Abstract
Oxidative stress regulates many physiological and pathological processes. Indeed, a low increase in the basal level of reactive oxygen species (ROS) is essential for various cellular functions, including signal transduction, gene expression, cell survival or death, as well as antioxidant capacity. However, if the amount of generated ROS overcomes the antioxidant capacity, excessive ROS results in cellular dysfunctions as a consequence of damage to cellular components, including DNA, lipids and proteins, and may eventually lead to cell death or carcinogenesis. Both in vitro and in vivo investigations have shown that activation of the mitogen-activated protein kinase kinase 5/extracellular signal-regulated kinase 5 (MEK5/ERK5) pathway is frequently involved in oxidative stress-elicited effects. In particular, accumulating evidence identified a prominent role of this pathway in the anti-oxidative response. In this respect, activation of krüppel-like factor 2/4 and nuclear factor erythroid 2-related factor 2 emerged among the most frequent events in ERK5-mediated response to oxidative stress. This review summarizes what is known about the role of the MEK5/ERK5 pathway in the response to oxidative stress in pathophysiological contexts within the cardiovascular, respiratory, lymphohematopoietic, urinary and central nervous systems. The possible beneficial or detrimental effects exerted by the MEK5/ERK5 pathway in the above systems are also discussed.
1. Introduction
The extracellular signal-regulated kinase 5 (ERK5), also referred to as big mitogen-activated kinase 1 (BMK1), is the last discovered member of the canonical mitogen-activated protein kinase (MAPK) [1,2,3]. As is the case with other MAPKs, a kinase cascade leads to ERK5 activation via a specific upstream dual-specificity kinase, MEK5. The MEK5/ERK5 pathway is involved in survival, antiapoptotic signalling, proliferation and differentiation of many cell types [3] and plays a role in the onset and progression of cancer [4].
Several lines of evidence point to an important role for ERK5 during oxidative stress in cells. In this review, we summarize: i. The molecular mechanisms through which oxidative stress influences MEK5/ERK5 activation; ii. The known downstream targets involved in biological outcomes following oxidative stress-dependent effects on MEK5/ERK5 activity; iii. Whether the MEK5/ERK5 pathway plays a protective or detrimental role in both physiological and pathological processes when cells are undergoing oxidative stress. The latter point is central, since targeting MEK5/ERK5 signalling, alone or in combination with chemotherapeutics or targeted drugs, is increasingly being considered as a possible treatment for cancer and inflammatory diseases [5].
2. Oxidative Stress
Oxidative stress occurs when the redox state is altered due to an imbalance between the produced reactive oxygen species (ROS) or reactive nitrogen species (RNS) and the endogenous antioxidant defence mechanisms [6,7,8]. ROS production relies on both enzymatic and non-enzymatic reactions. The superoxide radical (O2•−) can be generated from the mitochondrial electron transport chain, which is the main source of O2•− and many enzymes, including nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, lipoxygenase, cyclooxygenase and cytochrome P450 reductase [9]. Once formed, O2•− is involved in several reactions, which, in turn, may generate other ROS/RNS such as hydrogen peroxide (H2O2), hydroxyl radical (•OH), peroxynitrite (ONOO−) and hypochlorous acid (HOCl). Of note, O2•− generated in the mitochondria is not able to cross the inner mitochondrial membrane, thus it is confined to the matrix where it reacts rapidly with the enzyme manganese superoxide dismutase (MnSOD) to form H2O2. H2O2, a non-radical ROS, is the only freely diffusible form of ROS able to penetrate membranes, and is also produced by other oxidases, including amino acid oxidase and xanthine oxidase. Thus, on the basis of subcellular compartmentation, mitochondrial ROS (mROS) accumulation is predicted to elicit different signals from H2O2 stimulation. •OH, the most reactive among all free radical species, is generated by the reaction of O2•− with H2O2 in the presence of Fe2+ or Cu2+ in the Fenton reaction [10]. Regarding non-enzymatic reactions, free radical production may occur when oxygen reacts with organic compounds or when cells are exposed to ionizing radiation. Moreover, as stated above, non-enzymatic free radical production can occur also during mitochondrial respiration [7].
RNS refer to a number of nitrogenous products such as nitric oxide (NO), nitroxyl (HNO), nitrosonium cation (NO+), higher oxides of nitrogen, S-nitrosothiols (RSNOs), ONOO− and dinitrosyl iron complexes. NO is synthesized through arginine-to-citrulline oxidation by NO synthases (NOS) [11]. Three NOS isoforms have been identified and are named according to the cell type or the condition under which they were first described: endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) [11]. NO is generated by iNOS under many pathological conditions, and can rapidly react with O2•− to generate the more toxic ONOO− and the highly reactive •OH, which may then react with DNA, RNA, proteins and membrane lipids. ONOO− is a key factor in protein tyrosine nitration of important structural and functional domains, causing changes in protein functions [12].
The generation of ROS from molecular oxygen is a natural and indispensable part of aerobic life. Indeed, basal levels of ROS are essential for the exploitation of various cellular functions via the modulation of signal transduction pathways that regulate gene expression, support cell proliferation or induce cell death [13]. However, excessive levels of ROS induce cellular dysfunction and cause damage to cellular macromolecules such as DNA, lipids and proteins, eventually leading to cell death. Regarding ROS-dependent DNA damage, only ROS species produced within the nucleus or those capable of entering the nucleus, such as H2O2, can lead to DNA damage. In contrast, high O2•− levels in the mitochondria or lysosomes do not. Along this line, many studies have demonstrated that increased ROS/RNS production supports the progression of inflammatory diseases [14,15,16] and promotes carcinogenesis as well as cancer progression [6,17,18]. Additionally, oxidative stress-mediated chronic inflammation is a key risk factor for tumorigenesis [19,20].
At the cellular level, ROS/RNS production is supported by the onset of an antioxidant detoxification response that is central to controlling and/or adapting to increased ROS/RNS levels [21,22]. The endogenous antioxidant detoxification system is a very complex response that relies on: (i) enzymatic antioxidants such as copper-zinc superoxide dismutase (CuZnSOD), manganese superoxide dismutase (MnSOD), glutathione peroxidases (GPxs) and catalase. CuZnSOD is widely distributed in the cytosol and nucleus, while MnSOD is present in the mitochondrial matrix. Both are able to efficiently scavenge for O2•− and generate H2O2 [23,24,25], which, in turn, can be neutralized by GPxs and peroxiredoxins (thioredoxin-dependent peroxidases) [26,27]. (ii) Non-enzymatic antioxidants, such as vitamin E, vitamin C, carotenoids, flavonoids, selenium, thiol antioxidants (thioredoxin, lipoic acid and glutathione). (iii) Multiple regulatory factors, including the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) that induces gene expression by binding to the antioxidant responsive elements (ARE), nuclear factor-kB (NF-kB) and activator protein-1 (AP-1), which collectively regulate the expression of genes involved in the detoxification response [21,22]. The abnormal activity of the above enzymatic and non-enzymatic antioxidants is associated with the development of various human diseases, including cardiovascular, neurodegenerative and metabolic disorders as well as cancer [6,14,26,28,29,30].
3. The MEK5/ERK5 Signalling Pathway
The MAPK ERK5 is encoded by the MAPK7 gene [1,2] and is involved in survival, anti-apoptotic signalling, proliferation and differentiation of several types of cells [3]. In response to extracellular stimuli, such as growth factors [31,32,33,34] and cellular stresses [35,36,37], the Ser/Thr kinases MEKK2 and MEKK3 activate MEK5, a dual-specificity protein kinase that has ERK5 as its only known substrate. Once activated, MEK5 phosphorylates ERK5 at T219 and Y221 in the conserved threonine-glutamic acid-tyrosine (TEY) motif of the catalytic domain [38]. MEK5-dependent phosphorylation stimulates ERK5 nuclear translocation, which has been proposed as a key event in the regulation of cell proliferation [39,40,41]. Despite sharing high homology in the kinase domain with ERK2 and possessing a TEY motif identical to that of ERK1/2/8 in the activation loop, ERK5 has a long C-terminal tail that is unique among all MAPKs. The C-terminal tail includes a nuclear localization sequence (NLS) important for ERK5 nuclear shuttling, two proline-rich (PR) domains (PR1 and PR2) that are considered potential binding sites for src-homology 3 (SH3)-domain-containing proteins, a nuclear export sequence (NES) and a myocyte enhancer factor 2 (MEF2)-interacting region [42]. The C-terminus of ERK5 also possesses a transcriptional transactivation domain (TAD) [43] that undergoes autophosphorylation of serine and threonine residues, thereby enabling ERK5 to directly regulate gene transcription [44]. A number of C-terminal residues are also known to be phosphorylated by cyclin-dependent kinase (CDK) 1 and/or ERK1/2 [45,46,47,48,49]. Among the known substrates of ERK5 are the transcription factors c-FOS, c-MYC, SRF accessory protein-1a (Sap-1a), myocyte enhancer factor 2A (MEF2A), C and D, as well as other kinases such as ribosomal S6 kinase (RSK) and serum/glucocorticoid-regulated kinase (SGK) [50]. Several reports support the view that the kinase and transactivation activities of ERK5 can be uncoupled under certain circumstances. For example, it has been shown that the nuclear localization of an ERK5 mutant devoid of kinase activity results in the activation of transcription through the TAD located at the C-terminus [51]. On the other hand, some ERK5 inhibitors such as XMD17-109 and AX15836 have been reported to drive nuclear translocation of ERK5, leading to paradoxical activation of ERK5 transcriptional activity, despite effectively inhibiting its kinase activity [52]. On the other hand, some ERK5 inhibitors (e.g., AX15836 and BAY-885) do not show anti-proliferative or anti-inflammatory effects [53,54]. This fact may be due to the above-mentioned paradoxical ERK5 activation, or to the possibility that kinase-independent activities of ERK5 may play crucial roles in ERK5-elicited signals. However, several reports have clearly demonstrated inhibitory effects on cell proliferation upon ERK5 knockdown or knockout [3,4]. At variance with the above reports, Cook’s group observed that siRNA-mediated ERK5 knockdown had no effect on the proliferation of colon rectal cancer cell lines with either KRAS or BRAF mutations [55]. Moreover, it has been recently reported that INY-06-061, a potent and highly selective heterobifunctional degrader of ERK5, did not induce anti-proliferative effects in multiple cancer cell lines nor suppress inflammatory responses in primary endothelial cells (ECs) [56].
4. Activation of the MEK5/ERK5 Pathway by Oxidative Stress
The first evidence that ERK5 is a redox-sensitive kinase came from Abe and colleagues, who showed that H2O2 is a potent stimulus of ERK5 activation, as determined using a kinase assay (Figure 1). ERK5 activation following exposure to H2O2 turned out to be calcium-dependent, because depletion of intracellular calcium using thapsigargin prevented H2O2-induced ERK5 activation. These effects were observed in different cell types, including human umbilical vein endothelial cells (HUVECs), arterial smooth muscle cells and skin fibroblasts [35]. In a later study, the same authors demonstrated that SRC supports H2O2-induced ERK5 activation in mouse fibroblasts. Indeed, H2O2 failed to stimulate ERK5 activity in the presence of the src family kinase inhibitors (SFKi) herbimycin A and PP1, as well as in src−/− mouse embryonic fibroblasts [57]. The involvement of SFK in H2O2-induced ERK5 activation has also been reported in rat aortic smooth muscle cells, in which src inhibition using either SFKi PP2 or src specific siRNA attenuated H2O2-induced ERK5 activation [58]. We can include the lack of SFK inhibition by phosphatases such as the low molecular weight protein phosphatase, which is inactivated by H2O2, among the upstream mechanisms that may be responsible for SFK activation by H2O2 [59]. Additional SRC-dependent mechanisms may also be involved in ERK5 activation. Indeed, among the known upstream activators of MEK5/ERK5 signalling, a number of oncogenic signalling molecules and pathways are activated by SRC, including Abelson murine leukaemia viral oncogene homolog 1 (ABL1), PI3K/AKT, RAS/RAF/MEK/ERK and STAT3 [60,61]. A study conducted using pheochromocytoma 12 (PC12) cells later reported that treatment with the SFKi herbimycin A or PP2 or overexpression of a kinase-inactive SRC mutant (K297R) prevented H2O2-induced ERK5 activation, further supporting the involvement of SFK in this event. While studying upstream ERK5 activators, Sun and colleagues found that H2O2 caused significant activation of MEKK2, and that MEKK2’s interaction with the adaptor protein LAD is necessary for H2O2-induced ERK5 activation in CCL64 mink epithelial cells. Pre-treatment of the same cells with the SFKi PP1 prevented MEKK2 and ERK5 activation, indicating that H2O2 stimulates an SFK/LAD/MEKK2/MEK5/ERK5 cascade [62]. A recent study confirmed the involvement of MEKK2 in H2O2-induced ERK5 activation as H2O2-induced phosphorylation/activation of ERK5 was impaired in MEKK2−/− intestinal stromal cells [63].
Regarding additional mechanisms involved in ERK5 pathway activation by oxidative stress, it has been reported that exposure to H2O2 increases PI3K/AKT [64] and c-ABL activation [65,66]. On the other hand, H2O2 was reported to modulate specific cysteine residues within redox-sensitive proteins such as EGFR [67,68] and to stimulate the induction of VEGF, which, in turn, binds to and activates VEGFR [69]. Both PP1 and PP2A Ser/Thr phosphatases are involved in H2O2-induced ERK5 activation, since PP1/PP2Ai okadaic acid and calyculin A prevent this activation in HeLa and PC12 cells [70]. Besides the activation of ERK5 kinase activity, an additional mechanism that may be responsible for the activation of the MEK5/ERK5 pathway relies on the increase in ERK5 mRNA levels upon oxidative phosphorylation (OXPHOS) following pyruvate dehydrogenase kinase (PDK) inhibition by dichloroacetate (DCA) [71]. Finally, a recent study showed that laminar shear stress (LSS)-induced mtROS production leads to activation of the MEK5/ERK5/KLF2 pathway in ECs [72]. Interestingly, ionizing radiation, which is known to generate ROS, has been shown to cause sustained ERK5 activation and MEK5/ERK5 knockdown combined with irradiation markedly sensitizes one to radiotherapy by inducing strong inhibition of tumour growth in mouse xenografts [73,74].
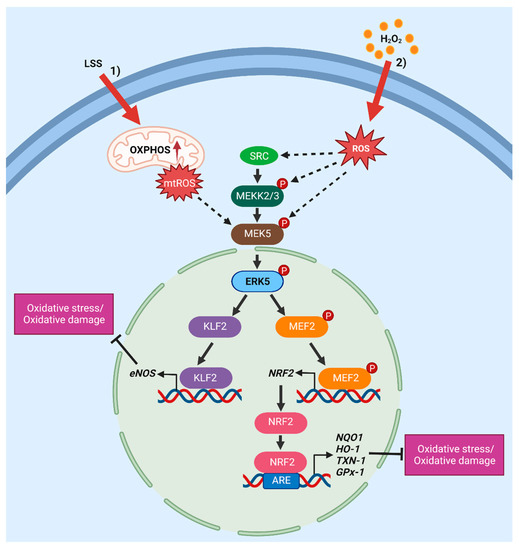
Figure 1.
Overview of the mechanisms of activation and downstream effectors of the MEK5/ERK5 pathway in response to oxidative stress. The rising in oxidative stress triggers MEK5/ERK5 activation, which, in turn, promotes an antioxidant response. (1) Laminar shear stress (LSS) induces mtROS production, which, in turn, leads to activation of the MEK5/ERK5/KLF2 pathway [72]. (2) H2O2 stimulation may activate the ERK5 pathway via SRC [36,57,58,62], MEKK2/3 [63] or MEK5 [75,76] activation. Continuous arrows indicate direct activation mechanisms and dashed arrows indicate demonstrated, but not direct, mechanisms. eNOS, endothelial nitric oxide synthase; NQO1, NAD(P)H quinone dehydrogenase 1; HO-1, heme oxygenase-1; TXN-1, thioredoxin-1; GPx-1, glutathione peroxidase (created using Biorender.com accessed on 23 March 2023).
5. Role of the MEK5/ERK5 Pathway in ROS-Dependent Effects on Human Diseases
5.1. Pathophysiology of Blood Vessels
5.1.1. Endothelial Cells
ECs play a critical role in cardiovascular homeostasis by regulating vascular tone and blood fluidity, fibrinolysis, angiogenesis as well as leukocyte and platelet adhesion [77]. NO has an important role in the regulation of vascular tone [78,79] and its deficiency, as much as excessive ROS (mainly O2•−), may promote endothelial dysfunction [80,81,82]. EC damage is associated with aging and the development of various cardiovascular disorders, including hypertension, atherosclerosis, heart failure and diabetes [83,84,85].
Erk5 knockout mice display cardiovascular defects, in particular those affecting heart development, vessel maturation, angiogenesis and endothelial integrity, which collectively lead to embryonic lethality at around stage E10 [86,87,88,89]. Analogous effects have been observed in mice in which Erk5 was selectively knocked out in ECs [87], thus confirming the critical role of ERK5 in normal cardiovascular development and vascular integrity. In this regard, in vitro studies have revealed that ERK5 is required to prevent apoptosis and mediate shear stress signalling in normal ECs, as well as to regulate tumour angiogenesis [90]. However, small molecule inhibitors of ERK5 do not seem to alter the vasculature in adult mice [91], leaving open the possibility of targeting ERK5 in human diseases.
The fact that ERK5 is activated by oxidative stress (i.e., H2O2) in ECs has been reported using HUVECs [39,75]. A later study provided evidence that ERK5 activation plays a protective role when ECs experience oxidative stress. Indeed, activation of ERK5 by overexpression of a constitutively active MEK5 mutant (MEK5-DD, S313D/T317D) protected HUVECs from H2O2-induced cell death. Depletion of NRF2 by specific siRNA abrogated these effects, pointing to the existence of an ERK5–NRF2 pathway. The same study also demonstrated that laminar flow induces ERK5–NRF2 pathway activation in HUVECs. Indeed, blocking MEK5/ERK5 using either siRNA (specific for ERK5) or BIX02189 (MEK5i [92]) inhibited laminar flow-induced upregulation of NRF2-dependent genes such as heme oxygenase-1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1). Additionally, MEK5-DD-induced activation of ERK5 increased the transcriptional activity and nuclear translocation of NRF2, whereas BIX02189 strongly suppressed the latter. Immunoprecipitation analysis showed that ERK5 binds to NRF2 and that this interaction is diminished by a mutant form of ERK5 (ERK5-AEF, T218A/Y220F) that cannot be phosphorylated by MEK5 [76]. Interestingly, using the osteoblastic MC3T3-E1 cell line, Xia and colleagues demonstrated the involvement of ERK5/NRF2 signalling in the protective effect of mangiferin, a naturally available polyphenol found in both mango and papaya [93], in response to ROS. Indeed, genetic inhibition of ERK5 using specific siRNA abolished the anti-apoptotic effect of mangiferin upon H2O2 treatment, pointing to the involvement of ERK5 in the protective effect of mangiferin [94]. Another study reported that sustained activation of ERK5 following the overexpression of MEK5-DD suppresses the generation of ROS/RNS caused by growth factor deprivation in HUVECs. The same study also demonstrated that shear stress-activated ERK5 signalling restores the redox state of ECs by reducing ROS production and increasing NO bioavailability [95]. Additional evidence of the protective role of ERK5 in HUVECs was provided by showing that two natural antioxidants, the stilbenes resveratrol and pterostilbene, activate MnSOD expression through the ERK5/HDAC5 pathway, thus alleviating mitochondrial oxidative stress in ECs that may be eventually associated with cardiovascular disease [96].
Overall, the above studies established that ERK5 plays a protective role against oxidative stress-induced damage of ECs.
5.1.2. Atherosclerosis
Atherosclerosis is a chronic inflammatory disease that is associated, in its early phase, with dyslipidaemia, increased expression of inflammatory factors and endothelial dysfunction, which collectively drive atherosclerotic lesion development in arteries [97,98].
Sugars have antiproliferative and proatherogenic effects on ECs and increase the risk of cardiovascular disease [99]. In order to better understand the mechanisms underlying hyperglycaemia-induced proatherogenic changes in ECs, Liu and colleagues found that glucose, and to a lesser extent raffinose, increase ERK5 activity in bovine pulmonary artery ECs. The involvement of ROS in glucose-induced ERK5 activation was proven by showing that N-acetylcysteine (NAC), a free radical scavenger, prevents the increase in ERK5 activation induced by glucose [100]. Another study conducted using ECs reported that despite activating ERK5 kinase activity, both H2O2 and advanced glycation end products (AGE) inhibited LSS-induced ERK5/MEF2/KLF2 and the subsequent eNOS expression through ERK5 SUMOylation. More importantly, ERK5 SUMOylation was increased in the aortas of diabetic mice in vivo. On that basis, the authors proposed that inhibiting ERK5 SUMOylation might be a new therapy for diabetes-mediated endothelial dysfunction and inflammation [101]. By contrast, Coon and colleagues found that LSS-induced mtROS production leads to activation, rather than inhibition, of the MEK5/ERK5/KLF2 pathway in ECs, pointing to the involvement of ERK5 in the anti-inflammatory response to LSS-dependent mtROS in ECs [72].
In another study conducted using human aortic ECs (HAECs), Wu and colleagues found that tumour necrosis factor-α (TNF-α) [102] plays a causal role in atherosclerosis by increasing intracellular ROS through the small GTPase Rac-1, which, in turn, stimulates the expression of VCAM-1 and ICAM-1—which play a major role in the initiation of early atherosclerosis—in HAECs via NF-kB. All these effects were reverted by treatment with atorvastatin, which was found to activate ERK5 in these cells in line with the well-known role of ERK5 in vasoprotection and the maintenance of endothelial integrity [86,87,88,89,103]. Moreover, inhibiting ERK5 using ERK5i XMD8-92 [91] or specific siRNA ablated the effects of atorvastatin and increased ROS production [104], further supporting the protective role of ERK5. Another study reported that TNF-α increases oxidative stress in HUVECs through generation of 4-hydroxy nonenal, a lipid peroxidation end product, and NADPH oxidase 4 (NOX4), which is involved in ROS production. TNF-α increased the levels of VCAM-1 and E-selectin, which, when overproduced, fuel the atherogenic process. Additionally, TNF-α reduced ERK5 phosphorylation and krüppel-like factor 2 (KLF2) mRNA and protein levels. Interestingly, butyrate, a short-chain fatty acid that has been shown to exert antioxidant, anti-inflammatory and other protective effects in atherosclerosis [105], prevented the proatherogenic effects of TNF-α (i.e., the increase in VCAM-1, E-selectin and oxidative stress) by reducing the levels of ROS—measured by quantifying 4-Hydroxynonenal (4-HNE)—and rescuing the activation of the ERK5/KLF2 pathway [106].
Lipopolysaccharides (LPSs) can stimulate immune cell activation and initiate inflammatory responses, contributing to endothelial dysfunction, an early event in atherosclerosis. Zhong and colleagues demonstrated that the antimalarial drug halofuginone suppressed LPS-induced oxidative stress in HUVECs, while stimulating the expression of the key atheroprotective transcription factor KLF2, a positive regulator of the antioxidant transcription factor NRF2 [107]. The latter effects were mediated by ERK5 because blocking ERK5 with XMD8-92 abolished the effect of halofuginone on KLF2 mRNA and protein [108]. Sun and colleagues showed that oscillatory shear stress (OSS), which is known to support the production of proinflammatory mediators in HUVECs, stimulates the production of mtROS (O2•−) and reduces the level of the lactate receptor GPR81, ERK5 phosphorylation and KLF2 mRNA and protein. Activation of GPR81 using physiological doses of lactate reduced oxidative stress by inhibiting the production of ROS and rescued OSS-induced reduced expression of KLF2, which was mediated by ERK5. Indeed, XMD8-92 abolished the rescue of KLF2 induced by lactate-mediated GPR81 activation [109].
Several lines of evidence indicate that hyperglycaemia enhances the proliferation of vascular smooth muscle cells (VSMCs), a critical step in the pathogenesis of atherosclerosis [110,111,112]. In this respect, Yu and colleagues found that intermittent high glucose enhances the proliferation and migration of primary rat VSMCs. The antioxidant rutin, a flavonoid found in many plants, inhibited the proliferation and migration of VSMCs by suppressing the phosphorylation of MEK1/2, ERK1/2, PI3K, NF-κB and ERK5, and the production of ROS under fluctuating glucose levels [113]. A later study identified the link between ERK5 and hyperglycaemia-related effects. Indeed, fluvastatin, the first fully synthetic statin that is effective at reducing total and low-density lipoprotein cholesterol [114], increased the mRNA and protein levels of NRF2 and NQO1 in VSMCs. This effect was dependent on MEK5/ERK5 because it was reduced upon MEK5 (BIX02189) or ERK5 (siRNA) inhibition. Treatment of VSMCs with fluvastatin suppressed AGE-dependent proliferation and this suppression was prevented by pre-treatment with BIX02189. Mechanistically, fluvastatin suppressed cyclin D1 and CDK4, and these effects were prevented by BIX02189 pre-treatment. In addition, overexpression of MEK5-DD diminished AGE-induced proliferation, while NRF2 depletion using specific siRNA restored AGE-induced proliferation. This study, therefore, showed that fluvastatin decreases AGE-dependent proliferation via the ERK5–NRF2 axis [115]. Another study conducted using primary rat VSMCs [116] reported that the ERK5 pathway may be involved in oxidant stress-stimulation of early growth response gene–1 (Egr-1), which has a key role in atherogenesis [117]. Indeed, insulin and oxidant stress, both as single agents and in combination, significantly increased the level of Egr-1 protein. In particular, inhibition of the ERK5 and ERK1/2 pathways with MEK1/2/5i UO126—but not inhibition of the ERK1/2 pathway alone with MEK1/2i PD98059—completely blocked the effect of oxidative stress on Egr-1 protein levels, thus suggesting that the MEK5–ERK5 pathway may be involved in oxidant stress stimulation of Egr-1 [116].
Altogether, the above studies point to a protective role for ERK5 against atherosclerosis.
5.1.3. Hypertension
Hypertension is a primary risk factor for cardiovascular diseases such as stroke, heart attack, heart failure and aneurysm, and increased oxidant stress is strongly implicated in the pathogenesis of hypertension. Angiotensin (Ang) II and endothelin-1 (ET-1) are two potent vasoconstrictors that mediate pleiotropic vascular actions through distinct receptors [118,119]. The activation of MAPK by Ang II and ET-1 in VSMCs plays an important role in the vascular changes associated with hypertension [120,121,122]. Touyz and colleagues showed that Ang II stimulated NADPH oxidase activity, and increased intracellular H2O2 production as well as p38MAPK and ERK5 phosphorylation in primary rat VSMCs in a dose-dependent manner [123]. These phosphorylation events were reduced by IGF-1Ri and EGFRi (AG1024 and AG1478, respectively), and were almost abolished by the NADPH oxidase inhibitor diphenyleneiodonium or by the intracellular scavenger, tiron. These findings suggested that Ang II activates p38MAP kinase and ERK5 via redox-dependent cascades that are regulated by IGF-1R and EGFR transactivation. In a subsequent study conducted using primary human VSMCs, the same authors reported that Ang II and ET-1 stimulate the phosphorylation of p38MAPK, JNK and ERK5, but not ERK1/2, via redox-sensitive processes. Ang II activated p38, JNK and ERK5 primarily through NAD(P)H oxidase-generated O2•−, while ET-1 stimulated these kinases via redox-sensitive processes that involve mtROS [124]. Besides VSMCs, the involvement of a p38/ERK5 axis in the oxidative stress response has been reported by Morimoto and colleagues, who demonstrated that a p38/ERK5/BCL6B pathway creates a positive feedback loop that drives self-renewal of spermatogonial stem cells via ROS amplification [125].
Collectively, the above studies suggest that ERK5-mediated signals may contribute to the pathogenesis of hypertension.
5.2. Pathophysiology of the Heart
In the heart, redox signalling is involved in physiological processes, including excitation-contraction coupling and cell differentiation, as well as, when unbalanced, pathological occurrences such as adverse cardiac remodelling, fibrosis and heart failure. NADPH oxidases, NO synthase and mitochondria are sources of ROS relevant to heart diseases, concurring during both myocardial and vascular dysfunction [126].
5.2.1. Ischemic Heart Disease
Ischemia-reperfusion (I/R) injury, an event occurring invariably in tissues when blood flow deprivation is followed by its restoration, is caused by excessive ROS, which are produced both during ischemia and upon reperfusion. Based on this, remarkable efforts have been directed to clarify the mechanisms that induce excess ROS production during I/R [127,128]. Using freshly explanted guinea pig hearts, it has been shown that ischemia stimulates the activation of p90RSK, src and ERK5, while reperfusion stimulates activation of p90RSK and ERK1/2. Ex vivo pre-treatment of the hearts with SFKi PP2 showed that SFKs are upstream activators of ERK5 in the context of ischemia. On the contrary, pre-treatment of the hearts with the antioxidant N-2-mercaptopropionyl glycine identified a major role for H2O2 in the stimulation of ERK1/2 and p90RSK after I/R, but only a partial role for H2O2 in ischemia-induced src and ERK5 activation [129].
5.2.2. Myocardial Disease
Mitochondrial dysfunction and metabolic cardiomyopathy are critical factors that lead to myocardial diseases. In the latter, unremitting metabolic stress leads to cardiac lipid overload, insulin resistance, energy deficiency, increased ROS production and apoptosis over time, finally leading to deterioration in contractility and heart failure [130]. Additionally, diabetic patients have a nearly threefold increased risk of developing heart failure compared with age-matched non-diabetics [131].
The first report to determine the role of ERK5 in cardiac dysfunction was by Yan and colleagues, who showed that ERK5 activation inhibits cardiac apoptosis and the subsequent dysfunction via inhibition of the feedback loop between phosphodiesterase 3A and inducible cAMP early repressor [132]. A later study using a cardiomyocyte-specific Erk5 knockout mouse model demonstrated, for the first time, the in vivo role of ERK5 in regulating hypertrophic growth and preventing heart failure. In the same paper, the authors identified the transcription factor MEF2 as a downstream effector of ERK5 in regulating the hypertrophic response [133].
A link between ERK5, ROS and cardiomyocyte health was first identified in a study showing that genetic inhibition of ERK5 using specific siRNA boosted hypothermia-induced apoptosis by increasing the levels of a series of ROS (O2•−, H2O2, •OH), the pro-apoptotic protein Bim and intracellular calcium in these cells [134]. A later study investigating the possible role of ERK5 in myocardial disease secondary to metabolic disorders found that expression of ERK5, MEF2A and MEF2D decreased in the hearts of obese (ob/ob) and diabetic (db/db) mice following a high-fat diet (HFD), eventually leading to cardiomyopathy. To identify the mechanisms linking ERK5 deficiency to cardiomyopathy, the authors used Erk5-CKO (cardiac knockout) and control (Erk5-Flox) mice and found that O2•− production was increased in HFD-Erk5-CKO hearts with respect to the controls. Interestingly, in the cardiac fibres of Erk5-CKO mice on HFD, OXPHOS capacity and mitochondria organization were reduced, while the density of lipid droplets was increased compared with HFD-treated Erk5-Flox mice. Finally, analysis of murine primary cardiomyocytes found that free fatty acid stimulation induced ERK5 degradation through calpain-1, a cysteine protease whose activation is dependent on Gp91phox—a component of the NADPH oxidase complex—and triggered by O2•− [135]. These results seem to indicate that the reduction in ERK5 levels that accompanies the onset of myocardial disease may be a consequence of ROS-induced calpain-1 activation following a HFD. In another study, Yu and colleagues studied myocardial hypertrophy induced by transverse aortic constriction in vivo, and H2O2 administration to H9C2 cells (derived from rat heart tissue) in vitro. These treatments induced oxidative stress and inflammation, which were associated with increased expression of miRNA (miR)-143-3p, a non-coding RNA that has been reported to regulate ERK5 expression [136]. However, whether or not ERK5 is involved in myocardial hypertrophy has not been determined [137].
Cameron and colleagues reported, for the first time, that ERK5 is expressed in platelets and functions as a redox switch to promote maladaptive platelet signalling during myocardial infarction (MI), a condition with greatly elevated ROS [138]. Indeed, they found that H2O2 activated ERK5 in isolated human platelets and that ERK5 is activated in platelets isolated from mice following MI induced by permanent ligation of the left anterior descending coronary artery. More importantly, platelet-specific ERK5−/− mice showed less platelet activation, reduced MI size and improved post-MI heart function. A later study provided evidence that the platelet scavenger receptor CD36 promotes thrombosis under atherogenic conditions by generating a redox-regulated signalling pathway requiring ERK5. Moreover, genetic deletion of ERK5 in megakaryocytes and platelets decreased platelet adhesion, activation and accumulation in vitro. Finally, in two different models of in vivo arterial thrombosis, ERK5 deficiency in platelets abrogated the enhanced thrombosis seen under hyperlipidaemic conditions [139].
The incidence of cardiovascular diseases (CVD) is higher in HIV+ patients subjected to chimeric antigen receptor T-cell (cART) therapy. Experiments carried out using primary macrophages, which are involved in the proinflammatory conditions that support CVD, showed that cART treatment increases mtROS, which, in turn, activate p90RSK, which is responsible for the subsequent phosphorylation of ERK5 at S496. This event reduced ERK5 transcriptional transactivation activity, resulting in reduced NRF2 activity on ARE, as measured by evaluating the expression of the NRF2-dependent antioxidant genes thioredoxin-1, HO-1 and GPx-1. Therefore, treatments directed at increasing ERK5–NRF2–ARE activity may offer promising approaches for overcoming the higher CVD incidence in cART patients [140]. In a more recent study, the same authors identified the possible mechanisms responsible for the increased incidence of CVD in cancer survivors with respect to the rest of the population. Using murine primary monocytes/macrophages, they found that doxorubicin and ionizing radiation, which is frequently used in the treatment of cancer, upregulates p90RSK phosphorylation, which, in turn, supports ERK5 phosphorylation at the inhibitory residue S496. In particular, these events increased the activity of poly (ADP-ribose) polymerase, a DNA damage response (DDR)-related molecule, and led to subsequent NAD+ depletion and an increase in mtROS production, ultimately resulting in mitochondrial dysfunction. These effects, including ERK5 phosphorylation, were reversed by the p90RSK inhibitor FMK-MEA [141] and by the overexpression of a dominant/negative p90RSK mutant (K94A/K447A) in primary macrophages. Importantly, doxorubicin- and ionizing radiation-induced mtROS production were inhibited in primary macrophages derived from mice in which wt ERK5 had been replaced with the ERK5-S496A mutant using CRISPR/Cas9 technique. This study, therefore, established the importance of ERK5 inhibitory phosphorylation at S496 and identified possible strategies for preventing CVD in cancer survivors [142].
Overall, the above studies established that ERK5 plays a protective role against oxidative stress-induced myocardial diseases.
5.3. Pathophysiology of the Lung
Accumulating evidence indicates that oxidative stress plays an important role in the pathogenesis of various lung disorders, including asthma, acute injury and pulmonary fibrosis, as well as cancer onset and progression [143,144]. The large surface area of the pulmonary tissue together with continuous exposure to high levels of O2 and endogenous and exogenous oxidants contribute to high ROS and RNS production. In fact, oxidants in this tissue may be generated endogenously through local metabolic reactions (e.g., from mitochondrial electron transport during respiration or the activation of phagocytes) or may be derived from exogenous sources such as air pollutants and cigarette smoke [145,146,147]. With regard to the former, using C10 murine lung epithelial cells, Scapoli and colleagues found that asbestos fibres, the phagocytosis of which is known to determine oxidant production [148], caused sustained ERK5 phosphorylation compared with treatment with EGF or H2O2, which were used as positive controls. Inhibition of MEK1/2/5 by UO126 or SFK by PP2—as well as inhibition by H2O2 or EGF—blocked asbestos-induced ERK5 activation in C10 cells. The involvement of src was confirmed in experiments showing that asbestos-induced ERK5 phosphorylation/activation was prevented by the overexpression of a dominant/negative src (K296R/Y528F) mutant. Additionally, pharmacological inhibition of src (using PP2) or overexpression of a dominant/negative MEK5 mutant (MEK5-AA, S311A/T315A [38]) significantly inhibited asbestos-induced cell proliferation, thus demonstrating that asbestos supports lung cell proliferation via an src/MEK5-dependent pathway. These results indicate that ERK5 inhibition may be a useful strategy against mineral fibre-induced carcinogenesis [149].
A possible protective role for ERK5 activation by oxidative stress in the lungs has been identified using A549 lung adenocarcinoma cells. Indeed, Wu and colleagues showed that ERK5 mediates antioxidant response to the oxidant-sensitive miR-200c, the expression of which is induced by H2O2 [150]. In these cells, overexpression of miR-200c reduced the expression of three antioxidant proteins—SOD2, HO-1 and sirtuin 1—with respect to control cells, under basal or H2O2-stimulated conditions. ERK5 KD using specific shRNA reduced the expression of sirtuin 1, in keeping with the notion that ERK5 is an upstream regulator of this gene [151]. This study therefore provided evidence that ERK5 participates in the modulation of redox homeostasis of normal and neoplastic lung cells [152]. Another study showed that upregulation of the MEK5/ERK5/NRF2 antioxidant pathway plays an indispensable role in regulating the antioxidant effect of the isoflavone biochanin A, alleviating lung injury induced by water-soluble components of urban particulate matter. [153].
The above studies seem to indicate that ERK5 plays a protective role against oxidative stress-induced lung injury.
5.4. Pathophysiology of the Lymphohematopoietic System
In recent years, ROS have been proven to participate in the regulation of the lymphohematopoietic system, which is responsible for the development of immune response and the production of blood cells. Indeed, low ROS levels are important for the maintenance of quiescence and multipotency of haematopoietic stem cells, whereas a higher level of ROS may support haematopoietic differentiation [154,155]. Furthermore, similar to what is well established in solid cancers, high ROS levels and oxidative stress play a key role in leukaemia onset and progression by stimulating genomic instability, cell survival and proliferation as well as drug resistance [154,156].
In Jurkat acute T-lymphoblastic leukaemia cells, ERK5 KD using specific shRNA impaired OXPHOS and reduced cell survival [157]. In further support of a pro-survival role for ERK5 in the above cellular model, a later study showed that Fas ligand (FasL)-induced apoptosis was attenuated by H2O2-induced ERK5 activation. The protective effect of H2O2 was abolished in Jurkat cells by the overexpression of MEK5-AA. On that basis, the authors proposed that inhibiting ERK5 signalling in conjunction with conventional chemotherapy, which is known to induce ROS production, might be more effective at maximizing leukemic cell death compared with chemotherapy alone [158]. Along this line, a study by Khan and colleagues shed light on the mechanisms through which ERK5 modulates the antioxidant response in leukemic cells. In human acute leukaemia cell lines (Jurkat, OCI-AML3, NB4 and MOLM-13), OXPHOS elicited using either DCA or an “OXPHOS medium” (e.g., glucose-free culture medium with glutamine and galactose) induced an increase in NQO-1, HO-1 and ERK5 mRNA levels, while decreasing the levels of Keap-1, a key sensor of oxidative stress that mediates ubiquitination and degradation of the NRF2 protein. Similar results were obtained upon DCA treatment of cells derived from AML patients, and in NOD/SCID-interleukin-2 receptor γ null (NSG) mice engrafted with primary human leukemic cells derived from patients with different haematological neoplasms (e.g., multiple myeloma, B-cell chronic lymphocytic leukaemia and T-cell lymphoma). The authors found that MEF2C, a downstream target of ERK5, binds to the promoter of miR-23a–27a–24-2 cluster and that miR-23a destabilizes the Keap-1 mRNA. These results suggest that, in leukemic cells undergoing OXPHOS, ERK5/MEF2/miR-23a signalling downregulates Keap-1, leading to activation of the NRF2/ARE pathway, which, in turn, generates an antioxidant response [159]. In a later study, the same authors demonstrated that in addition to increasing ERK5 mRNA, DCA-induced OXPHOS supports NRF2 expression in Jurkat, OCI-AML3 and NB4 cells. Similar results were obtained upon DCA treatment of cells derived from AML patients and NOD/SCID-NSG mice engrafted with AML cells. Indeed, ERK5 KD using ERK5-specific shRNA reduced the levels of DCA-induced NRF2 mRNA and protein, as well as expression of the NRF2-target genes NQO-1 and HO-1. Conversely, NRF2 mRNA levels increased following the expression of MEK5-DD. ERK5-dependent regulation of NRF2 upon DCA treatment was dependent on MEF2, because MEF2-targeting siRNA reduced DCA-induced NRF2 expression. Inhibition of mitochondrial complex I by metformin decreased ERK5, NRF2 and NQO-1 mRNA and protein levels. Conversely, complex I activity induced ERK5/MEF2-dependent NRF2 expression through fumarate accumulation, thus supporting the antioxidant response [71].
Collectively, the above studies indicate that ERK5 may reduce the burden of ROS that would result in the death of leukaemia cells, and ERK5-dependent antioxidant responses may prevent additional ROS-mediated mutagenesis that could support leukaemia progression.
5.5. Pathophysiology of the Kidney
The kidney is an organ characterized by a high metabolic rate, in particular oxidation reactions in the mitochondria, which make it vulnerable to damage caused by further oxidative stress. Oxidative stress is increased in patients with renal impairment as a result of enhanced oxidant activity and reduced antioxidant capacity, and inflammation further amplifies oxidant generation and impairs antioxidant systems, worsening patient conditions [160]. Moreover, consequences of oxidative stress in patients with chronic kidney disease include NO production, increased Ang II activity, hypertension, atherosclerosis and anaemia [161].
Glomerulonephritis (GN) encompasses a subset of renal diseases characterized by immuno-mediated damage to glomeruli. Acute GN forms can be either primary renal diseases or secondary to diabetes, hypertension, cardiovascular diseases, anaemia, metabolic acidosis and systemic immune-mediated diseases. Most acute GN are considered progressive disorders, which, without timely therapy, progress to chronic GN characterized by progressive glomerular damage and tubulo-interstitial fibrosis, leading to reduced glomerular filtration rates [162].
A link between oxidative stress-induced degenerative processes of the kidney and ERK5 was provided by a study showing that treatment with eplerenone (a selective mineralocorticoid receptor antagonist) or tempol (a cell membrane-permeable radical scavenger, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) prevented glomerular changes (i.e., cell proliferation and mesangial matrix expansion) and hypertension following the administration of aldosterone/1% NaCl to male Sprague-Dawley rats. Aldosterone/1% NaCl-treated animals had enhanced levels of ERK1/2, JNK and ERK5 phosphorylation. Both eplerenone and tempol prevented this enhancement, pointing to an ROS-dependent mechanism of activation of all the above MAPKs. However, whether ERK5 activation was associated with the observed detrimental changes or was an attempt to protect the cells from the ROS-induced effects was not addressed [163].
As mentioned above, Ang II activity may be increased as a consequence of oxidative stress. Using rat mesangial cells, Ishizawa and colleagues found that PDGF, a mitogen involved in the pathogenesis of GN, induced H2O2 and src-dependent ERK5 phosphorylation. Indeed, SFKi (herbimycin A, PP2) abolished PDGF-induced ERK5 activation. Pre-treatment with olmesartan, an Ang II type 1 (AT1) receptor blocker that provides renoprotection and suppresses oxidative stress, prevented PDGF-induced src and ERK5 activation. However, whether or not ERK5 plays a protective role following H2O2 induction was not addressed in the study. In the same study, ERK5 activation was shown to be necessary for PDGF-induced migration in rat mesangial cells, because ERK5 siRNA suppressed PDGF-stimulated cell migration [164]. Interestingly, in human hepatic stellate cells, ERK5 inhibition increases, rather than reduces, PDGF-induced migration [31]. In another study, Urushihara and colleagues investigated whether ERK5 is involved in the pathogenesis of chronic GN. Starting from the fact that the level of O2•− was significantly increased at the glomerular of nephritic rats (uninephrectomized rats treated with the nephritogenic anti-Thy-1 mAb) with respect to control rats, H2O2 induced ERK5 phosphorylation in rat mesangial cells in vitro in a dose-dependent manner. Genetic inhibition of ERK5 using specific siRNA decreased H2O2-induced cell viability and soluble collagen secretion in rat mesangial cells, pointing to the involvement of ERK5 in the pathogenesis of chronic GN [165].
High glucose is known to generate ROS—H2O2 in particular—in glomerular cells [166,167]. Along this line, Suzaki and colleagues found that high glucose levels activated ERK5 and ERK1/2, but not p38, in the glomeruli of Otsuka Long Evans Tokushima Fatty rats, which display diabetic nephropathy at 52 weeks of age. High glucose-induced ERK5 activation in cultured rat mesangial cells was reduced by inhibiting SFK (with herbimycin-A or PP2) or depleting PKC (with GF109203X- or prolonged PMA treatment). Taken together, these results indicate that high glucose induces SFK- and PKC-dependent ERK5 activation in mesangial cells, both in vivo and in vitro [168]. Another study showed that overexpression of ERK5 in mouse kidneys provides protection against renal I/R injury [169].
In conclusion, although several lines of evidence seem to indicate that ERK5 can support GN, further studies are needed to define the role of ERK5 in kidney pathophysiology.
5.6. Pathophysiology of the Liver
The role of oxidative stress has been extensively studied in the liver, a tissue at high risk of oxidative stress due to its high metabolic activity. Moreover, chronic liver diseases are nearly always characterized by increased oxidative stress, regardless of the aetiology [170].
As has been described for leukaemia cells (see above), activation of OXPHOS by DCA has been reported to induce an increase in NRF2 and ERK5, NQO-1 and HO-1 mRNA levels in two hepatocellular carcinoma (HCC) cell lines (Huh7 and HepG2) and in primary human hepatocytes. ERK5 KD (using siRNA) in primary human hepatocytes and HCC cells impaired DCA-induced expression of NRF2 and the NRF2-target genes NQO-1 and HO-1. ERK5-dependent regulation of NRF2 following DCA treatment was shown to depend on MEF2, because MEF2-specific siRNA reduced DCA-induced NRF2 expression. Finally, inhibition of mitochondrial complex I with metformin decreased the expression of ERK5, NRF2 and NQO-1 mRNA and proteins. The study showed that complex I activity induced ERK5 expression via fumarate accumulation, leading to MEF2/NRF2-mediated antioxidant response [71]. Different conclusions were reached in another study, where ERK5 mRNA levels decreased upon treatment with salidroside, a natural compound with ROS-scavenging activity that protects against CCl4-induced liver injury [171].
5.7. Pathophysiology of the Central Nervous System
The central nervous system (CNS) is one of the most metabolically active compartments in the body and, even at rest, consumes 20% of the body’s oxygen supply [172]. The presence of high concentrations of polyunsaturated fatty acids and the selectivity of the blood–brain barrier, which prevents the uptake of some antioxidants like vitamin E, make the CNS highly susceptible to oxidative stress [173]. More importantly, excessive ROS are generated after cerebral I/R, leading to severe damage to brain cells, including both neurons and glia [174].
The brain expresses the highest levels of ERK5 [89]. ERK5 expression is maximal during early embryonic development and declines as the brain matures [175]. Of note, ERK5 and ERK5-regulated MEF2 gene expression contribute to brain-derived neurotrophic factor-promoted survival of developing—but not mature—cortical and cerebellar neurons [175,176].
5.7.1. Degenerative Diseases
Oxidative stress has been implicated in the progression of neurodegenerative diseases, including Alzheimer’s [177] and Parkinson’s [178,179] diseases, as well as amyotrophic lateral sclerosis [180]. The first evidence of a link between ERK5 and oxidative stress in the CNS was reported by Suzaki and colleagues, who showed that ERK5 is activated by H2O2 in a concentration-dependent manner in PC12 cells, an established model of neurosecretion and neuronal differentiation. They also found that SFKi (herbimycin A and PP2) treatment or overexpression of a kinase-inactive src mutant (K297R) inhibits H2O2-induced ERK5 activation in PC12 cells. In addition, H2O2 treatment increased the ability of the ERK5-downstream target, MEF2C, to bind DNA. These findings suggest that c-src-mediated ERK5 activation by H2O2 may counteract oxidative stress-induced damage, likely through the activation of MEF2C transcription factor [36]. Another study conducted using PC12 cells reported that nerve growth factor (NGF) stimulation or low H2O2 dose preconditioning (PC) protects PC12 cells and mouse primary hippocampal neurons against high H2O2 dose-induced cell death. Both NGF and PC induced ERK5 phosphorylation and increased expression of the KLF4 transcription factor. Two MEK5i, BIX02188 [92] and BIX02189, abolished the neuroprotective effect of NGF and PC. Consistently, activation of ERK5 upon overexpression of MEK5-DD led to increased survival of cells experiencing H2O2-induced insult. The induction of KLF4 by NGF or PC was blocked by ERK5-targeting siRNA, pointing to the involvement of ERK5 in this induction. Importantly, overexpression of MEK5-DD or KLF4 in H2O2-stressed cells upregulated the expression of anti-apoptotic proteins such as Bcl-2 and neuronal apoptosis inhibitory protein-2, and downregulated the expression of pro-apoptotic proteins such as Bax and Caspase 1 [181]. Based on the above findings, it can be concluded that the ERK5/KLF4 pathway functions as a common mechanism for NGF and PC neuroprotection.
Another interesting aspect of the neuroprotective role of ERK5 in the CNS is its involvement in the action of melatonin, which is an antioxidant and anti-apoptotic agent that plays a neuroprotective role in different CNS injuries [182,183]. Liu and colleagues showed that melatonin prevented ROS and malondialdehyde production, induced SOD increase and prevented apoptosis in murine neuroblastoma N2a cells under hypoxia (i.e., 10 ppm O2; 0.001% O2). Furthermore, they found that melatonin induced ERK1/2 and ERK5 phosphorylation by upregulating the expression of the zinc uptake transporter Zip1, thus exerting a protective effect against hypoxia-induced apoptosis in these cells [184].
A possible role of ERK5 in the pathogenesis of Parkinson’s disease has also been identified. Potdar and colleagues showed that piceid, a resveratrol precursor, protects human dopaminergic-like SH-SY5Y cells from dopamine-induced cell death via activation of ERK1/2 and ERK5, which results in the inhibition of apoptosis, the latter being caused by oxidative stress following exposure to high dopamine concentrations. Piceid pre-treatment reduced caspase-3/7 activity and increased the expression of the anti-apoptotic Bcl-2 protein. Piceid-mediated protection against the dopamine-induced effect was attenuated by inhibition of ERK1/2 and ERK5 pathways using UO126 (MEK1/2/5i) or XMD8-92 (ERK5i). Thus, the protective effect of piceid seemed to be mediated by ERK1/2/5 activation [185]. Cavanaugh and colleagues investigated the contributions of ERK5 and ERK1/2 to the survival of the MN9D murine midbrain dopaminergic neuronal cell line under basal conditions and in response to 6-hydroxydopamine (6-OHDA), a neurotoxic synthetic organic compound used to induce lesions in the nigrostriatal dopaminergic system (a model of Parkinson’s disease). The authors observed that MEK1/2/5i UO126 decreased the survival of MN9D cells. Overexpression of a dominant/negative form of either ERK5 (ERK5-AEF) or MEK1 (K97A), the upstream activator of ERK1/2, mimicked the effect of UO126 on MN9D cells in reducing cell survival. Overexpression of MEK5-DD and wt ERK5 increased cell survival but did not inhibit 6-OHDA-induced toxicity. In contrast, overexpression of a constitutively active MEK1 mutant (S218D/S222D) decreased cell survival and inhibited 6-OHDA-induced cell death. Finally, overexpression of a constitutively active MEF2C mutant (S444D) increased cell survival and inhibited 6-OHDA-induced cell death, suggesting that MEF2C is important for MN9D cells survival, and is likely the downstream target of ERK5 in these events. Taken together, these results suggested that activation of both ERK5 and ERK1/2 promote MN9D cell survival, and that ERK1/2 also protects MN9D cells against oxidative stress [186]. Overexposure to Mn causes irreversible movement disorders with signs and symptoms similar, but not identical, to idiopathic Parkinson’s disease [187,188]. Using MN9D cells, Ding and colleagues found that Mn exposure increased apoptosis, ROS production and MEK5 and ERK5 protein expression. The inhibition of MEK5/ERK5 signalling using MEK5i BIX02189 increased Mn-dependent cell death and ROS production with respect to Mn treatment alone [173]. Additionally, MEK5/ERK5 signalling was proven to regulate cell responses to ROS and to exert a protective effect against Mn-induced cytotoxicity, likely via upregulation of Bcl-2 and downregulation of Bax [189].
5.7.2. Cerebrovascular Diseases
ROS produced upon cerebral I/R can have severe and irreversible detrimental effects on both neurons and glia [174]. In this regard, the first in vivo evidence of a link between ERK5 and oxidative stress in the CNS was provided using a four-vessel occlusion model of Sprague–Dawley rats. A study by Wang and colleagues showed that ERK5 was rapidly (from 10 min to 1 day, peaking at 30 min) activated (phosphorylation at TEY) upon reperfusion of the hippocampus following 15 min of ischemia. The involvement of ROS in this activation was demonstrated by the fact that NAC pre-treatment prevented I/R-induced ERK5 activation. Additionally, ERK5 activation upon reperfusion was suppressed by SFKi (PP2), nifedipine (an L-Type Voltage-Gated Calcium Channel (LVGCC) blocker) or dextromethorphan, an N-methyl-D-aspartate (NMDA) receptor antagonist. These results suggest that I/R-induced activation of ERK5 might be mediated by oxidative stress-activated SFK through NMDA receptor and LVGCC in the rat hippocampus [190].
Based on the above studies, it is clear that the MEK5–ERK5 pathway plays a protective role against oxidative stress-induced CNS damage.
6. Conclusions
Oxidative stress and free radicals are generally known for their detrimental effects on human health, as they contribute to the initiation and progression of several pathologies, ranging from inflammatory diseases to cancer. Regarding the role of ERK5 and the possible side effects occurring following its inhibition in case it is targeted therapeutically [5,41,191], accumulating evidence indicates that, overall, the MEK5/ERK5 cascade exerts a suppressive role on oxidative stress under both physiological and pathological processes (Table 1). Based on this, in inflammatory diseases, the inhibition of ERK5 or its downstream targets is very likely to result in a detrimental effect as a consequence of the impairment of the antioxidant response, with potential worsening of the severity of inflammation. Conversely, impairment of the antioxidant response following MEK5/ERK5 pathway inhibition may result in a desirable effect following chemotherapeutic treatments to boost oxidative stress-dependent cell death. Similarly, inhibition of ERK5 may ameliorate hypertension. Nevertheless, the above studies clearly indicate that the role of the MEK5–ERK5 pathway as a protective factor against oxidative-induced damage may result in complex outcomes that should be investigated further in view of MEK5/ERK5-targeted exploitation in clinics.

Table 1.
Role of the ERK5 pathway in response to oxidative stress in pathological contexts.
Author Contributions
Conceptualization, E.R.; writing-original draft preparation, I.T., A.M. and E.R.; writing-review and editing I.T., A.M., A.T. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research in Elisabetta Rovida’s lab is supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, IG-15282 and IG-21349), Ente Cassa di Risparmio di Firenze (ECRF), Università degli Studi di Firenze (“Progetto Competitivo di Ateneo per Ricercatori” funded by MUR and Next Generation EU). Alessandro Tubita is supported by a “Carlo Zanotti” Fondazione Italiana per la Ricerca sul Cancro (FIRC)-AIRC fellowship (ID-23847).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Persio Dello Sbarba for the helpful discussion on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, J.D.; Ulevitch, R.J.; Han, J. Primary structure of BMK1: A new mammalian map kinase. Biochem. Biophys. Res. Commun. 1995, 213, 715–724. [Google Scholar] [CrossRef]
- Zhou, G.; Bao, Z.Q.; Dixon, J.E. Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 1995, 270, 12665–12669. [Google Scholar] [CrossRef]
- Drew, B.A.; Burow, M.E.; Beckman, B.S. MEK5/ERK5 pathway: The first fifteen years. Biochim. Biophys. Acta 2012, 1825, 37–48. [Google Scholar] [CrossRef]
- Stecca, B.; Rovida, E. Impact of ERK5 on the Hallmarks of Cancer. Int. J. Mol. Sci. 2019, 20, 1426. [Google Scholar] [CrossRef]
- Paudel, R.; Fusi, L.; Schmidt, M. The MEK5/ERK5 Pathway in Health and Disease. Int. J. Mol. Sci. 2021, 22, 7594. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Zhan, X.; Desiderio, D.M. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal. Biochem. 2006, 354, 279–289. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y. Reactive Oxygen and Nitrogen Species in Carcinogenesis: Implications of Oxidative Stress on the Progression and Development of Several Cancer Types. Mini Rev. Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meyskens, F.L.; Estrela, J.M. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. 2019, 400, 589–612. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.T.; Carlson, E.J.; Melov, S.; Ursell, P.C.; Olson, J.L.; Noble, L.J.; Yoshimura, M.P.; Berger, C.; Chan, P.H.; et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995, 11, 376–381. [Google Scholar] [CrossRef]
- Melov, S.; Doctrow, S.R.; Schneider, J.A.; Haberson, J.; Patel, M.; Coskun, P.E.; Huffman, K.; Wallace, D.C.; Malfroy, B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 2001, 21, 8348–8353. [Google Scholar] [CrossRef]
- Elchuri, S.; Oberley, T.D.; Qi, W.; Eisenstein, R.S.; Jackson Roberts, L.; Van Remmen, H.; Epstein, C.J.; Huang, T.T. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 2005, 24, 367–380. [Google Scholar] [CrossRef]
- Chu, F.F.; Esworthy, R.S.; Chu, P.G.; Longmate, J.A.; Huycke, M.M.; Wilczynski, S.; Doroshow, J.H. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004, 64, 962–968. [Google Scholar] [CrossRef]
- Kang, S.W.; Rhee, S.G.; Chang, T.S.; Jeong, W.; Choi, M.H. 2-Cys peroxiredoxin function in intracellular signal transduction: Therapeutic implications. Trends Mol. Med. 2005, 11, 571–578. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Neumann, C.A.; Krause, D.S.; Carman, C.V.; Das, S.; Dubey, D.P.; Abraham, J.L.; Bronson, R.T.; Fujiwara, Y.; Orkin, S.H.; Van Etten, R.A. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 2003, 424, 561–565. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef]
- Rovida, E.; Spinelli, E.; Sdelci, S.; Barbetti, V.; Morandi, A.; Giuntoli, S.; Dello Sbarba, P. ERK5/BMK1 is indispensable for optimal colony-stimulating factor 1 (CSF-1)-induced proliferation in macrophages in a Src-dependent fashion. J. Immunol. 2008, 180, 4166–4172. [Google Scholar] [CrossRef]
- Cavanaugh, J.E.; Ham, J.; Hetman, M.; Poser, S.; Yan, C.; Xia, Z. Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons. J. Neurosci. 2001, 21, 434–443. [Google Scholar] [CrossRef]
- Finegan, K.G.; Wang, X.; Lee, E.J.; Robinson, A.C.; Tournier, C. Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ. 2009, 16, 674–683. [Google Scholar] [CrossRef]
- Kato, Y.; Tapping, R.I.; Huang, S.; Watson, M.H.; Ulevitch, R.J.; Lee, J.D. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 1998, 395, 713–716. [Google Scholar] [CrossRef]
- Abe, J.; Kusuhara, M.; Ulevitch, R.J.; Berk, B.C.; Lee, J.D. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem. 1996, 271, 16586–16590. [Google Scholar] [CrossRef]
- Suzaki, Y.; Yoshizumi, M.; Kagami, S.; Koyama, A.H.; Taketani, Y.; Houchi, H.; Tsuchiya, K.; Takeda, E.; Tamaki, T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: Potential role in cell survival following oxidative insults. J. Biol. Chem. 2002, 277, 9614–9621. [Google Scholar] [CrossRef]
- Yan, C.; Takahashi, M.; Okuda, M.; Lee, J.D.; Berk, B.C. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J. Biol. Chem. 1999, 274, 143–150. [Google Scholar] [CrossRef]
- Kato, Y.; Kravchenko, V.V.; Tapping, R.I.; Han, J.; Ulevitch, R.J.; Lee, J.D. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997, 16, 7054–7066. [Google Scholar] [CrossRef]
- Gomez, N.; Erazo, T.; Lizcano, J.M. ERK5 and Cell Proliferation: Nuclear Localization Is What Matters. Front. Cell Dev. Biol. 2016, 4, 105. [Google Scholar] [CrossRef]
- Erazo, T.; Espinosa-Gil, S.; Diéguez-Martínez, N.; Gómez, N.; Lizcano, J.M. SUMOylation Is Required for ERK5 Nuclear Translocation and ERK5-Mediated Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 2203. [Google Scholar] [CrossRef]
- Tubita, A.; Lombardi, Z.; Tusa, I.; Dello Sbarba, P.; Rovida, E. Beyond Kinase Activity: ERK5 Nucleo-Cytoplasmic Shuttling as a Novel Target for Anticancer Therapy. Int. J. Mol. Sci. 2020, 21, 938. [Google Scholar] [CrossRef]
- Yan, C.; Luo, H.; Lee, J.D.; Abe, J.; Berk, B.C. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J. Biol. Chem. 2001, 276, 10870–10878. [Google Scholar] [CrossRef]
- Kasler, H.G.; Victoria, J.; Duramad, O.; Winoto, A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol. Cell. Biol. 2000, 20, 8382–8389. [Google Scholar] [CrossRef]
- Morimoto, H.; Kondoh, K.; Nishimoto, S.; Terasawa, K.; Nishida, E. Activation of a C-terminal transcriptional activation domain of ERK5 by autophosphorylation. J. Biol. Chem. 2007, 282, 35449–35456. [Google Scholar] [CrossRef]
- Honda, T.; Obara, Y.; Yamauchi, A.; Couvillon, A.D.; Mason, J.J.; Ishii, K.; Nakahata, N. Phosphorylation of ERK5 on Thr732 is associated with ERK5 nuclear localization and ERK5-dependent transcription. PLoS ONE 2015, 10, e0117914. [Google Scholar] [CrossRef]
- Zhuang, K.; Zhang, J.; Xiong, M.; Wang, X.; Luo, X.; Han, L.; Meng, Y.; Zhang, Y.; Liao, W.; Liu, S. CDK5 functions as a tumor promoter in human colorectal cancer via modulating the ERK5-AP-1 axis. Cell Death Dis. 2016, 7, e2415. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, E.; Pandiella, A. Multisite phosphorylation of Erk5 in mitosis. J. Cell Sci. 2010, 123, 3146–3156. [Google Scholar] [CrossRef]
- Inesta-Vaquera, F.A.; Campbell, D.G.; Tournier, C.; Gomez, N.; Lizcano, J.M.; Cuenda, A. Alternative ERK5 regulation by phosphorylation during the cell cycle. Cell. Signal. 2010, 22, 1829–1837. [Google Scholar] [CrossRef]
- Tusa, I.; Gagliardi, S.; Tubita, A.; Pandolfi, S.; Urso, C.; Borgognoni, L.; Wang, J.; Deng, X.; Gray, N.S.; Stecca, B.; et al. ERK5 is activated by oncogenic BRAF and promotes melanoma growth. Oncogene 2018, 37, 2601–2614. [Google Scholar] [CrossRef]
- Hoang, V.T.; Yan, T.J.; Cavanaugh, J.E.; Flaherty, P.T.; Beckman, B.S.; Burow, M.E. Oncogenic signaling of MEK5-ERK5. Cancer Lett. 2017, 392, 51–59. [Google Scholar] [CrossRef]
- Erazo, T.; Moreno, A.; Ruiz-Babot, G.; Rodriguez-Asiain, A.; Morrice, N.A.; Espadamala, J.; Bayascas, J.R.; Gomez, N.; Lizcano, J.M. Canonical and kinase activity-independent mechanisms for extracellular signal-regulated kinase 5 (ERK5) nuclear translocation require dissociation of Hsp90 from the ERK5-Cdc37 complex. Mol. Cell. Biol. 2013, 33, 1671–1686. [Google Scholar] [CrossRef]
- Lochhead, P.A.; Tucker, J.A.; Tatum, N.J.; Wang, J.; Oxley, D.; Kidger, A.M.; Johnson, V.P.; Cassidy, M.A.; Gray, N.S.; Noble, M.E.M.; et al. Paradoxical activation of the protein kinase-transcription factor ERK5 by ERK5 kinase inhibitors. Nat. Commun. 2020, 11, 1383. [Google Scholar] [CrossRef]
- Lin, E.C.; Amantea, C.M.; Nomanbhoy, T.K.; Weissig, H.; Ishiyama, J.; Hu, Y.; Sidique, S.; Li, B.; Kozarich, J.W.; Rosenblum, J.S. ERK5 kinase activity is dispensable for cellular immune response and proliferation. Proc. Natl. Acad. Sci. USA 2016, 113, 11865–11870. [Google Scholar] [CrossRef]
- Nguyen, D.; Lemos, C.; Wortmann, L.; Eis, K.; Holton, S.J.; Boemer, U.; Moosmayer, D.; Eberspaecher, U.; Weiske, J.; Lechner, C.; et al. Discovery and Characterization of the Potent and Highly Selective (Piperidin-4-yl)pyrido [3,2-d]pyrimidine Based in Vitro Probe BAY-885 for the Kinase ERK5. J. Med. Chem. 2019, 62, 928–940. [Google Scholar] [CrossRef]
- Lochhead, P.A.; Clark, J.; Wang, L.Z.; Gilmour, L.; Squires, M.; Gilley, R.; Foxton, C.; Newell, D.R.; Wedge, S.R.; Cook, S.J. Tumor cells with KRAS or BRAF mutations or ERK5/MAPK7 amplification are not addicted to ERK5 activity for cell proliferation. Cell Cycle 2016, 15, 506–518. [Google Scholar] [CrossRef]
- You, I.; Donovan, K.A.; Krupnick, N.M.; Boghossian, A.S.; Rees, M.G.; Ronan, M.M.; Roth, J.A.; Fischer, E.S.; Wang, E.S.; Gray, N.S. Acute pharmacological degradation of ERK5 does not inhibit cellular immune response or proliferation. Cell Chem. Biol. 2022, 29, 1630–1638.e7. [Google Scholar] [CrossRef]
- Abe, J.; Takahashi, M.; Ishida, M.; Lee, J.D.; Berk, B.C. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J. Biol. Chem. 1997, 272, 20389–20394. [Google Scholar] [CrossRef]
- Zhao, J.; Kyotani, Y.; Itoh, S.; Nakayama, H.; Isosaki, M.; Yoshizumi, M. Big mitogen-activated protein kinase 1 protects cultured rat aortic smooth muscle cells from oxidative damage. J. Pharmacol. Sci. 2011, 116, 173–180. [Google Scholar] [CrossRef]
- Caselli, A.; Marzocchini, R.; Camici, G.; Manao, G.; Moneti, G.; Pieraccini, G.; Ramponi, G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J. Biol. Chem. 1998, 273, 32554–32560. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Buschbeck, M.; Hofbauer, S.; Di Croce, L.; Keri, G.; Ullrich, A. Abl-kinase-sensitive levels of ERK5 and its intrinsic basal activity contribute to leukaemia cell survival. EMBO Rep. 2005, 6, 63–69. [Google Scholar] [CrossRef]
- Sun, W.; Wei, X.; Kesavan, K.; Garrington, T.P.; Fan, R.; Mei, J.; Anderson, S.M.; Gelfand, E.W.; Johnson, G.L. MEK kinase 2 and the adaptor protein Lad regulate extracellular signal-regulated kinase 5 activation by epidermal growth factor via Src. Mol. Cell Biol. 2003, 23, 2298–2308. [Google Scholar] [CrossRef]
- Wu, N.; Sun, H.; Zhao, X.; Zhang, Y.; Tan, J.; Qi, Y.; Wang, Q.; Ng, M.; Liu, Z.; He, L.; et al. MAP3K2-regulated intestinal stromal cells define a distinct stem cell niche. Nature 2021, 592, 606–610. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. PI-103 and Quercetin Attenuate PI3K-AKT Signaling Pathway in T-Cell Lymphoma Exposed to Hydrogen Peroxide. PLoS ONE 2016, 11, e0160686. [Google Scholar] [CrossRef]
- Sun, X.; Majumder, P.; Shioya, H.; Wu, F.; Kumar, S.; Weichselbaum, R.; Kharbanda, S.; Kufe, D. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J. Biol. Chem. 2000, 275, 17237–17240. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, T.; Natsume, T.; Kufe, D.; Miki, Y. JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat. Cell Biol. 2005, 7, 278–285. [Google Scholar] [CrossRef]
- Heppner, D.E.; van der Vliet, A. Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol. 2016, 8, 24–27. [Google Scholar] [CrossRef]
- Truong, T.H.; Ung, P.M.; Palde, P.B.; Paulsen, C.E.; Schlessinger, A.; Carroll, K.S. Molecular Basis for Redox Activation of Epidermal Growth Factor Receptor Kinase. Cell Chem. Biol. 2016, 23, 837–848. [Google Scholar] [CrossRef]
- Ushio-Fukai, M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2007, 9, 731–739. [Google Scholar] [CrossRef]
- Garcia, L.; Garcia, F.; Llorens, F.; Unzeta, M.; Itarte, E.; Gómez, N. PP1/PP2A phosphatases inhibitors okadaic acid and calyculin A block ERK5 activation by growth factors and oxidative stress. FEBS Lett. 2002, 523, 90–94. [Google Scholar] [CrossRef]
- Khan, A.U.H.; Allende-Vega, N.; Gitenay, D.; Garaude, J.; Vo, D.N.; Belkhala, S.; Gerbal-Chaloin, S.; Gondeau, C.; Daujat-Chavanieu, M.; Delettre, C.; et al. Mitochondrial Complex I activity signals antioxidant response through ERK5. Sci. Rep. 2018, 8, 7420. [Google Scholar] [CrossRef]
- Coon, B.G.; Timalsina, S.; Astone, M.; Zhuang, Z.W.; Fang, J.; Han, J.; Themen, J.; Chung, M.; Yang-Klingler, Y.J.; Jain, M.; et al. A mitochondrial contribution to anti-inflammatory shear stress signaling in vascular endothelial cells. J. Cell Biol. 2022, 221, e202109144. [Google Scholar] [CrossRef]
- Broustas, C.G.; Duval, A.J.; Chaudhary, K.R.; Friedman, R.A.; Virk, R.K.; Lieberman, H.B. Targeting MEK5 impairs nonhomologous end-joining repair and sensitizes prostate cancer to DNA damaging agents. Oncogene 2020, 39, 2467–2477. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, G.; Cai, F.; Chen, X.; Cao, N.; Zhang, X.; Liu, J.; Chen, F.; Wang, F.; Dong, W.; et al. Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response. Exp. Mol. Med. 2019, 51, 1–20. [Google Scholar] [CrossRef]
- Ulrich-Merzenich, G.; Zeitler, H.; Panek, D.; Bokemeyer, D.; Vetter, H. Vitamin C promotes human endothelial cell growth via the ERK-signaling pathway. Eur. J. Nutr. 2007, 46, 87–94. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Lim, J.H.; Lee, C.; Choi, H.C.; Woo, C.H. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J. Biol. Chem. 2012, 287, 40722–40731. [Google Scholar] [CrossRef]
- Verhamme, P.; Hoylaerts, M.F. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin. Belg. 2006, 61, 213–219. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Kurisu, S.; Yoshimizu, A.; Sasaki, N.; Matsuura, H.; Kajiyama, G.; Oshima, T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: Role of endothelium-derived nitric oxide. Circulation 1999, 100, 1194–1202. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Kimura, M.; Noma, K.; Sasaki, S.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; et al. Low body mass index is a risk factor for impaired endothelium-dependent vasodilation in humans: Role of nitric oxide and oxidative stress. J. Am. Coll. Cardiol. 2003, 42, 256–263. [Google Scholar] [CrossRef]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef]
- Guzik, T.J.; West, N.E.; Black, E.; McDonald, D.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Vascular superoxide production by NAD(P)H oxidase: Association with endothelial dysfunction and clinical risk factors. Circ. Res. 2000, 86, E85–E90. [Google Scholar] [CrossRef]
- Sowers, J.R. Hypertension, angiotensin II, and oxidative stress. N. Engl. J. Med. 2002, 346, 1999–2001. [Google Scholar] [CrossRef]
- Leung, S.W.; Vanhoutte, P.M. Endothelium-dependent hyperpolarization: Age, gender and blood pressure, do they matter? Acta Physiol. 2017, 219, 108–123. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Boulanger, C.M. Endothelium. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e26–e31. [Google Scholar] [CrossRef]
- Sohn, S.J.; Sarvis, B.K.; Cado, D.; Winoto, A. ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J. Biol. Chem. 2002, 277, 43344–43351. [Google Scholar] [CrossRef]
- Hayashi, M.; Kim, S.W.; Imanaka-Yoshida, K.; Yoshida, T.; Abel, E.D.; Eliceiri, B.; Yang, Y.; Ulevitch, R.J.; Lee, J.D. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J. Clin. Investig. 2004, 113, 1138–1148. [Google Scholar] [CrossRef]
- Regan, C.P.; Li, W.; Boucher, D.M.; Spatz, S.; Su, M.S.; Kuida, K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc. Natl. Acad. Sci. USA 2002, 99, 9248–9253. [Google Scholar] [CrossRef]
- Yan, L.; Carr, J.; Ashby, P.R.; Murry-Tait, V.; Thompson, C.; Arthur, J.S. Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev. Biol. 2003, 3, 11. [Google Scholar] [CrossRef]
- Nithianandarajah-Jones, G.N.; Wilm, B.; Goldring, C.E.; Müller, J.; Cross, M.J. The role of ERK5 in endothelial cell function. Biochem. Soc. Trans. 2014, 42, 1584–1589. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, X.; Lu, B.; Cameron, M.; Fearns, C.; Patricelli, M.P.; Yates, J.R., III; Gray, N.S.; Lee, J.D. Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell 2010, 18, 258–267. [Google Scholar] [CrossRef]
- Tatake, R.J.; O’Neill, M.M.; Kennedy, C.A.; Wayne, A.L.; Jakes, S.; Wu, D.; Kugler, S.Z., Jr.; Kashem, M.A.; Kaplita, P.; Snow, R.J. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem. Biophys. Res. Commun. 2008, 377, 120–125. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Paim, B.A.; Castilho, R.F.; Velho, J.A.; Delgado, R.; Vercesi, A.E.; Oliveira, H.C. Mangifera indica L. extract (Vimang) and its main polyphenol mangiferin prevent mitochondrial oxidative stress in atherosclerosis-prone hypercholesterolemic mouse. Pharmacol. Res. 2008, 57, 332–338. [Google Scholar] [CrossRef]
- Xia, G.; Li, X.; Zhu, X.; Yin, X.; Ding, H.; Qiao, Y. Mangiferin protects osteoblast against oxidative damage by modulation of ERK5/Nrf2 signaling. Biochem. Biophys. Res. Commun. 2017, 491, 807–813. [Google Scholar] [CrossRef]
- Lee, E.S.; Boldo, L.S.; Fernandez, B.O.; Feelisch, M.; Harmsen, M.C. Suppression of TAK1 pathway by shear stress counteracts the inflammatory endothelial cell phenotype induced by oxidative stress and TGF-beta1. Sci. Rep. 2017, 7, 42487. [Google Scholar] [CrossRef]
- Gan, W.; Dang, Y.; Han, X.; Ling, S.; Duan, J.; Liu, J.; Xu, J.W. ERK5/HDAC5-mediated, resveratrol-, and pterostilbene-induced expression of MnSOD in human endothelial cells. Mol. Nutr. Food Res. 2016, 60, 266–277. [Google Scholar] [CrossRef]
- Fava, C.; Montagnana, M. Atherosclerosis Is an Inflammatory Disease which Lacks a Common Anti-inflammatory Therapy: How Human Genetics Can Help to This Issue. A Narrative Review. Front. Pharmacol. 2018, 9, 55. [Google Scholar] [CrossRef]
- Vaccarezza, M.; Balla, C.; Rizzo, P. Atherosclerosis as an inflammatory disease: Doubts? No more. Int. J. Cardiol. Heart Vasc. 2018, 19, 1–2. [Google Scholar] [CrossRef]
- Kamal, K.; Du, W.; Mills, I.; Sumpio, B.E. Antiproliferative effect of elevated glucose in human microvascular endothelial cells. J. Cell. Biochem. 1998, 71, 491–501. [Google Scholar] [CrossRef]
- Liu, W.; Schoenkerman, A.; Lowe, W.L., Jr. Activation of members of the mitogen-activated protein kinase family by glucose in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E782–E790. [Google Scholar] [CrossRef]
- Woo, C.H.; Shishido, T.; McClain, C.; Lim, J.H.; Li, J.D.; Yang, J.; Yan, C.; Abe, J. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ. Res. 2008, 102, 538–545. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Andersen-Ranberg, K.; Jeune, B.; Pedersen, A.N.; Skinhøj, P.; Pedersen, B.K. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, M357–M364. [Google Scholar] [CrossRef]
- Ohnesorge, N.; Viemann, D.; Schmidt, N.; Czymai, T.; Spiering, D.; Schmolke, M.; Ludwig, S.; Roth, J.; Goebeler, M.; Schmidt, M. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4). J. Biol. Chem. 2010, 285, 26199–26210. [Google Scholar] [CrossRef]
- Wu, K.; Tian, S.; Zhou, H.; Wu, Y. Statins protect human endothelial cells from TNF-induced inflammation via ERK5 activation. Biochem. Pharmacol. 2013, 85, 1753–1760. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Yang, M.; Zhang, M.; Xiao, M.; Li, X. Butyrate mitigates TNF-α-induced attachment of monocytes to endothelial cells. J. Bioenerg. Biomembr. 2020, 52, 247–256. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, S.; Du, Z.; Ke, A.; Liang, Q.; Li, X. KLF2 Protects against Osteoarthritis by Repressing Oxidative Response through Activation of Nrf2/ARE Signaling In Vitro and In Vivo. Oxid. Med. Cell. Longev. 2019, 2019, 8564681. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, X.; Shi, X.; Zheng, C. Halofuginone inhibits LPS-induced attachment of monocytes to HUVECs. Int. Immunopharmacol. 2020, 87, 106753. [Google Scholar] [CrossRef]
- Sun, Z.; Han, Y.; Song, S.; Chen, T.; Han, Y.; Liu, Y. Activation of GPR81 by lactate inhibits oscillatory shear stress-induced endothelial inflammation by activating the expression of KLF2. IUBMB Life 2019, 71, 2010–2019. [Google Scholar] [CrossRef]
- Alipui, C.; Ramos, K.; Tenner, T.E., Jr. Alterations of rabbit aortic smooth muscle cell proliferation in diabetes mellitus. Cardiovasc. Res. 1993, 27, 1229–1232. [Google Scholar] [CrossRef]
- Natarajan, R.; Gonzales, N.; Xu, L.; Nadler, J.L. Vascular smooth muscle cells exhibit increased growth in response to elevated glucose. Biochem. Biophys. Res. Commun. 1992, 187, 552–560. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- Yu, S.H.; Yu, J.M.; Yoo, H.J.; Lee, S.J.; Kang, D.H.; Cho, Y.J.; Kim, D.M. Anti-Proliferative Effects of Rutin on OLETF Rat Vascular Smooth Muscle Cells Stimulated by Glucose Variability. Yonsei Med. J. 2016, 57, 373–381. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Reckless, J.P. Fluvastatin. Expert Opin. Pharmacother. 2002, 3, 1631–1641. [Google Scholar] [CrossRef]
- Hwang, A.R.; Han, J.H.; Lim, J.H.; Kang, Y.J.; Woo, C.H. Fluvastatin inhibits AGE-induced cell proliferation and migration via an ERK5-dependent Nrf2 pathway in vascular smooth muscle cells. PLoS ONE 2017, 12, e0178278. [Google Scholar] [CrossRef]
- Wang, C.C.; Sharma, G.; Draznin, B. Early growth response gene-1 expression in vascular smooth muscle cells effects of insulin and oxidant stress. Am. J. Hypertens. 2006, 19, 366–372. [Google Scholar] [CrossRef]
- Blaschke, F.; Bruemmer, D.; Law, R.E. Egr-1 is a major vascular pathogenic transcription factor in atherosclerosis and restenosis. Rev. Endocr. Metab. Disord. 2004, 5, 249–254. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672. [Google Scholar]
- Schiffrin, E.L. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J. Cardiovasc. Pharmacol. 2001, 38 (Suppl. 2), S3–S6. [Google Scholar] [CrossRef]
- Kubo, T.; Ibusuki, T.; Chiba, S.; Kambe, T.; Fukumori, R. Mitogen-activated protein kinase activity regulation role of angiotensin and endothelin systems in vascular smooth muscle cells. Eur. J. Pharmacol. 2001, 411, 27–34. [Google Scholar] [CrossRef]
- Touyz, R.M.; He, G.; El Mabrouk, M.; Diep, Q.; Mardigyan, V.; Schiffrin, E.L. Differential activation of extracellular signal-regulated protein kinase 1/2 and p38 mitogen activated-protein kinase by AT1 receptors in vascular smooth muscle cells from Wistar-Kyoto rats and spontaneously hypertensive rats. J. Hypertens. 2001, 19, 553–559. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Gorospe, M.; Udelsman, R.; Holbrook, N.J. Acute hypertension activates mitogen-activated protein kinases in arterial wall. J. Clin. Investig. 1996, 97, 508–514. [Google Scholar] [CrossRef]
- Touyz, R.M.; Cruzado, M.; Tabet, F.; Yao, G.; Salomon, S.; Schiffrin, E.L. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: Role of receptor tyrosine kinase transactivation. Can. J. Physiol. Pharmacol. 2003, 81, 159–167. [Google Scholar] [CrossRef]
- Touyz, R.M.; Yao, G.; Viel, E.; Amiri, F.; Schiffrin, E.L. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J. Hypertens. 2004, 22, 1141–1149. [Google Scholar] [CrossRef]
- Morimoto, H.; Kanastu-Shinohara, M.; Ogonuki, N.; Kamimura, S.; Ogura, A.; Yabe-Nishimura, C.; Mori, Y.; Morimoto, T.; Watanabe, S.; Otsu, K.; et al. ROS amplification drives mouse spermatogonial stem cell self-renewal. Life Sci. Alliance 2019, 2, e201900374. [Google Scholar] [CrossRef]
- Münzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef]
- Devary, Y.; Gottlieb, R.A.; Smeal, T.; Karin, M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell 1992, 71, 1081–1091. [Google Scholar] [CrossRef]
- Baas, A.S.; Berk, B.C. Differential activation of mitogen-activated protein kinases by H2O2 and O2− in vascular smooth muscle cells. Circ. Res. 1995, 77, 29–36. [Google Scholar] [CrossRef]
- Takeishi, Y.; Abe, J.I.; Lee, J.D.; Kawakatsu, H.; Walsh, R.A.; Berk, B.C. Differential regulation of p90 ribosomal S6 kinase and big mitogen-activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ. Res. 1999, 85, 1164–1172. [Google Scholar] [CrossRef]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Devereux, R.B.; Roman, M.J.; Paranicas, M.; O’Grady, M.J.; Lee, E.T.; Welty, T.K.; Fabsitz, R.R.; Robbins, D.; Rhoades, E.R.; Howard, B.V. Impact of diabetes on cardiac structure and function: The strong heart study. Circulation 2000, 101, 2271–2276. [Google Scholar] [CrossRef]
- Yan, C.; Ding, B.; Shishido, T.; Woo, C.H.; Itoh, S.; Jeon, K.I.; Liu, W.; Xu, H.; McClain, C.; Molina, C.A.; et al. Activation of extracellular signal-regulated kinase 5 reduces cardiac apoptosis and dysfunction via inhibition of a phosphodiesterase 3A/inducible cAMP early repressor feedback loop. Circ. Res. 2007, 100, 510–519. [Google Scholar] [CrossRef]
- Kimura, T.E.; Jin, J.; Zi, M.; Prehar, S.; Liu, W.; Oceandy, D.; Abe, J.; Neyses, L.; Weston, A.H.; Cartwright, E.J.; et al. Targeted deletion of the extracellular signal-regulated protein kinase 5 attenuates hypertrophic response and promotes pressure overload-induced apoptosis in the heart. Circ. Res. 2010, 106, 961–970. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhou, J.; Liang, C.; Hong, K.; Cheng, X.S.; Wu, Z.G. ERK5 knock down aggravates detrimental effects of hypothermal stimulation on cardiomyocytes via Bim upregulation. Environ. Toxicol. Pharmacol. 2013, 36, 724–731. [Google Scholar] [CrossRef]
- Liu, W.; Ruiz-Velasco, A.; Wang, S.; Khan, S.; Zi, M.; Jungmann, A.; Dolores Camacho-Muñoz, M.; Guo, J.; Du, G.; Xie, L.; et al. Metabolic stress-induced cardiomyopathy is caused by mitochondrial dysfunction due to attenuated Erk5 signaling. Nat. Commun. 2017, 8, 494. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Naoe, T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol. Rep. 2006, 16, 845–850. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, Y.; Zhang, H.; Xie, D.; Nie, W.; Shi, K. Inhibition of microRNA-143-3p attenuates myocardial hypertrophy by inhibiting inflammatory response. Cell Biol. Int. 2018, 42, 1584–1593. [Google Scholar] [CrossRef]
- Cameron, S.J.; Ture, S.K.; Mickelsen, D.; Chakrabarti, E.; Modjeski, K.L.; McNitt, S.; Seaberry, M.; Field, D.J.; Le, N.T.; Abe, J.; et al. Platelet Extracellular Regulated Protein Kinase 5 Is a Redox Switch and Triggers Maladaptive Platelet Responses and Myocardial Infarct Expansion. Circulation 2015, 132, 47–58. [Google Scholar] [CrossRef]
- Yang, M.; Cooley, B.C.; Li, W.; Chen, Y.; Vasquez-Vivar, J.; Scoggins, N.O.; Cameron, S.J.; Morrell, C.N.; Silverstein, R.L. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood 2017, 129, 2917–2927. [Google Scholar] [CrossRef]
- Singh, M.V.; Kotla, S.; Le, N.T.; Ae Ko, K.; Heo, K.S.; Wang, Y.; Fujii, Y.; Thi Vu, H.; McBeath, E.; Thomas, T.N.; et al. Senescent Phenotype Induced by p90RSK-NRF2 Signaling Sensitizes Monocytes and Macrophages to Oxidative Stress in HIV-Positive Individuals. Circulation 2019, 139, 1199–1216. [Google Scholar] [CrossRef]
- Li, D.; Jin, L.; Alesi, G.N.; Kim, Y.M.; Fan, J.; Seo, J.H.; Wang, D.; Tucker, M.; Gu, T.L.; Lee, B.H.; et al. The prometastatic ribosomal S6 kinase 2-cAMP response element-binding protein (RSK2-CREB) signaling pathway up-regulates the actin-binding protein fascin-1 to promote tumor metastasis. J. Biol. Chem. 2013, 288, 32528–32538. [Google Scholar] [CrossRef]
- Kotla, S.; Zhang, A.; Imanishi, M.; Ko, K.A.; Lin, S.H.; Gi, Y.J.; Moczygemba, M.; Isgandarova, S.; Schadler, K.L.; Chung, C.; et al. Nucleus-mitochondria positive feedback loop formed by ERK5 S496 phosphorylation-mediated poly (ADP-ribose) polymerase activation provokes persistent pro-inflammatory senescent phenotype and accelerates coronary atherosclerosis after chemoradiation. Redox Biol. 2021, 47, 102132. [Google Scholar] [CrossRef]
- Henricks, P.A.; Nijkamp, F.P. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther. 2001, 14, 409–420. [Google Scholar] [CrossRef]
- MacNee, W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Nohl, H.; Kozlov, A.V.; Gille, L.; Staniek, K. Cell respiration and formation of reactive oxygen species: Facts and artefacts. Biochem. Soc. Trans. 2003, 31, 1308–1311. [Google Scholar] [CrossRef]
- Liu, P.L.; Chen, Y.L.; Chen, Y.H.; Lin, S.J.; Kou, Y.R. Wood smoke extract induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: Role of AIF and EndoG. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2005, 289, L739–L749. [Google Scholar] [CrossRef]
- Vayssier-Taussat, M.; Camilli, T.; Aron, Y.; Meplan, C.; Hainaut, P.; Polla, B.S.; Weksler, B. Effects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responses. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1293–H1300. [Google Scholar] [CrossRef]
- Mossman, B.T.; Kamp, D.W.; Weitzman, S.A. Mechanisms of carcinogenesis and clinical features of asbestos-associated cancers. Cancer Investig. 1996, 14, 466–480. [Google Scholar] [CrossRef]
- Scapoli, L.; Ramos-Nino, M.E.; Martinelli, M.; Mossman, B.T. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene 2004, 23, 805–813. [Google Scholar] [CrossRef]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef]
- Lopez-Royuela, N.; Rathore, M.G.; Allende-Vega, N.; Annicotte, J.S.; Fajas, L.; Ramachandran, B.; Gulick, T.; Villalba, M. Extracellular-signal-regulated kinase 5 modulates the antioxidant response by transcriptionally controlling Sirtuin 1 expression in leukemic cells. Int. J. Biochem. Cell Biol. 2014, 53, 253–261. [Google Scholar] [CrossRef]
- Wu, Y.H.; Lin, H.R.; Lee, Y.H.; Huang, P.H.; Wei, H.C.; Stern, A.; Chiu, D.T. A novel fine tuning scheme of miR-200c in modulating lung cell redox homeostasis. Free Radic. Res. 2017, 51, 591–603. [Google Scholar] [CrossRef]
- Xue, Z.; Gao, X.; Yu, W.; Zhang, Q.; Song, W.; Li, S.; Zheng, X.; Kou, X. Biochanin A alleviates oxidative damage caused by the urban particulate matter. Food Funct. 2021, 12, 1958–1972. [Google Scholar] [CrossRef]
- Prieto-Bermejo, R.; Romo-González, M.; Pérez-Fernández, A.; Ijurko, C.; Hernández-Hernández, Á. Reactive oxygen species in haematopoiesis: Leukaemic cells take a walk on the wild side. J. Exp. Clin. Cancer Res. 2018, 37, 125. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, Y.; Cai, J.; Li, X.; Xu, F.; Yuan, N.; Zhang, S.; Wang, J. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology 2016, 21, 613–618. [Google Scholar] [CrossRef]
- Hole, P.S.; Darley, R.L.; Tonks, A. Do reactive oxygen species play a role in myeloid leukemias? Blood 2011, 117, 5816–5826. [Google Scholar] [CrossRef]
- Charni, S.; de Bettignies, G.; Rathore, M.G.; Aguiló, J.I.; van den Elsen, P.J.; Haouzi, D.; Hipskind, R.A.; Enriquez, J.A.; Sanchez-Beato, M.; Pardo, J.; et al. Oxidative phosphorylation induces de novo expression of the MHC class I in tumor cells through the ERK5 pathway. J. Immunol. 2010, 185, 3498–3503. [Google Scholar] [CrossRef]
- Susuki, T.; Yang, J. Hydrogen peroxide activation of ERK5 confers resistance to Jurkat cells against apoptosis induced by the extrinsic pathway. Biochem. Biophys. Res. Commun. 2014, 444, 248–253. [Google Scholar] [CrossRef]
- Khan, A.U.H.; Rathore, M.G.; Allende-Vega, N.; Vo, D.N.; Belkhala, S.; Orecchioni, S.; Talarico, G.; Bertolini, F.; Cartron, G.; Lecellier, C.H.; et al. Human Leukemic Cells performing Oxidative Phosphorylation (OXPHOS) Generate an Antioxidant Response Independently of Reactive Oxygen species (ROS) Production. eBioMedicine 2015, 3, 43–53. [Google Scholar] [CrossRef]
- Morena, M.; Cristol, J.P.; Senécal, L.; Leray-Moragues, H.; Krieter, D.; Canaud, B. Oxidative stress in hemodialysis patients: Is NADPH oxidase complex the culprit? Kidney Int. 2002, 61, S109–S114. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K.L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Nishiyama, A.; Yao, L.; Nagai, Y.; Miyata, K.; Yoshizumi, M.; Kagami, S.; Kondo, S.; Kiyomoto, H.; Shokoji, T.; Kimura, S.; et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 2004, 43, 841–848. [Google Scholar] [CrossRef]
- Ishizawa, K.; Izawa-Ishizawa, Y.; Dorjsuren, N.; Miki, E.; Kihira, Y.; Ikeda, Y.; Hamano, S.; Kawazoe, K.; Minakuchi, K.; Tomita, S.; et al. Angiotensin II receptor blocker attenuates PDGF-induced mesangial cell migration in a receptor-independent manner. Nephrol. Dial. Transplant. 2010, 25, 364–372. [Google Scholar] [CrossRef]
- Urushihara, M.; Takamatsu, M.; Shimizu, M.; Kondo, S.; Kinoshita, Y.; Suga, K.; Kitamura, A.; Matsuura, S.; Yoshizumi, M.; Tamaki, T.; et al. ERK5 activation enhances mesangial cell viability and collagen matrix accumulation in rat progressive glomerulonephritis. Am. J. Physiol. Renal. Physiol. 2010, 298, F167–F176. [Google Scholar] [CrossRef]
- Ha, H.; Lee, H.B. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int. Suppl. 2000, 58, S19–S25. [Google Scholar] [CrossRef]
- Tomlinson, D.R. Mitogen-activated protein kinases as glucose transducers for diabetic complications. Diabetologia 1999, 42, 1271–1281. [Google Scholar] [CrossRef]
- Suzaki, Y.; Yoshizumi, M.; Kagami, S.; Nishiyama, A.; Ozawa, Y.; Kyaw, M.; Izawa, Y.; Kanematsu, Y.; Tsuchiya, K.; Tamaki, T. BMK1 is activated in glomeruli of diabetic rats and in mesangial cells by high glucose conditions. Kidney Int. 2004, 65, 1749–1760. [Google Scholar] [CrossRef]
- Kawakami, T.; Park, S.W.; Kaku, R.; Yang, J. Extracellular-regulated-kinase 5-mediated renal protection against ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2012, 418, 603–608. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Lin, S.Y.; Dan, X.; Du, X.X.; Ran, C.L.; Lu, X.; Ren, S.J.; Tang, Z.T.; Yin, L.Z.; He, C.L.; Yuan, Z.X.; et al. Protective Effects of Salidroside against Carbon Tetrachloride (CCl4)-Induced Liver Injury by Initiating Mitochondria to Resist Oxidative Stress in Mice. Int. J. Mol. Sci. 2019, 20, 3187. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Hüttemann, M. Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef]
- Liu, L.; Cavanaugh, J.E.; Wang, Y.; Sakagami, H.; Mao, Z.; Xia, Z. ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc. Natl. Acad. Sci. USA 2003, 100, 8532–8537. [Google Scholar] [CrossRef]
- Shalizi, A.; Lehtinen, M.; Gaudilliere, B.; Donovan, N.; Han, J.; Konishi, Y.; Bonni, A. Characterization of a neurotrophin signaling mechanism that mediates neuron survival in a temporally specific pattern. J. Neurosci. 2003, 23, 7326–7336. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Nikam, S.; Nikam, P.; Ahaley, S.K.; Sontakke, A.V. Oxidative stress in Parkinson’s disease. Indian J. Clin. Biochem. 2009, 24, 98–101. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Przedborski, S. Oxidative stress in Parkinson’s disease: A mechanism of pathogenic and therapeutic significance. Ann. N. Y. Acad. Sci. 2008, 1147, 93–104. [Google Scholar] [CrossRef]
- Chi, L.; Ke, Y.; Luo, C.; Gozal, D.; Liu, R. Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience 2007, 144, 991–1003. [Google Scholar] [CrossRef]
- Su, C.; Sun, F.; Cunningham, R.L.; Rybalchenko, N.; Singh, M. ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. Age 2014, 36, 9685. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–357. [Google Scholar] [CrossRef]
- Liu, X.W.; Zi, Y.; Liu, Y.E.; Zhang, Y.B.; Xiang, L.B.; Hou, M.X. Melatonin exerts protective effect on N2a cells under hypoxia conditions through Zip1/ERK pathway. Neurosci. Lett. 2015, 595, 74–80. [Google Scholar] [CrossRef]
- Potdar, S.; Parmar, M.S.; Ray, S.D.; Cavanaugh, J.E. Protective effects of the resveratrol analog piceid in dopaminergic SH-SY5Y cells. Arch. Toxicol. 2018, 92, 669–677. [Google Scholar] [CrossRef]
- Cavanaugh, J.E.; Jaumotte, J.D.; Lakoski, J.M.; Zigmond, M.J. Neuroprotective role of ERK1/2 and ERK5 in a dopaminergic cell line under basal conditions and in response to oxidative stress. J. Neurosci. Res. 2006, 84, 1367–1375. [Google Scholar] [CrossRef]
- Racette, B.A.; Aschner, M.; Guilarte, T.R.; Dydak, U.; Criswell, S.R.; Zheng, W. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology 2012, 33, 881–886. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Ding, H.; Wang, F.; Su, L.; Zhao, L.; Hu, B.; Zheng, W.; Yao, S.; Li, Y. Involvement of MEK5/ERK5 signaling pathway in manganese-induced cell injury in dopaminergic MN9D cells. J. Trace Elem. Med. Biol. 2020, 61, 126546. [Google Scholar] [CrossRef]
- Wang, R.M.; Zhang, Q.G.; Zhang, G.Y. Activation of ERK5 is mediated by N-methyl-D-aspartate receptor and L-type voltage-gated calcium channel via Src involving oxidative stress after cerebral ischemia in rat hippocampus. Neurosci. Lett. 2004, 357, 13–16. [Google Scholar] [CrossRef]
- Tubita, A.; Tusa, I.; Rovida, E. Playing the Whack-A-Mole Game: ERK5 Activation Emerges Among the Resistance Mechanisms to RAF-MEK1/2-ERK1/2-Targeted Therapy. Front. Cell Dev. Biol. 2021, 9, 647311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).