The Ability of Airborne Microalgae and Cyanobacteria to Survive and Transfer the Carcinogenic Benzo(a)pyrene in Coastal Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Selecting Experimental Organisms from Airborne Microalgae and Cyanobacteria
2.2. Experimental Investigation of B(a)P Effects on Cyanobacteria and Microalgae in Laboratory Cultures
2.3. Calculation of Cell Density for Cyanobacteria and Microalgae
2.4. Determination of Chlorophyll a
2.5. Determination of the Chlorophyll a Fluorescence in Cyanobacteria and Microalgae
2.6. B(a)P Analysis Using High-Performance Liquid Chromatography
2.7. Statistical Analyses
3. Results
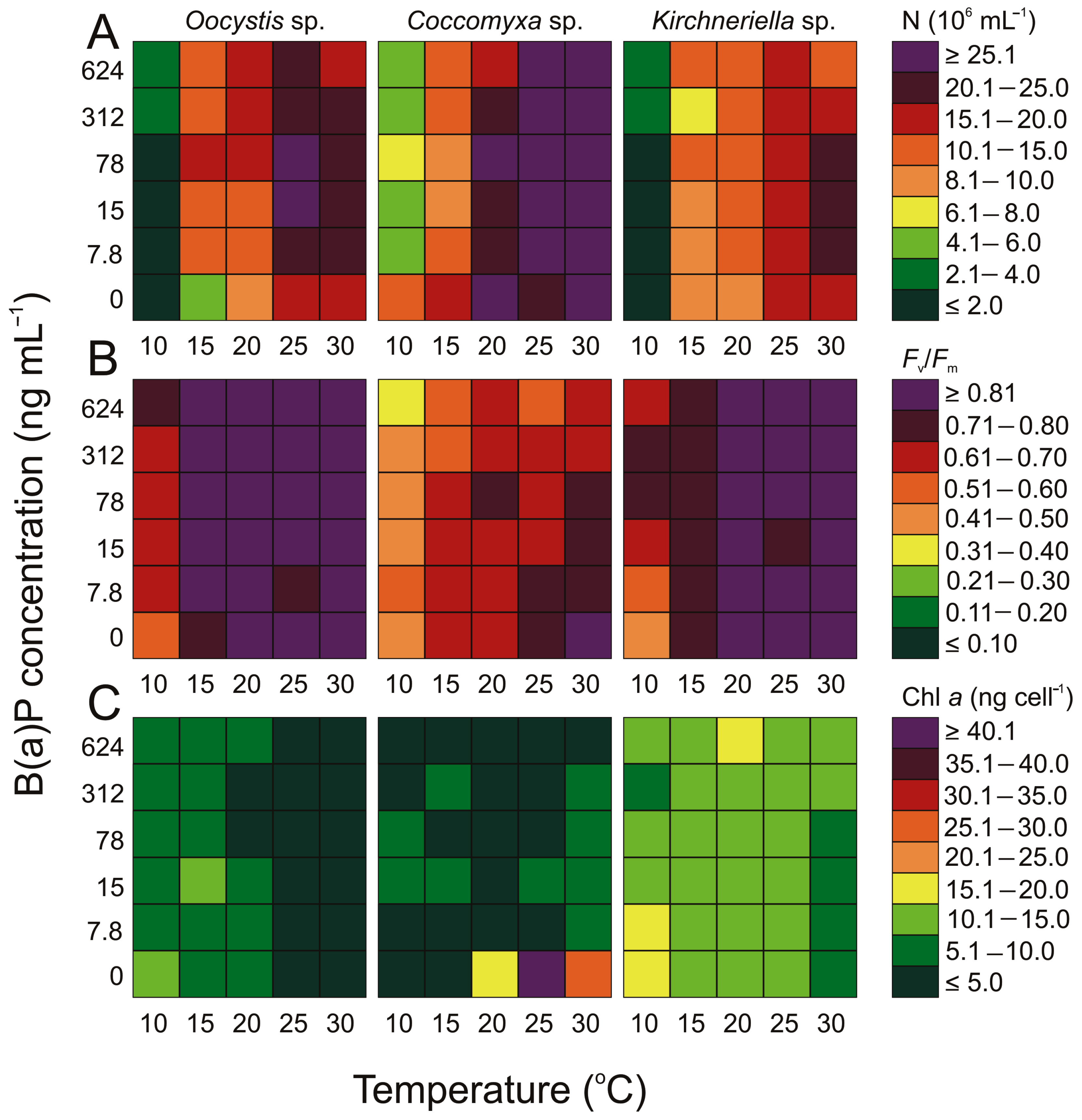
3.1. Variability in the Number of Cyanobacterial and Microalgal Cells
3.2. Variability in Chlorophyll a Concentration
3.3. Variability in the Maximum Quantum Efficiency of PSII Photochemistry (Fv/Fm)
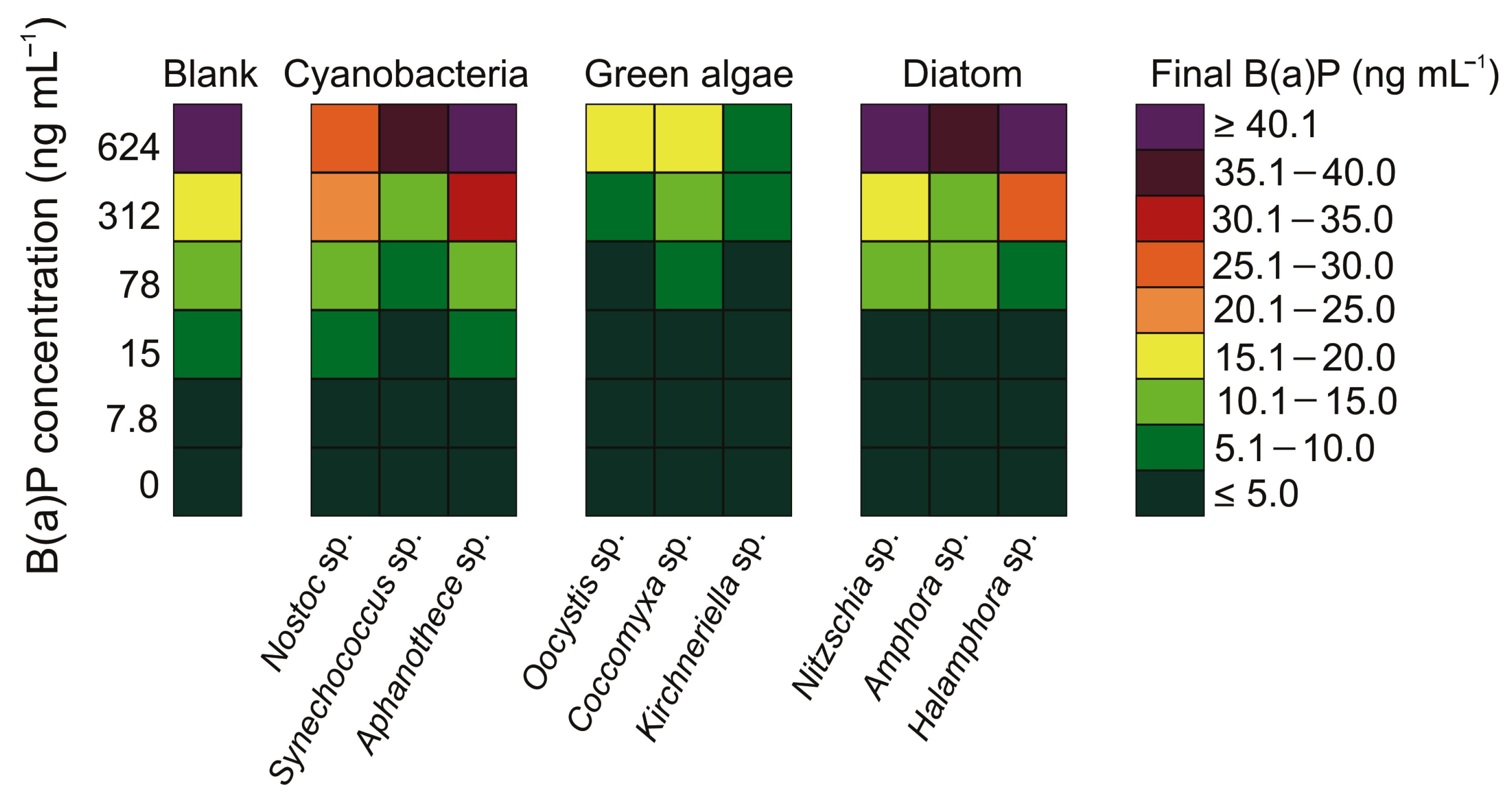
3.4. Variability in the Concentration of Benzo(a)pyrene after a 7-Day Exposure
4. Discussion
4.1. Cyanobacteria
4.2. Ochrophyta
4.3. Chlorophyta
4.4. The Role of Microalgae and Cyanobacterial Cell in the Degradation of B(a)P
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Jang, G.I.; Hwang, C.Y.; Cho, B.C. Effects of heavy rainfall on the composition of airborne bacterial communities. Front. Environ. Sci. Eng. 2018, 12, 12. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Lewandowska, A.U.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment–Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Z.-F.; Froines, J.R.; Zhao, J.; Wang, H.; Yu, S.-Z.; Detels, R. Air pollution and case fatality of SARS in the People’s Republic of China: An ecologic study. Environ. Health 2003, 2, 15. [Google Scholar] [CrossRef]
- Su, W.; Wu, X.; Geng, X.; Zhao, X.; Liu, Q.; Liu, T. The short-term effects of air pollutants on influenza-like illness in Jinan, China. BMC Public Health 2019, 19, 1319. [Google Scholar] [CrossRef] [PubMed]
- Frontera, A.; Cianfanelli, L.; Vlachos, K.; Landoni, G.; Cremona, G. Severe air pollution links to higher mortality in COVID-19 patients: The “double-hit” hypothesis. J. Infect. 2020, 81, 255–259. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, X.; Tao, Y.; Mi, S.; Huang, J.; Zhang, Q. The effects of air pollution and meteorological factors on measles cases in Lanzhou, China. Environ. Sci. Pollut. Res. 2020, 27, 13524–13533. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, J.; Wang, W.; Liu, Z.; Kan, H.; Qiu, Y.; Meng, X.; Wang, W. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020, 741, 140396. [Google Scholar] [CrossRef]
- Annesi-Maesano, I.; Maesano, C.N.; D’Amato, M.; D’Amato, G. Pros and cons for the role of air pollution on COVID-19 development. Allergy 2021, 76, 2647–2649. [Google Scholar] [CrossRef]
- Pansini, R.; Fornacca, D. COVID-19 Higher Mortality in Chinese Regions With Chronic Exposure to Lower Air Quality. Front. Public Health 2021, 8, 597753. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.; Śliwińska-Wilczewska, S.; Savoie, M.; Lewandowska, A.U. Quantitative and qualitative variability of airborne cyanobacteria and microalgae and their toxins in the coastal zone of the Baltic Sea. Sci. Total Environ. 2022, 826, 154152. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rai, A.K.; Singh, S. Meteorological factors affecting the diversity of airborne algae in an urban atmosphere. Ecography 2006, 29, 766–772. [Google Scholar] [CrossRef]
- El-Gamal, A.D. Aerophytic Cyanophyceae (cyanobacteria) from some Cairo districts, Egypt. Pak. J. Biol. Sci. 2008, 11, 1293–1302. [Google Scholar] [CrossRef]
- Genitsaris, S.; Kormas, K.A.; Moustaka-Gouni, M. Airborne algae and cyanobacteria: Occurrence and related health effects. Front. Biosci.-Elite 2011, 3, 772–787. [Google Scholar]
- Ng, E.H.P.; Chu, W.L.; Ambu, S. Occurrence of airborne algae within the township of Bukit Jalil in Kuala Lumpur, Malaysia. Grana 2011, 50, 217–227. [Google Scholar] [CrossRef]
- Chu, W.L.; Tneh, S.Y.; Ambu, S. A survey of airborne algae and cyanobacteria within the indoor environment of an office building in Kuala Lumpur, Malaysia. Grana 2013, 52, 207–220. [Google Scholar] [CrossRef]
- Sahu, N.; Tangutur, A.D. Airborne algae: Overview of the current status and its implications on the environment. Aerobiologia (Bologna) 2015, 31, 89–97. [Google Scholar] [CrossRef]
- Lewandowska, A.U.; Śliwińska-Wilczewska, S.; Woźniczka, D. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea. Mar. Pollut. Bull. 2017, 125, 30–38. [Google Scholar] [CrossRef]
- Singh, H.W.; Wade, R.M.; Sherwood, A.R. Diurnal patterns of airborne algae in the Hawaiian Islands: A preliminary study. Aerobiologia (Bologna) 2018, 34, 363–373. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Mostert, M.M.; Ayoko, G.A.; Kokot, S. Application of chemometrics to analysis of soil pollutants. Trends Anal. Chem. 2010, 29, 430–445. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Tsapakis, M.; Stephanou, E.G. Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: Study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ. Pollut. 2005, 133, 147–156. [Google Scholar] [CrossRef]
- Alves, C.A.; Vicente, A.M.; Custódio, D.; Cerqueira, M.; Nunes, T.; Pio, C.; Lucarelli, F.; Calzolai, G.; Nava, S.; Diapouli, E.; et al. Polycyclic aromatic hydrocarbons and their derivatives (nitro-PAHs, oxygenated PAHs, and azaarenes) in PM2. 5 from Southern European cities. Sci. Total Environ. 2017, 595, 494–504. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic aromatic hydrocarbons: A critical review of environmental occurrence and bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef] [PubMed]
- Warshawsky, D.; Cody, T.; Radike, M.; Reilman, R.; Schumann, B.; LaDow, K.; Schneider, J. Biotransformation of benzo [a] pyrene and other polycyclic aromatic hydrocarbons and heterocyclic analogs by several green algae and other algal species under gold and white light. Chem. Biol. Interact. 1995, 97, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Narro, M.L.; Cemiglia, C.E.; Van Baalen, C.; Gibson, D.T. Evidence of NIH shift in naphthalene oxidation by the marine cyanobacterium, Osciiiatoria species strain JCM. Appl. Environ. Microbiol. 1992, 58, 1360–1363. [Google Scholar] [CrossRef]
- Narro, M.L.; Cemiglia, C.E.; Van Baalen, C.; Gibson, D.T. Metabolism of phenanthrene by the marine cyanobacterium Agmenellum quadruplicatum, strain PR-6. Appl. Environ. Microbiol. 1992, 58, 1351–1359. [Google Scholar] [CrossRef]
- Chan, S.M.N.; Luan, T.; Wong, M.H. Removal and biodegradation of polycyclic aromatic hydrocarbons by Selenastrum capricornutum. Environ. Toxicol. Chem. 2006, 25, 1772–1779. [Google Scholar] [CrossRef]
- Ke, L.; Luo, L.; Wang, P.; Luan, T.; Tam, N.F. Effects of metals on biosorption and biodegradation of mixed polycyclic aromatic hydrocarbons by a freshwater green alga Selenastrum capricornutum. Bioresour. Technol. 2010, 101, 6950–6961. [Google Scholar] [CrossRef]
- Luo, L.; Wang, P.; Lin, L.; Luan, T.; Ke, L.; Tam, N.F. Removal and transformation of high molecular weight polycyclic aromatic hydrocarbons in water by live and dead microalgae. Process Biochem. 2014, 49, 1723–1732. [Google Scholar] [CrossRef]
- Luo, L.; Lai, X.; Chen, B.; Lin, L.; Fang, L.; Tam, N.F.; Luan, T. Chlorophyll catalyse the photo-transformation of carcinogenic benzo [a] pyrene in water. Sci. Rep. 2015, 5, 12776. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Tiwari, K.K. Remediation of Acenaphthene and Fluoranthene by Chlorella vulgaris Beijerinck: FTIR based study. Int. J. Biosci. 2015, 8, 5–9. [Google Scholar]
- Wiśniewska, K.A.; Śliwińska-Wilczewska, S.; Lewandowska, A.U. Airborne microalgal and cyanobacterial diversity and composition during rain events in the southern Baltic Sea region. Sci. Rep. 2022, 12, 2029. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Śliwińska-Wilczewska, S.; Lewandowska, A.; Konik, M. The effect of abiotic factors on abundance and photosynthetic performance of airborne cyanobacteria and microalgae isolated from the Southern Baltic Sea region. Cells 2021, 10, 103. [Google Scholar] [CrossRef]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Staniszewska, M.; Graca, B.; Bełdowska, M.; Saniewska, D. Factors controlling benzo (a) pyrene concentration in aerosols in the urbanized coastal zone. A case study: Gdynia, Poland (Southern Baltic Sea). Environ. Sci. Pollut. Res. 2013, 20, 4154–4163. [Google Scholar] [CrossRef]
- Gaffke, J.; Lewandowska, A.; Bartkowski, K. Polycyclic Aromatic Hydrocarbons (PAHs) in the atmosphere of the Baltic Sea Region. Ecocycles 2015, 1, 51–55. [Google Scholar] [CrossRef]
- Lewandowska, A.U.; Staniszewska, M.; Witkowska, A.; Machuta, M.; Falkowska, L. Benzo (a) pyrene parallel measurements in PM1 and PM2. 5 in the coastal zone of the Gulf of Gdansk (Baltic Sea) in the heating and non-heating seasons. Environ. Sci. Pollut. Res. 2018, 25, 19458–19469. [Google Scholar] [CrossRef]
- Skalska, K.; Lewandowska, A.U.; Staniszewska, M.; Reindl, A.; Witkowska, A.; Falkowska, L. Sources, deposition flux and carcinogenic potential of PM2. 5-bound polycyclic aromatic hydrocarbons in the coastal zone of the Baltic Sea (Gdynia, Poland). Air Qual. Atmos. Health 2019, 12, 1291–1301. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Lewandowska, A.U.; Staniszewska, M. Air quality at two stations (Gdynia and Rumia) located in the region of Gulf of Gdansk during periods of intensive smog in Poland. Air Qual. Atmos. Health 2019, 12, 879–890. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Felpeto, A.B.; Maculewicz, J.; Sobczyk, A.; Vasconcelos, V.; Latała, A. Allelopathic activity of the picocyanobacterium Synechococcus sp. on unicellular eukaryote planktonic microalgae. Mar. Freshw. Res. 2018, 69, 1472–1479. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Latała, A. Allelopathic activity of the bloom-forming picocyanobacterium Synechococcus sp. on the coexisting microalgae: The role of eutrophication. Int. Rev. Hydrobiol. 2018, 103, 37–47. [Google Scholar] [CrossRef]
- Jeffrey, S.T.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Strickland, J.D.; Parsons, T.R. A practical handbook of seawater analysis. Fish. Res. Bd. Can. Bull. 1972, 167, 310. [Google Scholar]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human-and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef]
- Zhu, X.; Kong, H.; Gao, Y.; Wu, M.; Kong, F. Low concentrations of polycyclic aromatic hydrocarbons promote the growth of Microcystis aeruginosa. J. Hazard. Mater. 2012, 237, 371–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, C.; Wang, Z.; Zhang, J.; Li, L.; Huang, S.; Li, D. The species-specific responses of freshwater diatoms to elevated temperatures are affected by interspecific interactions. Microorganisms 2018, 6, 82. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Börner, T.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.X.; Shi, X.L.; Yu, Y.; Zhang, M. Effects of UV-B radiation on microcystin production of a toxic strain of Microcystis aeruginosa and its competitiveness against a non-toxicstrain. J. Hazard. Mater. 2015, 283, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Bopp, S.K.; Lettieri, T. Gene regulation in the marine diatom Thalassiosira pseudonana upon exposure to polycyclic aromatic hydrocarbons (PAHs). Gene 2007, 396, 293–302. [Google Scholar] [CrossRef]
- Othman, H.B.; Pick, F.R.; Hlaili, A.S.; Leboulanger, C. Effects of polycyclic aromatic hydrocarbons on marine and freshwater microalgae–A review. J. Hazard. Mater. 2022, 441, 129869. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.N.; Lettieri, T. Proteomic analysis of the marine diatom Thalassiosira pseudonana upon exposure to benzo(a)pyrene. BMC Genom. 2011, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Spilling, K.; Olli, K.; Lehtoranta, J.; Kremp, A.; Tedesco, L.; Tamelander, T.; Klais, R.; Peltonen, H.; Tamminen, T. Shifting diatom—Dinoflagellate dominance during spring bloom in the Baltic Sea and its potential effects on biogeochemical cycling. Front. Mar. Sci. 2018, 5, 327. [Google Scholar] [CrossRef]
- Vieira, L.R.; Guilhermino, L. Multiple stress effects on marine planktonic organisms: Influence of temperature on the toxicity of polycyclic aromatic hydrocarbons to Tetraselmis chuii. J. Sea Res. 2012, 72, 94–98. [Google Scholar] [CrossRef]
- Takáčová, A.; Smolinská, M.; Ryba, J.; Mackuľak, T.; Jokrllová, J.; Hronec, P.; Čík, G. Biodegradation of Benzo [a] Pyrene through the use of algae. Cent. Eur. J. Chem. 2014, 12, 1133–1143. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, K.A.; Lewandowska, A.U.; Śliwińska-Wilczewska, S.; Staniszewska, M.; Budzałek, G. The Ability of Airborne Microalgae and Cyanobacteria to Survive and Transfer the Carcinogenic Benzo(a)pyrene in Coastal Regions. Cells 2023, 12, 1073. https://doi.org/10.3390/cells12071073
Wiśniewska KA, Lewandowska AU, Śliwińska-Wilczewska S, Staniszewska M, Budzałek G. The Ability of Airborne Microalgae and Cyanobacteria to Survive and Transfer the Carcinogenic Benzo(a)pyrene in Coastal Regions. Cells. 2023; 12(7):1073. https://doi.org/10.3390/cells12071073
Chicago/Turabian StyleWiśniewska, Kinga A., Anita U. Lewandowska, Sylwia Śliwińska-Wilczewska, Marta Staniszewska, and Gracjana Budzałek. 2023. "The Ability of Airborne Microalgae and Cyanobacteria to Survive and Transfer the Carcinogenic Benzo(a)pyrene in Coastal Regions" Cells 12, no. 7: 1073. https://doi.org/10.3390/cells12071073
APA StyleWiśniewska, K. A., Lewandowska, A. U., Śliwińska-Wilczewska, S., Staniszewska, M., & Budzałek, G. (2023). The Ability of Airborne Microalgae and Cyanobacteria to Survive and Transfer the Carcinogenic Benzo(a)pyrene in Coastal Regions. Cells, 12(7), 1073. https://doi.org/10.3390/cells12071073






