target gene expression in human microglia. J. Neuroinflamm. 2017, 14, 132. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, I.T.; Wu, W.B.; Liu, C.J.; Hsieh, H.L.; Hsiao, L.D.; Yang, C.C.; Yang, C.M. Thrombin mediates migration of rat brain astrocytes via PLC, Ca2+, CaMKII, PKCalpha, and AP-1-dependent matrix metalloproteinase-9 expression. Mol. Neurobiol. 2013, 48, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, K.P.; Gurwitz, D.; Cunningham, D.D.; Bradshaw, R.A. Reciprocal modulation of astrocyte stellation by thrombin and protease nexin-1. J. Neurochem. 1990, 54, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Birukova, A.A.; Birukov, K.G.; Smurova, K.; Adyshev, D.; Kaibuchi, K.; Alieva, I.; Garcia, J.G.; Verin, A.D. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004, 18, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Ander, B.P.; Xu, H.; Shen, Y.; Kaur, P.; Deng, W.; Sharp, F.R. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann. Neurol. 2010, 67, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Zhou, Y.P.; Yang, H. Donepezil combined with natural hirudin improves the clinical symptoms of patients with mild-to-moderate Alzheimer’s disease: A 20-week open-label pilot study. Int. J. Med. Sci. 2012, 9, 248–255. [Google Scholar] [CrossRef]
- Mongkhon, P.; Naser, A.Y.; Fanning, L.; Tse, G.; Lau, W.C.Y.; Wong, I.C.K.; Kongkaew, C. Oral anticoagulants and risk of dementia: A systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci. Biobehav. Rev. 2019, 96, 1–9. [Google Scholar] [CrossRef]
- Ding, M.; Fratiglioni, L.; Johnell, K.; Santoni, G.; Fastbom, J.; Ljungman, P.; Marengoni, A.; Qiu, C. Atrial fibrillation, antithrombotic treatment, and cognitive aging: A population-based study. Neurology 2018, 91, e1732–e1740. [Google Scholar] [CrossRef]
- Field, T.S.; Weijs, B.; Curcio, A.; Giustozzi, M.; Sudikas, S.; Katholing, A.; Wallenhorst, C.; Weitz, J.I.; Cohen, A.T.; Martinez, C. Incident Atrial Fibrillation, Dementia and the Role of Anticoagulation: A Population-Based Cohort Study. Thromb. Haemost. 2019, 119, 981–991. [Google Scholar] [CrossRef]
- Silva, R.; Miranda, C.M.; Liu, T.; Tse, G.; Roever, L. Atrial Fibrillation and Risk of Dementia: Epidemiology, Mechanisms, and Effect of Anticoagulation. Front. Neurosci. 2019, 13, 18. [Google Scholar] [CrossRef]
- Bergamaschini, L.; Rossi, E.; Storini, C.; Pizzimenti, S.; Distaso, M.; Perego, C.; De Luigi, A.; Vergani, C.; De Simoni, M.G. Peripheral treatment with enoxaparin, a low molecular weight heparin, reduces plaques and beta-amyloid accumulation in a mouse model of Alzheimer’s disease. J. Neurosci. 2004, 24, 4181–4186. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Kruyer, A.; Fernandez-Nueda, I.; Marcos-Diaz, A.; Ceron, C.; Richards, A.T.; Jno-Charles, O.C.; Rodriguez, I.; Callejas, S.; Norris, E.H.; et al. Long-Term Dabigatran Treatment Delays Alzheimer’s Disease Pathogenesis in the TgCRND8 Mouse Model. J. Am. Coll. Cardiol. 2019, 74, 1910–1923. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, J.; Johnson, S.L.; Majchrzak, M.; Barlock, B.J.; Akhlaghi, F.; Seeram, N.P.; Sen, A.; Grammas, P. Short-term treatment with dabigatran alters protein expression patterns in a late-stage tau-based Alzheimer’s disease mouse model. Biochem. Biophys. Rep. 2020, 24, 100862. [Google Scholar] [CrossRef]
- Friberg, L.; Rosenqvist, M. Less dementia with oral anticoagulation in atrial fibrillation. Eur. Heart J. 2018, 39, 453–460. [Google Scholar] [CrossRef]
- Mongkhon, P.; Fanning, L.; Lau, W.C.Y.; Tse, G.; Lau, K.K.; Wei, L.; Kongkaew, C.; Wong, I.C.K. Oral anticoagulant and reduced risk of dementia in patients with atrial fibrillation: A population-based cohort study. Heart Rhythm 2020, 17, 706–713. [Google Scholar] [CrossRef]
- Jacobs, V.; May, H.T.; Bair, T.L.; Crandall, B.G.; Cutler, M.J.; Day, J.D.; Mallender, C.; Osborn, J.S.; Stevens, S.M.; Weiss, J.P.; et al. Long-Term Population-Based Cerebral Ischemic Event and Cognitive Outcomes of Direct Oral Anticoagulants Compared with Warfarin among Long-term Anticoagulated Patients for Atrial Fibrillation. Am. J. Cardiol. 2016, 118, 210–214. [Google Scholar] [CrossRef]
- Cadogan, S.L.; Powell, E.; Wing, K.; Wong, A.Y.; Smeeth, L.; Warren-Gash, C. Anticoagulant prescribing for atrial fibrillation and risk of incident dementia. Heart 2021, 107, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, W.; Li, B.; Li, D. Relationship of Anticoagulant Therapy with Cognitive Impairment among Patients with Atrial Fibrillation: A Meta-Analysis and Systematic Review. J. Cardiovasc. Pharmacol. 2018, 71, 380–387. [Google Scholar] [CrossRef]
- Zeng, D.; Jiang, C.; Su, C.; Tan, Y.; Wu, J. Anticoagulation in atrial fibrillation and cognitive decline: A systematic review and meta-analysis. Medicine 2019, 98, e14499. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.L.; Hsieh, S.W.; Chou, P.S.; Yang, Y.H. Effects of Dabigatran on Dementia Pathogenesis and Neuropsychological Function: A Review. J. Alzheimer’s Dis. 2022, 86, 1589–1601. [Google Scholar] [CrossRef]
- Grossmann, K. Direct Oral Anticoagulants (DOACs) for Therapeutic Targeting of Thrombin, a Key Mediator of Cerebrovascular and Neuronal Dysfunction in Alzheimer’s Disease. Biomedicines 2022, 10, 1890. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Zhang, R.; Southworth, M.R.; Levenson, M.; Sheu, T.C.; Mott, K.; Goulding, M.R.; Houstoun, M.; et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015, 131, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Hsueh, Y.H.; Izem, R.; Southworth, M.R.; Wei, Y.; Liao, J.; Goulding, M.R.; Mott, K.; et al. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Beneficiaries Treated with Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA Intern. Med. 2016, 176, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Solkhon, F.; Jalal, Z.; Al-Hamid, A.M.; Jones, A.M. Relevance of physicochemical properties and functional pharmacology data to predict the clinical safety profile of direct oral anticoagulants. Pharmacol. Res. Perspect. 2020, 8, e00603. [Google Scholar] [CrossRef]
- Marinescu, M.; Sun, L.; Fatar, M.; Neubauer, A.; Schad, L.; van Ryn, J.; Lehmann, L.; Veltkamp, R. Cerebral Microbleeds in Murine Amyloid Angiopathy: Natural Course and Anticoagulant Effects. Stroke 2017, 48, 2248–2254. [Google Scholar] [CrossRef]
- Michael, N.; Grigoryan, M.M.; Kilday, K.; Sumbria, R.K.; Vasilevko, V.; van Ryn, J.; Cribbs, D.H.; Paganini-Hill, A.; Fisher, M.J. Effects of Dabigatran in Mouse Models of Aging and Cerebral Amyloid Angiopathy. Front. Neurol. 2019, 10, 966. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimer’s Dement. 2020, 16, 1553–1560. [Google Scholar] [CrossRef]
- Servick, K. Alzheimer’s drug approved despite murky results. Science 2021, 372, 1141. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Yang, F.; Chu, T.; Chen, P.; Beech, W.; Teter, B.; Tran, T.; Ubeda, O.; Ashe, K.H.; Frautschy, S.A.; et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000, 20, 5709–5714. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Schulzer, M.; McGeer, E.G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology 1996, 47, 425–432. [Google Scholar] [CrossRef]
- Stewart, W.F.; Kawas, C.; Corrada, M.; Metter, E.J. Risk of Alzheimer’s disease and duration of NSAID use. Neurology 1997, 48, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Hayden, K.M.; Zandi, P.P.; Khachaturian, A.S.; Szekely, C.A.; Fotuhi, M.; Norton, M.C.; Tschanz, J.T.; Pieper, C.F.; Corcoran, C.; Lyketsos, C.G.; et al. Does NSAID use modify cognitive trajectories in the elderly? The Cache County study. Neurology 2007, 69, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vlad, S.C.; Miller, D.R.; Kowall, N.W.; Felson, D.T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008, 70, 1672–1677. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Wang, D.; Zhang, J.; Zhang, F. NSAID Exposure and Risk of Alzheimer’s Disease: An Updated Meta-Analysis from Cohort Studies. Front. Aging Neurosci. 2018, 10, 83. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannucci, J.; Grammas, P. Thrombin, a Key Driver of Pathological Inflammation in the Brain. Cells 2023, 12, 1222. https://doi.org/10.3390/cells12091222
Iannucci J, Grammas P. Thrombin, a Key Driver of Pathological Inflammation in the Brain. Cells. 2023; 12(9):1222. https://doi.org/10.3390/cells12091222
Chicago/Turabian StyleIannucci, Jaclyn, and Paula Grammas. 2023. "Thrombin, a Key Driver of Pathological Inflammation in the Brain" Cells 12, no. 9: 1222. https://doi.org/10.3390/cells12091222
APA StyleIannucci, J., & Grammas, P. (2023). Thrombin, a Key Driver of Pathological Inflammation in the Brain. Cells, 12(9), 1222. https://doi.org/10.3390/cells12091222






