Modeling the Effects of Cypermethrin Toxicity on Ovalbumin-Induced Allergic Pneumonitis Rats: Macrophage Phenotype Differentiation and p38/STAT6 Signaling Are Candidate Targets of Pirfenidone Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Preparation and Dosage of Pirfenidone, Dexamethasone, Ovalbumin, and Cypermethrin
2.3. Induction of Allergic Asthma
2.4. Animals
2.5. Study Groups
2.6. Blood Sampling
2.7. Bronchoalveolar Lavage Fluid (BALF) Preparation
2.8. Lung Tissue Sampling
2.9. Tissue Homogenate Analysis of TNF-α, MDA, SOD
2.10. PCR Assay for Quantification of STAT6, p38MAPK, MUC5AC, and IL-13 Gene Expressions
2.11. Histological Procedure
2.12. Immunohistochemical Identification of CD86, CD206, and p38 MAPK
2.13. Morphometric Study
2.14. Statistical Analysis
3. Results
3.1. Pirfenidone Reversed the Serum Level of IgE
3.2. Pirfenidone Suppressed Inflammatory Cytokines, Eosinophilic and Neutrophilic Count in BALF
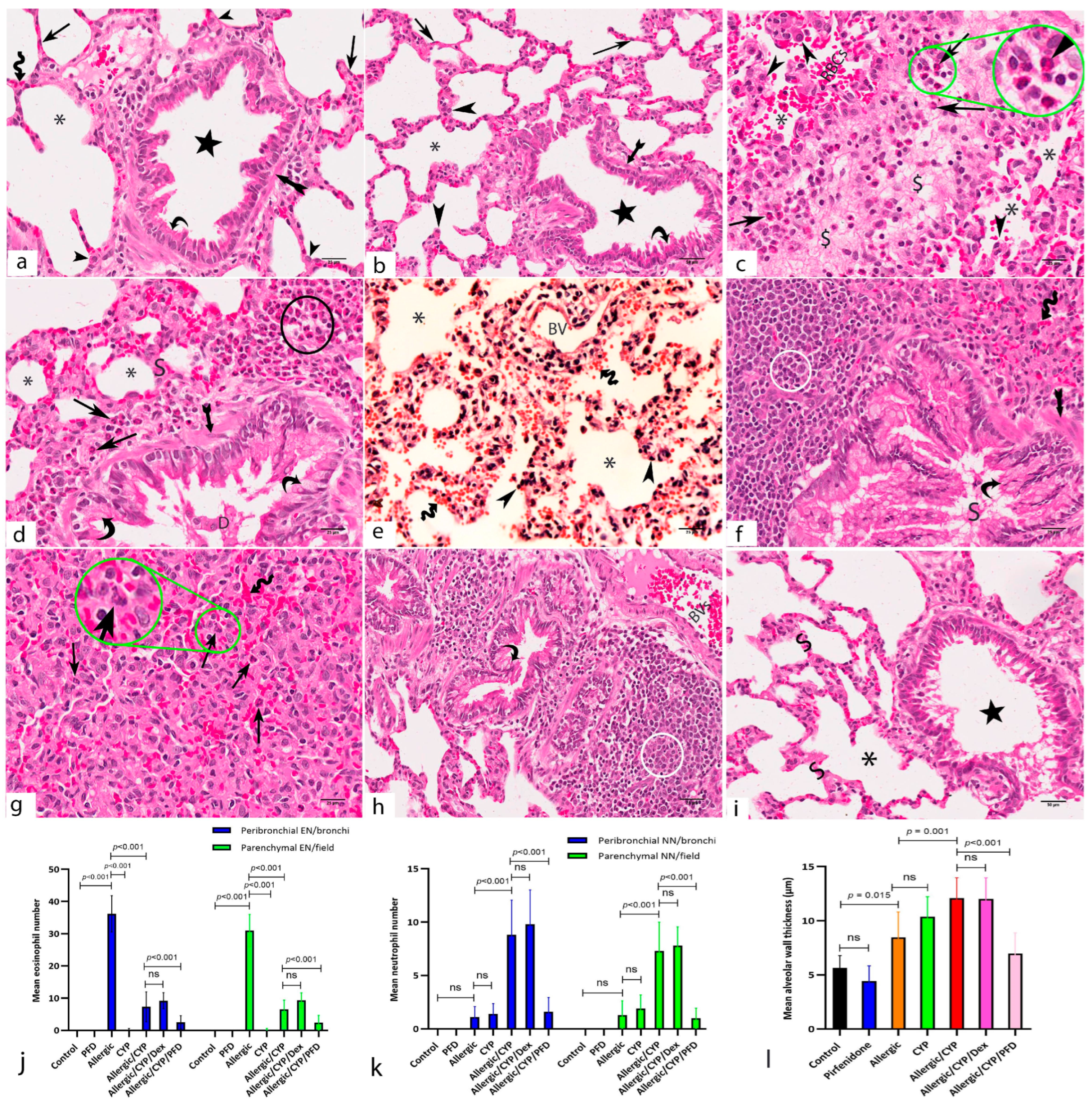
3.3. Pirfenidone Alleviated Inflammation and Oxidative Stress in the Lung Tissues
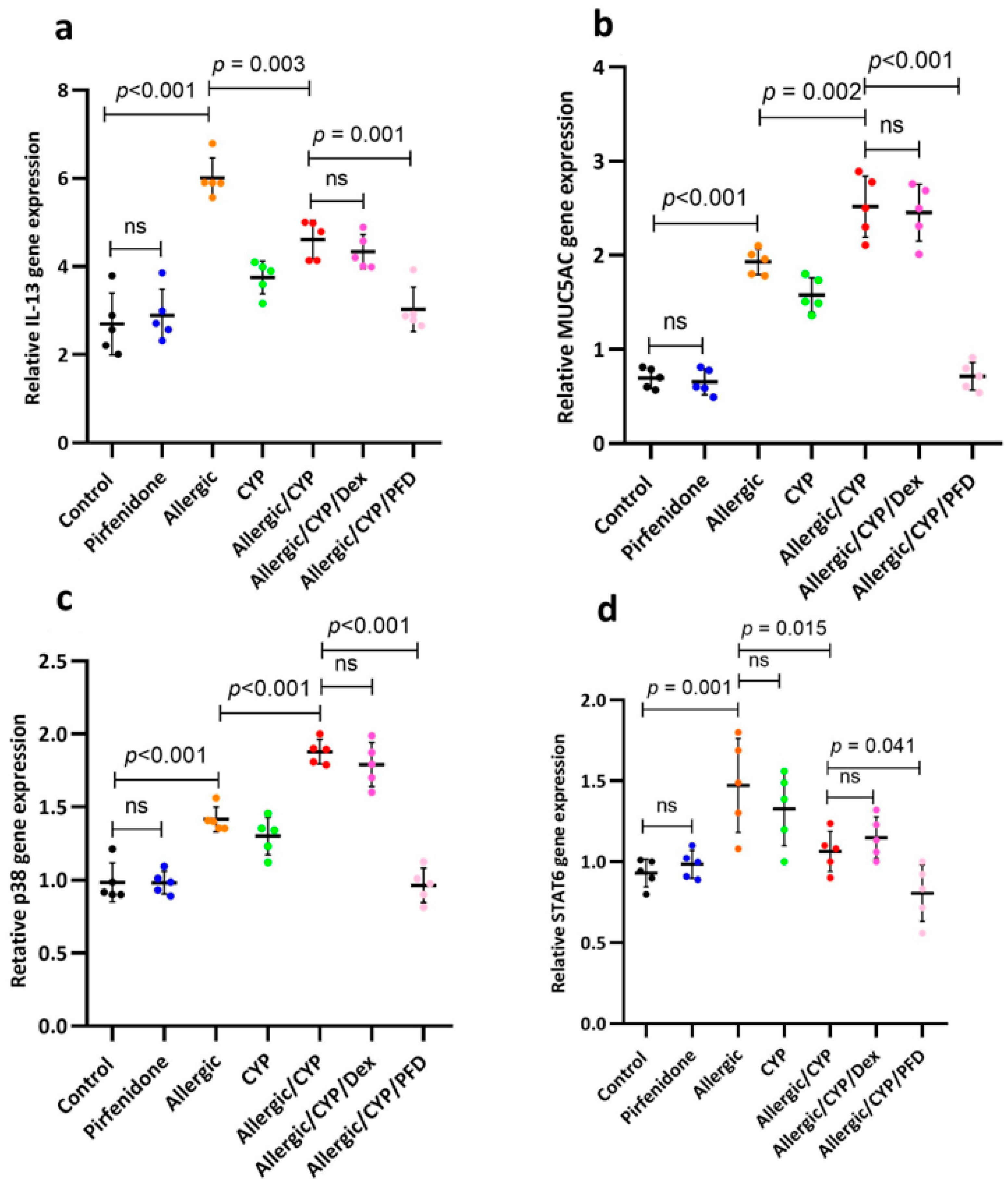
3.4. Pirfenidone Modulated STAT6, p38MAPK, MUC5AC, and IL-13 Gene Expression
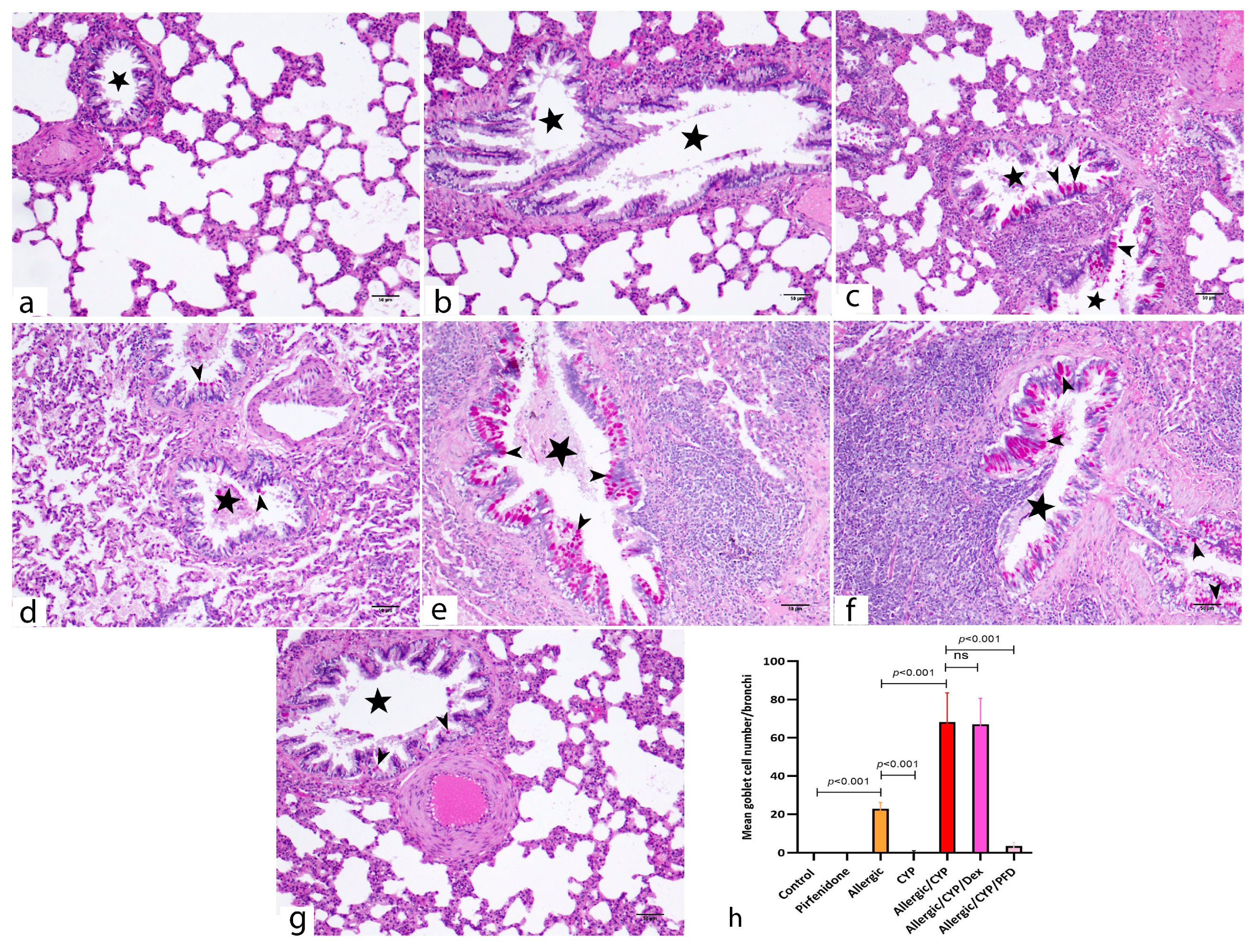
3.5. Pirfenidone Improved the Histological Structure of the Lungs
3.6. Pirfenidone Inhibited the Lung Immunoexpression of p38 MAPK
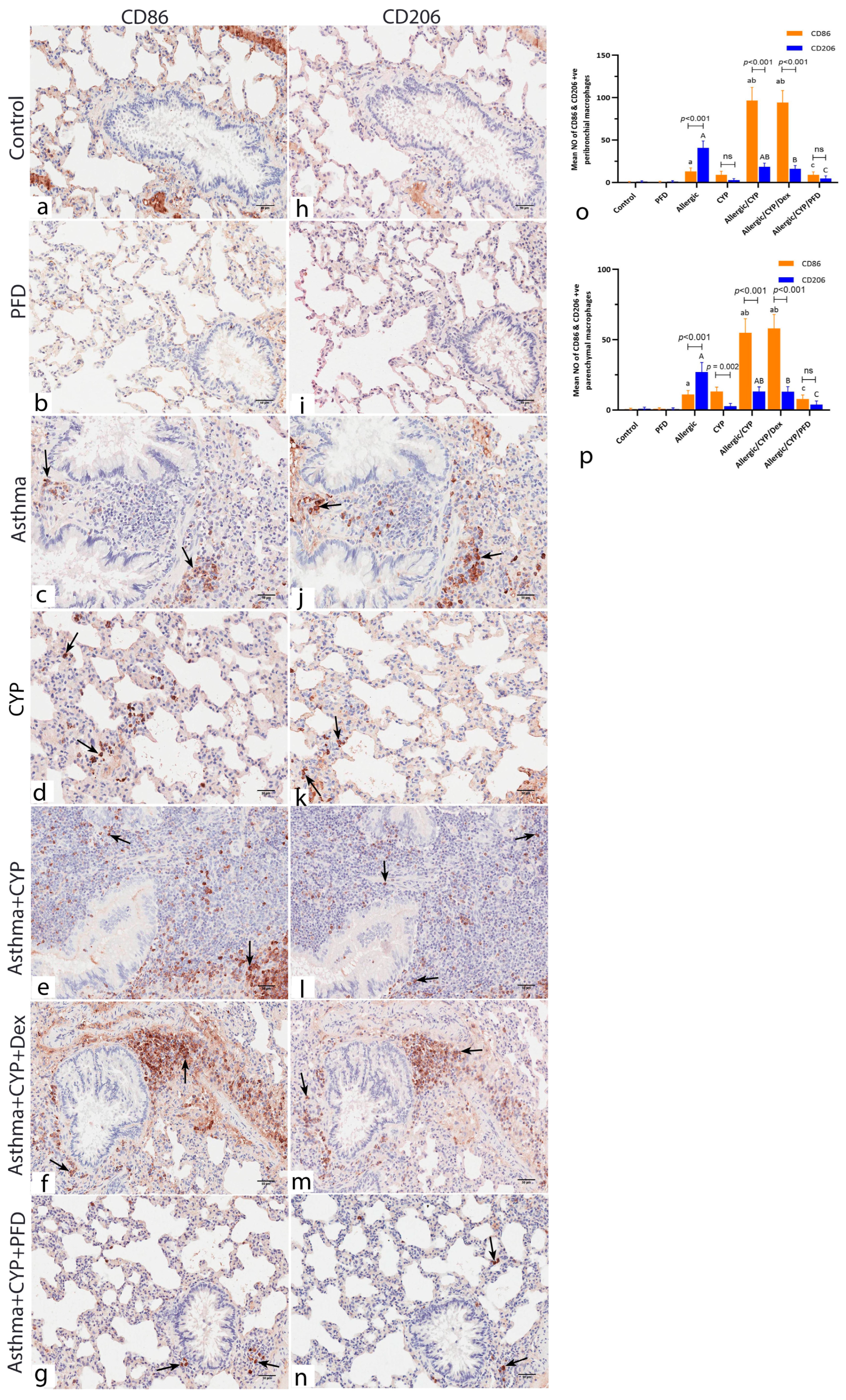
3.7. Pirfenidone Modulated the Immunoexpression of Macrophage Markers, CD86 and CD206
3.8. Pirfenidone Reversed the Histomorphometric Measurements in the Lung Tissues
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, R.; Ahmed, N.; Salman, M.; Daudpota, S.; Masroor, M.; Nasir, M. Assessment of Quality of Life in Bronchial Asthma Patients. Cureus 2020, 12, e10845. [Google Scholar] [CrossRef]
- Rahbarghazi, R.; Keyhanmanesh, R.; Aslani, M.R.; Hassanpour, M.; Ahmadi, M. Bone Marrow Mesenchymal Stem Cells and Condition Media Diminish Inflammatory Adhesion Molecules of Pulmonary Endothelial Cells in an Ovalbumin-Induced Asthmatic Rat Model. Microvasc. Res. 2019, 121, 63–70. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Wen, X.; Huang, G.; Nian, S.; Li, L.; Guo, X.; Ye, Y.; Yuan, Q. The Onset, Development and Pathogenesis of Severe Neutrophilic Asthma. Immunol. Cell Biol. 2022, 100, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Athari, S.S. Targeting Cell Signaling in Allergic Asthma. Signal Transduct. Target. Ther. 2019, 4, 45. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their Role, Activation and Polarization in Pulmonary Diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.; Yang, H.; Ji, J.; Chen, Y.; Bao, W.; Li, F.; Zhang, M.; Zhou, X.; Li, Q.; Ben, S. Involvements of P38 MAPK and Oxidative Stress in the Ozone-Induced Enhancement of AHR and Pulmonary Inflammation in an Allergic Asthma Model. Respir. Res. 2017, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Yatera, K. The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases. Int. J. Environ. Res. Public Health 2022, 19, 2788. [Google Scholar] [CrossRef]
- Ghazouani, L.; Feriani, A.; Mufti, A.; Tir, M.; Baaziz, I.; Mansour, H.B.; Mnafgui, K. Toxic Effect of Alpha Cypermethrin, an Environmental Pollutant, on Myocardial Tissue in Male Wistar Rats. Environ. Sci. Pollut. Res. 2020, 27, 5709–5717. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Wu, N.; Gao, M.; Tan, Y. Combined Toxicity of Pyrethroid Insecticides and Heavy Metals: A Review. Environ. Chem. Lett. 2019, 17, 1693–1706. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, Y.; Zhang, Z.; Yang, P.; Li, Z.; Song, M.; Qi, X.; Han, Z.; Pang, J.; Li, B.; et al. Pirfenidone Ameliorates Silica-Induced Lung Inflammation and Fibrosis in Mice by Inhibiting the Secretion of Interleukin-17A. Acta Pharmacol. Sin. 2022, 43, 908–918. [Google Scholar] [CrossRef]
- Bast, A.; Semen, K.O.; Drent, M. Pulmonary Toxicity Associated with Occupational and Environmental Exposure to Pesticides and Herbicides. Curr. Opin. Pulm. Med. 2021, 27, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, S.V.; Khazaei, H.; Fakhri, S.; Mohammadi-Noori, E.; Farzaei, M.H. Naringenin Improves Ovalbumin-Induced Allergic Asthma in Rats through Antioxidant and Anti-Inflammatory Effects. Evid. Based Complement. Altern. Med. 2022, 2022, 9110798. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Han, X.; Li, T.; Feng, Y.; Sun, J. Protective Effect of MiR-138-5p Inhibition Modified Human Mesenchymal Stem Cell on Ovalbumin-induced Allergic Rhinitis and Asthma Syndrome. J. Cell. Mol. Med. 2021, 25, 5038–5049. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.K.; Aderibigbe, F.A.; Folarin, D.T.; Arinola, A.; Wusu, A.D. Oxidative Stress and Inflammation Following Sub-Lethal Oral Exposure of Cypermethrin in Rats: Mitigating Potential of Epicatechin. Heliyon 2019, 5, e02274. [Google Scholar] [CrossRef] [PubMed]
- Ezz-Eldin, Y.M.; Aboseif, A.A.; Khalaf, M.M. Potential Anti-Inflammatory and Immunomodulatory Effects of Carvacrol against Ovalbumin-Induced Asthma in Rats. Life Sci. 2020, 242, 117222. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tataurov, A.V.; You, Y.; Owczarzy, R. Predicting Ultraviolet Spectrum of Single Stranded and Double Stranded Deoxyribonucleic Acids. Biophys. Chem. 2008, 133, 66–70. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. Connective and Other Mesenchymal Tissues and Their Stains. In Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; pp. 153–175. [Google Scholar]
- Kiernan, J.A. Immunohistochemistry. In Histological and Histochemical Methods. Theory and Practice; Kiernan, J., Ed.; Scion Publishing Ltd.: Banbury, UK, 2015; pp. 454–490. [Google Scholar]
- Rasband, W.S. ImageJ (1997–2007); US National Institutes of Health: Bethesda, MD, USA, 2007. Available online: https://imagej.nih.gov/ij/ (accessed on 1 January 2023).
- Tang, X.; Nian, H.; Li, X.; Yang, Y.; Wang, X.; Xu, L.; Shi, H.; Yang, X.; Liu, R. Effects of the Combined Extracts of Herba Epimedii and Fructus Ligustrilucidi on Airway Remodeling in the Asthmatic Rats with the Treatment of Budesonide. BMC Complement. Altern. Med. 2017, 17, 112. [Google Scholar] [CrossRef]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-Resident Eosinophils Represent a Distinct Regulatory Eosinophil Subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef]
- Wu, Z.; Mehrabi Nasab, E.; Arora, P.; Athari, S.S. Study Effect of Probiotics and Prebiotics on Treatment of OVA-LPS-Induced of Allergic Asthma Inflammation and Pneumonia by Regulating the TLR4/NF-KB Signaling Pathway. J. Transl. Med. 2022, 20, 130. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G* Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Liang, L.; Gu, X.; Shen, H.J.; Shi, Y.H.; Li, Y.; Zhang, J.; Chen, Y.Y.; Chen, Z.H.; Ma, J.Y.; Li, Q.Y. Chronic Intermittent Hypoxia Reduces the Effects of Glucosteroid in Asthma via Activating the P38 MAPK Signaling Pathway. Front. Physiol. 2021, 12, 703281. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.I.; El-Desouky, M.; Nahas, A.E.-H.A.E.-M. Cypermethrin-Induced Lung Damage in Albino Rats: The Preventive Impact of Moringa Oleifera. Egypt. J. Chem. 2021, 64, 5585–5595. [Google Scholar] [CrossRef]
- Pelaia, C.; Vatrella, A.; Gallelli, L.; Lombardo, N.; Sciacqua, A.; Savino, R.; Pelaia, G. Role of P38 Mitogen-Activated Protein Kinase in Asthma and COPD: Pathogenic Aspects and Potential Targeted Therapies. Drug Des. Dev. Ther. 2021, 15, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-H.; Lin, S.-Y.; Chiang, B.-L. STAT6 Pathway Is Critical for the Induction and Function of Regulatory T Cells Induced by Mucosal B Cells. Front. Immunol. 2021, 11, 615868. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jeong, J.J.; Nyenhuis, S.; Berdyshev, E.; Chung, S.; Ranjan, R.; Karpurapu, M.; Deng, J.; Qian, F.; Kelly, E.A.B. Recruited Alveolar Macrophages, in Response to Airway Epithelial–Derived Monocyte Chemoattractant Protein 1/CCL2, Regulate Airway Inflammation and Remodeling in Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2015, 52, 772–784. [Google Scholar] [CrossRef]
- Draijer, C.; Robbe, P.; Boorsma, C.E.; Hylkema, M.N.; Melgert, B.N. Dual Role of YM1+ M2 Macrophages in Allergic Lung Inflammation. Sci. Rep. 2018, 8, 5105. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, B.; Nie, Y.; Wen, J.; Wang, Q.; Gu, C. Pirfenidone Suppresses MAPK Signalling Pathway to Reverse Epithelial-mesenchymal Transition and Renal Fibrosis. Nephrology 2017, 22, 589–597. [Google Scholar] [CrossRef]
- Fois, A.G.; Posadino, A.M.; Giordo, R.; Cossu, A.; Agouni, A.; Rizk, N.M.; Pirina, P.; Carru, C.; Zinellu, A.; Pintus, G. Antioxidant Activity Mediates Pirfenidone Antifibrotic Effects in Human Pulmonary Vascular Smooth Muscle Cells Exposed to Sera of Idiopathic Pulmonary Fibrosis Patients. Oxid. Med. Cell. Longev. 2018, 2018, 2639081. [Google Scholar] [CrossRef]
- Hirano, A.; Kanehiro, A.; Ono, K.; Ito, W.; Yoshida, A.; Okada, C.; Nakashima, H.; Tanimoto, Y.; Kataoka, M.; Gelfand, E.W.; et al. Pirfenidone Modulates Airway Responsiveness, Inflammation, and Remodeling after Repeated Challenge. Am. J. Respir. Cell Mol. Biol. 2006, 35, 366–377. [Google Scholar] [CrossRef]
- Mansoor, J.K.; Decile, K.C.; Giri, S.N.; Pinkerton, K.E.; Walby, W.F.; Bratt, J.M.; Grewal, H.; Margolin, S.B.; Schelegle, E.S. Influence of Pirfenidone on Airway Hyperresponsiveness and Inflammation in a Brown-Norway Rat Model of Asthma. Pulm. Pharmacol. Ther. 2007, 20, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Zeyen, L.; Seternes, O.M.; Mikkola, I. Crosstalk between P38 MAPK and GR Signaling. Int. J. Mol. Sci. 2022, 23, 3322. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively Activated Macrophages; a Double-Edged Sword in Allergic Asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef] [PubMed]


| Gene | Accession Number | Primer Sequence (5′ → 3′) |
|---|---|---|
| Signal transducer and activator of transcription 6 (STAT6) | NM_001044250.1 | F: GAGCTACTGGTCAGATCGGC R: GGTTCCATCTGGCTCGTTGA |
| Mitogen-activated protein kinase p38 (p38 MAPK) | NM_031020.3 | F: TCGGCACACTGATGACGAAA R: TCATGGCTTGGCATCCTGTT |
| Mucin 5AC (MUC5AC) | XM_039101269.1 | F: GTTTCTGCACCATGTCAGGC R: TGGGGCGGTAGATGTGGATA |
| Interleukin 13 (IL-13) | NM_053828.1 | F: TCTCGCTTGCCTTGGTGG R: CATTCAATATCCTCTGGGTCCTGT |
| Glyceraldehyde 3- phosphate dehydrogenase (GAPDH) | NM_001394060.2 | F: GGTGCTGAGTATGTCGTGGAG R: ACAGTCTTCTGAGTGGCAGTGAT |
| Serum IgE (ng/mL) | BALF TNF-α (pg/mL) | BALF IL13 (pg/mL) | BALF IL17 (pg/mL) | BALF INF-γ (pg/mL) | BALF Neutrophil Count/mm3 | BALF Eosinophil Count/mm3 | |
|---|---|---|---|---|---|---|---|
| Control | 12.7 ± 2.02 | 22.02 ± 2.2 | 37.1 ± 2.4 | 53.6 ± 4.9 | 8.7 ± 1.1 | 0.25 ± 0.5 | 0.38 ± 0.7 |
| Pirfenidone (PFD) | 13.1 ± 2.2 | 23.04 ± 2.9 | 37.3 ± 2.9 | 51 ± 3.7 | 9 ± 1.1 | 0.13 ± 0.4 | 0.63 ± 0.7 |
| Allergic pneumonitis | 95 ± 6.1 a (p < 0.01) | 83 ± 8.4 a (p < 0.01) | 184.5 ± 9 a (p < 0.01) | 90.2 ± 3.1 a (p < 0.01) | 11.8 ± 1.5 a (p = 0.01) | 0.75 ± 0.7 | 120.9 ± 6.2 a (p < 0.01) |
| CYP | 12.3 ± 2.3 | 58.9 ± 6.1 a (p < 0.01) | 44.7 ± 3.6 | 57.5 ± 5.6 | 12.4 ± 1.5 a (p =0.01) | 1 ± 0.8 | 1.9 ± 1.8 |
| Allergic/CYP | 83.7 ± 7.3 ab (p < 0.01) | 69.8 ± 9.4 ab (p < 0.01, = 0.003) | 132.4 ± 6.9 ab (p < 0.01) | 172.4 ± 16 ab (p < 0.01) | 18.9 ± 1.5 ab (p < 0.01) | 18.6 ± 4.3 ab (p < 0.01) | 15.5 ± 3.5 ab (p < 0.01) |
| Allergic/CYP/Dex | 77.9 ± 3.2 | 69.9 ± 8.3 | 123.9 ± 5.9 | 174 ± 12.8 | 19.7 ± 2 | 16.6 ± 5.2 | 11.5 ± 2.3 |
| Allergic/CYP/PFD | 18.6 ± 3.3 bc (p < 0.01) | 21.5 ± 3.7 bc (p < 0.01) | 36.2 ± 4.9 bc (p < 0.01) | 50.9 ± 4 bc (p < 0.01) | 9.9 ± 2.1 bc (p = 0.03, < 0.01) | 2.6 ± 1.6 c (p < 0.01) | 1 ± 0.8 bc (p < 0.01) |
| MDA (µmol/mL) | SOD (mmol/min/mg) | TNF-α (pg/100 μg Protein) | |
|---|---|---|---|
| Control | 17.2 ± 2 | 96.9 ±9.4 | 11.9 ± 2.7 |
| Pirfenidone (PFD) | 17.4 ± 2.1 | 98.1 ± 7 | 12.9 ± 1.8 |
| Allergic | 34.7 ± 4.7 a (p < 0.01) | 25.6 ± 3.4 a (p < 0.01) | 19.3 ± 2.5 a (p = 0.01) |
| CYP | 42.1 ± 3.8 a (p < 0.01) | 34.8 ± 9.1 a (p < 0.01) | 32.2 ± 2.3 a (p < 0.01) |
| Allergic/CYP | 77.07 ± 4.2 ab (p < 0.01) | 12.8 ± 2.4 ab (p < 0.01, = 0.03) | 42.6 ± 3.6 ab (p < 0.01) |
| Allergic/CYP/Dex | 81.6 ± 8.3 | 14.8 ± 3.4 | 43.4 ± 1.1 |
| Allergic/CYP/PFD | 22.7 ± 5.4 bc (p < 0.01) | 73 ± 7.7 abc (p < 0.01) | 12.6 ± 2.8 bc (p = 0.004, < 0.01)) |
| Peribronchiolar Inflammation | Perivascular Inflammation | |
|---|---|---|
| Control | 0.7 ± 0.67 | 0.4 ± 0.52 |
| Pirfenidone (PFD) | 0.9 ± 0.74 | 0.8 ± 0.79 |
| Allergic | 1.4 ± 0.84 a (p = 0.03) | 1.2 ± 0.92 a (p = 0.04) |
| CYP | 0.9 ± 0.73 | 0.6 ± 0.69 |
| Allergic/CYP | 3.1 ± 0.99 ab (p < 0.001, = 0.001) | 2 ± 0.82 ab (p = 0.001, = 0.03) |
| Allergic/CYP/Dex | 2.8 ± 0.92 ab (p < 0.001, = 0.008) | 2.1 ± 0.88 ab (p = 0.001, = 0.01) |
| Allergic/CYP/PFD | 1.4 ± 0.97 c (p = 0..008) | 1.1 ± 0.99 c (p = 0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsi, A.A.; Faruk, E.M.; Mogahed, M.M.; Baioumy, B.; Hussein, A.Y.A.; El-shafey, R.S.; Mersal, E.A.; Abdelmoneim, A.M.; Alanazi, M.M.; Elshazly, A.M.E. Modeling the Effects of Cypermethrin Toxicity on Ovalbumin-Induced Allergic Pneumonitis Rats: Macrophage Phenotype Differentiation and p38/STAT6 Signaling Are Candidate Targets of Pirfenidone Treatment. Cells 2023, 12, 994. https://doi.org/10.3390/cells12070994
Morsi AA, Faruk EM, Mogahed MM, Baioumy B, Hussein AYA, El-shafey RS, Mersal EA, Abdelmoneim AM, Alanazi MM, Elshazly AME. Modeling the Effects of Cypermethrin Toxicity on Ovalbumin-Induced Allergic Pneumonitis Rats: Macrophage Phenotype Differentiation and p38/STAT6 Signaling Are Candidate Targets of Pirfenidone Treatment. Cells. 2023; 12(7):994. https://doi.org/10.3390/cells12070994
Chicago/Turabian StyleMorsi, Ahmed A., Eman Mohamed Faruk, Mysara Mohamed Mogahed, Bodour Baioumy, Asmaa Y. A. Hussein, Rabab Shaban El-shafey, Ezat A. Mersal, Ahmed M. Abdelmoneim, Mohammed M. Alanazi, and Amal Mahmoud ElSafy Elshazly. 2023. "Modeling the Effects of Cypermethrin Toxicity on Ovalbumin-Induced Allergic Pneumonitis Rats: Macrophage Phenotype Differentiation and p38/STAT6 Signaling Are Candidate Targets of Pirfenidone Treatment" Cells 12, no. 7: 994. https://doi.org/10.3390/cells12070994
APA StyleMorsi, A. A., Faruk, E. M., Mogahed, M. M., Baioumy, B., Hussein, A. Y. A., El-shafey, R. S., Mersal, E. A., Abdelmoneim, A. M., Alanazi, M. M., & Elshazly, A. M. E. (2023). Modeling the Effects of Cypermethrin Toxicity on Ovalbumin-Induced Allergic Pneumonitis Rats: Macrophage Phenotype Differentiation and p38/STAT6 Signaling Are Candidate Targets of Pirfenidone Treatment. Cells, 12(7), 994. https://doi.org/10.3390/cells12070994







