The Multifaceted Role of Annexin A1 in Viral Infections
Abstract
1. Introduction
2. Inflammation, the Resolution Phase and Its Importance during Infections
3. Annexin A1
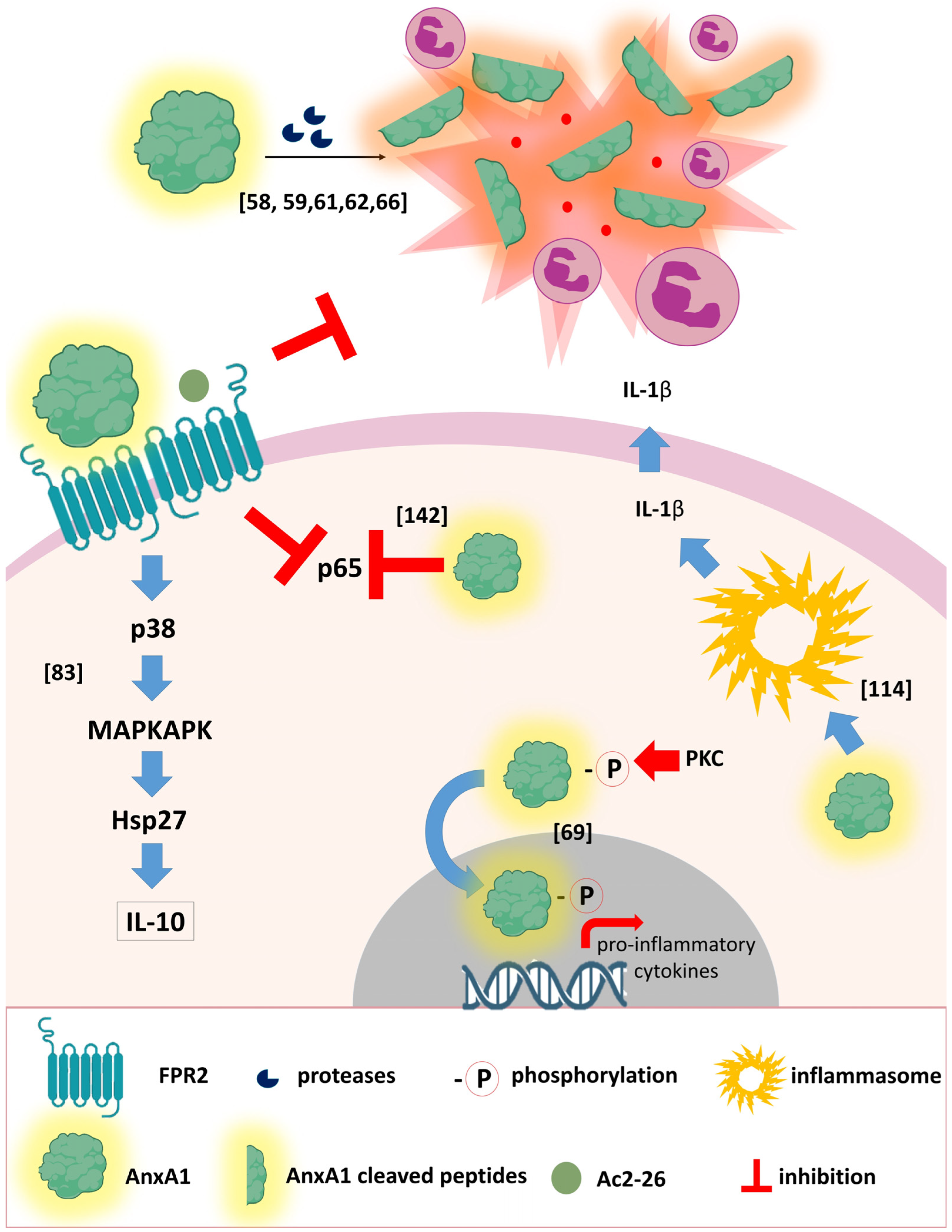
3.1. The AnxA1 Receptor
3.2. Annexin A1 during Respiratory Viral Infections
3.2.1. SARS-CoV-2 (COVID-2019)
3.2.2. Influenza Virus (IAV)
3.3. Annexin A1 during Non-Respiratory Viral Infections
3.3.1. Arbovirus
3.3.2. Hepatitis C Virus (HCV)
3.3.3. Human Papillomavirus (HPV)
3.3.4. Simian Immunodeficiency Virus (SIV)
3.3.5. Herpes Simplex Virus 1 (HSV-1)
3.3.6. Human T-Lymphotropic Virus 1 (HTLV-1)
3.3.7. Human Immunodeficiency Virus (HIV)
3.3.8. Measles/Reovirus
3.3.9. Foot and Mouth Disease Virus (FMDV)
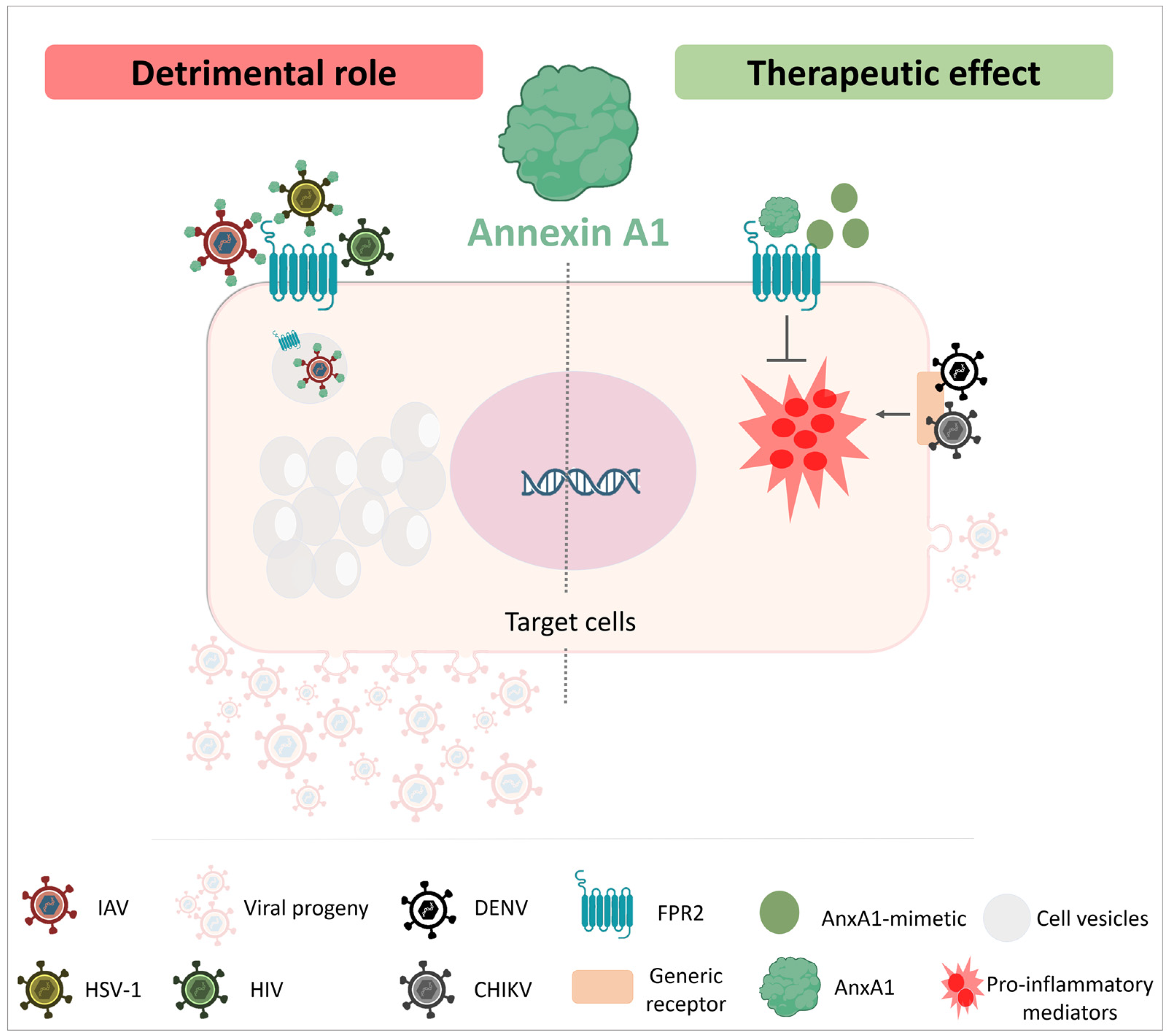
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–21. Lancet 2022, 399, 1513–1536, Erratum in Lancet 2022, 399, 1468. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, M.; Dhull, D.; Sharma, Y.; Kaushik, S.; Kaushik, S. Zika virus: An emerging challenge to public health worldwide. Can. J. Microbiol. 2020, 66, 87–98. [Google Scholar] [CrossRef]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C.; Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A., 2nd; Hewlett, A.; Kraft, C.S.; Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Prim. 2020, 6, 13. [Google Scholar] [CrossRef]
- Lanrewaju, A.A.; Enitan-Folami, A.M.; Sabiu, S.; Edokpayi, J.N.; Swalaha, F.M. Global public health implications of human exposure to viral contaminated water. Front. Microbiol. 2022, 13, 981896. [Google Scholar] [CrossRef] [PubMed]
- Torres-Flores, J.M.; Reyes-Sandoval, A.; Salazar, M.I. Dengue Vaccines: An Update. Biodrugs 2022, 36, 325–336. [Google Scholar] [CrossRef]
- Stephenson, K.E. Novel chikungunya vaccine shows promise for durable protection. Lancet Infect. Dis. 2022, 22, 1259–1261. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Maskin, L.P.; Bonelli, I.; Olarte, G.L.; Palizas, J.F.; Velo, A.E.; Lurbet, M.F.; Lovazzano, P.; Kotsias, S.; Attie, S.; Saubidet, I.L.; et al. High- Versus Low-Dose Dexamethasone for the Treatment of COVID-19-Related Acute Respiratory Distress Syndrome: A Multicenter, Randomized Open-Label Clinical Trial. J. Intensiv. Care Med. 2022, 37, 491–499. [Google Scholar] [CrossRef] [PubMed]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Gilroy, D.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J. Immunol. Res. 2016, 2016, 8239258. [Google Scholar] [CrossRef]
- Sousa, L.P.; Pinho, V.; Teixeira, M.M. Harnessing inflammation resolving-based therapeutic agents to treat pulmonary viral infections: What can the future offer to COVID-19? Br. J. Pharmacol. 2020, 177, 3898–3904. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the Resolution of the Inflammatory Response. Trends Immunol. 2019, 40, 212–227. [Google Scholar] [CrossRef]
- Tavares, L.P.; Melo, E.M.; Sousa, L.P.; Teixeira, M.M. Pro-resolving therapies as potential adjunct treatment for infectious diseases: Evidence from studies with annexin A1 and angiotensin-(1-7). Semin. Immunol. 2022, 59, 101601. [Google Scholar] [CrossRef] [PubMed]
- Gavins, F.N.E.; Yona, S.; Kamal, A.M.; Flower, R.J.; Perretti, M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood 2003, 101, 4140–4147. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Caloi, C.M.; Pimenta, S.T.S.; Seshayyan, S.; Govindarajulu, S.; Souto, F.J.D.; Damazo, A.S. Expression of annexin-A1 in blood and tissue leukocytes of leprosy patients. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200277. [Google Scholar] [CrossRef]
- Schloer, S.; Hübel, N.; Masemann, D.; Pajonczyk, D.; Brunotte, L.; Ehrhardt, C.; Brandenburg, L.; Ludwig, S.; Gerke, V.; Rescher, U. The annexin A1/FPR2 signaling axis expands alveolar macrophages, limits viral replication, and attenuates pathogenesis in the murine influenza A virus infection model. FASEB J. 2019, 33, 12188–12199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Wu, S.-R.; Yao, H.-W.; Ling, P.; Perng, G.-C.; Chiu, Y.-C.; Hsu, S.-M.; Chen, S.-H. Suppression of annexin A1 and its receptor reduces herpes simplex virus 1 lethality in mice. PLoS Pathog. 2022, 18, e1010692. [Google Scholar] [CrossRef]
- Yap, G.L.R.; Sachaphibulkij, K.; Foo, S.L.; Cui, J.; Fairhurst, A.-M.; Lim, L.H.K. Annexin-A1 promotes RIG-I-dependent signaling and apoptosis via regulation of the IRF3–IFNAR–STAT1–IFIT1 pathway in A549 lung epithelial cells. Cell Death Dis. 2020, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.; Guabiraba, R.; Soriani, F.M.; Teixeira, M.M. The development of anti-inflammatory drugs for infectious diseases. Discov. Med. 2010, 10, 479–488. [Google Scholar]
- Machado, M.G.; Tavares, L.P.; Souza, G.V.S.; Queiroz-Junior, C.M.; Ascenção, F.R.; Lopes, M.E.; Garcia, C.C.; Menezes, G.B.; Perretti, M.; Russo, R.C.; et al. The Annexin A1/FPR2 pathway controls the inflammatory response and bacterial dissemination in experimental pneumococcal pneumonia. FASEB J. 2020, 34, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.V.; Sugimoto, M.A.; Hubner, J.; Bonilha, C.S.; Queiroz-Junior, C.M.; Gonçalves-Pereira, M.H.; Chen, J.; Gobbetti, T.; Rodrigues, G.O.L.; Bambirra, J.L.; et al. Targeting the Annexin A1-FPR2/ALX pathway for host-directed therapy in dengue disease. Elife 2022, 11, e73853. [Google Scholar] [CrossRef]
- Vago, J.P.; Nogueira, C.R.C.; Tavares, L.P.; Soriani, F.M.; Lopes, F.; Russo, R.C.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Annexin A1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J. Leukoc. Biol. 2012, 92, 249–258. [Google Scholar] [CrossRef]
- de Araújo, S.; Costa, V.R.d.M.; Santos, F.M.; de Sousa, C.D.F.; Moreira, T.P.; Gonçalves, M.R.; Félix, F.B.; Queiroz-Junior, C.M.; Campolina-Silva, G.H.; Nogueira, M.L.; et al. Annexin A1-FPR2/ALX Signaling Axis Regulates Acute Inflammation during Chikungunya Virus Infection. Cells 2022, 11, 2717. [Google Scholar] [CrossRef]
- Tracy, R.P. The Five Cardinal Signs of Inflammation: Calor, Dolor, Rubor, Tumor ... and Penuria (Apologies to Aulus Cornelius Celsus, De medicina, c. A.D. 25). J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1051–1052. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Abe, T.; Marutani, Y.; Shoji, I. Cytosolic DNA-sensing immune response and viral infection. Microbiol. Immunol. 2019, 63, 51–64. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef] [PubMed]
- Mdkhana, B.; Saheb-Sharif Askari, N.; Ramakrishnan, R.K.; Goel, S.; Hamid, Q.; Halwani, R. Nucleic Acid-Sensing Pathways During SARS-CoV-2 Infection: Expectations versus Reality. J. Inflamm. Res. 2021, 14, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.J.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of in flammation: State of the art, definitions and terms. FASEB J. 2007, 21, 325–332. [Google Scholar] [CrossRef]
- Perretti, M.; Leroy, X.; Bland, E.J.; Montero-Melendez, T. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Hajishengallis, G.; Chavakis, T. Phagocytosis of Apoptotic Cells in Resolution of Inflammation. Front. Immunol. 2020, 11, 553. [Google Scholar] [CrossRef]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Vago, J.P.; Tavares, L.P.; Riccardi, C.; Teixeira, M.M.; Sousa, L.P. Exploiting the pro-resolving actions of glucocorticoid-induced proteins Annexin A1 and GILZ in infectious diseases. Biomed. Pharmacother. 2020, 133, 111033. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.J.; Blackwell, G.J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature 1979, 278, 456–459. [Google Scholar] [CrossRef]
- Flower, R. Eleventh Gaddum memorial lecture. Lipocortin and the mechanism of action of the glucocorticoids. Br. J. Pharmacol. 1988, 94, 987–1015. [Google Scholar] [CrossRef] [PubMed]
- D’Acunto, C.W.; Gbelcova, H.; Festa, M.; Ruml, T. The complex understanding of Annexin A1 phosphorylation. Cell Signal. 2014, 26, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.D.; Hoye, E.; Blackwood, R.A.; Jaye, D. Purification and characterization of an abundant cytosolic protein from human neutrophils that promotes Ca2(+)-dependent aggregation of isolated specific granules. J. Clin. Investig. 1990, 85, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Morand, E.; Hutchinson, P.; Hargreaves, A.; Goulding, N.J.; Boyce, N.W.; Holdsworth, S.R. Detection of intracellular lipocortin 1 in human leukocyte subsets. Clin. Immunol. Immunopathol. 1995, 76, 195–202. [Google Scholar] [CrossRef]
- Perretti, M.; Christian, H.; Wheller, S.K.; Aiello, I.; Mugridge, K.G.; Morris, J.F.; Flower, R.J.; Goulding, N.J. Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol. Int. 2000, 24, 163–174. [Google Scholar] [CrossRef]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef]
- Roviezzo, F.; Getting, S.J.; Paul-Clark, M.J.; Yona, S.; Gavins, F.N.; Perretti, M.; Hannon, R.; Croxtall, J.D.; Buckingham, J.C.; Flower, R.J. The annexin-1 knockout mouse: What it tells us about the inflammatory response. J. Physiol. Pharmacol. 2002, 53, 541–553. [Google Scholar]
- Hannon, R.; Croxtall, J.D.; Getting, S.J.; Roviezzo, F.; Yona, S.; Paul-Clark, M.J.; Gavins, F.N.E.; Perretti, M.; Morris, J.F.; Buckingham, J.C.; et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1-/- mouse. FASEB J. 2002, 17, 253–255. [Google Scholar] [CrossRef]
- Grewal, T.; Rentero, C.; Enrich, C.; Wahba, M.; Raabe, C.; Rescher, U. Annexin Animal Models—From Fundamental Principles to Translational Research. Int. J. Mol. Sci. 2021, 22, 3439. [Google Scholar] [CrossRef]
- Perretti, M.; Ahluwalia, A.; Harris, J.G.; Goulding, N.J.; Flower, R.J. Lipocortin-1 fragments inhibit neutrophil accumulation and neu-trophil-dependent edema in the mouse. A qualitative comparison with an anti-CD11b monoclonal antibody. J. Immunol. 1993, 151, 4306–4314. [Google Scholar] [CrossRef]
- Yang, Y.H.; Morand, E.F.; Getting, S.J.; Paul-Clark, M.; Liu, D.L.; Yona, S.; Hannon, R.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Modulation of inflammation and response to dexamethasone by Annexin 1 in antigen-induced arthritis. Arthritis Rheum. 2004, 50, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S.; Yazid, S.; Flower, R.J. Increased susceptibility of annexin-A1 null mice to nociceptive pain is indicative of a spinal antinociceptive action of annexin-A1. Br. J. Pharmacol. 2008, 154, 1135–1142. [Google Scholar] [CrossRef]
- Chatterjee, B.E.; Yona, S.; Rosignoli, G.; Young, R.E.; Nourshargh, S.; Flower, R.J.; Perretti, M. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J. Leukoc. Biol. 2005, 78, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Murata, H.; Sonegawa, H.; Sakaguchi, Y.; Futami, J.-I.; Kitazoe, M.; Yamada, H.; Huh, N.-H. Truncation of annexin A1 Is a regulatory lever for linking epidermal growth factor signaling with cytosolic phospholipase A2 in normal and malignant squamous epithelial cells. J. Biol. Chem. 2007, 282, 35679–35686. [Google Scholar] [CrossRef]
- Wang, W.; Creutz, C.E. Role of the amino-terminal domain in regulating interactions of annexin I with membranes: Effects of amino-terminal truncation and mutagenesis of the phosphorylation sites. Biochemistry 1994, 33, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Rescher, U.; Goebeler, V.; Wilbers, A.; Gerke, V. Proteolytic cleavage of annexin 1 by human leukocyte elastase. Biochim. Biophys. Acta. 2006, 1763, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; D’Acquisto, F.; Pederzoli-Ribeil, M.; Lavagno, L.; Flower, R.J.; Witko-Sarsat, V.; Perretti, M. Annexin 1 cleavage in activated neutrophils: A pivotal role for proteinase 3. J. Biol. Chem. 2007, 282, 29998–30004. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Oh, H.Y.; Kim, J.Y.; Kim, J.H.; Kim, I.Y. Allergen-induced proteolytic cleavage of annexin-1 and activation of cytosolic phospholipase A2 in the lungs of a mouse model of asthma. Proteomics 2004, 4, 3328–3334. [Google Scholar] [CrossRef]
- Tsao, F.H.C.; Meyer, K.C.; Chen, X.; Rosenthal, N.S.; Hu, J. Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 1998, 18, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Tsao, F.H.C.; Xiang, Z.; Abbasi, A.; Meyer, K.C. Neutrophil necrosis and annexin 1 degradation associated with airway inflammation in lung transplant recipients with cystic fibrosis. BMC Pulm. Med. 2012, 12, 44. [Google Scholar] [CrossRef]
- Smith, S.F.; Tetley, T.D.; Guz, A.; Flower, R.J. Detection of lipocortin 1 in human lung lavage fluid: Lipocortin degradation as a possible proteolytic mechanism in the control of inflammatory mediators and inflammation. Environ. Health Perspect. 1990, 85, 135–144. [Google Scholar] [CrossRef]
- Vago, J.P.; Tavares, L.P.; Sugimoto, M.A.; Lima, G.L.N.; Galvão, I.; de Caux, T.R.; Lima, K.M.; Ribeiro, A.L.C.; Carneiro, F.S.; Nunes, F.F.C.; et al. Proresolving Actions of Synthetic and Natural Protease Inhibitors Are Mediated by Annexin A1. J. Immunol. 2016, 196, 1922–1932. [Google Scholar] [CrossRef]
- Perretti, M.; Wheller, S.K.; Flower, R.J.; Wahid, S.; Pitzalis, C. Modulation of cellular annexin I in human leukocytes infiltrating DTH skin reactions. J. Leukoc. Biol. 1999, 65, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.M.; Vago, J.P.; Caux, T.R.; Negreiros-Lima, G.L.; Sugimoto, M.A.; Tavares, L.P.; Arribada, R.G.; Carmo, A.A.F.; Galvão, I.; Costa, B.R.C.; et al. The resolution of acute inflammation induced by cyclic AMP is dependent on annexin A1. J. Biol. Chem. 2017, 292, 13758–13773. [Google Scholar] [CrossRef]
- Galvão, I.; Vago, J.P.; Barroso, L.C.; Tavares, L.P.; Queiroz-Junior, C.M.; Costa, V.V.; Carneiro, F.S.; Ferreira, T.P.; Silva, P.M.R.; Amaral, F.A.; et al. Annexin A1 promotes timely resolution of inflammation in murine gout. Eur. J. Immunol. 2017, 47, 585–596. [Google Scholar] [CrossRef]
- Williams, S.L.; Milne, I.R.; Bagley, C.J.; Gamble, J.R.; Vadas, M.A.; Pitson, S.M.; Khew-Goodall, Y. A proinflammatory role for proteolytically cleaved annexin A1 in neutrophil transendothelial migration. J. Immunol. 2010, 185, 3057–3063. [Google Scholar] [CrossRef]
- Rüger, M.; Kipp, E.; Schubert, N.; Schröder, N.; Pufe, T.; Stope, M.B.; Kipp, M.; Blume, C.; Tauber, S.C.; Brandenburg, L.-O. The formyl peptide receptor agonist Ac2-26 alleviates neuroinflammation in a mouse model of pneumococcal meningitis. J. NeuroInflamm. 2020, 17, 325. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N.; Dahlén, S.E.; Drazen, J.M.; Hay, D.W.P.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006, 58, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, J.; Liu, L.; Li, X.; Liu, S.; Xia, Q.; Shi, J. Annexin A1 translocates to nucleus and promotes the expression of pro-inflammatory cytokines in a PKC-dependent manner after OGD/R. Sci. Rep. 2016, 6, 27028. [Google Scholar] [CrossRef] [PubMed]
- McArthur, S.; Juban, G.; Gobbetti, T.; Desgeorges, T.; Theret, M.; Gondin, J.; Toller-Kawahisa, J.E.; Reutelingsperger, C.P.; Chazaud, B.; Perretti, M.; et al. Annexin A1 drives macrophage skewing to accelerate muscle regeneration through AMPK activation. J. Clin. Investig. 2020, 130, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Leoni, G.; Hinkel, R.; Ormanns, S.; Paulin, N.; Ortega-Gomez, A.; Viola, J.R.; de Jong, R.; Bongiovanni, D.; Bozoglu, T.; et al. Pro-Angiogenic Macrophage Phenotype to Promote Myocardial Repair. J. Am. Coll. Cardiol. 2019, 73, 2990–3002. [Google Scholar] [CrossRef] [PubMed]
- Oliani, S.M.; Ciocca, G.A.; Pimentel, T.A.; Damazo, A.S.; Gibbs, L.; Perretti, M. Fluctuation of annexin-A1 positive mast cells in chronic granulomatous inflammation. Inflamm. Res. 2008, 57, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Sinniah, A.; Yazid, S.; Bena, S.; Oliani, S.M.; Perretti, M.; Flower, R.J. Endogenous Annexin-A1 Negatively Regulates Mast Cell-Mediated Allergic Reactions. Front. Pharmacol. 2019, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Rivière, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 2009, 459, 574–577. [Google Scholar] [CrossRef]
- Qin, C.X.; Norling, L.V.; Vecchio, E.A.; Brennan, E.P.; May, L.T.; Wootten, D.; Godson, C.; Perretti, M.; Ritchie, R.H. Formylpeptide receptor 2: Nomenclature, structure, signalling and translational perspectives: IUPHAR review 35. Br. J. Pharmacol. 2022, published online ahead of print. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, M.; Zong, X.; Ge, Y.; Zhang, H.; Wang, M.; Han, G.W.; Yi, C.; Ma, L.; Ye, R.D.; et al. Structural basis of ligand binding modes at the human formyl peptide receptor 2. Nat. Commun. 2020, 11, 1208. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, H.; Zhou, X.E.; Verma, R.K.; de Waal, P.W.; Jang, W.; Xu, T.-H.; Wang, L.; Meng, X.; Zhao, G.; et al. Structure of formylpeptide receptor 2-Gi complex reveals insights into ligand recognition and signaling. Nat. Commun. 2020, 11, 885. [Google Scholar] [CrossRef]
- He, H.Q.; Troksa, E.L.; Caltabiano, G.; Pardo, L.; Ye, R.D. Structural determinants for the interaction of formyl peptide receptor 2 with peptide ligands. J. Biol. Chem. 2014, 289, 2295–2306, Erratum in J. Biol. Chem. 2014, 289, 4814. [Google Scholar] [CrossRef]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International union of basic and clinical pharmacology. LXXIII. nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, L.; Guo, J.; Sun, D.; Wang, Y.; Liu, W.; Xu, H.E.; Zhang, C. Molecular recognition of formylpeptides and diverse agonists by the formylpeptide receptors FPR1 and FPR2. Nat. Commun. 2022, 13, 1054. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, S.; Wang, J.; Xia, F.; Wan, J.; Lu, J.; Ye, R.D. Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J. 2020, 34, 6920–6933. [Google Scholar] [CrossRef]
- Ge, Y.J.; Liao, Q.W.; Xu, Y.C.; Zhao, Q.; Wu, B.L.; Ye, R.D. Anti-inflammatory signaling through G protein-coupled receptors. Acta Pharmacol. Sin. 2020, 41, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.L.; Flower, R.J.; Perretti, M. Ligand-specific conformational change of the G-protein–coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef]
- Han, P.F.; Che, X.D.; Li, H.Z.; Gao, Y.Y.; Wei, X.C.; Li, P.C. Annexin A1 involved in the regulation of inflammation and cell signaling pathways. Chin. J. Traumatol. 2020, 23, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Tcherniuk, S.; Cenac, N.; Comte, M.; Frouard, J.; Errazuriz-Cerda, E.; Galabov, A.; Morange, P.-E.; Vergnolle, N.; Si-Tahar, M.; Alessi, M.-C.; et al. Formyl Peptide Receptor 2 Plays a Deleterious Role During Influenza A Virus Infections. J. Infect. Dis. 2016, 214, 237–247. [Google Scholar] [CrossRef]
- Bist, P.; Shu, S.; Lee, H.; Arora, S.; Nair, S.; Lim, J.Y.; Dayalan, J.; Gasser, S.; Biswas, S.K.; Fairhurst, A.-M.; et al. Annexin-A1 regulates TLR-mediated IFN-β production through an interaction with TANK-binding kinase 1. J. Immunol. 2013, 191, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Ampomah, P.B.; Moraes, L.A.; Lukman, H.M.; Lim, L.H.K. Formyl peptide receptor 2 is regulated by RNA mimics and viruses through an IFN-β-STAT3-dependent pathway. FASEB J. 2018, 32, 1468–1478. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Li, X.; Zheng, L.; Lei, L. ANXA1 silencing increases the sensitivity of cancer cells to low-concentration arsenic trioxide treatment by inhibiting ERK MAPK activation. Tumori. J. 2015, 101, 360–367. [Google Scholar] [CrossRef]
- Canacik, O.; Sabirli, R.; Altintas, E.; Karsli, E.; Karis, D.; Kaymaz, B.; Sabirli, G.T.; Kurt, Ö.; Koseler, A. Annexin A1 as a potential prognostic biomarker for COVID-19 disease: Case–control study. Int. J. Clin. Pract. 2021, 75, e14606. [Google Scholar] [CrossRef]
- Busch, M.H.; Timmermans, S.A.M.E.G.; Aendekerk, J.P.; Ysermans, R.; Amiral, J.; Damoiseaux, J.G.M.C.; Reutelingsperger, C.P.; van Paassen, P. Annexin A1 Is Associated with Adverse Clinical Outcomes in Patients with COVID-19. J. Clin. Med. 2022, 11, 7486. [Google Scholar] [CrossRef]
- Arora, S.; Lim, W.; Bist, P.; Perumalsamy, R.; Lukman, H.M.; Li, F.; Welker, L.B.; Yan, B.; Sethi, G.; Tambyah, P.A.; et al. Influenza A virus enhances its propagation through the modulation of Annexin-A1 dependent endosomal trafficking and apoptosis. Cell Death Differ. 2016, 23, 1243–1256. [Google Scholar] [CrossRef]
- Rahman, F.; Chebbo, M.; Courtin, N.; Fotso, A.F.; Alessi, M.-C.; Riteau, B. The Annexin A1 Receptor FPR2 Regulates the Endosomal Export of Influenza Virus. Int. J. Mol. Sci. 2018, 19, 1400. [Google Scholar] [CrossRef]
- Molás, R.B.; Ribeiro, M.R.; Ramalho Dos Santos, M.J.C.; Borbely, A.U.; Oliani, D.V.; Oliani, A.H.; Nadkarni, S.; Nogueira, M.L.; Moreli, J.B.; Oliani, S.M. The involvement of annexin A1 in human placental response to maternal Zika virus infection. Antivir. Res. 2020, 179, 104809. [Google Scholar] [CrossRef]
- Hiramoto, H.; Dansako, H.; Takeda, M.; Satoh, S.; Wakita, T.; Ikeda, M.; Kato, N. Annexin A1 negatively regulates viral RNA replication of hepatitis C virus. Acta Med. Okayama 2015, 69, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Calmon, M.F.; Mota, M.T.; Babeto, É.; Candido, N.M.; Girol, A.P.; Mendiburu, C.F.; Bonilha, J.L.; Silvestre, R.V.D.; Rosa, B.M.; Thome, J.A.; et al. Overexpression of ANXA1 in penile carcinomas positive for high-risk HPVs. PLoS ONE 2013, 8, e53260. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.J.; Nakata, C.M.; Solito, E.; Damazo, A.S. Relationship between HPV and the biomarkers annexin A1 and p53 in oropharyngeal cancer. Infect. Agents Cancer 2014, 9, 13. [Google Scholar] [CrossRef]
- Sena, A.A.; Glavan, T.; Jiang, G.; Sankaran-Walters, S.; Grishina, I.; Dandekar, S.; Goulart, L.R. Divergent Annexin A1 expression in periphery and gut is associated with systemic immune activation and impaired gut immune response during SIV infection. Sci. Rep. 2016, 6, 31157. [Google Scholar] [CrossRef] [PubMed]
- Nedellec, R.; Coetzer, M.; Shimizu, N.; Hoshino, H.; Polonis, V.R.; Morris, L.; Mårtensson, U.E.A.; Binley, J.; Overbaugh, J.; Mosier, D.E. Virus entry via the alternative coreceptors CCR3 and FPRL1 differs by human immunodeficiency virus type 1 subtype. J. Virol. 2009, 83, 8353–8363. [Google Scholar] [CrossRef]
- Jiang, C.; Parrish, N.F.; Wilen, C.B.; Li, H.; Chen, Y.; Pavlicek, J.W.; Berg, A.; Lu, X.; Song, H.; Tilton, J.C.; et al. Primary infection by a human immunodeficiency virus with atypical coreceptor tropism. J. Virol. 2011, 85, 10669–10681. [Google Scholar] [CrossRef] [PubMed]
- Cashin, K.; Jakobsen, M.R.; Sterjovski, J.; Roche, M.; Ellett, A.; Flynn, J.K.; Borm, K.; Gouillou, M.; Churchill, M.J.; Gorry, P.R. Linkages between HIV-1 specificity for CCR5 or CXCR4 and in vitrousage of alternative coreceptors during progressive HIV-1 subtype C infection. Retrovirology 2013, 10, 98. [Google Scholar] [CrossRef]
- Shimizu, N.; Tanaka, A.; Oue, A.; Mori, T.; Apichartpiyakul, C.; Hoshino, H. A short amino acid sequence containing tyrosine in the N-terminal region of G protein-coupled receptors is critical for their potential use as co-receptors for human and simian immunodeficiency viruses. J. Gen. Virol. 2008, 89, 3126–3136. [Google Scholar] [CrossRef]
- Santana, B.B.; Queiroz, M.A.F.; Cerveira, R.A.; Rodrigues, C.M.; Amoras, E.D.S.G.; da Costa, C.A.; de Sousa, M.S.; Ishak, R.; Goulart, L.R.; Vallinoto, A.C.R. Low Annexin A1 level in HTLV-1 infected patients is a potential biomarker for the clinical progression and diagnosis of HAM/TSP. BMC Infect. Dis. 2021, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Caricchio, R.; Gallucci, M.; Dass, C.; Zhang, X.; Gallucci, S.; Fleece, D.; Bromberg, M.; Criner, G.J. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021, 80, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.J.; Peltan, I.D.; Jensen, P.; Hoda, D.; Hunter, B.; Silver, A.; Starr, N.; Buckel, W.; Grisel, N.; Hummel, E.; et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: A cohort study. Lancet Rheumatol. 2020, 2, e754–e763. [Google Scholar] [CrossRef]
- Corral-Gudino, L.; Bahamonde, A.; Arnaiz-Revillas, F.; Gómez-Barquero, J.; Abadía-Otero, J.; García-Ibarbia, C.; Mora, V.; Cerezo-Hernández, A.; Hernández, J.L.; López-Muñíz, G.; et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: An open-label randomized trial (GLUCOCOVID). Wien. Klin. Wochenschr. 2021, 133, 303–311. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, R.J.; Mafham, M.; Bell, L.J.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Vital, S.A.; Senchenkova, E.Y.; Ansari, J.; Gavins, F.N.E. Targeting AnxA1/Formyl Peptide Receptor 2 Pathway Affords Protection against Pathological Thrombo-Inflammation. Cells 2020, 9, 2473. [Google Scholar] [CrossRef] [PubMed]
- Ural, O.; Kıratlı, H.E.; Sümer, Ş.; Demir, N.A.; Kırık, S.Y.; Vatansev, H.; Akyürek, F.; Cebeci, H.; Arslan, U.; Demir, L.S. COVID-19 Tanısıyla Takip Edilen Hastalarda Annexin-1 (ANXA-1), Annexin-2 (ANXA-2) ve Kemik Morfogenetik Protein-7 (BMP-7) Serum Düzeyinin Değerlendirilmesi [Evaluation of Annexin-1 (ANXA-1), Annexin-2 (ANXA-2) and Bone Morpho-genetic Protein-7 (BMP-7) Serum Levels in Patients Followed Up With A Diagnosis of COVID-19]. Mikrobiyol. Bul. 2022, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.D.; Weinberg, S.E.; Swaminathan, S.; Chaudhuri, S.; Mubarak, H.F.; Schipma, M.J.; Mao, C.; Wang, X.; El-Shennawy, L.; Dashzeveg, N.K.; et al. Unique molecular signatures sustained in circulating monocytes and reg-ulatory T cells in Convalescent COVID-19 patients. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bonavita, A.G. Ac2-26 mimetic peptide of annexin A1 to treat severe COVID-19: A hypothesis. Med. Hypotheses 2020, 145, 110352. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939. [Google Scholar] [CrossRef]
- Ni, C.; Gao, S.; Zheng, Y.; Liu, P.; Zhai, Y.; Huang, W.; Jiang, H.; Lv, Q.; Kong, D.; Jiang, Y. Annexin A1 Attenuates Neutrophil Migration and IL-6 Expression through Fpr2 in a Mouse Model of Streptococcus suis-Induced Meningitis. Infect. Immun. 2021, 89, e00680-20. [Google Scholar] [CrossRef]
- Cui, J.; Morgan, D.; Cheng, D.H.; Foo, S.L.; Yap, G.L.R.; Ampomah, P.B.; Arora, S.; Sachaphibulkij, K.; Periaswamy, B.; Fairhurst, A.-M.; et al. RNA-Sequencing-Based Transcriptomic Analysis Reveals a Role for Annexin-A1 in Classical and Influenza A Virus-Induced Autophagy. Cells 2020, 9, 1399. [Google Scholar] [CrossRef]
- Courtin, N.; Fotso, A.F.; Fautrad, P.; Mas, F.; Alessi, M.-C.; Riteau, B. Antiviral activity of formyl peptide receptor 2 antagonists against influenza viruses. Antivir. Res. 2017, 143, 252–261. [Google Scholar] [CrossRef]
- Hossain, S.; Choudhury, M.R.; Islam, M.A.; Hassan, M.; Yeasmin, S.; Hossain, F.; Zaman, M.M. Post-chikungunya arthritis: A longitudinal study in a tertiary care hospital in Bangladesh. Trop. Med. Health 2022, 50, 21. [Google Scholar] [CrossRef]
- Cunha, M.S.; de Moura Coletti, T.; Guerra, J.M.; Ponce, C.C.; Fernandes, N.C.C.A.; Résio, R.A.; Claro, I.M.; Salles, F.; Neto, D.F.L.; Sabino, E. A fatal case of dengue hemorrhagic fever associated with dengue virus 4 (DENV-4) in Brazil: Genomic and histopathological findings. Braz. J. Microbiol. 2022, 53, 1305–1312. [Google Scholar] [CrossRef]
- Cavalcante, T.B.; Ribeiro, M.R.C.; Sousa, P.D.S.; Costa, E.D.P.F.; Alves, M.T.S.S.D.B.E.; Simões, V.M.F.; Batista, R.F.L.; Takahasi, E.H.M.; Amaral, G.A.; Khouri, R.; et al. Congenital Zika syndrome: Growth, clinical, and motor development outcomes up to 36 months of age and differences according to microcephaly at birth. Int. J. Infect. Dis. 2021, 105, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Cirino, G.; Flower, R.J.; Browning, J.L.; Sinclair, L.K.; Pepinsky, R.B. Recombinant human lipocortin 1 inhibits thromboxane release from guinea-pig isolated perfused lung. Nature 1987, 328, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Puttamallesh, V.N.; Sreenivasamurthy, S.K.; Singh, P.K.; Harsha, H.C.; Ganjiwale, A.; Broor, S.; Pandey, A.; Narayana, J.; Prasad, T.S.K. Proteomic profiling of serum samples from chikungunya-infected patients provides insights into host response. Clin. Proteom. 2013, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Galvão, I.; de Carvalho, R.V.H.; Vago, J.P.; Silva, A.L.N.; Carvalho, T.G.; Antunes, M.M.; Ribeiro, F.; Menezes, G.B.; Zamboni, D.; Sousa, L.P.; et al. The role of annexin A1 in the modulation of the NLRP3 inflammasome. Immunology 2020, 160, 78–89. [Google Scholar] [CrossRef]
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef]
- Sejima, H.; Mori, K.; Ariumi, Y.; Ikeda, M.; Kato, N. Identification of host genes showing differential expression profiles with cell-based long-term replication of hepatitis C virus RNA. Virus Res. 2012, 167, 74–85. [Google Scholar] [CrossRef]
- Lehtinen, M.; Pimenoff, V.N.; Nedjai, B.; Louvanto, K.; Verhoef, L.; Heideman, D.A.M.; El-Zein, M.; Widschwendter, M.; Dillner, J. Assessing the risk of cervical neoplasia in the post-HPV vaccination era. Int. J. Cancer 2023, 152, 1060–1068. [Google Scholar] [CrossRef]
- Zawacka-Pankau, J.E. The Role of p53 Family in Cancer. Cancers 2022, 14, 823. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol. Cell. Biol. 1993, 13, 775–784. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef]
- Calmon, M.F.; Sichero, L.; Boccardo, E.; Villa, L.L.; Rahal, P. HPV16 E6 regulates annexin 1 (ANXA1) protein expression in cervical carcinoma cell lines. Virology 2016, 496, 35–41. [Google Scholar] [CrossRef]
- Mellors, J.W.; Rinaldo, C.R., Jr.; Gupta, P.; White, R.M.; Todd, J.A.; Kingsley, L.A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996, 272, 1167–1170, Erratum in Science 1997, 275, 14. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; Lackner, A.A. Getting to the guts of HIV pathogenesis. J. Exp. Med. 2004, 200, 697–700. [Google Scholar] [CrossRef]
- Bookstaver, P.B.; Mohorn, P.L.; Shah, A.; Tesh, L.D.; Quidley, A.M.; Kothari, R.; Bland, C.; Weissman, S. Management of Viral Central Nervous System Infections: A Primer for Clinicians. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517703342. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhou, Y.; Liu, Z.; Zhang, P. Interaction between ANXA1 and GATA-3 in Immunosuppression of CD4+ T Cells. Mediat. Inflamm. 2016, 2016, 1701059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Song, W.; Deane, J.A.; Kao, W.; Ooi, J.D.; Ngo, D.; Kitching, A.R.; Morand, E.F.; Hickey, M.J. Deficiency of annexin A1 in CD4+ T cells exacerbates T cell-dependent inflammation. J. Immunol. 2013, 190, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yoshihara, E.; Son, A.; Matsuo, Y.; Masutani, H.; Sugie, K.; Maeda, M.; Yodoi, J. Differential roles of Annexin A1 (ANXA1/lipocortin-1/lipomodulin) and thioredoxin binding protein-2 (TBP-2/VDUP1/TXNIP) in glucocorticoid signaling of HTLV-I-transformed T cells. Immunol. Lett. 2010, 131, 11–18. [Google Scholar] [CrossRef]
- Fan, Y.; Wei, C.; Xiao, W.; Zhang, W.; Wang, N.; Chuang, P.Y.; He, J.C. Temporal profile of the renal transcriptome of HIV-1 transgenic mice during disease progression. PLoS ONE 2014, 9, e93019. [Google Scholar] [CrossRef]
- Ciechonska, M.; Key, T.; Duncan, R. Efficient reovirus- and measles virus-mediated pore expansion during syncytium formation is dependent on annexin A1 and intracellular calcium. J. Virol. 2014, 88, 6137–6147. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, K.; Luo, Z.; Nian, X.; Choudhury, S.K.M.; Zhu, Z.; Song, R.; Pei, J.; Huo, Y.; Li, Y.; et al. FMDV 3A Antagonizes the Effect of ANXA1 to Positively Modulate Viral Replication. J. Virol. 2022, 96, e0031722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, L.; Zhao, W.; Rigas, B. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: Anticancer effects in vitro and in vivo. Cancer Res. 2010, 70, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
| |||
|---|---|---|---|
| Virus | In Vivo (Model); In Vitro (Cell Type) | Comment | Reference |
| SARS-CoV-2 (COVID-19) | Case-control study with analysis of clinic-based blood samples | Lower AnxA1 levels were found in the severe/critical disease group compared with the control and moderate disease groups | [89] |
| Prospective cohort study with analysis of clinic-based blood samples | Elevated levels of AnxA1 were observed in cases of moderate and severe disease | [90] | |
| Influenza A virus (IAV) | Human epithelial cell line (A549) or MDCK cells; C57BL/6 (WT mice) | Activation of FPR2 stimulated viral replication through an ERK-dependent pathway, resulting in reduced survival in mice | [85] |
| AnxA1-KO and WT mice; A549 cells | AnxA1 increased viral replication, affected virus binding, and promoted the trafficking of endosomal viruses to the nucleus | [91] | |
| A549 cells | The blocking of FPR2 signaling inhibited viral replication and interfered with the endosomal trafficking of IAV | [92] | |
| C57BL/6 (WT) mice; samples: lungs of mice | The activation of the AnxA1/FPR2 pathway demonstrated beneficial effects for the host, improving survival, inhibiting viral replication and expanding alveolar macrophages | [19] | |
| A549 cells | AnxA1 plays a regulatory role in RIG-I signaling and induces apoptotic cell death upon infection | [21] | |
| |||
| Virus | In vivo (model); in vitro (cell type) | Comment | Reference |
| Chikungunya (CHIKV) | AnxA1-KO; FPR2-KO and WT mice | The activation of the AnxA1/FPR2 pathway demonstrated beneficial effects for the host by reducing the inflammatory response | [26] |
| Dengue virus (DENV) | AnxA1-KO; FPR2-KO; A129, and WT mice | The activation of the AnxA1/FPR2 pathway demonstrated beneficial effects for the host | [24] |
| Zika virus (ZIKV) | Cross-sectional study with analysis of clinic-based samples: placental fragments of pregnant women with suspected Zika virus infection | AnxA1 is involved in modulating inflammation in response to ZIKV infection | [93] |
| Hepatitis C virus (HCV) | Human hepatoma cell line Li23 and Li23-derived D7 cells | AnxA1 negatively regulates the step of viral RNA replication, but does not regulate viral entry | [94] |
| Human papillomavirus (HPV) | Analysis of clinic-based samples: tissue sections from penile squamous cell carcinoma | AnxA1 is overexpressed in high-risk HPV-positive penile carcinoma patients | [95] |
| Analysis of clinic-based samples: tumor and adjacent mucosa from patients with squamous cell carcinoma of the oropharynx | Increased expression of AnxA1 in HPV+ samples suggests that the protein is involved in the early stages of HPV-driven carcinogenesis | [96] | |
| Simian immunodeficiency virus (SIV) | Rhesus macaques | During early stages of infection, AnxA1 expression decreased in the gut and increased in the blood, while during chronic infection, AnxA1 expression increased in both compartments | [97] |
| Human immunodefiency virus (HIV) | NP-2 cells; C8166 cells; T CD4+ cells obtained from human donors | HIV strains used FPR2 efficiently as a co-receptor; 22 envelope proteins can bind to FPR2 | [98,99,100,101] |
| Herpes simplex virus 1 (HSV-1) | AnxA1-KO and WT mice; A549 cells | Similar to IAV, viral particles of HSV-1 hijack the AnxA1/FPR2 pathway to increase endocytosis by the cells via FPR2 receptor | [20] |
| Human T-lymphotropic virus 1 (HTLV-1) | Analysis of clinic-based samples: blood | An imbalance in the expression of ANXA1 may contribute to the development of chronic neurodegenerative diseases caused by HTLV-1 | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, F.; Araújo, S.d.; Tavares, L.P.; Teixeira, M.M.; Costa, V.V. The Multifaceted Role of Annexin A1 in Viral Infections. Cells 2023, 12, 1131. https://doi.org/10.3390/cells12081131
Resende F, Araújo Sd, Tavares LP, Teixeira MM, Costa VV. The Multifaceted Role of Annexin A1 in Viral Infections. Cells. 2023; 12(8):1131. https://doi.org/10.3390/cells12081131
Chicago/Turabian StyleResende, Filipe, Simone de Araújo, Luciana Pádua Tavares, Mauro Martins Teixeira, and Vivian Vasconcelos Costa. 2023. "The Multifaceted Role of Annexin A1 in Viral Infections" Cells 12, no. 8: 1131. https://doi.org/10.3390/cells12081131
APA StyleResende, F., Araújo, S. d., Tavares, L. P., Teixeira, M. M., & Costa, V. V. (2023). The Multifaceted Role of Annexin A1 in Viral Infections. Cells, 12(8), 1131. https://doi.org/10.3390/cells12081131







